Abstract
Mycobacterium avium subsp. paratuberculosis (MAP) and Mycobacterium bovis (BCG) have been associated to several human autoimmune diseases such as multiple sclerosis (MS), but there are conflicting evidence on the issue. The objective of this study is to evaluate their role in Japanese patients affected by inflammatory demyelinating disorders of the central nervous system (IDDs). A total of 97 IDDs subjects including 51 MS and 46 neuromyelitis optica spectrum disorder (NMOSD) patients, and 34 healthy controls (HCs) were tested for the detection of IgG, IgM and IgA against mycobacterial antigens by indirect ELISA. The levels of anti-MAP IgG were higher in MS patients compared to NMOSD patients (AUC = 0.59, p = 0.02) and HCs (AUC = 0.67, p = 0.01), and the anti-MAP antibodies were more prevalent in MS patients treated with interferon-beta (OR = 11.9; p = 0.004). Anti-BCG IgG antibodies were detected in 8% of MS, 32% of NMOSD and 18% of HCs, the difference between MS and NMOSD groups was statistically significant (AUC = 0.66, p = 0.005). Competition experiments showed that nonspecific IgM were elicited by common mycobacterial antigens. Our study provided further evidence for a possible association between MAP and MS, while BCG vaccination seemed to be inversely related to the risk of developing MS.
Introduction
Multiple sclerosis (MS) is the most common inflammatory demyelinating disease (IDDs) of the central nervous system (CNS) and it is mainly caused by T cells reactive against components of myelin1. Neuromyelitis optica spectrum disorder (NMOSD) is characterized by the development of recurrent optic neuritis and/or longitudinally extensive transverse myelitis2. Astrocytopathy and secondary demyelination is mediated by antibodies (Abs) targeting aquaporin 4 (AQP4) protein, but exist also a variant AQP4-negative such as myelin oligodendrocyte glycoprotein (MOG) positive. The origins of the pathogenic autoimmune attack in MS and how the Abs against AQP4 appear in NMOSD are not known, and the pathogenesis of both diseases results from complex interactions between genetic and environmental factors3. Different studies pointed out the possibility that one or more infectious pathogens might trigger autoimmunity4, and the immune response against Mycobacterium avium subsp. paratuberculosis (MAP) and Mycobacterium bovis strain bacille Calmette-Guérin (BCG) has been associated with several human diseases such as MS, however, their role in the pathologic process has been controversial and sometimes opposite5, 6.
In two recent studies conducted on MS and healthy Japanese subjects, a statistically significant percentage of MS patients resulted Ab-positive against MAP_2694295-303 peptide7 and MAP surface antigens8. This finding highlighted the possibility that Japanese could be exposed to MAP antigens, and a small fraction of these people might be genetically susceptible to the development of autoimmune disorders8. On the other side, BCG vaccine seems to have a beneficial effect on MS development. Different clinical trials proved that BCG vaccination may be able to reduce the magnetic resonance imaging activity in patients with relapsing-remitting (RRMS) and clinically isolated syndrome (CIS) in Italy6.
For this reason, we aimed to evaluate for the first time the humoral response to different mycobacteria in Japanese MS patients compared to NMOSD and healthy controls (HCs).
Results
Anti-MAP IgG Ab-titer is increased in MS patients
Based on the determined cut-off point, 9 out of 51 MS patients (18%, 95% CI: 7.5–28.5%), none of NMOSD patients and none of the HCs were positive for anti-MAP IgG Abs (Fig. 1A). The difference between MS and NMOSD patients, and between MS and HCs was statistically significant (AUC = 0.59, p = 0.02; AUC = 0.67, p = 0.01; respectively).
Figure 1.
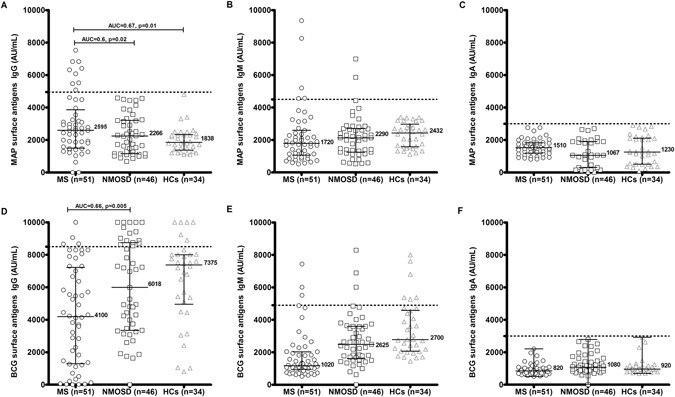
ELISA-based analysis. Fifty-one MS, 46 NMOSD and 34 HCs were screened for Abs reactivity against MAP IgG (A), MAP IgM (B), MAP IGA (C), BCG IgG (D), BCG IgM (E) and BCG IgA (F) by indirect ELISA. The horizontal black bars represent median plus interquartile range, while the dotted lines indicate the cut off for positivity as calculated by ROC analysis. Area under ROC curve (AUC) and P values, significant if <0.05, are indicate by two headed arrows.
If we analyze MS clinical characteristic in relation to the IgG-positivity towards MAP, we find that among 9 MAP IgG positive MS patients: high levels of IgG1 were observed in 7 [6 RRMS and 1 secondary progressive MS (SPMS)] sera (55.5%, 95% CI: 23–88%), and 2 primary progressive MS (PPMS) sera (22.2%, 95% CI: −5–49%) were positive for IgG4. Since IgG4 expression is predominantly under the condition of chronic antigenic stimulation9, we can hypothesize that an isotype switching from IgG1 to IgG4 occurred in patients with longer exposure to MAP antigens.
All MAP positive patients were not in relapsing phase at the sampling time. No substantial levels of IgG2, IgG3, IgM (Fig. 1B) and IgA (Fig. 1C) were detected in all sera.
Anti-BCG IgG and IgM Abs prevalence is lower in MS patients compared to NMOSD and HCs
Despite all participants were BCG-vaccinated, difference in the humoral responses were observed between the three groups. Anti-BCG IgG Abs were found in 4 out of 51 MS (8%, 95% CI: −1.4–9%), in 15 out of 46 NMOSD (32%, 95% CI: 18.5–45.4%), and in 6 out of 34 HCs (18%, 95% CI: 5.1–30.9%). The difference between NMOSD and MS was statistically significant (AUC = 0.66, p = 0.005) (Fig. 1D). MS positive subjects were RRMS (2), PPMS (1) and SPMS (1). Amongst NMOSD Ab-positive patients, 3 were AQP4-positive and 12 were AQP4 negative. As regards anti-BCG IgM and IgA Abs, there was no statistically significant difference when comparing MS, NMOSD and HCs groups (Fig. 1E,F).
Overall, the Ab titer against BCG lipophilic antigens was elevated but in MS groups, the titer of the majority of subjects falls below the cut-off point.
In a followed up study conducted on 449 Japanese people analyzed before and for 1 year after BCG vaccination, it was detected a general low Ab-response to anti- tuberculous glycolipid antigens Abs in the serum of the vaccinated subjects10. Glycolipid antigens are components derived from the cell wall of Mycobacterium tuberculosis used for the serodiagnosis of tuberculosis, which are also present in other mycobacteria species included BCG10.
Similar to our results, this mechanism might reflect anergy after BCG vaccination. It would be related to the environmental influences such as previous mycobacterial infections, as well as genetic factors11.
Assessment of the MAP and BCG IgG-level in relation to clinical and therapeutic variables
The presence of anti-MAP and anti-BCG Abs was not related to age, age at onset, disease duration, EDSS, disease phenotype, IgG index, oligoclonal bands positivity or the presence of anti-AQP4 Abs. Patients who received oral steroids and those who were treated with steroid pulse therapy were excluded from the study. All 4 MS patients positive for BCG IgG did not received disease-modified immunomodulatory agents at least 6 months before sampling.
Regarding MAP Ab-seropositivity, the mean total serum level in the MS patients was 1128 ± 193 mg/dL, whereas in the NMOSD it was 1266 ± 398 mg/dL (Table 1). Chi square showed that MAP positivity was more frequent in patients under interferon-beta therapy at the time of the blood collection (p = 0.005). Logistic regression confirmed a significant association between the presence of anti-MAP IgG Abs and interferon-beta therapy at study time (OR = 11.9; confidence interval (CI) 95% = 2.2–63; p = 0.004) (Table 2).
Table 1.
Baseline characteristics of study population.
| MS | NMOSD | HCs | |
|---|---|---|---|
| Number | 51 | 46 | 34 |
| Female/male ratio | 38/13 (74%/26%) | 28/18 (61%/39%) | 25/9 (73%/27%) |
| Age, years (SD) | 41.1 ± 11.3 | 49.6 ± 15.2 | 40.0 ± 10.1 |
| Age at onset, years (SD) | 32.8 ± 9.8 | 42.3 ± 15.2 | |
| Duration of disease, years (SD) | 8.4 ± 6.8 | 8.5 ± 12.1 | |
| Oligoclonal bands positivity | 31/49 (63%) | 8/33 (24%) | |
| IgG index ≥ 0.7 | 30/44 (68%) | 5/33 (15%) | |
| Total serum IgG (mg/dL) | 1128 ± 193 | 1266 ± 398 | |
| EDSS score at onset | 2.0 (0–7) | 2.9 (0–7.5) |
MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorders; HCS, healthy controls; EDSS, Expanded Disability Status Scale.
Table 2.
Relationship between mycobacterial IgG positivity and interferon-beta (IFN) therapy.
| features | OR | 95%CI | p | |||
|---|---|---|---|---|---|---|
| IFN-beta neg | IFN-beta pos | 34 | ||||
| MS (n = 51) | MAP IgG pos | 4 | 5 | 11.9 | 2.2–63 | 0.004* |
| MAP IgG neg | 38 | 4 | ||||
| IFN-beta neg | IFN-beta pos | |||||
| MS (n = 51) | BCG IgG pos | 4 | 0 | 1.6 | 0.1–17 | 0.6 |
| BCG IgG neg | 39 | 8 | ||||
MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorders; OR, odds ratio; CI, confidence interval; * statistically significant.
When we only considered those MS patients treated with interferon-beta, 5 out of 9 (55%) RRMS patients were MAP IgG positive. These results are in agreement with those of Frau et al., who observed a prevalence of total IgG Abs against MAP_2694 protein in 30.7% of MS patients six months after initiating interferon-beta treatment12.
The mechanism of interferon-beta in MS and its effects in the modulation of innate and adaptive immune response to pathogens are not fully understood. On the one hand this disease modifying drug seems to enhance the humoral response to MAP12. If, however, we consider the mean Ab-titer of the MAP positive MS subjects (n = 9), the interferon-beta-treated patients (n = 5) did not have a statistically significant difference compared to the drug-free ones (n = 4) (6574 ± 747 AU/mL vs 5811 ± 520 AU/mL, p = 0.13), probably because interferon-beta treatment causes a Th1 to Th2 shift or class switching.
Evaluation of cross-reactivity between anti-MAP and anti-BCG antibody-titers
To determine if the Ab-response observed to MAP surface antigens was specific, 2 MS IgG positive and 2 NMOSD IgM positive sera for MAP were pre-adsorbed with saturating concentrations (titrated for each individual serum) of MAP lipophilic antigen, Mycobacterium phlei and BCG lipophilic antigen. Sera were then subjected to ELISA on MAP lipophilic antigen-coated plates.
In the case of MS sera, both Mycobacterium phlei (negative control) and BCG antigens did not cause any decrease in signal, indicating the absence of cross recognition IgG Abs between MAP and BCG (Fig. 2). Besides, regression analysis indicated no correlation between MAP and BCG antigens in MS (rs = 0.04), NMOSD (rs = 0.17) and HCs group (rs = 0.01) (Fig. 3A–C).
Figure 2.
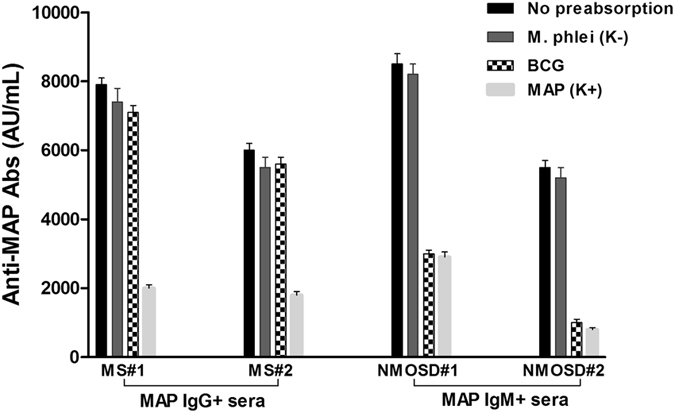
Competition assay. Bars depict means ± standard deviation of triplicate wells and results are representative of two separate experiments.
Figure 3.
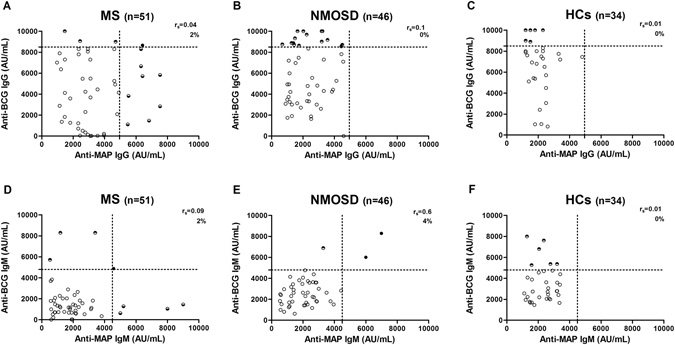
Correlation analysis. Correlation between Abs recognizing anti-MAP and anti-BCG specific IgG in MS (A), NMOSD (B) and HCs (C); and Abs recognizing anti-MAP and anti-BCG specific IgM in MS (D), NMOSD (E) and HCs (F). Each filled black circle represents the titer of a patient with double positivity for MAP and BCG Abs, while each half-filled circle represents the titer of a patients with specie-specific Abs. Empty circles are Ab-negative patients. The dotted line lines designate the cutoff for positivity used in each assay, as calculated by ROC analysis.
Concerning NMOSD subjects, competition experiments showed that nonspecific IgM were elicited by common mycobacterial glycolipid antigens, probably because of the low affinity and cross reactivity of IgM isotype (Fig. 2)13. In fact, when pre-adsorbing the sera with BCG antigen, the latter was capable of blocking the binding of the Abs to the targeted MAP antigen-coated, strongly inhibiting the MAP sero-reactivity in the sera (decline in signal between 70–90%) (Fig. 2). Additionally, IgM binding profile of MAP correlate significantly (rs = 0.6) with the profile of BCG lipophilic antigens (Fig. 3E).
Discussion
T and B cell responses are influenced by the bacterial site in which the antigen is expressed14, and bacterial proteins can be expressed in the cytoplasm, bound to the surface of the bacteria or secreted. The majority of the MS-related MAP antigens identified share sequence homology with intracellular human proteins15–17. It was hypothesized that the phenomenon of the epitope spreading may contribute to the production of Abs against previously sequestered antigens, leading to a secondary autoimmune response against the newly released intracellular antigens15.
In this study we analyzed the humoral response mounted against surface-exposed antigens, which are the primary target of the host immune system18.
We found a significantly higher Ab response against MAP IgG Abs in MS patients compared to the other groups. The main IgG subclass elicited by MAP was IgG1, and two MS patients diagnosed with PPMS were positive for IgG4 Abs. These findings are congruent with the previous results obtained in a MAP-based seroprevalence study conducted in Japanese subjects, in which elevated IgG1 response against MAP lipophilic antigens was detected in the sera of these subjects8. However, at present we are unable to establish if MAP is a potential risk factor of conversion from CIS to clinically definite MS, an accelerator of the pathogenesis, or maybe it plays only a peripheral role.
We also evaluated the Ab-prevalence to BCG, and the differences in the Ab-profiles to BCG antigens between the groups were remarkable. BCG antigen was capable to ignite a strong humoral response in NMOSD subjects, while in MS group we observed a low Ab-titer against BCG antigen, indicating that this factor was inversely related to the relative risk of developing MS.
The difference in the anti-BCG and anti- MAP Ab-level between MS and NMOSD groups, may be explained in different ways. First, the different route of transmission/administration of foreign antigens, oral for MAP and intradermal for BCG, might have significant effects in influencing the immune response19. Second, it may be that some individuals have, due to a different genetic predisposition, an increased/decreased reactivity to the mycobacterial cell wall-derived antigens. Indeed HLA restriction analysis revealed that depending on the individual’s HLA haplotype, the specific adaptive immune responses to distinct mycobacterial antigens that mimic self-protein could be restricted to HLA products encoded by HLA-DRB1, HLA-DRB3 or HLA-DRB420. Further, it is well known the existence of different genetic and infectious profiles between MS and NMOSD patients in Japan21, 22. HLA-DRB1*0405 and HLA-DPB1*0301 were found to be MS susceptibility genes21, whereas HLA-DRB1*1602 and HLA-DPB1*0501 conferred susceptibility only to anti-AQP4 antibody-positive NMO22. Third, even if there is a considerable overlap in lipid content between MAP and BCG, the low-cross reactivity observed in the competition experiments between anti-MAP and anti-BCG IgG Abs suggests the existence of an antigen-specific humoral immune response. MAP lipopentapetide (L5P) is a specific cell wall component of MAP capable to induce a strong host humoral response, and able to distinguish MAP from other mycobacteria including BCG23. A highly specific IgG responses to L5P were detected in patients affected by Crohn’s disease and in children at risk for type 1 diabetes24, 25.
Lastly, Ab production is also influenced by interaction between T and B cells and by alteration of cytokine milieu. In NMO there is an enhancement of humoral (Th2) immune response with elevated Il-4 and Il-10 levels compared with levels in MS subjects26. Therefore, it is reasonable that the Ab titer against BCG was higher in NMOSD patients. Instead, the higher MAP Ab response in MS compared to NMOSD might be due to CD1 NKT cell recognition. Evidence suggests that clonal population of CD1a-, CD1b- and CD1c restricted T cells specific for mycobacterial lipid antigens can respond differently to MAP and BCG27. To note, lipopeptides such as L5P are recognized by CD1a T cells28, and polymorphisms of CD1a and CD1e genes have been associated with susceptibility to MS29.
In conclusion, the mechanism by which mycobacteria and in particular BCG exert their effect in patients is unknown, and further research is warranted to explore the relationship between Ab prevalence and genotype in MS and NMOSD groups. For example, it will be useful whether serum BCG versus MAP IgG reactivity are able to differentiate MS from NMOSD in the initial attack.
Methods
Ethical considerations
The study protocol was approved by the National Ethical Committee of the Juntendo University School of Medicine (Approved No. 205), Sangenjyaya hospital (Approved No. 2014.3.7) and Tohto College of Health Sciences (Approved No. H2511). All the methods were carried out in “accordance” with the approved guidelines. All subjects provided written informed consent for participation.
Subjects
Baseline characteristics and clinical demographic data of the study population are shown in Table 1. All participating subjects were Japanese and BCG vaccinated. At the time of the sampling, all the subjects were HIV negative and with no history of past tuberculosis (TB) infection.
Fifty-one MS patients fulfilling the revised McDonald diagnostic criteria were enrolled in this study30. The clinical characteristic of the MS cases were the following: 46 RRMS, 3 SPMS, 2 PPMS and all AQP4 negative.
Forty-six NMOSD were also included. NMOSD group includes patients with NMO-like symptoms that fulfill the 2015 diagnostic criteria for NMOSD2. Thirty-four sex and age-matched healthy volunteers without history of autoimmune diseases were also included as control group.
Aquaporin 4 cell based assay
AQP4 live transfected human embryonic kidney cells (HEK293) expressing the untagged AQP4-M23 isoform were incubated in 4x diluted serum for 1 h, washed and incubated in fluorescein-conjugated goat anti-human IgG (MP Biomedicals, Aurora, OH, USA) for 30 min. Cells were fixed in 95% ethanol and mounted in the prolong antifade mounted media Permafluor (Beckman Coulter, Fullerton, Ca, USA). Image were captured by confocal microscopy Fluoview (Olympus Tokyo). Ab-seropositivity was based on comparison with mock-transfected cells that did not express AQP4.
Mycobacterial antigens
Preparation of MAP (strain ATCC 19698) and BCG (strain Tokyo-172) lipophilic antigen was performed as described in detail elsewhere8. MAP and BCG surface antigens are a crude extract of the mycobacteria and the lipid fractions were used as an antigen after removing cross-reactive component by absorbing the serum with Mycobacterium phlei 8. The lyophilized bacteria was obtained from the commercially available ELISA kit, Johnelisa II kit (Kyoritsu Seiyaku Corporation, Tokyo, Japan).
ELISA
Optimal conditions for the indirect ELISAs were established by titration experiment following our previously published methodology8. BSA-coated wells were included as a negative control, and the mean value obtained was subtracted from all other data points. A serum sample from a patient with high IgG anti-MAP was included in all experiments and used as the internal laboratory positive control. All test serum samples were therefore normalized against this sample, the reactivity of which was set at 10,000 arbitrary units (AU)/mL. Experimental samples were analyzed in triplicate.
Inhibition assay
In order to assess potential cross-reactivity between mycobacterial Abs, a competitive ELISA was performed. Briefly, diluted MAP Ab-positive serum samples were pre-absorbed with saturating concentrations [10–15 mM] of BCG lipophilic antigen for 2 h at room temperature, and then sample where transferred in plates coated with MAP lipophilic antigens and subjected to indirect ELISA as previously described8, 15. MAP lipophilic antigen was used as a positive control for a true Ab interaction, and Mycobacterium phlei antigen was applied as negative control for nonspecific reactions.
Statistical analysis
Statistical analysis was performed using Graphpad Prism 6.0 software (San Diego, CA, USA). Receiver operating characteristic (ROC) curves were used to evaluate the diagnostic accuracy of the ELISA to detect specific Abs against the mycobacterial antigens. Comparison of the ELISA results between patient and HCs was statistically analyzed using Mann-Whitney’s nonparametric, unpaired, two-tailed test, Chi square and Fisher’s exact probability test. Spearman correlation analysis was performed in order to test cross-reactivity between mycobacterial Abs. Logistic regression-derived odds ratio (OR) was used to evaluate Ab-positivity with respect to clinical independent variables.
Acknowledgements
Davide Cossu is an “overseas researcher under Postdoctoral Fellowship of Japan Society for the Promotion of Sciences”.
Author Contributions
K.Y. and N.H. conceived and designed the experiments; D.C. and E.M. conducted the experiments; N.H., K.Y. and Y.T contributed reagents/materials/analysis tools; D.C. and K.Y. interpreted the results and wrote the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hohlfeld R, Dornmair K, Meinl E, Wekerle H. The search for the target antigens of multiple sclerosis, part 2: CD8+ T cells, B cells, and antibodies in the focus of reverse-translational research. Lancet Neurol. 2016;15:317–331. doi: 10.1016/S1474-4422(15)00313-0. [DOI] [PubMed] [Google Scholar]
- 2.Wingerchuk DM, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morandi E, Tarlinton RE, Gran B. Multiple Sclerosis between Genetics and Infections: Human Endogenous Retroviruses in Monocytes and Macrophages. Front Immunol. 2015;6:647. doi: 10.3389/fimmu.2015.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mameli G, Cocco E, Frau J, Marrosu MG, Sechi LA. Epstein Barr Virus and Mycobacterium avium subsp. paratuberculosis peptides are recognized in sera and cerebrospinal fluid of MS patients. Sci Rep. 2016;6:22401. doi: 10.1038/srep22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cossu D, et al. Evaluation of the humoral response against mycobacterial peptides, homologous to MOG(3)(5)(-)(5)(5), in multiple sclerosis patients. J Neurol Sci. 2014;347:78–81. doi: 10.1016/j.jns.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Ristori G, et al. Effects of Bacille Calmette-Guerin after the first demyelinating event in the CNS. Neurology. 2014;82:41–48. doi: 10.1212/01.wnl.0000438216.93319.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cossu D, et al. Humoral response against host-mimetic homologous epitopes of Mycobacterium avium subsp. paratuberculosis in Japanese multiple sclerosis patients. Sci Rep. 2016;6:29227. doi: 10.1038/srep29227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otsubo S, et al. Seroprevalence of IgG1 and IgG4 class antibodies against Mycobacterium avium subsp. paratuberculosis in Japanese population. Foodborne Pathog Dis. 2015;12:851–856. doi: 10.1089/fpd.2015.1956. [DOI] [PubMed] [Google Scholar]
- 9.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–551. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 10.Nabeshima S, et al. Serum antibody response to tuberculosis-associated glycolipid antigen after BCG vaccination in adults. J Infect Chemother. 2005;11:256–258. doi: 10.1007/s10156-005-0398-7. [DOI] [PubMed] [Google Scholar]
- 11.Black GF, et al. Patterns and implications of naturally acquired immune responses to environmental and tuberculous mycobacterial antigens in northern Malawi. J Infect Dis. 2001;184:322–329. doi: 10.1086/322042. [DOI] [PubMed] [Google Scholar]
- 12.Frau J, et al. Role of interferon-beta in Mycobacterium avium subspecies paratuberculosis antibody response in Sardinian MS patients. J Neurol Sci. 2015;349:249–250. doi: 10.1016/j.jns.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Vollmers HP, Brandlein S. Natural IgM antibodies: from parias to parvenus. Histol Histopathol. 2006;21:1355–1366. doi: 10.14670/HH-21.1355. [DOI] [PubMed] [Google Scholar]
- 14.Sali M, et al. Surface expression of MPT64 as a fusion with the PE domain of PE_PGRS33 enhances Mycobacterium bovis BCG protective activity against Mycobacterium tuberculosis in mice. Infect Immun. 2010;78:5202–5213. doi: 10.1128/IAI.00267-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cossu D, et al. Human interferon regulatory factor 5 homologous epitopes of Epstein-Barr virus and Mycobacterium avium subsp. paratuberculosis induce a specific humoral and cellular immune response in multiple sclerosis patients. Mult Scler. 2015;21:984–995. doi: 10.1177/1352458514557304. [DOI] [PubMed] [Google Scholar]
- 16.Cossu D, et al. Are Mycobacterium avium subsp. paratuberculosis and Epstein-Barr virus triggers of multiple sclerosis in Sardinia? Mult Scler. 2012;18:1181–1184. doi: 10.1177/1352458511433430. [DOI] [PubMed] [Google Scholar]
- 17.Cossu D, et al. Anti Mycobacterium avium subsp. paratuberculosis heat shock protein 70 antibodies in the sera of Sardinian patients with multiple sclerosis. J Neurol Sci. 2013;335:131–133. doi: 10.1016/j.jns.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 18.McNamara M, Tzeng SC, Maier C, Zhang L, Bermudez LE. Surface proteome of “Mycobacterium avium subsp. hominissuis” during the early stages of macrophage infection. Infect Immun. 2012;80:1868–1880. doi: 10.1128/IAI.06151-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belyakov IM, Ahlers JD. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J Immunol. 2009;183:6883–6892. doi: 10.4049/jimmunol.0901466. [DOI] [PubMed] [Google Scholar]
- 20.Mustafa AS. HLA-restricted immune response to mycobacterial antigens: relevance to vaccine design. Hum Immunol. 2000;61:166–171. doi: 10.1016/S0198-8859(99)00137-8. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimura S, et al. Genetic and infectious profiles influence cerebrospinal fluid IgG abnormality in Japanese multiple sclerosis patients. PLoS One. 2014;9:e95367. doi: 10.1371/journal.pone.0095367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshimura S, et al. Distinct genetic and infectious profiles in Japanese neuromyelitis optica patients according to anti-aquaporin 4 antibody status. J Neurol Neurosurg Psychiatry. 2013;84:29–34. doi: 10.1136/jnnp-2012-302925. [DOI] [PubMed] [Google Scholar]
- 23.Biet F, et al. Lipopentapeptide induces a strong host humoral response and distinguishes Mycobacterium avium subsp. paratuberculosis from M. avium subsp. avium. Vaccine. 2008;26:257–268. doi: 10.1016/j.vaccine.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 24.Verdier J, et al. Specific IgG response against Mycobacterium avium paratuberculosis in children and adults with Crohn’s disease. PLoS One. 2013;8:e62780. doi: 10.1371/journal.pone.0062780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niegowska M, et al. Seroreactivity against Specific L5P Antigen from Mycobacterium avium subsp. paratuberculosis in Children at Risk for T1D. PLoS One. 2016;11:e0157962. doi: 10.1371/journal.pone.0157962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang KC, et al. Distinct serum cytokine profiles in neuromyelitis optica and multiple sclerosis. J Interferon Cytokine Res. 2013;33:58–64. doi: 10.1089/jir.2012.0040. [DOI] [PubMed] [Google Scholar]
- 27.Van Rhijn I, et al. Low cross-reactivity of T-cell responses against lipids from Mycobacterium bovis and M. avium paratuberculosis during natural infection. Eur J Immunol. 2009;39:3031–3041. doi: 10.1002/eji.200939619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moody DB, et al. T cell activation by lipopeptide antigens. Science. 2004;303:527–531. doi: 10.1126/science.1089353. [DOI] [PubMed] [Google Scholar]
- 29.Caporale CM, et al. CD1A and CD1E gene polymorphisms are associated with susceptibility to multiple sclerosis. Int J Immunopathol Pharmacol. 2011;24:175–183. doi: 10.1177/039463201102400120. [DOI] [PubMed] [Google Scholar]
- 30.Milo R, Miller A. Revised diagnostic criteria of multiple sclerosis. Autoimmun Rev. 2014;13:518–524. doi: 10.1016/j.autrev.2014.01.012. [DOI] [PubMed] [Google Scholar]


