Abstract
Short telomere length (TL) occurs in individuals under psychological stress, and with various psychiatric diseases. Recent studies have also reported mitochondrial DNA copy number (mtDNAcn) alterations under several neuropsychiatric conditions. However, no study has examined whether aberrant TL or mtDNAcn occur in completed suicide, one of the most serious outcomes of mental illnesses. TL and mtDNAcn in post-mortem samples from 528 suicide completers without severe physical illness (508 peripheral bloods; 20 brains) and 560 samples from control subjects (peripheral bloods from 535 healthy individuals; 25 post-mortem brains) were analysed by quantitative polymerase chain reaction. Suicide completers had significantly shorter TL and higher mtDNAcn of peripheral bloods with sex/age-dependent differences (shorter TL was more remarkably in female/young suicides; higher mtDNAcn more so in male/elderly suicides). The normal age-related decline of TL and mtDNAcn were significantly altered in suicide completers. Furthermore, shorter TL and lower mtDNAcn of post-mortem prefrontal cortex were seen in suicide completers compared to controls. This study shows the first association of aberrant telomeres and mtDNA content with suicide completion. Our results indicate that further research on telomere shortening and mitochondrial dysfunction may help elucidate the molecular underpinnings of suicide-related pathophysiology.
Introduction
Suicide is a significant public health problem that accounts for nearly 1 million deaths worldwide each year. Previous data have implicated abnormalities in the hypothalamic-pituitary-adrenal (HPA) axis, noradrenergic, and serotonergic systems in suicide1, 2. The involvement of genetic factors has also been demonstrated in suicidal behaviour by family studies, twin and adoption studies, candidate gene analyses and genome-wide association studies (GWAS)3, 4. These diverse pieces of evidence suggest that suicide is caused by the accumulation of stress, traumatic events, and/or illnesses in individuals with neurobiological changes and genetic risk. However, biological insights into suicidal behaviour lag behind those of other mental problems, and no useful genetic biomarker of suicide risk has been found. This could be because suicidal behaviour varies in terms of degree of lethality and suicidal intent, and it is very difficult to obtain viable biological samples from suicide completers, which is the most serious phenotype.
Telomeres, repetitive nucleotide sequences located at the ends of chromosomes, have gained special attention in the field of stress biomarkers. Telomere shortening to a critical length triggers genomic instability and cellular apoptosis. In somatic cells such as leukocytes, telomeres shorten with advancing age and cell divisions because of low telomerase activity5. Therefore, telomere length (TL) largely reflects the cellular biological age6. However, TL shortening has also been associated with the presence of stressors, such as various physical diseases and mental illnesses7. Indeed, several studies have documented short TL in individuals with psychological stress, and with various psychiatric diseases including major depressive disorder (MDD)8, 9, schizophrenia10, 11, anxiety disorders12, posttraumatic stress disorder (PTSD)13 and bipolar disorder14; however, these associations have not always been observed15, 16. Recently, a significant meta-analysis demonstrated that short TL was found in MDD, anxiety disorders and PTSD, but not in schizophrenia and bipolar disorder17. In addition, several reports have shown shorter telomeres in white matter oligodendrocytes, and in the hippocampus of post-mortem MDD brains18, 19.
Increasing interest has developed in the association of mitochondrial dysfunctions with neuropsychiatric conditions. Mitochondria are ubiquitous organelles in eukaryotic systems that play crucial roles in cellular energy production, calcium signalling, cell growth and differentiation, cell cycle control, and cell death20. Functional changes in mitochondria appear to be associated with aging and age-related disorders21. Mitochondrial biogenesis can be indirectly assessed by measuring mitochondrial DNA (mtDNA) copy number (cn), the number of mtDNA molecules per cell. Although alterations of mtDNAcn are considered an index of mitochondrial dysfunction22, only limited studies have examined mtDNAcn changes in psychological stress or psychiatric disorders23–27.
Published evidence has demonstrated the link between telomere shortening and mitochondrial dysfunction. For example, telomere shortening leads to subsequent p53-mediated repression of peroxisome proliferator-activated receptor gamma and coactivator-1α and 1β (PGC-1α and PGC-1β), which can lead to mitochondrial dysfunction22, 28, 29. Recently, two reports showed evidence that shorter TL and higher mtDNAcn occurred in the same cohorts with MDD or other neuropsychiatric conditions (e.g., childhood adversity)30, 31.
Telomere shortening and mitochondrial dysfunction have attracted much attention as key processes in cellular aging and neuropsychiatric conditions. However, no previous study has been conducted to examine whether aberrant telomeres or mtDNA are linked to completed suicide, which is the most serious outcome of psychological stress and psychiatric diseases.
Here, we report on the first investigation of TL and mtDNAcn in post-mortem bloods and brains from suicide completers without severe physical illness, and compared these results to those of control subjects.
Results
TL and mtDNAcn in blood samples of suicide completers and controls
The results of regression analyses of TL and mtDNAcn in blood samples of suicide completers (n = 508) and healthy controls (n = 535) are shown in Table 1. Demographic data of sex and age (≤34 years old, young; 35–59 years old, middle; ≥60 years old, elderly)-based subgroup analyses are shown in Table S1. Significantly shorter telomeres in suicide completers were observed (β = −0.0928, p < 0.0001) (Table 1 and Fig. 1a); this effect appeared to be greater in female suicide completers (male, β = −0.0684, p = 0.0618; female, β = −0.1238, p = 0.0025) (Table S1). Significantly shorter TL was seen in young suicide completers in both sexes (male, β = −0.3224, p < 0.0001; female, β = −0.3120, p < 0.0001). In middle-age suicide completers, this shortening was specific to women (male, β = 0.0786, p = 0.1546; female, β = −0.1566, p = 0.0099); it was not found at all in elderly suicide completers (Fig. 1c,e and Table S1). A significantly higher mtDNAcn than that in controls was observed in suicide completers (β = 0.1038, p < 0.0001) (Table 1 and Fig. 1b). This effect appeared to be greater in men than women (male, β = 0.1320, p = 0.0003; female, β = 0.0671, p = 0.0584) (Table S1). Elderly suicide completers had significantly higher mtDNAcn than the other age groups in both sexes (male, β = 0.2750, p < 0.0001; female, β = 0.1855, p = 0.0036), whereas this increase was specific to middle-aged men (male, β = 0.1309, p = 0.0151; female, β = −0.0114, p = 0.8282) and absent in young suicide completers (Fig. 1d,f and Table S1). Even when suicide completers with current/past psychiatric disorders and/or psychotropic medication use were excluded, TL was significantly shorter for the suicide completers (male, β = −0.1081, p = 0.0156; female, β = −0.1595, p = 0.0381) than the controls, and male, but not female, suicide completers showed a significantly higher mtDNAcn (male, β = 0.1639, p = 0.0002; female, β = −0.0123, p = 0.8463) (Fig. 1g,h; The results of regression analyses are shown in Table S2). We gathered the presence/absence of suicide attempt history for 471 suicide completers, and conducted an additional analysis among only those samples. However, there was no association between suicide attempt history and either TL or mtDNAcn (Table S3). Furthermore, we investigated whether or not TL and mtDNAcn were biochemically stable post-mortem. There was no association between post-mortem interval (PMI) and TL or mtDNAcn in post-mortem blood samples (Table S4).
Table 1.
Regression analyses of telomere length and mtDNA copy number of blood in suicide completers and controls.
| Telomere length | mtDNA copy number | |||||||
|---|---|---|---|---|---|---|---|---|
| βa | s.e. | t | p valueb | βa | s.e. | t | p valueb | |
| Total samples (n = 1043); Suicide completers (n = 508) and Controls (n = 535) | ||||||||
| —Phenotype (Suicide vs. Control) | −0.0928 | 0.0272 | −3.411 | <0.0001 | 0.1038 | 0.0255 | 4.078 | <0.0001 |
| —Age | −0.0077 | 0.0008 | −9.950 | <0.0001 | −0.0048 | 0.0007 | −6.631 | <0.0001 |
| —Sex (Male vs. Female) | −0.0358 | 0.0274 | −1.308 | 0.1912 | −0.0703 | 0.0256 | −2.745 | 0.0062 |
Abbreviation: s.e., standard error. aβ means regression coefficient derived from generalized linear models. bp values shown in bold are significant at <0.05.
Figure 1.
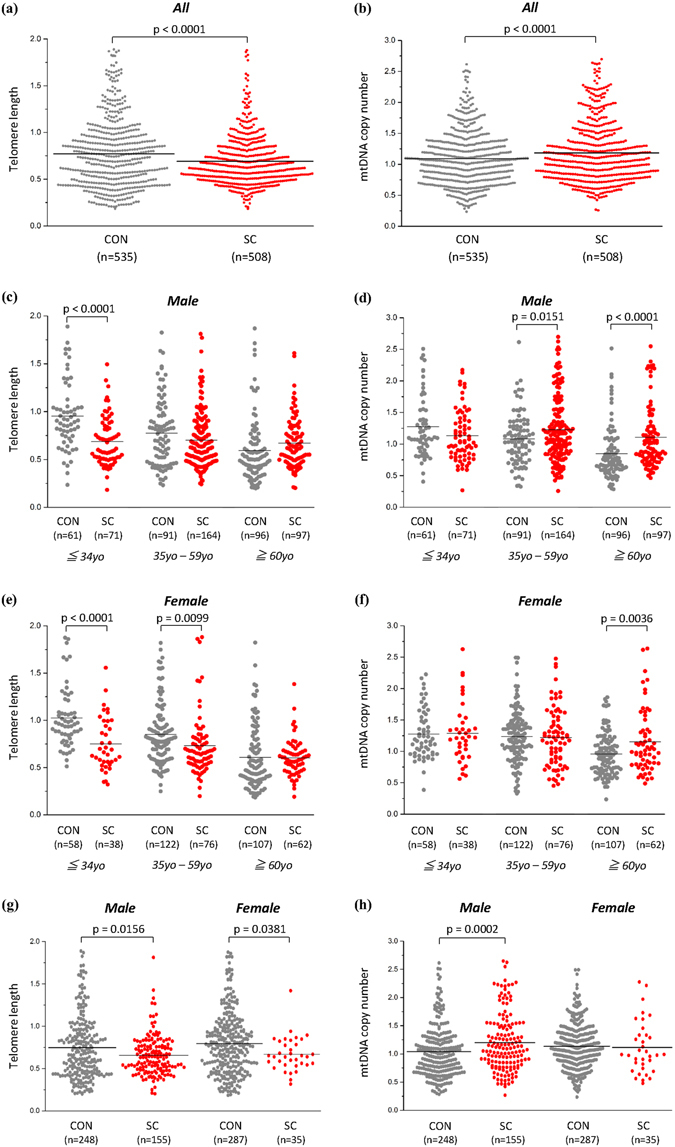
Dot plot of Telomere length (TL) and mtDNA copy number (mtDNAcn) of bloods in suicide completers and healthy controls. (a) TL in all suicide completers and controls. (b) mtDNAcn in all suicide completers and controls. (c) TL in male suicide completers and male controls. (d) mtDNAcn in male suicide completers and male controls. (e) TL in female suicide completers and female controls. (f) mtDNAcn in female suicide completers and female controls. (g) TL in suicide completers without psychiatric disorders and/or psychotropic medication and controls. (h) mtDNAcn in suicide completers without psychiatric disorders and/or psychotropic medication and controls. Figure (c–f) were divided into three age groups (≤34 years old, young; 35–59 years old, middle; ≥60 years old, elderly). All p values were adjusted by age and sex, or with age as covariates. Horizontal lines represent mean values for each group. Abbreviations: CON, control; SC, suicide; yo, years old.
There was a weak trend of sex effect on TL (p = 0.0663), and a statistically significant effect on mtDNAcn (p = 0.0016) in healthy controls; no sex effect on either TL or mtDNAcn was found in suicide completers (Table S5).
Healthy controls showed the normal age-related reduction in TL and mtDNAcn (TL, r = −0.4849, p < 0.0001; mtDNAcn, r = −0.3830, p < 0.0001) (Fig. 2a,c). In suicide completers, however, age-related attrition was significantly milder compared to controls (r = −0.1282, p = 0.0038), and the negative correlation between mtDNAcn and age was not observed (r = −0.0727, p = 0.1020) (Fig. 2b,d).
Figure 2.
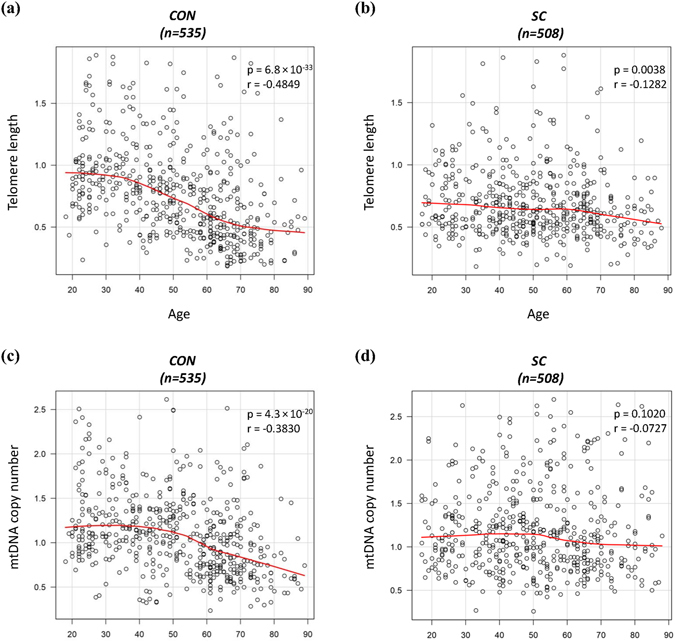
Age-TL/mtDNAcn relationship in suicide completers and healthy controls. (a) Correlation between TL and age in controls. (b) Correlation between TL and age in suicide completers. (c) Correlation between mtDNAcn and age in controls. (d) Correlation between mtDNAcn and age in suicide completers. Red line fitting a smooth spline showed non-linear relationship between TL/mtDNAcn and age. All p values and r values were calculated by Spearman’s rho tests. Abbreviations: CON, control; SC, suicide.
TL and mtDNAcn in brain samples of suicide completers and controls
Regression analyses of TL and mtDNAcn in dorsolateral prefrontal cortex (DLPFC) samples of suicide completers (n = 20) and controls (n = 25) are shown in Table 2. We noted a significantly shorter TL and lower mtDNAcn in the DLPFC of suicide completers compared to controls (β = −0.3006, p = 0.0014 and β = −0.2803, p = 0.0044, respectively) (Fig. 3a,b). Regression analysis showed that PMI had no significant effect on TL and mtDNAcn in any brain sample (Table 2).
Table 2.
Regression analyses of telomere length and mtDNA copy number of post-mortem dorsolateral prefrontal cortex (DLPFC) in suicide completers and controls.
| Telomere length | mtDNA copy number | |||||||
|---|---|---|---|---|---|---|---|---|
| βa | s.e. | t | p valueb | βa | s.e. | t | p valueb | |
| Total samples (n = 45); Suicide completers (n = 20) and Controls (n = 25) | ||||||||
| —Phenotype (Suicide vs. Control) | −0.3006 | 0.0875 | −3.436 | 0.0014 | −0.2803 | 0.0929 | −3.018 | 0.0044 |
| —Age | −0.0064 | 0.0026 | −2.462 | 0.0182 | −0.0045 | 0.0028 | −1.633 | 0.1102 |
| —Sex (Male vs. Female) | −0.0552 | 0.0918 | −0.601 | 0.5510 | −0.1333 | 0.0975 | −1.368 | 0.1790 |
| —PMI | 0.0035 | 0.0050 | 0.707 | 0.4835 | −0.0001 | 0.0053 | −0.006 | 0.9951 |
Abbreviations: PMI, post-mortem interval; s.e., standard error. aβ means regression coefficient derived from generalized linear models. bp values shown in bold are significant at <0.05.
Figure 3.
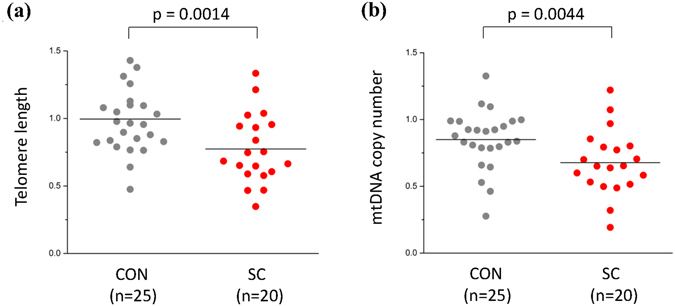
Dot plot of Telomere length (TL) and mtDNA copy number (mtDNAcn) of post-mortem dorsolateral prefrontal cortex (DLPFC) in suicide completers and controls. (a) TL of DLPFC in suicide completers and controls. (b) mtDNAcn of DLPFC in suicide completers and controls. All p values were adjusted by age, sex and post-mortem interval as covariates. Horizontal lines represent mean values for each group. Abbreviations: CON, control; SC, suicide.
Discussion
This is the first study to investigate TL and mtDNAcn in post-mortem blood and brain samples of suicide completers. First, we found significantly shorter TL and higher mtDNAcn in blood samples from suicide completers, which remained significant after excluding subjects with psychiatric disorders and/or psychotropic medication use. Our results were similar to prior findings of shortened TL and increased mtDNAcn in cohorts with MDD or other neuropsychiatric conditions30, 31. The results from one of these studies revealed two additional findings in laboratory mice: (i) behavioural stress led to decreased TL and increased mtDNAcn, and (ii) mice injected with corticosterone showed shortened telomeres and increased mtDNAcn following behavioural stress30. According to other findings, high cortisol reactivity to stress is associated with shorter telomeres in children of depressed mothers32. Cortisol also inhibits telomerase activity in vitro 33. These data suggest that alterations of TL and mtDNA content after stress are at least partially caused by activation of the HPA axis. Several lines of evidence, especially resistance to the dexamethasone suppression test (DST), also indicate that HPA axis abnormalities might be the main diathesis for suicide2. Further investigation of the HPA axis may help clarify the association between alterations of TL/mtDNAcn and suicide. In fact, a recent report showed significantly higher plasma levels of free-circulating mtDNA in medication-free suicide attempters compared to healthy controls; notably, the higher free plasma mtDNA levels were associated with resistance to the DST in suicide attempters34.
In addition, we found significant sex-dependent differences in aberrant TL/mtDNAcn. Shorter TL was more divergent from controls in female suicide completers, and the significantly higher mtDNAcn was higher still in male suicides. These sex-dependent differences likely led to the loss of the sex effects on blood cell TL and mtDNAcn in suicide completers. Similarly to our control samples, previous studies in the general population reported longer TL in women than in men, and a slower rate of telomere attrition in women35, 36. This difference is thought to be partially related to female hormones such as estrogen, which may reduce oxidative stress and stimulate the production of telomerase37, 38. Other reports showed the relationship between lower levels of progesterone and susceptibility to suicide attempts in women39, 40. Further research into telomere shortening and disturbed levels of female hormones in suicide will be needed to better elucidate the underlying mechanism of this association. In studying mtDNA, several studies of the general population showed a higher level of mtDNA content in women than in men, consistent with our findings in healthy controls41, 42. In addition, Borras et al. reported that female rats were less prone to mtDNA damage by reactive oxygen species43. These characteristics of female mtDNA may lead to the less significant results found in this study.
We also found age-dependent differences in aberrant TL/mtDNAcn; shorter TL was significant only in suicide completers under 60 years old, particularly in the young (under 34 years old), and higher mtDNAcn was far more remarkable in elderly suicide completers. These results indicated that the biological basis for completed suicide appears to be different between young, middle-aged, and elderly individuals. In addition, aberrant TL/mtDNAcn in completed suicide may be more easily detectable in comparison with healthy young people with longer telomeres and healthy elderly with lower mtDNA content, respectively.
We also performed further investigation about the association of age with TL and mtDNAcn. The results of many studies have shown an age-related decline in TL29, 44; several studies have shown that mtDNAcn did not decline until around 50 years of age, after which an age-related decline was observed in healthy subjects42, 45. In our study, the control subjects also showed an age dependency for both TL and mtDNAcn similar to that seen in previous reports. In suicide completers, however, TL was significantly less correlated with age compared to healthy controls, and mtDNAcn showed no negative association. These altered correlations were at least partially caused by the presence of young suicide completers with shorter TL, and elderly suicide completers with higher mtDNAcn.
DLPFC is one of the brain regions which previous studies indicate is related to suicide vulnerability. Structural and functional neuroimaging studies in suicidal populations revealed abnormal volume reductions and dysfunction in the frontal cortex, primarily in the DLPFC as well as the orbitofrontal cortex (OFC) and ventrolateral PFC46. The DLPFC plays an important role in cognitive control and emotional regulation, both of which are involved in suicide-related pathophysiology47. In addition, elevated corticotropin-releasing hormone levels have been found in the post-mortem DLPFC of patients who committed suicide relative to controls, which suggests that HPA axis hyperactivity was present48. In this study, we identified shorter telomeres in the post-mortem DLPFC of suicide completers. A previous study reported that TL was not changed in the post-mortem DLPFC region of patients with MDD, contrary to our findings19. These inconsistent results may derive in part from the difference between MDD and completed suicide, in that the severity of or duration of exposure to the psychological stressor causing suicidal ideation may have been greater in patients who completed suicide, which thus increased the shortening effect. Interestingly, one large cohort study showed that peripheral blood cell TL, but not brain tissue TL, was positively associated with total cerebral volume, as well as that of some distinct regions in the PFC49. In future studies, it would be interesting to analyse this direct association between brain region/cell-specific telomere length and brain volume in the post-mortem brains of both of suicide completers and controls. We also found lower mtDNA content in the post-mortem DLPFC of suicide completers. Hunter et al. reported that acute stress in adult rats induced down-regulation of mtDNA-encoded genes, and that corticosteroids regulated rat hippocampal mtDNA gene expression via glucocorticoid receptors50. They also determined that the glucocorticoid receptor can translocate into mitochondria in neurons of the PFC51. HPA axis abnormalities in the brains of suicidal individuals may cause the lower mtDNA content of the PFC reported here. Only a few studies have investigated mtDNA content in human brain samples from patients with psychiatric disorders (MDD, schizophrenia and bipolar disorder), and no differences were reported relative to control samples in any of these studies52–54. In addition, no study provides clues as to why the data herein demonstrated disturbances in mtDNAcn that were the opposite of those in blood and DLPFC in this study. Further studies focused on mtDNA content and psychiatric conditions in distinct brain regions are required to clarify these findings.
A major strength of this study is the large sample size of suicide completers. Indeed, most previous candidate gene analyses and GWAS for completed suicide were limited by a small sample size2, 4, 55. In addition, we obtained information regarding age, comorbid diseases (severe physical illness and psychiatric disease) and psychotropic medication use, to exclude factors that can alter TL and mtDNAcn as much as possible. Therefore, the significant alterations in TL and mtDNAcn reported here should be robustly linked to the molecular signature of suicidal behaviour with the intention of completion. However, there are some limitations to consider as well. First, the condition of post-mortem samples should be considered. Although we selected only subjects with certain clinical information prior to death, the influence of PMI, pH changes and protein degradation on our results cannot be controlled. In this study, there was no association between PMI and TL or mtDNAcn in post-mortem blood and brain samples. Furthermore, both the shorter telomeres and aberrant mtDNAcn in suicide completers reported here showed significant sex-dependent differences. According to one forensic study, the age-related decline of TL also remained strong in healthy cadaver blood56. These findings may provide evidence that our results were not influenced by the acquisition of samples post-mortem. Second, the criteria underlying the exclusion of psychiatric cases from analysis are insufficient, since we only investigated the cases through their medical records and bereaved family interviews. Thus, some suicide completers who we considered to be without psychiatric cases might have indeed experienced psychiatric symptoms that met the criteria of specific disorders. Third, we could not exclude other potential confounders (e.g. smoking status, BMI) that are known to affect TL and mtDNAcn as well57–59. Fourth, TL and mtDNAcn of other brain regions correlated with suicidal vulnerability (e.g., OFC, ventral PFC, hippocampus or amygdala) should be explored in the post-mortem brains of suicide completers.
In conclusion, we report the first association of aberrant telomeres and mtDNAcn with suicide completion. Our results raise the possibility that further research into telomere shortening and mtDNA dysfunction may elucidate the molecular underpinnings of suicide-related pathophysiology.
Methods
Subjects
The entire study design and procedures were performed in accordance with the Declaration of Helsinki. This study was approved by the Ethical Committee for Genetic Studies of the Kobe University Graduate School of Medicine. All subjects were of Japanese descent and ranged from 18 years to 89 years of age. Autopsies on suicide victims were conducted at the Division of Legal Medicine in the Department of Community Medicine and Social Health Science at the Kobe University Graduate School of Medicine. The verdict of “completed suicide” was made through discussion with the Medical Examiner’s Office of Hyogo Prefecture and the Division of Legal Medicine in the Kobe University Graduate School of Medicine. Informed consent was obtained from all of the participants and from the families of the subjects used for post-mortem blood and brain experiments.
Blood and brain sampling
Peripheral blood samples were obtained from all of suicide completers post-mortem (n = 508), and from healthy living controls (n = 535). The demographics of the subjects are shown in Table S6. The age distribution was matched among suicide completers and controls in each sex group. We excluded suicide victims with severe physical illnesses (cardiovascular diseases, cerebral infarction, diabetes, bone marrow diseases, and cancer) because they can affect TL and mtDNAcn60–65. All of the healthy volunteers were recruited from the main islands of Japan, including medical students, hospital workers, and the general population. No control subjects were related to each other, or manifested psychiatric problems in unstructured interviews conducted by two psychiatrists using the Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSM-IV) criteria. During the interview, all control subjects were checked for a personal and family history of psychiatric disorders and/or suicidal behaviours. We excluded control subjects with personal and/or familial history of psychiatric disorders and/or suicidal behaviours.
Autopsied brains were obtained from 20 suicide victims and 25 control subjects. Demographic data are shown in Table S7. DLPFC was dissected on dry ice for subsequent DNA extraction.
Measurement of TL and mtDNAcn
Blood and brain samples were stored at −80 °C before use. DNA was extracted using the QIAamp DNA Blood Midi Kit and DNeasy Blood & Tissue Kit (Qiagen Inc., Valencia, CA) as appropriate. Each DNA sample was quantified and qualified using a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE).
TL was measured using quantitative polymerase chain reaction (qPCR), by applying the telomere/single-copy gene ratio method66 with minor modifications. Briefly, the method was used to measure the ratio between the number of telomere repeats and that of a single-copy gene (β-haemoglobin [HGB]) used as a quantitative control, relative to a reference sample. mtDNAcn was calculated based on measuring the amount of mtDNA (NADH dehydrogenase, subunit 1 [ND1]) relative to that of a nuclear gene (HGB). All qPCR experiments were performed using a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA), with SYBR Green Master Mix (Applied Biosystems, Foster City, CA). The primer sequences and cycling conditions are described in Table S8. Each sample was run in triplicate, using 10 ng of DNA. Amplification of telomeres, ND1, and the single-copy gene HGB were performed in separate runs, using the same reference sample in the same well positions. TL and mtDNAcn were determined by measuring the ratio of telomeric and mtDNA content to that of a reference single-copy gene in each sample, relative to the reference gene; the standard curve method using a 5-point serial-dilution series with reference DNA was employed. Laboratory personnel were blinded with regard to case-control status and the sample order was randomized in each batch.
Statistical analysis
Statistical analysis was performed using R Version 3.2.2 and EZR67, 68. Student’s t-tests were performed to analyse between-group comparisons of the continuous variables. Regression analyses using generalized linear models with a gamma distribution and log link were applied to analyse between-group comparisons of TL and mtDNAcn, with covariates (age, sex, suicide attempt history and PMI) as needed. Spearman’s rho tests were performed to assess the relationships between TL/mtDNAcn and age. Dummy variables were used as necessary (phenotype, control = 0 and suicide = 1; sex, male = 0 and female = 1; suicide attempt history, no = 0 and yes = 1). Statistical significance was defined as two-tailed p < 0.05.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Electronic supplementary material
Acknowledgements
This work was supported in part by JSPS KAKENHI Grant Number JP26461718 and JP16K19766, and GSK Japan Research Grant 2016. We thank Yasuko Nagashima for providing technical assistance.
Author Contributions
A.H. designed the study. A.H., T.I. and I.S. managed the research. I.O., T.I., S.B., A.K., Y.Z. and K.S. performed experiments. I.O., M.T., Y.U., O.S. and A.H. collected human samples. M.K. and S.O. undertook the statistical analysis. I.O. wrote the first draft of this paper. All authors contributed to and have approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-03599-8
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coryell W, Schlesser M. The dexamethasone suppression test and suicide prediction. Am J Psychiatry. 2001;158:748–753. doi: 10.1176/appi.ajp.158.5.748. [DOI] [PubMed] [Google Scholar]
- 2.Oquendo MA, et al. Toward a biosignature for suicide. Am J Psychiatry. 2014;171:1259–1277. doi: 10.1176/appi.ajp.2014.14020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bondy B, Buettner A, Zill P. Genetics of suicide. Mol Psychiatry. 2006;11:336–351. doi: 10.1038/sj.mp.4001803. [DOI] [PubMed] [Google Scholar]
- 4.Mirkovic B, et al. Genetic Association Studies of Suicidal Behavior: A Review of the Past 10 Years, Progress, Limitations, and Future Directions. Front Psychiatry. 2016;7:158. doi: 10.3389/fpsyt.2016.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13:693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mather KA, Jorm AF, Parslow RA, Christensen H. Is telomere length a biomarker of aging? A review. J Gerontol A Biol Med Sci. 2011;66:202–213. doi: 10.1093/gerona/glq180. [DOI] [PubMed] [Google Scholar]
- 7.Lindqvist D, et al. Psychiatric disorders and leukocyte telomere length: Underlying mechanisms linking mental illness with cellular aging. Neurosci Biobehav Rev. 2015;55:333–364. doi: 10.1016/j.neubiorev.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon NM, et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60:432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Verhoeven JE, et al. Major depressive disorder and accelerated cellular aging: results from a large psychiatric cohort study. Mol Psychiatry. 2014;19:895–901. doi: 10.1038/mp.2013.151. [DOI] [PubMed] [Google Scholar]
- 10.Kao HT, et al. Rapid telomere erosion in schizophrenia. Mol Psychiatry. 2008;13:118–119. doi: 10.1038/sj.mp.4002105. [DOI] [PubMed] [Google Scholar]
- 11.Polho GB, De-Paula VJ, Cardillo G, dos Santos B, Kerr DS. Leukocyte telomere length in patients with schizophrenia: A meta-analysis. Schizophr Res. 2015;165:195–200. doi: 10.1016/j.schres.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 12.Hoen PW, et al. Association between anxiety but not depressive disorders and leukocyte telomere length after 2 years of follow-up in a population-based sample. Psychol Med. 2013;43:689–697. doi: 10.1017/S0033291712001766. [DOI] [PubMed] [Google Scholar]
- 13.O’Donovan A, et al. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011;70:465–471. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizzo LB, et al. Immunosenescence is associated with human cytomegalovirus and shortened telomeres in type I bipolar disorder. Bipolar Disord. 2013;15:832–838. doi: 10.1111/bdi.12121. [DOI] [PubMed] [Google Scholar]
- 15.Needham BL, et al. Depression, anxiety and telomere length in young adults: evidence from the National Health and Nutrition Examination Survey. Mol Psychiatry. 2015;20:520–528. doi: 10.1038/mp.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nieratschker V, et al. Longer telomere length in patients with schizophrenia. Schizophr Res. 2013;149:116–120. doi: 10.1016/j.schres.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 17.Darrow SM, et al. The Association Between Psychiatric Disorders and Telomere Length: A Meta-Analysis Involving 14,827 Persons. Psychosom Med. 2016;78:776–787. doi: 10.1097/PSY.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szebeni A, et al. Shortened telomere length in white matter oligodendrocytes in major depression: potential role of oxidative stress. Int J Neuropsychopharmacol. 2014;17:1579–1589. doi: 10.1017/S1461145714000698. [DOI] [PubMed] [Google Scholar]
- 19.Mamdani F, et al. Variable telomere length across post-mortem human brain regions and specific reduction in the hippocampus of major depressive disorder. Transl Psychiatry. 2015;5:e636. doi: 10.1038/tp.2015.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johannsen DL, Ravussin E. The role of mitochondria in health and disease. Curr Opin Pharmacol. 2009;9:780–786. doi: 10.1016/j.coph.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagouge M, Larsson NG. The role of mitochondrial DNA mutations and free radicals in disease and ageing. J Intern Med. 2013;273:529–543. doi: 10.1111/joim.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahin E, DePinho RA. Axis of ageing: telomeres, p53 and mitochondria. Nat Rev Mol Cell Biol. 2012;13:397–404. doi: 10.1038/nrm3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giulivi C, et al. Mitochondrial dysfunction in autism. JAMA. 2010;304:2389–2396. doi: 10.1001/jama.2010.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z, et al. Association of telomere length and mitochondrial DNA copy number with risperidone treatment response in first-episode antipsychotic-naïve schizophrenia. Sci Rep. 2015;5:18553. doi: 10.1038/srep18553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang CC, Jou SH, Lin TT, Liu CS. Mitochondrial DNA variation and increased oxidative damage in euthymic patients with bipolar disorder. Psychiatry Clin Neurosci. 2014;68:551–557. doi: 10.1111/pcn.12163. [DOI] [PubMed] [Google Scholar]
- 26.Bersani FS, et al. Mitochondrial DNA copy number is reduced in male combat veterans with PTSD. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:10–17. doi: 10.1016/j.pnpbp.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Kim JH, Kim HK, Ko JH, Bang H, Lee DC. The relationship between leukocyte mitochondrial DNA copy number and telomere length in community-dwelling elderly women. PLoS One. 2013;8:e67227. doi: 10.1371/journal.pone.0067227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sahin E, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyrka AR, et al. Association of telomere length and mitochondrial DNA copy number in a community sample of healthy adults. Exp Gerontol. 2015;66:17–20. doi: 10.1016/j.exger.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai N, et al. Molecular signatures of major depression. Curr Biol. 2015;25:1146–1156. doi: 10.1016/j.cub.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tyrka AR, et al. Alterations of mitochondrial DNA copy number and telomere length with early adversity and psychopathology. Biol Psychiatry. 2016;79:78–86. doi: 10.1016/j.biopsych.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gotlib IH, et al. Telomere length and cortisol reactivity in children of depressed mothers. Mol Psychiatry. 2015;20:615–620. doi: 10.1038/mp.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi J, Fauce SR, Effros RB. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav Immun. 2008;22:600–605. doi: 10.1016/j.bbi.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindqvist D, et al. Increased plasma levels of circulating cell-free mitochondrial DNA in suicide attempters: associations with HPA-axis hyperactivity. Transl Psychiatry. 2016;6:e971. doi: 10.1038/tp.2016.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanders JL, Newman AB. Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol Rev. 2013;35:112–131. doi: 10.1093/epirev/mxs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Needham BL, et al. A test of biological and behavioral explanations for gender differences in telomere length: the multi-ethnic study of atherosclerosis. Biodemography Soc Biol. 2014;60:156–173. doi: 10.1080/19485565.2014.947471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/S0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 38.Bayne S, Jones ME, Li H, Liu JP. Potential roles for estrogen regulation of telomerase activity in aging. Ann N Y Acad Sci. 2007;1114:48–55. doi: 10.1196/annals.1396.023. [DOI] [PubMed] [Google Scholar]
- 39.Baca-García E, Díaz-Sastre C, de Leon J, Saiz-Ruiz J. The relationship between menstrual cycle phases and suicide attempts. Psychosom Med. 2000;62:50–60. doi: 10.1097/00006842-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Mousavi SG, Bateni S, Maracy MR, Mardanian F, Mousavi SH. Recurrent suicide attempt and female hormones. Adv Biomed Res. 2014;3:201. doi: 10.4103/2277-9175.142046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curran JE, et al. Genetic determinants of mitochondrial content. Hum Mol Genet. 2007;16:1504–1514. doi: 10.1093/hmg/ddm101. [DOI] [PubMed] [Google Scholar]
- 42.Knez J, et al. Correlates of Peripheral Blood Mitochondrial DNA Content in a General Population. Am J Epidemiol. 2016;183:138–146. doi: 10.1093/aje/kwv175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borrás C, et al. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med. 2003;34:546–552. doi: 10.1016/S0891-5849(02)01356-4. [DOI] [PubMed] [Google Scholar]
- 44.Müezzinler A, Zaineddin AK, Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing Res Rev. 2013;12:509–519. doi: 10.1016/j.arr.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Mengel-From J, et al. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Hum Genet. 2014;133:1149–1159. doi: 10.1007/s00439-014-1458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding Y, et al. Prefrontal cortex markers of suicidal vulnerability in mood disorders: a model-based structural neuroimaging study with a translational perspective. Transl Psychiatry. 2015;5:e516. doi: 10.1038/tp.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pu S, et al. Suicidal ideation is associated with reduced prefrontal activation during a verbal fluency task in patients with major depressive disorder. J Affect Disord. 2015;181:9–17. doi: 10.1016/j.jad.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 48.Merali Z, et al. Corticotropin-releasing hormone, arginine vasopressin, gastrin-releasing peptide, and neuromedin B alterations in stress-relevant brain regions of suicides and control subjects. Biol Psychiatry. 2006;59:594–602. doi: 10.1016/j.biopsych.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 49.King KS, et al. Effect of leukocyte telomere length on total and regional brain volumes in a large population-based cohort. JAMA Neurol. 2014;71:1247–1254. doi: 10.1001/jamaneurol.2014.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hunter RG, et al. Stress and corticosteroids regulate rat hippocampal mitochondrial DNA gene expression via the glucocorticoid receptor. Proc Natl Acad Sci USA. 2016;113:9099–9104. doi: 10.1073/pnas.1602185113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Du J, et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci USA. 2009;106:3543–2548. doi: 10.1073/pnas.0812671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vawter MP, et al. Mitochondrial-related gene expression changes are sensitive to agonal-pH state: implications for brain disorders. Mol Psychiatry. 2006;11:663–679. doi: 10.1038/sj.mp.4001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sabunciyan S, et al. Quantification of total mitochondrial DNA and mitochondrial common deletion in the frontal cortex of patients with schizophrenia and bipolar disorder. J Neural Transm. 2007;114:665–674. doi: 10.1007/s00702-006-0581-8. [DOI] [PubMed] [Google Scholar]
- 54.Torell H, et al. Mitochondrial DNA (mtDNA) in brain samples from patients with major psychiatric disorders: gene expression profiles, mtDNA content and presence of the mtDNA common deletion. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:213–223. doi: 10.1002/ajmg.b.32134. [DOI] [PubMed] [Google Scholar]
- 55.Galfalvy H, et al. A genome-wide association study of suicidal behavior. Am J Med Genet B Neuropsychiatr Genet. 2015;168:557–563. doi: 10.1002/ajmg.b.32330. [DOI] [PubMed] [Google Scholar]
- 56.Srettabunjong S, Satitsri S, Thongnoppakhun W, Tirawanchai N. The study on telomere length for age estimation in a Thai population. Am J Forensic Med Pathol. 2014;35:148–153. doi: 10.1097/PAF.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 57.Suen Q, et al. Healthy lifestyle and leukocyte telomere length in U.S. women. PLoS One. 2012;7:e38374. doi: 10.1371/journal.pone.0038374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meng S, et al. Leukocyte mitochondrial DNA copy number, anthropometric indices, and weight change in US women. Oncotarget. 2016;7:60676–60686. doi: 10.18632/oncotarget.10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Müezzinler A, et al. Smoking habits and leukocyte telomere length dynamics among older adults: Results from the ESTHER cohort. Exp Gerontol. 2015;70:18–25. doi: 10.1016/j.exger.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 60.Fitzpatrick AL, et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 61.Ding H, et al. Telomere length and risk of stroke in Chinese. Stroke. 2012;43:658–663. doi: 10.1161/STROKEAHA.111.637207. [DOI] [PubMed] [Google Scholar]
- 62.Sampson MJ, Winterbone MS, Hughes JC, Dozio N, Hughes DA. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care. 2006;29:283–289. doi: 10.2337/diacare.29.02.06.dc05-1715. [DOI] [PubMed] [Google Scholar]
- 63.Savage SA. Human telomeres and telomere biology disorders. Prog Mol Biol Transl Sci. 2014;125:41–66. doi: 10.1016/B978-0-12-397898-1.00002-5. [DOI] [PubMed] [Google Scholar]
- 64.Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA. The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20:1238–1250. doi: 10.1158/1055-9965.EPI-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meng S, et al. Pre-diagnostic leukocyte mitochondrial DNA copy number and risk of lung cancer. Oncotarget. 2016;7:27307–27312. doi: 10.18632/oncotarget.8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. (Available at: https://www.R-project.org; Accessed on: February 3, 2016).
- 68.Kanda Y. Investigation of the freely available easy-to-use software/‘EZR/’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).


