Abstract
Sand flies inject saliva while feeding in the vertebrate host and anti-saliva antibodies can be used as biomarkers of exposure to Leishmania vectors. We expressed recombinant salivary proteins from Lutzomyia intermedia, a vector of Leishmania braziliensis, and evaluated the seroreactivity in exposed individuals in search for exposure markers. We found a strong correlation among positive serology to recombinant proteins LinB-13, 26, 15, 21 and to salivary proteins: rLinB-13 was the top performing molecule; IgG4 was the most predominant antibody subclass and antibodies to rLinB-13 did not cross react with Lu. longipalpis salivary proteins. By evaluating a cohort of contacts of CL patients, we confirmed that rLinB-13, an antigen 5-related protein, is a marker of exposure to Lu. intermedia with high degree of accuracy. In a 5-year follow up, we determined that individuals who developed CL presented higher anti-rLinB13 IgG responses, before the appearance of clinical symptoms. They also presented a lower frequency of cellular responses to the parasite (DTH). Our results show that seroconversion to a salivary molecule, rLinB-13, is a marker of risk for CL development caused by Leishmania braziliensis. This highlight the possibility of developing tools based on vector molecules to manage the disease in endemic areas.
Introduction
In the Americas, cutaneous leishmaniasis (CL) is caused mainly by Leishmania braziliensis, a parasitic protozoan transmitted by Lutzomyia intermedia sand flies. During Leishmania sp. transmission, the sand fly inoculates the vertebrate host with both Leishmania parasites and with salivary molecules. These molecules aid in blood feeding and some of these salivary proteins are immunogenic (rev. in ref. 1).
The humoral and cellular immune response to sand fly salivary proteins in animals including humans has been well documented (rev. in refs 2, 3) and the idea or concept of using sand fly salivary proteins as markers of vector exposure has been reported and used successfully for Lutzomyia longipalpis 4–6 and Phlebotomus papatasi 7–10. Therefore, the antibody response to salivary antigens can be used to probe for exposure to other sand flies and, hence, to assess the possibility of developing leishmaniasis associated to that sand fly species. However, the use of salivary gland sonicate (SGS) in large-scale epidemiologic assays is challenging due the difficulty inherent to sand fly capture or rearing, both necessary for salivary gland antigen preparation. Furthermore, SGS is a combination of various antigens, limiting therefore the purity or specificity of the antigen. To overcome this obstacle, transcriptomics and proteomics approaches have been effectively used in identifying and characterizing a number of sand fly salivary proteins (rev. in ref. 11). Sand fly salivary transcripomic approaches have been followed by the production of recombinant sand fly salivary proteins in heterologous systems and the use of these recombinant proteins for serologic testing. Sand fly recombinant salivary proteins LJM17 and LJM11 have been validated in the field as markers of exposure to Lu. longipalpis in Brazil6, 12 and the recombinant salivary protein PpSP32 has been successfully used as a marker for Ph. papatasi exposure in Tunisia and Saudi Arabia13, 14. These works underline the feasibility of using sand fly salivary recombinant proteins as epidemiological tools for vector exposure.
In addition to using anti-saliva antibodies to probe for sand fly exposure in animal reservoirs and humans, the anti-saliva immune response has also been associated with the risk of developing leishmaniasis upon infection with an infected sand fly9, 15. In Brazil, we observed that patients with CL caused by Leishmania braziliensis presented a significantly higher IgG response to Lu. intermedia SGS compared to individuals with subclinical L. braziliensis infection8 and natural exposure to Lu. intermedia bites indeed increases the risk of developing CL caused by L. braziliensis 16.
Based on the availability of the Lu. intermedia salivary gland transcriptome17, we expressed the most abundant salivary proteins from this sand fly species and searched for antigens that could act as exposure markers to Lu. intermedia in an area where CL is caused by L. braziliensis. Besides validating the top-performing antigen in a large cohort of household contacts, we also performed a 5-year follow up study of exposed individuals. We probed for serology to rLinB-13 and CL development and we show that individuals seropositive to a defined Lu. intermedia salivary recombinant protein are at increased risk of developing disease.
Results
Screening Lu. intermedia salivary proteins for markers of vector exposure
Eleven Lu. intermedia transcripts coding for secreted salivary proteins (LinB-8; LinB-13; LinB-14; LinB-15; LinB-17; LinB-21; LinB-26; LinB-28; LinB-37; LinB-38; LinB-44) were cloned and expressed as recombinant proteins in HEK cells (Table 1). Lu. intermedia recombinant proteins were tested with human sera (n = 89) from residents of a CL endemic area to determine IgG levels specific to these recombinant proteins (Fig. 1). We found a positive IgG response against all the recombinant proteins tested, however, only for LinB-13, LinB-21, LinB-26 and LinB-38 IgG levels were not statistically different to those detected against Lu. intermedia SGS (Fig. 1A). Using a correlation matrix to evaluate the response to Lu. intermedia SGS and the recombinant salivary proteins, we showed the presence of three major clusters: one cluster contained LinB-38, LinB-44, LinB-28 and LinB-37 and a second cluster contained LinB-14 and LinB-17, indicating that the IgG response to these proteins did not correlate to that observed for Lu. intermedia SGS (Fig. 1B). SGS was present in the third cluster together with LinB-8, LinB-13, LinB-15, LinB-21, LinB-26 salivary proteins confirming that the response to these salivary proteins is similar to that observed for Lu. intermedia SGS (Fig. 1A). Among these proteins, the response to rLinB-13 displayed the strongest correlation to SGS (P < 0.0001, r = 0.78) (Supplemental Table 1).
Table 1.
Panel of Lu. intermedia recombinant salivary proteins.
| Name | Accession Number | Weight (kDa) | Comment |
|---|---|---|---|
| LinB-8 | KA660054 | 14.06 | SP15 family member |
| LinB-13 | KA660053 | 28.4 | Antigen 5-related |
| LinB-14 | KA660066 | 17.65 | C-type lectin |
| LinB-15 | KA660065 | 16.38 | C-type lectin |
| LinB-17 | KA660055 | 33.5 | Similar to Factor Xa inhibitor |
| LinB-21 | KA660057 | 44 | Yellow related-protein |
| LinB-26 | KA660060 | 22.9 | 30-kDa Phlebotomine |
| LinB-28 | KA660061 | 13.8 | SP15 family member |
| LinB-37 | KA660070 | 15.41 | ML domain salivary peptide |
| LinB-38 | KA660071 | 12.34 | 10-kDa family member |
| LinB-44 | KA660075 | 10.53 | 10-kDa family member |
Figure 1.

Screening Lu. intermedia salivary proteins for markers of vector exposure. (A) Total IgG response against Lu. intermedia SGS and recombinant salivary proteins was measured by ELISA in residents of a CL-endemic area (n = 89). Data are shown individually and black lines represent the median OD value for each antigen. All statistical analysis were performed in comparison to Lu. intermedia SGS. (B) Correlation matrix of IgG antibodies against Lu. intermedia SGS and recombinant salivary proteins in the same individuals represented in (A). Circles represent individual OD values, as shown in (A) Blue circles depict a positive Spearman correlation. Circle diameter and intensity of blue color depict higher Spearman r values. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
We then evaluated whether these five recombinant proteins (LinB-8, LinB-21, LinB-15, LinB-26 and LinB-13) could predict seroconversion to Lu. intermedia SGS using a panel of seropositive (n = 63) and seronegative (n = 26) individuals. ROC analysis showed that the IgG response to LinB-13, LinB-26 and LinB-15 correctly discriminated individuals exposed to Lu. intermedia whereas LinB-21 and LinB-8 failed to do so (Fig. 2A,B). However, LinB-13 had a superior performance (AUC = 0.89, P < 0.0001) when compared to LinB-21 and LinB-8, displaying the highest sensitivity [87.3% (76.5–94.3)] and specificity [73% (52.2–88.4)], considering a cut off value of 0.033 (Fig. 2B). These results show that, among the 11 recombinant proteins tested, LinB-13, was the top-performing molecule in predicting exposure to Lu. intermedia sand flies.
Figure 2.

ROC antibody threshold level predicting ELISA positivity against Lu. intermedia SGS. (A) ROC curves were built using serology data from individuals seropositive (n = 63) or seronegative (n = 26) against Lu. intermedia salivary proteins. (B) Detailed information obtained from each ROC curve is shown: Area Under Curve (AUC), P values of the ROC curves, the cut-off values chosen, and sensitivity and specificity with the 95% confidence interval (CI).
LinB-13 is a 28 kDa salivary protein, member of the family of antigen 5-related proteins, with ubiquitous distribution in sand flies [rev. in ref. 11]. Although its function is still unknown, it belongs to the cysteine rich secretory proteins, present in the saliva of most blood-sucking insects and in hookworms18. Multiple sequence alignment revealed that LinB-13 and homologues present in Lu. longipalpis, Lu. ayacuchensis, Ph. duboscqi, Ph. papatasi, Ph. argentipes, Ph. perniciosus, Ph. orientalis, Ph. ariasi and Ph. sergenti have a high degree of conservation at the amino acid level (Supplemental Fig. 1A). Phylogenetic analysis confirmed the clustering of LinB-13 and its homologues in Lu. longipalpis and Lu. ayacuchensis sand flies confirming the similarity between these molecules (Supplemental Fig. 1B).
Validation of rLinB-13 as an exposure marker in a L. braziliensis transmission area
Next, we validated rLinB-13 as marker of exposure to Lu. intermedia by, evaluating the IgG response against this protein and against Lu. intermedia SGS. Among the household contacts (n = 264) of CL patients tested 136 (51.5%) had positive serology to rLinB-13 and 150 individuals (56.8%) had positive serology to SGS, indicating a similar degree of reactivity (Fig. 3A). Dot Blot analysis confirmed that rLinB-13 and Lu. intermedia SGS were equally recognized by individuals seropositive for both SGS and rLinB-13 but not by seronegative subjects (Fig. 3B). We also did not observe significant differences among seropositive or seronegative individuals to rLinB-13 regarding epidemiological characteristics such as age, gender, occupation, etc, (Supplemental Table 2). One exception was that subjects seropositive to rLinB-13 arrived more frequently at home after 4 pm. Furthermore, we found a strong correlation between IgG to rLinB-13 and to Lu. intermedia SGS (P < 0.0001, r = 0.74) (Fig. 3C) and ROC analysis confirmed, in this larger cohort, that antibodies against rLinB-13 discriminate a positive response to SGS (AUC = 0.86, P < 0.0001) (Fig. 3D). Therefore, rLinB-13 can predict exposure to Lu. intermedia with 90% sensitivity and 74.5% specificity (cut-off value of 0.018) (Fig. 3D). With regards to IgG subclasses, IgG4 was the most predominant isotype followed by IgG3 (Fig. 4A) and a significant correlation was found between anti-LinB-13 total IgG and anti-LinB-13 IgG4 (P < 0.001, r = 0.47) (Fig. 4B).
Figure 3.

Validation of LinB-13 as a vector exposure maker in an area of L. braziliensis transmission. (A) Total IgG response against Lu. intermedia SGS and LinB-13 was measured by ELISA in household contacts (n = 264) of CL patients. Black lines represent the median OD value for each antigen. (B) Dot blot analysis against Lu. intermedia SGS and LinB-13 using serum samples from subjects with positive serology to SGS and to LinB-13 (n = 5) and from controls with negative serology (n = 4). (C) Spearman correlation between IgG to Lu. intermedia SGS and LinB-13 in the same individuals represented in (A). (D) ROC antibody threshold levels predicting positivity to LinB-13 and accompanying detailed information. Data are shown individually.
Figure 4.
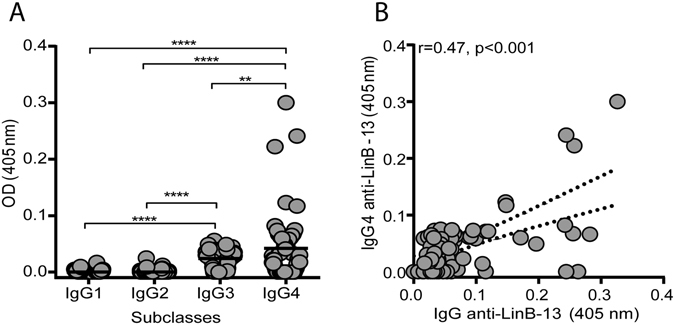
Anti-LinB-13 IgG subclasses detected in Lu. intermedia-exposed individuals. (A) IgG subclasses against LinB-13 were measured by ELISA using sera from residents of a CL-endemic area (n = 89). Black lines represent the median OD value for each antigen. (B) Spearman correlation between anti-LinB-13 IgG and IgG4 in the same individuals represented in (A). Data are shown individually **P < 0.01; ****P < 0.0001.
To evaluate cross-reactivity between anti-LinB-13 antibodies and IgG to salivary antigens from a related sand fly, we probed sera from individuals (n = 20) exposed to Lu. longipalpis against rLinB-13 and against Lu. intermedia SGS. Seventeen individuals (85%) presented positive serology to Lu. longipalpis SGS and one individual was seropositive to Lu. intermedia SGS but none of the sera reacted against rLinB-13 (Fig. 5A). On the contrary, 20 (100%) control sera (obtained from a CL endemic area) reacted against both Lu. intermedia SGS and rLinB-13 whereas two sera (10%) reacted against Lu. longipalpis SGS (Fig. 5A). Hierarchical clustering confirmed that the seroreactivity pattern of individuals from a CL endemic area is similar for both Lu. intermedia SGS and rLinB-13 whereas it is clearly distinct from that observed for individuals from a VL endemic area (Fig. 5B). These results highlight the potential of rLinB-13 to act as a marker of vector exposure in an area of L. braziliensis transmission.
Figure 5.

Cross-reactivity between serological responses to LinB-13 and to Lu. longipalpis saliva. Serum samples (n = 20) from residents of a CL endemic or of a VL endemic area were tested by ELISA against Lu. intermedia SGS, Lu. longilpalpis SGS and LinB-13. (A) Number of subjects with positive serology to each antigen, comparing the CL and the VL endemic areas. (B) Heat map depicting the pattern of Lu. intermedia SGS, Lu. longilpalpis SGS and LinB-13 recognition using Z-score normalized serology.
Antibodies to rLinB-13 as a risk marker of CL development
We previously showed that individuals with CL caused by L. braziliensis have a higher IgG response to Lu. intermedia SGS8 and this increases the risk of developing CL16. However, specific salivary molecule(s) within SGS that may play a role in this outcome remained unknown and we hypothesized that LinB-13 was a likely candidate based on its performance in predicting exposure to Lu. intermedia. We performed a 5-year follow up study in which 264 household contacts of CL patients were evaluated for presence of IgG to LinB-13. These evaluation were performed at the beginning of the study (Baseline) and at the 2nd and 4th years of follow-up. In parallel, individuals were evaluated yearly for the appearance of clinical signs of CL. At the time of enrollment in the study, we observed that 136/264 (51.5%) individuals developed anti-LinB13 antibodies whereas 128/264 (48.5%) did not (Fig. 6A). During the 5-year follow up, 38/264 (14.3%) subjects developed CL and, among these, 28/38 (73.7%) were seropositive to rLinB-13 whereas 10/38 (26.3%) were seronegative to rLinB-13 (Fig. 6A). In fact, the subjects who developed CL displayed a higher (p < 0.0001) anti-LinB-13 IgG response compared to those who remained free of disease. Also, the elevated anti-LinB-13 IgG response was detected before the development signs of the disease (Fig. 6B). Thus, individuals seropositive to LinB-13 are 2.6 times more likely to develop CL caused by L. braziliensis (RR = 2.6; 95% confidence interval = 1.3–5.2; P < 0.01) compared to seronegative subjects. Of note, seropositivity against LinB-13 did not change over time: individuals who presented IgG to LinB-13 at the time of enrollment in the study (Baseline) continued to display such response during the follow up period (Supplemental Fig. 2).
Figure 6.

LinB-13 acts as risk factor for development of Cutaneous Leishmaniasis. (A) Total IgG response against LinB-13 was measured by ELISA in residents of a CL-endemic area who developed CL (n = 38) or who did not developed CL (n = 226). Data are shown individually. Black lines represent the median OD value for each antigen and dotted line represents the cut off value. (B) Flow chart showing the number of household contacts of CL patients who developed disease during the 5-year follow-up period, in both LinB-13 seropositive and seronegative individuals. ****P < 0.0001.
Presence of a delayed type hypersensitivity (DTH) reaction to Leishmania antigen is associated with protection against infection19. Among those individuals who seroconverted to rLinB-13, we detected a lower frequency (p < 0.05) of DTH + subjects (Fig. 7A). A positive DTH is a marker of subclinical infection in areas of L. braziliensis transmission20 but we observed that DTH + individuals displayed lower levels (p < 0.01) of anti-rLinB-13 IgG (Fig. 7B). Therefore, we show that development of antibodies to rLinB-13 is not concurrent with development of a cellular response to Leishmania.
Figure 7.

Lower response to Leishmania antigen in seropositive subjects to LinB-13. (A) Frequency of DTH reaction in subjects with positive or negative serology to LinB-13. (B) Total IgG response against LinB-13 was measured by ELISA in residents of a CL-endemic area who had positive (n = 43) or negative (n = 221) DTH to Leishmania. Data are shown individually. Black lines represent the median OD values for each group of individuals. *P < 0.05; **P < 0.01.
Discussion
Antibody responses to antigens present in arthropod saliva have been explored as vector exposure monitoring tool21–23, including vectors of leishmaniasis4, 5, 7, 24. This task has been enormously facilitated by the characterization and production of recombinant salivary proteins. In the present work we searched for immunodominant antigens in Lu. intermedia saliva using a panel of sand fly recombinant salivary proteins. We further validated the top-performing molecule in household contacts of CL patients and determined whether exposure to rLinB-13 could be used as a risk assessment tool.
Following the initial screening to identify the immunodominant molecules within Lu. intermedia salivary proteins, we found a strong correlation among anti-LinB-13 and anti-Lu. intermedia SGS antibodies, a result corroborated by ROC curve analysis. Similarly, antibody responses to LJM11 and LJM1712 also correlated with reactivity to Lu. longipalpis SGS, albeit with an inferior performance compared to rLinB-13. Besides LJM11 and LJM17 present in Lu. longipalpis, PpSP32 present in Ph. papatasi SP3213–15, SP03B present in Ph. perniciosus 25, 26 and rPorSP24 present in Ph. orientalis (rPorSP24)27 were identified as immunodominant salivary antigens and validated as markers of vector exposure in different endemic areas. In this work we identified rLinB-13 as a marker of Lu. intermedia exposure, a salivary antigen different in sequence and size to any other marker of exposure described so far. rLinB-13 was validated as a marker of sand fly exposure in a large cohort of individuals residing in a L. braziliensis transmission area.
As seen with Lu. intermedia SGS16 and in a study conducted with Ph. papatasi-exposed individuals in Egypt and Jordan, authors also found IgG4 to be the dominant subclass28. In the later, there was a positive correlation between concentrations of IgG4 and IgE, in agreement with reports showing concurrent IgE and IgG4 responses following exposure to Aedes sp.29, 30. Chronic exposure to Aedes sp. bites induces higher IgG4 antibodies against salivary proteins compared to other IgG subclasses30 and it has been suggested that IgG4 is a marker of intense exposure to arthropod vectors31. B-cell class switching to IgG4 and IgE is induced by IL-1332 but IL-10, in the presence of IL-4, can also drive IgG4 class switch in the absence of IgE33. Herein we have not addressed the presence of anti-LinB13 IgE but both IL-10 and IL-13 were detected upon stimulation of peripheral blood cells from Lu. intermedia exposed individuals with SGS16, suggesting a mechanistic explanation for the presence of IgG4 antibodies in Lu. intermedia-exposed individuals.
Besides the possibility of using sand fly salivary proteins to measure vector exposure, these molecules have also been employed to access the risk of disease development. In Tunisia, individuals who developed CL caused by L. major displayed significantly higher antibodies levels against Ph. papatasi saliva compared to subjects that remained disease-free9. In Turkey, patients with L. tropica lesions also showed higher levels of IgG against Ph. sergenti saliva compared to control individuals7. We corroborated these findings in Brazil: patients with CL caused by L. braziliensis presented a higher IgG response to Lu. intermedia SGS compared to individuals with subclinical L. braziliensis infection8, indicated by a positive DTH to Leishmania, in the absence of clinical signs. Indeed, higher serology to Lu. intermedia SGS increases the risk of developing CL16. These studies were performed using SGS and the specific salivary molecule or molecules associated with such outcome remained unknown. By following up individuals living in a CL endemic area and constantly exposed to Lu. intermedia we detected seroconversion to rLinB-13 before the appearance of clinical signs of CL. Importantly, positive serology to rLinb-13 was higher individuals who later developed CL, showing that seroconversion to rLinB-13 increases the risk of developing disease. This increased risk of disease was paralleled by a significantly lower frequency of a positive DTH response to Leishmania in seropositive subjects, suggesting that exposure to Lu. intermedia LinB-13 may negatively impact a cellular responses to the parasite. We previously showed that individuals exposed to Lu. intermedia present a higher frequency of CD4+CD25+IL-10-secreting cells and, in vitro, such response enabled parasite replication16. We speculate that LinB-13 may induce a similar response thereby promoting a parasite-friendly environment at the moment of transmission. However, we cannot rule out the hypothesis that a higher antibody response to LinB-13 reflects an increased exposure to Lu. intermedia and, in consequence, and increased probability of contact with infected sand flies and increased risk of developing CL. Since we do not know when infection occurred in subjects who presented CL, the latter is difficult to be addressed in human studies.
Experimental studies addressing how exposure to sand fly saliva could promote protection from leishmaniasis34–37 enabled the formulation of the following hypothesis: exposure to sand fly salivary proteins primes the development of CD4+IFN-γ-secreting T cells that, upon the bite of an infected sand fly, are rapidly mobilized to the inoculation site leading to macrophage activation and parasite killing (rev. in ref. 2). Our results, however, suggest that this response may not occur as such in areas of L. braziliensis transmission by Lu. intermedia, especially given our finding that a positive serology to LinB-13 is dissociated from DTH conversion to Leishmania. In Saudi Arabia15 and in Tunisia38, higher serology to salivary antigens has been reported in CL patients also suggesting a possible detrimental effect of exposure to sand fly saliva on human cutaneous leishmaniasis.
Sand fly salivary proteins are useful and practical tool to understand the dynamics of leishmaniasis transmission. Herein, we identified a single salivary molecule in Lu. intermedia saliva, LinB-13, that accurately predicts exposure to sand flies in a CL endemic area. Moreover, antibodies to rLinB-13 are a biomarker of risk of developing leishmaniasis caused by L. braziliensis. Host factors have been associated with susceptibility to CL39–41 and our study now identifies a sand fly salivary component that may also be decisive in the outcome of disease. We thus envisage a translational approach in which clusters of individuals with high titers of LinB-13 could be targeted for Insecticide Residual Spraying interventions, especially in areas where L. braziliensis transmission is peridomiciliar.
Methods
Study design and selection of individuals
This study was conducted in Corte de Pedra, municipality of Tancredo Neves, BA, Brazil, an area of L. braziliensis transmission by Lu. intermedia and it consisted of three phases (Supplemental Fig. 3). In Phase 1, we screened Lu. intermedia recombinant salivary proteins (n = 11) using sera (n = 89) from residents of the endemic area. In Phase 2, we validated the top-performing Lu. intermedia salivary molecule, as indicated by ROC analysis, in a larger cohort of household contacts of CL patients (n = 264). We also evaluated cross-reactivity of LinB-13 against sera from individuals residing in a VL endemic area (n = 20) (Vila Nova and Bom Viver), municipality of Raposa, São Luis, MA, Brazil24. In Phase 3, we evaluated whether exposure to LinB-13 is a risk marker for CL development. We tested sera from the cohort of household contacts of CL patients (n = 264). The cohort was established in 2010 and followed up to January 201542. Inclusion criteria consisted of a negative history of any type of Leishmania infection, established after a medical interview and physical examination for signs consistent with active or previous CL or ML. At the moment of enrollment, blood was collected for serological assays against LinB-13. At the same time, the Leishmania Skin Test (DTH) was performed, as described below. The serological surveys were repeated at the 2nd and 4th year of follow up. Among the 264 household contacts, 237 were evaluated at the 2nd year and 224 were evaluated at the 4th year of follow up. The individuals not evaluated either developed CL or refused to participate. During follow-up, one visit was performed annually for active monitoring of CL development and subjects suspected of having CL were diagnosed by parasite isolation or by a positive PCR for L. braziliensis. This research was conducted with the approval of the Ethical Committee of the Hospital Prof. Edgard Santos (Salvador, Bahia, Brazil; 240/2009) and Comissão Nacional de Ética em Pesquisa (CEP, Brazilian National Ethics Committee, Brazil). Informed consent was obtained from each participant. All methods were performed in accordance with the guidelines and regulations determined by CEP.
Leishmania Skin Test
The Leishmania Skin Test was performed at the moment of enrollment in the study and at the 2nd and 4th years of follow up. For skin test, 0,1 mL (15 μg/mL) of Soluble Leishmania Antigen, prepared as described elsewhere43, was injected intracutaneously on the volar surface of the forearm, and the greater diameter of induration was measured 48–72 h later. Induration of ≥ 5 mm was defined as a positive reaction.
Sand flies and preparation of SGS
Adult Lu. intermedia or Lu. longipalpis sand flies were captured in Corte de Pedra and Cavunge, BA, Brazil, respectively. Sand fly identification and Salivary Gland Sonicate (SGS) preparation were performed as described elsewhere44.
Cloning and expression of Lu. intermedia salivary transcripts
Eleven Lu. intermedia cDNA coding for secreted proteins (LinB-8, LinB-13, LinB-14, LinB-15, LinB-17, LinB-21, LinB-26, LinB-28, LinB-37, LinB-38, LinB-44) (Table 1)17 were amplified by PCR, cloned into VR2010-TOPO and sequenced as described45. Recombinant proteins were produced by transfection of 293-F cells (Invitrogen) with VR2010-TOPO plasmids coding for different Lu. intermedia salivary proteins by the Protein Expression Laboratory at the Frederick National Laboratory for Cancer Research (Frederick, MD). High-performance liquid chromatography purification of His-tagged Lu. intermedia salivary proteins was performed as described6 using an NGC Chromatography System (BioRad). Eluted proteins were detected at 280 nm and collected every minute on a 96-well microtiter plate using a BioFrac Fraction Collector (BioRad). Recombinant proteins were tested for endotoxin using a QCL-1000 LAL assay (Lonza). Endotoxin-free recombinant proteins were used in all assays. Aliquots of eluted proteins were checked by SDS-PAGE developed by silver staining. Edman sequencing of the N-terminus region of the purified recombinant proteins was performed at the Protein Chemistry Section, Research Technology Branch, NIAID, NIH (Rockville, MD). Proteins were stored at −70 °C until use.
Analysis of anti–Lu. intermedia SGS antibodies and anti–Lu. intermedia salivary proteins antibodies
Humoral (IgG and IgG subclasses) response to Lu. intermedia SGS and to recombinant salivary proteins was determined as described16, 46. Recombinant proteins were used at 1 µg/ml. In all ELISA assays, the cut-off value was established employing sera from healthy volunteers (n = 30) from a non-endemic area and was determined as the mean optical density (OD) value plus 2.5 standard deviations.
Dot Blot Analysis
Dot blot analysis was performed by spotting 1 µg of rLinB-13 or Lu. intermedia SGS onto nitrocellulose membrane (Invitrogen). Membranes were blocked with 5% BSA in PBS-T (0.1% Tween 20) for two hours. Serum samples (1/100) from individuals (n = 5) with positive serology for both LinB-13 and Lu. intermedia SGS or from non-exposed subjects (n = 4) were diluted in PBS-T with 0.1% BSA and incubated with spotted membranes for 45 minutes. Following another round of washing, anti-human HRP-conjugated IgG (Sigma) was added (1/20000) and membranes were incubated for 30 minutes. Signal detection was carried out using ECL system (Amersham) on an Image analyzer (ImageQuant Las 4000).
Statistical analysis
Comparisons between 2 groups were performed by Mann–Whitney test and among 3 or more groups by Kruskal–Wallis test followed by Dunn multiple comparison tests. Categorical data were compared using the Fisher exact test. The matrix based on non-parametric Spearman correlation was performed using R version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria). ROC analysis was used to establish sensitivity and specificity of recombinant proteins in predicting anti- Lu. intermedia SGS positivity. Correlation between IgG to LinB-13 and Lu. intermedia SGS or between IgG and IgG4 to LinB-13 were checked using non-parametric Spearman test. Hierarchical cluster analysis of Z-score normalized serology response to Lu. intermedia SGS, Lu. longipalpis SGS and rLinB-13 using the Ward’s method with bootstrap (100x) was performed using JMP Statistical Discovery (V. 12). Statistical analyses were conducted using Prism (V. 5.0) (GraphPad Software) and differences were considered significant when P < 0.05. The relative risk (RR) was calculated using the following formula: RR = I1/I0, where I1 is the incidence of CL in exposed individuals (seropositive to LinB-13 salivary protein), and I0 the incidence of CL in non-exposed individuals (seronegative to LinB-13 salivary protein).
Electronic supplementary material
Acknowledgements
This work was supported by the National Institutes of Health (AI 30639 to E.M.C.), by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases (J.G.V., and F.O.), and by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (564689/2008-4 to C.I.O.). E.M.C., A.B. and C.I.O. are senior researchers at CNPq. A.M.C. received fellowship from Fundação de Amparo à Pesquisa da Bahia (FAPESB).
Author Contributions
A.M.C., F.O. and C.I.O. designed the study; A.M.C., R.S. and R.P.C., J.C.M., J.V. and A.B. and contributed reagents. A.M.C., K.F.F., E.M.C., F.O. and C.I.O. analyzed data and wrote the manuscript. All authors read and approved the final version of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-03345-0
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fabiano Oliveira, Email: loliveira@niaid.nih.gov.
Camila I. de Oliveira, Email: camila@bahia.fiocruz.br
References
- 1.Oliveira F, de Carvalho AM, de Oliveira CI. Sand-Fly Saliva–Man: The Trigger Trio. Front Immunol. 2013;4:375. doi: 10.3389/fimmu.2013.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomes R, Oliveira F. The immune response to sand fly salivary proteins and its influence on leishmania immunity. Front Immunol. 2012;3:110. doi: 10.3389/fimmu.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clements MF, et al. Measurement of recent exposure to Phlebotomus argentipes, the vector of Indian visceral Leishmaniasis, by using human antibody responses to sand fly saliva. Am J Trop Med Hyg. 2010;82:801–807. doi: 10.4269/ajtmh.2010.09-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barral A, et al. Human immune response to sand fly salivary gland antigens: a useful epidemiological marker? Am J Trop Med Hyg. 2000;62:740–745. doi: 10.4269/ajtmh.2000.62.740. [DOI] [PubMed] [Google Scholar]
- 5.Gomes RB, et al. Seroconversion against Lutzomyia longipalpis saliva concurrent with the development of anti-Leishmania chagasi delayed-type hypersensitivity. J Infect Dis. 2002;186:1530–1534. doi: 10.1086/344733. [DOI] [PubMed] [Google Scholar]
- 6.Teixeira C, et al. Discovery of markers of exposure specific to bites of Lutzomyia longipalpis, the vector of Leishmania infantum chagasi in Latin America. PLoS Negl Trop Dis. 2010;4:e638. doi: 10.1371/journal.pntd.0000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohousova I, Ozensoy S, Ozbel Y, Volf P. Detection of species-specific antibody response of humans and mice bitten by sand flies. Parasitology. 2005;130:493–499. doi: 10.1017/S003118200400681X. [DOI] [PubMed] [Google Scholar]
- 8.de Moura TR, et al. Enhanced Leishmania braziliensis infection following pre-exposure to sandfly saliva. PLoS Negl Trop Dis. 2007;1:e84. doi: 10.1371/journal.pntd.0000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marzouki S, et al. Characterization of the antibody response to the saliva of Phlebotomus papatasi in people living in endemic areas of cutaneous leishmaniasis. Am J Trop Med Hyg. 2011;84:653–661. doi: 10.4269/ajtmh.2011.10-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin-Martin I, et al. High levels of anti-Phlebotomus perniciosus saliva antibodies in different vertebrate hosts from the re-emerging leishmaniosis focus in Madrid, Spain. Vet Parasitol. 2014;202:207–216. doi: 10.1016/j.vetpar.2014.02.045. [DOI] [PubMed] [Google Scholar]
- 11.Abdeladhim M, Kamhawi S, Valenzuela JG. What’s behind a sand fly bite? The profound effect of sand fly saliva on host hemostasis, inflammation and immunity. Infect Genet Evol. 2014;28:691–703. doi: 10.1016/j.meegid.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Souza AP, et al. Using recombinant proteins from Lutzomyia longipalpis saliva to estimate human vector exposure in visceral Leishmaniasis endemic areas. PLoS Negl Trop Dis. 2010;4:e649. doi: 10.1371/journal.pntd.0000649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marzouki S, et al. Salivary antigen SP32 is the immunodominant target of the antibody response to Phlebotomus papatasi bites in humans. PLoS Negl Trop Dis. 2012;6:e1911. doi: 10.1371/journal.pntd.0001911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marzouki S, et al. Validation of Recombinant Salivary Protein PpSP32 as a Suitable Marker of Human Exposure to Phlebotomus papatasi, the Vector of Leishmania major in Tunisia. PLoS Negl Trop Dis. 2015;9:e0003991. doi: 10.1371/journal.pntd.0003991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mondragon-Shem K, et al. Severity of old world cutaneous leishmaniasis is influenced by previous exposure to sandfly bites in Saudi Arabia. PLoS Negl Trop Dis. 2015;9:e0003449. doi: 10.1371/journal.pntd.0003449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carvalho, A. M. et al. Interleukin 10-Dominant Immune Response and Increased Risk of Cutaneous Leishmaniasis After Natural Exposure to Lutzomyia intermedia Sand Flies. J Infect Dis (2015). [DOI] [PMC free article] [PubMed]
- 17.de Moura TR, et al. Functional transcriptomics of wild-caught Lutzomyia intermedia salivary glands: identification of a protective salivary protein against Leishmania braziliensis infection. PLoS Negl Trop Dis. 2013;7:e2242. doi: 10.1371/journal.pntd.0002242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma D, Francischetti IM, Ribeiro JM, Andersen JF. The structure of hookworm platelet inhibitor (HPI), a CAP superfamily member from Ancylostoma caninum. Acta Crystallogr F Struct Biol Commun. 2015;71:643–649. doi: 10.1107/S2053230X1500271X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben Salah A, et al. The predictive validity of naturally acquired delayed-type hypersensitivity to leishmanin in resistance to Leishmania major-associated cutaneous leishmaniasis. J Infect Dis. 2005;192:1981–1987. doi: 10.1086/498042. [DOI] [PubMed] [Google Scholar]
- 20.Follador I, et al. Epidemiologic and immunologic findings for the subclinical form of Leishmania braziliensis infection. Clin Infect Dis. 2002;34:E54–58. doi: 10.1086/340261. [DOI] [PubMed] [Google Scholar]
- 21.Brummer-Korvenkontio H, Lappalainen P, Reunala T, Palosuo T. Detection of mosquito saliva-specific IgE and IgG4 antibodies by immunoblotting. J Allergy Clin Immunol. 1994;93:551–555. doi: 10.1016/S0091-6749(94)70066-4. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz BS, Ford DP, Childs JE, Rothman N, Thomas RJ. Anti-tick saliva antibody: a biologic marker of tick exposure that is a risk factor for Lyme disease seropositivity. Am J Epidemiol. 1991;134:86–95. doi: 10.1093/oxfordjournals.aje.a115996. [DOI] [PubMed] [Google Scholar]
- 23.Orlandi-Pradines E, et al. Antibody response against saliva antigens of Anopheles gambiae and Aedes aegypti in travellers in tropical Africa. Microbes Infect. 2007;9:1454–1462. doi: 10.1016/j.micinf.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Aquino DM, et al. Epidemiological study of the association between anti-Lutzomyia longipalpis saliva antibodies and development of delayed-type hypersensitivity to Leishmania antigen. Am J Trop Med Hyg. 2010;83:825–827. doi: 10.4269/ajtmh.2010.10-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drahota J, et al. Recombinant antigens from Phlebotomus perniciosus saliva as markers of canine exposure to visceral leishmaniases vector. PLoS Negl Trop Dis. 2014;8:e2597. doi: 10.1371/journal.pntd.0002597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kostalova T, et al. Canine Antibodies against Salivary Recombinant Proteins of Phlebotomus perniciosus: A Longitudinal Study in an Endemic Focus of Canine Leishmaniasis. PLoS Negl Trop Dis. 2015;9:e0003855. doi: 10.1371/journal.pntd.0003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sima M, Ferencova B, Warburg A, Rohousova I, Volf P. Recombinant Salivary Proteins of Phlebotomus orientalis are Suitable Antigens to Measure Exposure of Domestic Animals to Sand Fly Bites. PLoS Negl Trop Dis. 2016;10:e0004553. doi: 10.1371/journal.pntd.0004553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geraci NS, et al. Profiling of human acquired immunity against the salivary proteins of Phlebotomus papatasi reveals clusters of differential immunoreactivity. Am J Trop Med Hyg. 2014;90:923–938. doi: 10.4269/ajtmh.13-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shan EZ, et al. Immunoglobulins specific to mosquito salivary gland proteins in the sera of persons with common or hypersensitive reactions to mosquito bites. J Dermatol. 1995;22:411–418. doi: 10.1111/j.1346-8138.1995.tb03415.x. [DOI] [PubMed] [Google Scholar]
- 30.Reunala T, et al. Frequent occurrence of IgE and IgG4 antibodies against saliva of Aedes communis and Aedes aegypti mosquitoes in children. Int Arch Allergy Immunol. 1994;104:366–371. doi: 10.1159/000236693. [DOI] [PubMed] [Google Scholar]
- 31.Remoue F, et al. IgE and IgG4 antibody responses to Aedes saliva in African children. Acta Trop. 2007;104:108–115. doi: 10.1016/j.actatropica.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Punnonen J, et al. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci USA. 1993;90:3730–3734. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satoguina JS, Weyand E, Larbi J, Hoerauf A. T regulatory-1 cells induce IgG4 production by B cells: role of IL-10. J Immunol. 2005;174:4718–4726. doi: 10.4049/jimmunol.174.8.4718. [DOI] [PubMed] [Google Scholar]
- 34.Kamhawi S, Belkaid Y, Modi G, Rowton E, Sacks D. Protection against cutaneous leishmaniasis resulting from bites of uninfected sand flies. Science. 2000;290:1351–1354. doi: 10.1126/science.290.5495.1351. [DOI] [PubMed] [Google Scholar]
- 35.Valenzuela JG, et al. Toward a defined anti-Leishmania vaccine targeting vector antigens: characterization of a protective salivary protein. J Exp Med. 2001;194:331–342. doi: 10.1084/jem.194.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collin N, et al. Sand fly salivary proteins induce strong cellular immunity in a natural reservoir of visceral leishmaniasis with adverse consequences for Leishmania. PLoS Pathog. 2009;5:e1000441. doi: 10.1371/journal.ppat.1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliveira F, et al. A sand fly salivary protein vaccine shows efficacy against vector-transmitted cutaneous leishmaniasis in nonhuman primates. Sci Transl Med. 2015;7:290. doi: 10.1126/scitranslmed.aaa3043. [DOI] [PubMed] [Google Scholar]
- 38.Abdeladhim M, et al. Human cellular immune response to the saliva of Phlebotomus papatasi is mediated by IL-10-producing CD8 + T cells and Th1-polarized CD4 + lymphocytes. PLoS Negl Trop Dis. 2011;5:e1345. doi: 10.1371/journal.pntd.0001345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramasawmy R, et al. The -2518bp promoter polymorphism at CCL2/MCP1 influences susceptibility to mucosal but not localized cutaneous leishmaniasis in Brazil. Infect Genet Evol. 2010;10:607–613. doi: 10.1016/j.meegid.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castellucci L, et al. IL6 -174 G/C promoter polymorphism influences susceptibility to mucosal but not localized cutaneous leishmaniasis in Brazil. J Infect Dis. 2006;194:519–527. doi: 10.1086/505504. [DOI] [PubMed] [Google Scholar]
- 41.Castellucci L, et al. FLI1 polymorphism affects susceptibility to cutaneous leishmaniasis in Brazil. Genes Immun. 2011;12:589–594. doi: 10.1038/gene.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muniz, A. C. et al. Immunologic Markers of Protection in Leishmania (Viannia) braziliensis Infection: A 5-Year Cohort Study. J Infect Dis (2016). [DOI] [PMC free article] [PubMed]
- 43.Reed SG, et al. Selection of a skin test antigen for American visceral leishmaniasis. Am J Trop Med Hyg. 1986;35:79–85. doi: 10.4269/ajtmh.1986.35.79. [DOI] [PubMed] [Google Scholar]
- 44.de Moura TR, et al. Immunity to Lutzomyia intermedia saliva modulates the inflammatory environment induced by Leishmania braziliensis. PLoS Negl Trop Dis. 2010;4:e712. doi: 10.1371/journal.pntd.0000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oliveira F, et al. From transcriptome to immunome: identification of DTH inducing proteins from a Phlebotomus ariasi salivary gland cDNA library. Vaccine. 2006;24:374–390. doi: 10.1016/j.vaccine.2005.07.085. [DOI] [PubMed] [Google Scholar]
- 46.Vinhas V, et al. Human anti-saliva immune response following experimental exposure to the visceral leishmaniasis vector, Lutzomyia longipalpis. Eur J Immunol. 2007;37:3111–3121. doi: 10.1002/eji.200737431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


