Abstract
Although pain is frequently accompanied with depression, little is known about the risk factors contributing to individual differences to the comorbidity of pain and depression. In this study, we examined whether cytokines and brain-derived neurotrophic factor (BDNF) might contribute to the individual differences in the development of neuropathic pain-induced depression. Rats were randomly subjected to spared nerved ligation (SNI) or sham surgery. The SNI rats were divided into two groups by the data from depression-related behavioral tests. Rats with depression-like phenotype displayed higher levels of pro-inflammatory cytokines (e.g., interleukin (IL)-1β, IL-6) as well as imbalance of pro/anti-inflammatory cytokines compared with rats without depression-like phenotype and sham-operated rats. Levels of BDNF in the prefrontal cortex of rats with depression-like phenotype were lower than those of rats without depression-like phenotype and sham-operated rats. A single dose of ketamine ameliorated depression-like behaviors in the rats with depression-like phenotype. Interestingly, higher serum levels of IL-1β and IL-6 in the rat with depression-like phenotype were normalized after a single dose of ketamine. These findings suggest that alterations in the inflammatory cytokines and BDNF might contribute to neuropathic pain-induced depression, and that serum cytokines may be predictable biomarkers for ketamine’s antidepressant actions.
Introduction
Depression and pain are frequently comorbid in clinics. Epidemiological studies show that the prevalence of pain in depressed patients is up to 50%1, 2. Meanwhile, pain is a risk factor for depression, and the prevalence of painful patients with depression is around 30%3, 4. These epidemiological studies suggest that individual differences exist in the development of the comorbid pain and depression2, 5–8. Depression and pain share biological pathways, which have implications for the treatment of both concurrently. However, the precise mechanisms underlying the comorbidity of pain and depression are unknown. In addition, the possible predisposing factors for individual differences in this comorbidity are still poorly understood.
Neuro-immune system plays a critical role in the development of neuropathic pain9, 10 and depression11–13. Higher levels of pro-inflammatory cytokines (e.g., tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-1β) in the central nervous system (CNS) contribute to the pathophysiology of neuropathic pain and depression14–16. In contrast, anti-inflammatory cytokines (e.g., IL-4 and IL-10) can influence nociceptive and depressive behaviors as a failure to counterbalance the over-expressed pro-inflammatory cytokines17, 18. Furthermore, imbalance of pro/anti-inflammatory cytokines is also observed in depressed patients19 and painful neuropathy patients20 who can be improved after antidepressant and analgesic treatment21, 22. Recent studies show that activated inflammatory response serves as a key mechanism for the comorbidity of pain and depression23–26. Furthermore, researchers shed more light on the mechanisms of individual differences in the pathogenesis of depression27. It is reported that vulnerability to depression might be closely related to exaggerated inflammatory response28, 29. Besides, the ratio of IL-6 to IL-10, which is the balance between proinflammatory (IL-6) and anti-inflammatory (IL-10) cytokines, is elevated in the individuals vulnerable to depression30. Therefore, the neuro-immune system may play a key role in vulnerability or resilience to inflammation (or stress). However, it is currently unknown how inflammation plays a role in the individual differences in the comorbidity of neuropathic pain and depression.
Brain-derived neurotrophic factor (BDNF) plays a key role in the pathogenesis of depression31, which is down-regulated in the prefrontal cortex (PFC) two weeks after neuropathic pain32. Alterations in the BDNF expression are implicated in the pathogenesis of depression and antidepressant mechanisms33, 34. We reported down-regulation of hippocampal BDNF in a chronic unpredictable stress35, inflammation36 and social defeat stress models37, 38. Several lines of evidence have suggested that BDNF can regulate the resilience to stress-induced depression-like phentype36, 39–41. However, little is known about the effect of BDNF on individual emotional response to peripheral nerve injury.
Ketamine, the N-methyl-D-aspartate (NMDA) receptors antagonist, showed rapid onset and long-lasting antidepressant effects in patients with treatment-resistant major depressive disorder (MDD)42, 43. A single sub-anesthetic dose (0.5 mg/kg) of ketamine relieves depressive symptoms in two-thirds of the MDD patients, lasting for over a week44, 45. Furthermore, ketamine has been reported to relieve pain-induced depression, which is independent of its antinociceptive effect46, 47. Although stimulation of neurogenesis and neuroplasticity via modulating of glutamatergic signaling as well as the mammalian target of rapamycin is involved antidepressant effects of ketamine48, it seems that inhibition of inflammatory response may account for antidepressant effects of ketamine49, 50. Interestingly, we also reported that serum IL-6 is a predictive biomarker for ketamine’s antidepressant effect in patients with MDD51, 52. However, the roles of cytokines in ketamine’s antidepressant response have not been explored in rodent models of the comorbid neuropathic pain and depression.
The purpose of the present study was undertaken to examine whether cytokines and BDNF might contribute to the individual differences in rats after spared nerve injury (SNI). Furthermore, we examined whether ketamine could improve the comorbid neuropathic pain and depression after SNI.
Results
SNI induced long-lasting mechanical hyperalgesia and depression-like behaviors
In the experiment 1 (Fig. 1), SNI surgery induced mechanical hyperalgesia compared with sham surgery on days 7, 14 and 21 after surgery (P < 0.001) while the baseline mechanical threshold exhibited no difference between them (Fig. S1a). Furthermore, SNI rats displayed less weight gain compared with sham-operated rats on days 14 and 21 after surgery (P < 0.01; Fig. S1b). In the SPT, SNI rats showed decreased sucrose preference on days 14 (P < 0.01, vs baseline and sham-operated rats) and 21 (P < 0.05, vs baseline) after surgery (Fig. S1c), although total fluid intake was similar in the two groups (Fig. S1d). In the FST, the immobility time of SNI rats on days 14 and 21 after SNI surgery was higher than sham-operated rats (P < 0.05; Fig. S1e). Total distance traveled in the open field had no difference between sham-operated and SNI rats before and after surgery, which indicated that the locomotor ability was not affected by the surgery (Fig. S1f). These findings show that SNI produced mechanical hyperalgesia and depression-like behaviors in rats, consistent with previous report46.
Figure 1.
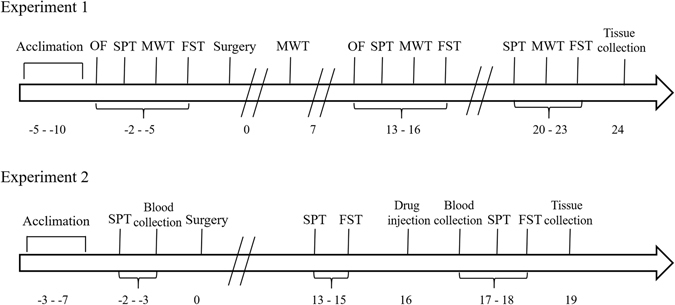
Schedule of behavioral tests and drug treatment.
Classification of SNI rats with or without depression-like phenotype
We applied hierarchical cluster analysis to classify the SNI rats into two clusters. In order to distinguish between the depression and not-depression phenotypes, the results in the SPT and FST were analyzed by two-way ANOVA. Then, 13 of 32 (40%) rats showing depression-like phenotypes were regarded as “rats with depression-like phenotype” (with depression), and the others were “rats without depression-like phenotype” (without depression) (Fig. S2). Both rats with or without depression-like phenotype showed mechanical hyperalgesia and decreased weight gain on days 14 and 21 after SNI surgery compared with sham-operated rats (P < 0.001), although there was no difference in mechanical thresholds or weight gain between two SNI groups (Fig. 2a,b). In the SPT, rats with depression-like phenotype displayed reduced sucrose preference compared with sham-operated rats and rats without depression-like phenotype on days 14 (P < 0.001) and 21 (P < 0.05) (Fig. 2c). In the FST, rats with depression-like phenotype had increased immobility time on day 14 (P < 0.05, vs sham-operated and rats without depression-like phenotype, respectively) or 21 (P < 0.05, vs sham-operated rats) after surgery (Fig. 2d).
Figure 2.
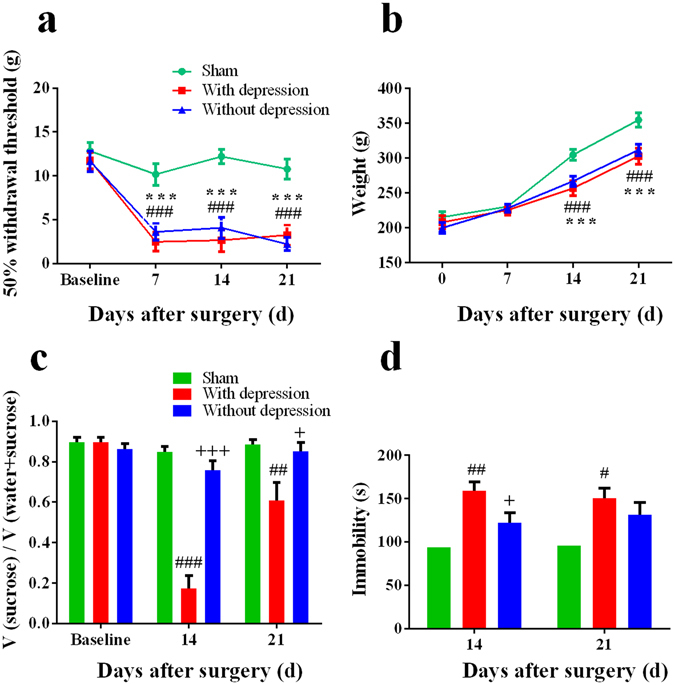
Behavior assessments of rats with or without depression-like phenotype. (a) In the MWT, both rats with or without depression-like phenotype showed mechanical hyperalgesia compared with sham group. There was no difference between two groups. (b) Both rats with or without depression-like phenotype showed less weight gain compared with sham group. There was no difference between two groups. (c) In the SPT, rats with depression-like phenotype displayed reduced sucrose preference compared with the sham-operated rats and rats without depression-like phenotype on days 14 and 21 after surgery. (d) In the FST, rats with depression-like phenotype (n = 13) displayed increased immobility time compared with sham-operated rats (n = 14) and rats without depression-like phenotype (n = 19). # P < 0.05, ## P < 0.01 and ### P < 0.001 for rats with depression-like phenotype vs sham group; *P < 0.05 and ***P < 0.001 for rats without depression-like phenotype vs sham group; + P < 0.05 and +++ P < 0.001 for rats with depression-like phenotype vs rats without depression-like phenotype.
Different levels of cytokines and BDNF in the prefrontal cortex (PFC) in rats with or without depression-like phenotype
To evaluate the role of neuroinflammation in the individual differences of the comorbidity, we measured the levels of proinflammatory cytokines (IL-1β, IL-6 and TNF-α) and anti-inflammatory cytokines (IL-4, IL-10) in the prefrontal cortex (PFC) using ELISA. Rats with depression-like phenotype displayed increased levels of cytokines (IL-1β, IL-6, TNF-α, IL-4 and IL-10) in the PFC compared with sham-operated and rats without depression-like phenotype (P < 0.05) on day 21 after SNI surgery. In addition, the levels of these cytokines showed no difference between rats without depression-like phenotype and sham-operated rats (Fig. 3a–e).
Figure 3.
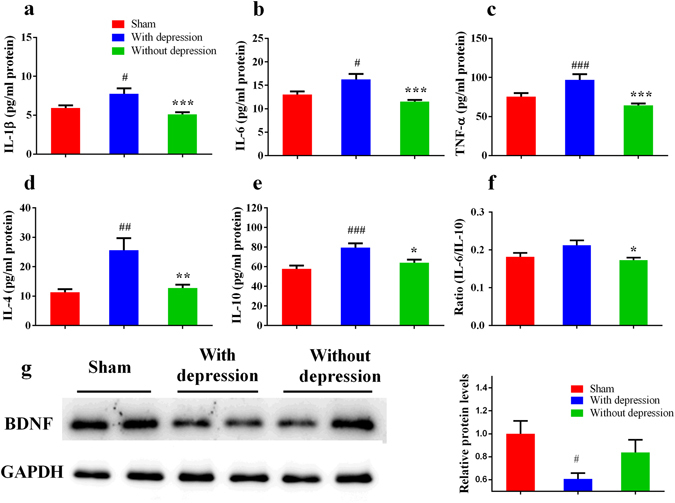
Expression of inflammatory cytokines in the PFC. (a) The levels of IL-1β in the PFC from rats with depression-like phenotype were higher than sham-operated rats and rats without depression-like phenotype. (b) The levels of IL-6 in the PFC from rats with depression-like phenotype were higher than sham-operated rats and rats without depression-like phenotype. (c) The levels of TNF-α in the PFC from rats with depression-like phenotype were higher than sham-operated rats and rats without depression-like phenotype. (d) The levels of IL-4 in the PFC from rats with depression-like phenotype were higher than sham-operated rats and rats without depression-like phenotype. (e) The levels of IL-10 in the PFC from rats with depression-like phenotype were higher than sham-operated rats and rats without depression-like phenotype. (f) There was no difference of the ratio of IL-6 to IL-10 between rats with depression-like phenotype and sham-operated rats. The ratio of IL-6 to IL-10 in the rats without depression-like phenotype was lower than rats with depression-like phenotype. (g) Western blot analysis of BDNF in the PFC after SNI surgery. # P < 0.05, ## P < 0.01 and ### P < 0.001 vs sham group; *P < 0.05, **P < 0.01 and ***P < 0.001 vs rats without depression-like phenotype. Each group, n = 6 or 7 for Elisa and n = 4 for western blot.
To ascertain the balance between pro- and anti-inflammation, we assessed the ratio of IL-6 to IL-10 in the PFC. The ratio of rats with depression-like phenotype was higher than that of rats without depression-like phenotype (P < 0.05; Fig. 3f).
To determine the role BDNF in the individual differences of the comorbidity, we performed Western blot analysis of BDNF in the PFC. Levels of BDNF in the PFC of rats with depression-like phenotype were significantly lower than those of rats without depression-like phenotype and sham-operated rats (P < 0.05; Fig. 3g).
Individual differences of ketamine’s antidepressant effect on day 14 after SNI surgery
In the experiment 2 (Fig. 1), we examined the effects of ketamine on the comorbidity after SNI surgery. Using the equation from discrimination analysis, 12 of 30 (40%) SNI rats were classified as “with depression-like phenotype”, and the others were regarded as “without depression-like phenotype”. Saline or ketamine (20 mg/kg) was injected into rats. In the SPT, rats with depression-like phenotype displayed less sucrose preference than rats without depression-like phenotype (P < 0.05, Fig. 4a). A single dose of ketamine (20 mg/kg) significantly increased decreased sucrose preference of rats with depression-like phenotype (P < 0.05, Fig. 4a). In contrast, ketamine displayed no difference in sucrose preference of rats without depression-like phenotype (Fig. 4a). In the FST, rats with depression-like phenotype displayed longer immobility time than rats without depression-like phenotype (P < 0.05, Fig. 4b). A single dose of ketamine (20 mg/kg) significantly decreased an increased immobility time of rats with depression-like phenotype (P < 0.05, Fig. 4b). In contrast, ketamine displayed no difference in immobility time of rats without depression-like phenotype (P < 0.05, Fig. 4b). These results show that ketamine can show a rapid antidepressant effect in rats with depression-like phenotype, but not rats without depression-like phenotype, on day14 after SNI surgery.
Figure 4.
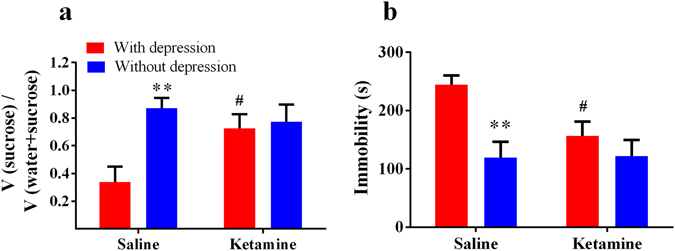
Ketamine’s antidepressant effects on day 14 after SNI surgery. (a) In the SPT, rats with depression-like phenotype displayed decreased sucrose preference than rats without depression-like phenotype rats after saline injection. A single injection of ketamine (20 mg/kg) attenuated decreased sucrose preference in the rats with depression-like phenotype. (b) In the FST, rats with depression-like phenotype displayed longer immobility time than rats without depression-like phenotype after saline injection. A single injection of ketamine (20 mg/kg) attenuated increased immobility time in the rats with depression-like phenotype. **P < 0.01 for rats without depression-like phenotype vs rats with depression-like phenotype after saline treatment. # P < 0.01 for ketamine treatment vs saline treatment in rats with depression-like phenotype.
Role of serum cytokines in ketamine’s antidepressant efficacy
There were no differences of serum baseline cytokines between rats with or without depression-like phenotype (Fig. 5a–c). To determine the role of serum cytokines in antidepressant effect of ketamine, we measured serum levels of cytokines IL-1β, IL-6 and TNF-α 3 days after a single dose of ketamine (20 mg/kg). Rats with depression-like phenotype showed higher serum levels of IL-1β, IL-6 and TNF-α compared with sham-operated group (P < 0.05, Fig. 5d–f). Furthermore, ketamine could decrease serum levels of IL-1β and IL-6 in rats with depression-like phenotype compared with saline treatment (P < 0.05, Fig. 5d,e). Rats with depression-like phenotype displayed higher serum levels of IL-1β, IL-6 and TNF-α than rats without depression-like phenotype (P < 0.05, Fig. 5d–f).
Figure 5.
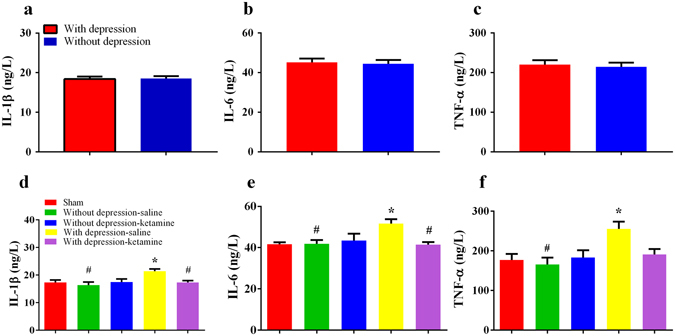
Serum levels of pro-inflammatory cytokines. Serum levels of (a) IL-1β, (b) IL-6 and (c) TNF-α at baseline were not different between rats with or without depression-like phenotype. Saline-treated rats with depression-like phenotype displayed higher serum levels of (d) IL-1β, (e) IL-6 and (f) TNF-α compared with sham-operated rats and rats without depression-like phenotype. A single dose of ketamine (20 mg/kg) reduced the serum levels of IL-1β and IL-6 in the rats with depression-like phenotype. *P < 0.05 vs with sham group, # P <0.05 vs depression-saline group.
To determine the ketamine’s response in rats with depression-like phenotype, discrimination analysis was applied to differentiate between responder group and non-responder group. Three of 8 rats with depression-like phenotype were classified as “non-responder group”, and the other 5 rats were regarded as “responder group”. Responder group displayed increased sucrose preference in the SPT (P < 0.001) and decreased immobility time (P < 0.05) in the FST after a single dose of ketamine compared with non-responder group. There was no difference between two groups at baseline and before ketamine injection (Fig. 6a,b).
Figure 6.
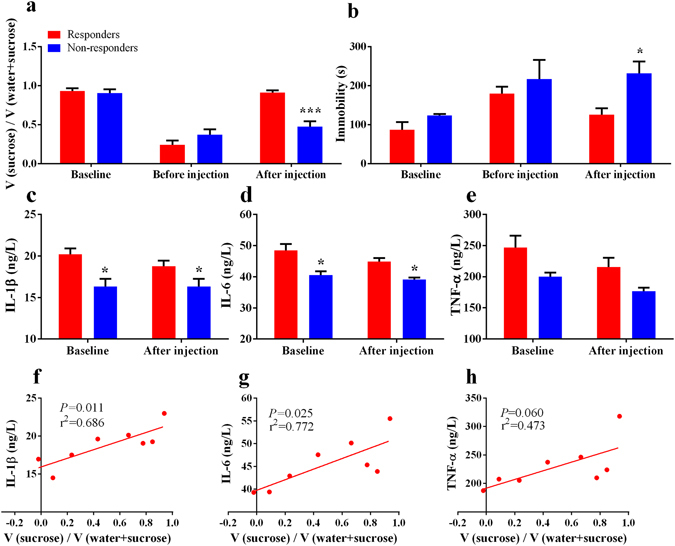
Serum pro-inflammatory cytokines for ketamine’s antidepressant actions. (a,b) Ketamine non-responder group showed decreased sucrose preference and increased immobility time after a single dose of ketamine (20 mg/kg). (c–e) Ketamine non-responder group showed decreased serum levels of IL-1β and IL-6 at baseline and after ketamine injection. There was no difference of serum levels of TNF-α between ketamine-responder group and non-responder group at baseline and after ketamine injection. (f,g) There were positive correlations between the changes of sucrose preference after ketamine injection and serum levels of IL-1β and IL-6 or TNF-α at baseline. (h) There was no correlation between the changes of sucrose preference after ketamine injection and serum levels of TNF-α at baseline. * P < 0.01 and *** P < 0.001 vs ketamine-responder group.
Responder group showed higher serum levels of IL-1β and IL-6 than non-responder group at baseline and after ketamine injection (P < 0.05). Responder group had similar serum levels of IL-1β and IL-6 between baseline and after ketamine injection (Fig. 6c,d). No difference of TNF-α levels was observed at baseline and after ketamine injection between responders and non-responders (Fig. 6e).
To validate whether serum levels of cytokines at baseline can predict ketamine’s antidepressant action, we conducted a correlation analysis between the changes of sucrose preference after ketamine injection and serum levels of cytokines at baseline. Interestingly, there were positive correlations between the changes of sucrose preference and the baseline levels of serum IL-1β and IL-6 (Fig. 6f, r2 = 0.686, P = 0.011; Fig. 6g, r2 = 0.772, P = 0.025). However, there was no correlation between the changes of sucrose preference and serum levels of TNF-α (Fig. 6h, r2 = 0.473, P = 0.060).
Discussion
The major findings of this study are as follows: Although SNI rats suffered near identical nociceptive damage, there were rats with or without depression-like phenotype. Rats with depression-like phenotype are susceptible, but rats without depression-like phenotype are resilience to SNI. Rats with depression-like phenotype had elevated neuroinflammtory response and imbalance of pro/anti-inflammatory compared with rats without depression-like phenotype and sham-operated rats. Tissue levels of BDNF in the PFC from rats with depression-like phenotype were lower than those of rats without depression-like phnotype and sham-operated rats. Furthermore, a subanethetic single dose of ketamine ameliorated depression-like behaviors in the rats with depression-like phenotype on day 14 after SNI surgery. Interestingly, higher serum levels of IL-1β and IL-6 may accout for ketamine’s antidepressant effect in the rat with depression-like phenotype after SNI. To our best knowledge, this is the first study showing the role of neuroinflammation and BDNF for individual differences of depression induced after neuropathic pain. In addition, this is also the first study showing the role of serum IL-1β and IL-6 in the individual differences of ketamine’s antidepressant response in the comorbidity of neuropahtic pain and depression in rodents.
In the preclinical studies, hyperalgesia rats were highly heterogeneous in depression-related behaviors. Keay and colleagues reported that chronic constriction injury (CCI) induces a subgroup (around 30%) of rats which were altered in dominant behavior7 and sleep-wake cycles53 using resident-intruder social interaction and sleep-wake analysis. In this study, the hierarchical cluster analysis divided SNI rats into two clusters: one group (40%, ‘depression-like phenotype’) with increased immobility time in the FST and reduced sucrose preference in the SPT, the other group (60%, ‘without depression-like phenotype’) with similar immobility time and sucrose preference compared with sham-operated rats. Previous clinical studies demonstrate that the incidence of comorbid chronic pain and depression is around 30% to 50%1–4, which is in line with the present study. In this study, we found that rats with or without depression-like phenotype showed similar mechanical withdrawal threshold, suggesting that the alterations in mood-related behaviors were independent of the degree of nociceptive damage, consistent with previous studies7, 53–55.
Elevated inflammatory response may account for the different alterations in mood-related behaviors induced by SNI. It is well recognized that neuroimmune alterations play vital roles in the course of pain and depression9, 11, 55. Nerve injury results in mood disturbance associated with up-regulation of IL-1β24, IL-626 and TNF-α56 in the PFC, and inhibition of inflammation rescues mood disturbance. Inflammatory signaling is triggered after peripheral nerve injury, which projects to the several supraspinal regions (including the PFC) via neural, humoral or cellular pathways57. In this study, we observed increased levels of proinflammatory cytokines of IL-6, IL-1β, TNF-α as well as anti-inflammatory cytokines of IL-4 and IL-10 in the rats with depression-like phenotype, but not rats without depression-like phenotype. Wood et al.30 reported that exaggerated inflammatory response may elicit individual differences in risks for stress-induced depression. Pro-inflammatory (IL-1β) genetic variation and associated higher IL-1β expression increase risks for stress-induced depression58. Taken together, it is likely that elevated inflammatory response might contribute to vulnerability to depression-like phenotype induced by neuropathic pain.
The imbalance between pro- and anti-inflammatory cytokines may contribute to mood disorders59, 60. In this study, the ratio of IL-6 to IL-10 in the PFC of rats with depression-like phenotype was higher than rats without depression-like phenotype. A study using 66 patients demonstrated that depression group had higher ratio of IL-6 to IL-10 than control group61. Furthermore, it is also shown that increased ratio of pro- to anti-inflammatory is detected in animals with stress vulnerability27, 30. Additionally, the M1 and M2 polarization state of microglia, which is associated with increased pro- and anti-inflammatory cytokines, respectively, might be involved in the development of depression and neuropathic pain26, 62. Enhanced IL-6/IL-10 could be explained by more M1 than M2 phenotype of microglia, which would produce more pro-inflammatory cytokines and lead to a sick state. Therefore, it seems that the imbalance of pro/anti-inflammatory response might be an important factor for individual differences of the comorbidity.
We found the decreased levels of BDNF in the PFC on day 21 after SNI, consistent with the previous study32. Furthermore, we also found lower expression of BDNF in the rats with depression-like phenotype than rats without depression-like phenotype. Low levels of BDNF in the PFC are associated with the development of depression-like phenotype in rodents36, 41, 63, 64. BDNF plays a key role in promoting neurogenesis, resilience to damage, as well as neural plasticity31, 65, 66. In addition, pro-inflammatory cytokines may damage neuronal structure and function by suppression of BDNF67, 68. By contrast, anti-inflammatory cytokine IL-4 promotes glia to release BDNF69. Together, it is likely that reduced levels of BDNF in the PFC might be implicated in the depression vulnerability in the comorbid neuropathic pain and depression.
Ketamine is well known to improve both depression and neuropathic pain. Sub-anesthetic ketamine relives depression-like behaviors35 and the dose of ketamine above 25 mg/kg is necessary for analgesia70. A single dose (20 mg/kg) of ketamine ameliorated neuropathic pain-induced depression but had no analgesia effect46. In addition, ketamine (20 mg/kg) showed antidepressant effects in rat learned helplessness model of depression37. Although some studies have focused on ketamine’s antidepressant effects in the comorbidity of pain and depression46, 47, 51, there was no study showing individual differences of ketamine’s antidepressant effects in the comorbidity of pain and depression. Sub-anesthetic dose of ketamine increased social interaction of stress susceptible, but not stress resilient rats, after social defeat stress71, consistent with our study. In this study, we found that ketamine could attenuate decreased serum levels of IL-1β and IL-6, but not TNF-α level, in rats with depression-like phenotype. Moreover, we observed that 3 of 8 depression rats regarded as ketamine non-responder group displayed lower serum levels of IL-1β and IL-6. Interestingly, we reported that ketamine responders had higher levels of IL-1β and IL-6 at baseline compared with non-responders in the treatment-resistant patients with MDD51. Ketamine’s antidepressant effects may dependent on regulation of Th2-mediated humoral immunity. The imbalance between Th1 (e.g., TNF-α) and Th2 (e.g., IL-6) immunity may play vital role in the pathophysiology of MDD since the higher levels of IL-6 might predict ketamine’s antidepressant efficacy52. In this study, we found lower serum levels of IL-1β and IL-6 in non-responders at baseline, consistent with report using depressed patients51.
In this study, we found that SNI rats with depression-like phenotype showed higher inflammatory cytokines than SNI rats without depression-like phenotype. Previously, it was reported that peripheral IL-6 may contribute to resilience versus susceptible to inescapable stress72. Thus, it is likely that elevated inflammatory responses may play a role in the depression-like phenotype after SNI. However, the precise mechanisms underlying the relationship between elevated inflammatory responses and susceptibility to SNI are currently unknown. Interestingly, we reported that alterations in the composition of gut-microbiota may confer resilience to chronic social defeat stress73. Given the key role of gut-microbiota-brain axis in the psychiatric and neurological disorders74, 75, it is likely that alterations in the gut-microbiota may contribute to susceptibility to SNI surgery. Further detailed studies on the role of gut-microbiota in SNI model will be needed.
In the preliminary experiments, we performed the novelty-suppressed feeding (NSF) test to assess the depressive symptoms76, 77. However, we did not find any difference in the latency to feed after ketamine injection. Although the reasons underlying the negative effect of ketamine are unknown, the experimental protocols (e.g., different animal models, experimental apparatus, dose of ketamine, and observational protocol) may contribute to the negative result. In addition, we did not perform the OF test after ketamine injection in the experiment 2 because the total distance, indicating the motor function in the OF test, is not affected by a single sub-anesthetic dose of ketamine in our previous studies35, 47, 64 and other reports46, 78. Together, it seems that ketamine may not have beneficial effects on motor function deficits after SNI surgery. Nonetheless, further studies on the effects of ketamine in the SNI surgery model are needed.
In conclusion, the present study suggests that elevated inflammatory response, especially pro-inflammatory cytokines, and reduced levels of BDNF in the PFC play key roles in individual differences of comorbid neuropathic pain and depression. Furthermore, serum levels of IL-1β and IL-6 at baseline may predict ketamine’s antidepressant effects in pain-induced depression. Therefore, inflammatory cytokines may play a role in the depression vulnerability and ketamine’s antidepressant efficacy in the comorbid neuropathic pain and depression.
Materials and Methods
Animals
Male Sprague-Dawley rats (6–8 weeks, 150–180 g) were purchased from the Animal Center of Jingling Hospital, Nanjing, China. All procedures in this study were performed in accordance with the Guideline for the Care and Use of Laboratory Animals from the National Institutes of Health, USA. The protocol was approved by the Southeast University Institutional Animal Care and Use Committee. Rats were housed with water and food ad libitum under a 12-h light/dark cycle in a temperature-controlled room at 24 ± 1 °C.
Experimental design
Experiment 1 (Fig. 1) Rats were acclimated to environment and sucrose intake for 5 days. We performed the OF, SPT and MWT from days 2 to 5 before surgery (baselines), the OF, SPT, MWT and FST from days 13 to 16 after surgery (test phase 1), and the SPT, MWT and FST from days 20 to 23 after surgery (test phase 2). Then the brain tissues were harvested and the PFC tissues were dissected. In addition, rats were weighted each week.
Experiment 2 (Fig. 1) rats were acclimated to environment and sucrose intake for 5 days. The SPT was performed and blood samples from orbital venous sinus were obtained on day 2 before surgery, and the SPT and FST were conducted from days 13 to 15 after surgery. A single dose of ketamine (20 mg/kg in 1 ml, Hengrui Pharmaceutical Company, Jiangsu, China) or the same volume of saline 1 ml was intraperitoneally injected on day 16 after surgery. Blood samples from orbital venous sinus were collected 24 h after intraperitoneal injection. The SPT and FST were performed to investigate the antidepressant effect of ketamine 48 h after intraperitoneal injection. Then, the PFC tissues were collected.
Spared Nerve Injury (SNI)
The SNI surgery was performed as previously described79. With the anesthesia of intraperitoneal injection of 2% sodium pentobarbital (60 mg/kg, Sigma, St Louise, MO, USA), rats were incised from skin on the right thigh and bluntly dissected biceps femoris muscle. Sciatic nerve and its three terminal branches were exposed: the sural, common peroneal and tibial nerves. The common peroneal and tibial nerves were tight-ligated with 4–0 silk and sectioned the distal to the ligation. In the sham surgery, rats only received blunt dissection of the muscle and exposed nerve, but without ligation and cut of the nerve. Muscle and skin layers were then closed by 4–0 silk.
Mechanical withdrawal test (MWT)
A traditional Dixon up-down method with von Frey filaments was used to measure mechanical allodynia80, 81. Before the test, rats were individually placed into transparent plexiglass chambers over a mesh table and habituated for about 20 min. Von Frey filaments started with 2.0 g were applied to the lateral 1/3 of right paws (in the distribution of the sural nerve) of rats. The maximum score was 15.10 g, and the minimum was 0.25 g. Paw withdrawals in response to the stimuli were considered positive, and no withdrawal was negative. Depending on the positive or negative result, descending or ascending intensity of subsequent filament was applied, respectively. We calculated 50% withdrawal thresholds, as described previously80.
Sucrose preference test (SPT)
In the acclimation phase, animals drank two identical bottles (100 ml in each bottle) for 5 days: one contained 1% sucrose solution and the other contained water. For avoiding side preference, we alternated sides of the bottles every day. Before surgery, baseline sucrose preference was determined and only rats that displayed a sucrose preference ≥65% were included in this study25. During test phases 1 and 2, two bottles were provided for each animal for 24 h. At the end of each test, sucrose preference was calculated as volume of sucrose consumed divided by total liquid consumption for each rat.
Forced swim test (FST)
The FST was applied as documented in the previous study82. The FST was conducted in a transparent plexiglas cylinder (65 cm height, 30 cm diameter) filled with water (23–24 °C) to a depth of 45 cm for 6 min. Water in the cylinder was changed for each rat. After test, the animal was taken out of the cylinder, dried and put back into its home cage. Immobility time during last 5 min of the 6-min test was calculated as floating in the water without struggling except making necessary movements to keep its head above water.
Open field (OF)
On day 14 after surgery, rats were individually placed in the center of a gray polyvinyl chloride box (100 × 100 × 40 cm). Each rat was allowed to explore in the box for 5 min while the exploratory behavior and spontaneous motor activity were automatically recorded by a video tracking system.
Western blot analysis
Rats were sacrificed after anesthetized with sodium pentobarbital (80 mg/kg) and the PFC tissues were collected on ice plate. Tissues were homogenated in RIPA buffer and protease inhibitors. Homogenate was centrifuged at 12000 rpm at 4 °C centrifuge for 15 min, and the supernatant was retained. The protein concentration was determined by Bradford method using bovine serum albumin as a standard. The normalized protein samples were separated on 12% SDS-PAGE and transferred to polyvinylidene fluoride membrane. Membranes were then blocked with 5% non-fat milk at room temperature for 1 h and incubated overnight at 4 °C with rabbit anti-BDNF (1:1000, Abcam, ab203573) and rabbit anti-GAPDH (1:5000, Millipore). Membranes were washed 3 × 10 min with PBST and incubated with secondary antibody (goat anti-mouse and goat anti-rabbit, Bioworld Technology) diluted in 1:5000 for 1 h at room temperature. After washing the membranes 3 × 10 min, bands were visualized using chemiluminescence quantitated with Image J software (Wayne Rasband, National Institute of Health, MA, USA).
Enzyme-linked immunosorbent assay (ELISA)
Tissue levels of IL-1β, IL-6 and TNF-α in the PFC were quantified by the ELISA (n = 6 or 7 for each group, twice quantifications for each tissue) according to the protocol provided by the manufacturer (Jiancheng, China). Rats were killed by decapitation with the anesthesia of sodium pentobarbital (80 mg/kg), and the PFC tissues were collected. The tissue was homogenized with each 1 mg tissue added 9 uL saline. Homogenate was centrifuged for 10 min at 2500 rpm at 4 °C centrifuge, and the supernatant was obtained. Then each protein concentration was quantified by Bradford method. The standard curves for the cytokines were generated using Optical Density (OD) tested at 450 nm. The concentration of the cytokines was calculated by the standard curves.
Statistical analyses
In the experiment 1, the data were standardized by z scores. Then, hierarchical cluster analysis was performed using Ward’s method and applying squared Euclidean distance as the distance measure, and rats were classified as “rats with depression-like phenotype” or “rats without depression-like phenotype” clusters.
In the experiment 2, a two-group discriminant function analysis was performed to predict the rats with or without depression-like phenotype. A discriminant function analysis compared the two groups defined by the previous hierarchical cluster analysis in the experiment 1. The canonical discriminant function coefficients can be seen as an indication of the contribution each variable makes to the equation. Based on the discriminant function scores, rats were classified as “ketamine responder group” or “ketamine non-responder group” clusters.
Data are presented as the mean ± standard error of the mean (S.E.M.). Repeated measured one-way ANOVA, followed by Tukey test was used to detect interactions between test time points and groups. Data of Western blot and ELISA were analyzed using one-way ANOVA, followed by the Tukey test. Pearson correlation analysis was performed to examine the correlation between the changes of sucrose preference after ketamine injection and serum levels of cytokines at baseline. Statistical analyses were performed using SPSS16.0, with a significance level of P < 0.05.
Electronic supplementary material
Acknowledgements
This study was supported by the grants from the National Natural Science Foundation of China (to Z.Q.Z. grant nos. 81571083 and J.J.Y. grant nos. 81471105) and the Strategic Research Program for Brain Sciences from Japan Agency for Medical Research and Development, AMED (to K.H.)
Author Contributions
Z.M.X. and J.W. carried out the behavioral study and drafted the manuscript. X.M.W. and W.P. performed the Western blotting analysis. N.X. and X.H.T. carried out the enzyme-linked immunosorbent assay. Z.M.X. and Z.Q.Z. performed the statistical analysis. J.J.Y. and K.H. designed the study and helped to draft the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-03590-3
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kenji Hashimoto, Email: hashimoto@faculty.chiba-u.jp.
Jian-Jun Yang, Email: yjyangjj@126.com.
References
- 1.Maletic V, Raison CL. Neurobiology of depression, fibromyalgia and neuropathic pain. Front Biosci (Landmark Ed) 2009;14:5291–5338. doi: 10.2741/3598. [DOI] [PubMed] [Google Scholar]
- 2.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 3.Radat F, Margot-Duclot A, Attal N. Psychiatric co-morbidities in patients with chronic peripheral neuropathic pain: a multicentre cohort study. Eur J Pain. 2013;17:1547–1557. doi: 10.1002/j.1532-2149.2013.00334.x. [DOI] [PubMed] [Google Scholar]
- 4.Gustorff B, et al. Prevalence of self-reported neuropathic pain and impact on quality of life: a prospective representative survey. Acta Anaesthesiol Scand. 2008;52:132–136. doi: 10.1111/j.1399-6576.2007.01486.x. [DOI] [PubMed] [Google Scholar]
- 5.Uceyler N, Rogausch JP, Toyka KV, Sommer C. Differential expression of cytokines in painful and painless neuropathies. Neurology. 2007;69:42–49. doi: 10.1212/01.wnl.0000265062.92340.a5. [DOI] [PubMed] [Google Scholar]
- 6.Keay KA, Monassi CR, Levison DB, Bandler R. Peripheral nerve injury evokes disabilities and sensory dysfunction in a subpopulation of rats: a closer model to human chronic neuropathic pain? Neurosci Lett. 2004;361:188–191. doi: 10.1016/j.neulet.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Monassi CR, Bandler R, Keay KA. A subpopulation of rats show social and sleep-waking changes typical of chronic neuropathic pain following peripheral nerve injury. Eur J Neurosci. 2003;17:1907–1920. doi: 10.1046/j.1460-9568.2003.02627.x. [DOI] [PubMed] [Google Scholar]
- 8.Luedtke, K. et al. Assessment of depression in a rodent model of spinal cord injury. J Neurotrauma31, 1107–1121; doi:0.1089/neu.2013.3204 (2014). [DOI] [PMC free article] [PubMed]
- 9.Walters ET. Neuroinflammatory contributions to pain after SCI: roles for central glial mechanisms and nociceptor-mediated host defense. Exp Neurol. 2014;258:48–61. doi: 10.1016/j.expneurol.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Sacerdote P, et al. Cytokine modulation is necessary for efficacious treatment of experimental neuropathic pain. J Neuroimmune Pharmacol. 2013;8:202–211. doi: 10.1007/s11481-012-9428-2. [DOI] [PubMed] [Google Scholar]
- 11.Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426–436. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark AK, Old EA, Malcangio M. Neuropathic pain and cytokines: current perspectives. J Pain Res. 2013;6:803–814. doi: 10.2147/JPR.S53660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dowlati Y, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 16.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 17.Kwilasz AJ, Grace PM, Serbedzija P, Maier SF, Watkins LR. The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology. 2015;96:55–69. doi: 10.1016/j.neuropharm.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roque S, Correia-Neves M, Mesquita AR, Palha JA, Sousa N. Interleukin-10: a key cytokine in depression? Cardiovasc Psychiatry Neurol. 2009;2009:187894. doi: 10.1155/2009/187894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song C, Halbreich U, Han C, Leonard BE, Luo H. Imbalance between pro- and anti-inflammatory cytokines, and between Th1 and Th2 cytokines in depressed patients: the effect of electroacupuncture or fluoxetine treatment. Pharmacopsychiatry. 2009;42:182–188. doi: 10.1055/s-0029-1202263. [DOI] [PubMed] [Google Scholar]
- 20.DeVon HA, Piano MR, Rosenfeld AG, Hoppensteadt DA. The association of pain with protein inflammatory biomarkers: a review of the literature. Nurs Res. 2014;63:51–62. doi: 10.1097/NNR.0000000000000013. [DOI] [PubMed] [Google Scholar]
- 21.Han A, et al. IL-4/10 prevents stress vulnerability following imipramine discontinuation. J Neuroinflammation. 2015;12:197. doi: 10.1186/s12974-015-0416-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maes M, et al. Negative immunoregulatory effects of antidepressants: inhibition of interferon-gamma and stimulation of interleukin-10 secretion. Neuropsychopharmacology. 1999;20:370–379. doi: 10.1016/S0893-133X(98)00088-8. [DOI] [PubMed] [Google Scholar]
- 23.Kim H, et al. Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression. J Clin Invest. 2012;122:2940–2954. doi: 10.1172/JCI61884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norman GJ, et al. Stress and IL-1beta contribute to the development of depressive-like behavior following peripheral nerve injury. Mol Psychiatry. 2010;15:404–414. doi: 10.1038/mp.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dellarole A, et al. Neuropathic pain-induced depressive-like behavior and hippocampal neurogenesis and plasticity are dependent on TNFR1 signaling. Brain Behav Immun. 2014;41:65–81. doi: 10.1016/j.bbi.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burke NN, Kerr DM, Moriarty O, Finn DP, Roche M. Minocycline modulates neuropathic pain behaviour and cortical M1-M2 microglial gene expression in a rat model of depression. Brain Behav Immun. 2014;42:147–156. doi: 10.1016/j.bbi.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Felger JC, Haroon E, Miller AH. Risk and Resilience: Animal Models Shed Light on the Pivotal Role of Inflammation in Individual Differences in Stress-Induced Depression. Biol Psychiatry. 2015;78:7–9. doi: 10.1016/j.biopsych.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodes GE, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci USA. 2014;111:16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fagundes CP, Glaser R, Hwang BS, Malarkey WB, Kiecolt-Glaser JK. Depressive symptoms enhance stress-induced inflammatory responses. Brain Behav Immun. 2013;31:172–176. doi: 10.1016/j.bbi.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wood SK, et al. Inflammatory Factors Mediate Vulnerability to a Social Stress-Induced Depressive-like Phenotype in Passive Coping Rats. Biol Psychiatry. 2015;78:38–48. doi: 10.1016/j.biopsych.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mossner R, et al. Consensus paper of the WFSBP Task Force on Biological Markers: biological markers in depression. World J Biol Psychiatry. 2007;8:141–174. doi: 10.1080/15622970701263303. [DOI] [PubMed] [Google Scholar]
- 32.Al-Amin H, Sarkis R, Atweh S, Jabbur S, Saade N. Chronic dizocilpine or apomorphine and development of neuropathy in two animal models II: effects on brain cytokines and neurotrophins. Exp Neurol. 2011;228:30–40. doi: 10.1016/j.expneurol.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu E, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003;54:70–75. doi: 10.1016/S0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- 34.Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res. 2005;136:29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 35.Liu WX, et al. Regulation of glutamate transporter 1 via BDNF-TrkB signaling plays a role in the anti-apoptotic and antidepressant effects of ketamine in chronic unpredictable stress model of depression. Psychopharmacology (Berl) 2016;233:405–415. doi: 10.1007/s00213-015-4128-2. [DOI] [PubMed] [Google Scholar]
- 36.Zhang, J. C. et al. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int J Neuropsychopharmacol18, doi:10.1093/ijnp/pyu077 (2014). [DOI] [PMC free article] [PubMed]
- 37.Yang C, Shirayama Y, Zhang JC, Ren Q. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:e632. doi: 10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang LM, et al. Anxiolytic effects of ketamine in animal models of posttraumatic stress disorder. Psychopharmacology (Berl) 2015;232:663–672. doi: 10.1007/s00213-014-3697-9. [DOI] [PubMed] [Google Scholar]
- 39.Taliaz D, et al. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J Neurosci. 2011;31:4475–4483. doi: 10.1523/JNEUROSCI.5725-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krishnan V, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 41.Yang B, et al. Regional differences in the expression of brain-derived neurotrophic factor (BDNF) pro-peptide, proBDNF and preproBDNF in the brain confer stress resilience. Eur Arch Psychiatry Clin Neurosci. 2016;266:765–769. doi: 10.1007/s00406-016-0693-6. [DOI] [PubMed] [Google Scholar]
- 42.Zhang GF, et al. Repeated ketamine administration redeems the time lag for citalopram’s antidepressant-like effects. Eur Psychiatry. 2015;30:504–510. doi: 10.1016/j.eurpsy.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Berman RM, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/S0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 44.Murrough JW, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry. 2013;73:1133–1141. doi: 10.1016/j.biopsych.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, et al. A single subanesthetic dose of ketamine relieves depression-like behaviors induced by neuropathic pain in rats. Anesthesiology. 2011;115:812–821. doi: 10.1097/ALN.0b013e31822f16ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang GF, et al. Acute single dose of ketamine relieves mechanical allodynia and consequent depression-like behaviors in a rat model. Neurosci Lett. 2016;631:7–12. doi: 10.1016/j.neulet.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Li N, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-alpha) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. 2012;139:230–239. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 50.Yang, J.J., Zhou, Z.Q. & Yang, C. Letter to the editor: does ketamine exert a fast-acting antidepressant effect via inhibition of pro-inflammatory cytokines? Psychol Med41, 1787; author reply 1787–1789, doi:10.1017/S0033291711000699 (2011). [DOI] [PubMed]
- 51.Yang JJ, et al. Serum interleukin-6 is a predictive biomarker for ketamine’s antidepressant effect in treatment-resistant patients with major depression. Biol Psychiatry. 2015;77:e19–20. doi: 10.1016/j.biopsych.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 52.Hashimoto K. Inflammatory biomarkers as differential predictors of antidepressant response. Int J Mol Sci. 2015;16:7796–7801. doi: 10.3390/ijms16047796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Austin PJ, et al. Evidence for a distinct neuro-immune signature in rats that develop behavioural disability after nerve injury. J Neuroinflammation. 2015;12:96. doi: 10.1186/s12974-015-0318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gui, W.S. et al. Interleukin-1beta overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents. Mol Pain12, doi:10.1177/1744806916646784 (2016). [DOI] [PMC free article] [PubMed]
- 55.Austin PJ, Beyer K, Bembrick AL, Keay KA. Peripheral nerve injury differentially regulates dopaminergic pathways in the nucleus accumbens of rats with either ‘pain alone’ or ‘pain and disability’. Neuroscience. 2010;171:329–343. doi: 10.1016/j.neuroscience.2010.08.040. [DOI] [PubMed] [Google Scholar]
- 56.Nascimento FP, et al. Thalidomide reduces mechanical hyperalgesia and depressive-like behavior induced by peripheral nerve crush in mice. Neuroscience. 2015;303:51–58. doi: 10.1016/j.neuroscience.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 57.Fiore NT, Austin PJ. Are the emergence of affective disturbances in neuropathic pain states contingent on supraspinal neuroinflammation? Brain Behav Immun. 2016;56:397–411. doi: 10.1016/j.bbi.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 58.Tartter M, Hammen C, Bower JE, Brennan PA, Cole S. Effects of chronic interpersonal stress exposure on depressive symptoms are moderated by genetic variation at IL6 and IL1beta in youth. Brain Behav Immun. 2015;46:104–111. doi: 10.1016/j.bbi.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.You Z, et al. Pro- and anti-inflammatory cytokines expression in rat’s brain and spleen exposed to chronic mild stress: involvement in depression. Behav Brain Res. 2011;225:135–141. doi: 10.1016/j.bbr.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 60.Magaki S, Mueller C, Dickson C, Kirsch W. Increased production of inflammatory cytokines in mild cognitive impairment. Exp Gerontol. 2007;42:233–240. doi: 10.1016/j.exger.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tavakoli-Ardakani M, Mehrpooya M, Mehdizadeh M, Hajifathali A, Abdolahi A. Association between Interlukin-6 (IL-6), Interlukin-10 (IL-10) and depression in patients undergoing Hematopoietic stem cell transplantation. Int J Hematol Oncol Stem Cell Res. 2015;9:80–87. [PMC free article] [PubMed] [Google Scholar]
- 62.Hu X, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- 63.Yang C, Shirayama Y, Zhang JC, Ren Q, Hashimoto K. Regional differences in brain-derived neurotrophic factor levels and dendritic spine density confer resilience to inescapable stress. Int J Neuropsychopharmacol. 2015;18:pyu121. doi: 10.1093/ijnp/pyu121. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Sun HL, et al. Role of hippocampal p11 in the sustained antidepressant effect of ketamine in the chronic unpredictable mild stress model. Transl Psychiatry. 2016;6:e741. doi: 10.1038/tp.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu J, et al. BDNF pathway is involved in the protective effects of SS-31 on isoflurane-induced cognitive deficits in aging mice. Behav Brain Res. 2016;305:115–121. doi: 10.1016/j.bbr.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 66.Lu B, Nagappan G, Guan X, Nathan PJ, Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:401–416. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- 67.Calabrese F, et al. Brain-derived neurotrophic factor: a bridge between inflammation and neuroplasticity. Front Cell Neurosci. 2014;8:430. doi: 10.3389/fncel.2014.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goshen I, et al. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13:717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- 69.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qian J, Brown SD, Carlton SM. Systemic ketamine attenuates nociceptive behaviors in a rat model of peripheral neuropathy. Brain Res. 1996;715:51–62. doi: 10.1016/0006-8993(95)01452-7. [DOI] [PubMed] [Google Scholar]
- 71.Zanos P, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang C, Shirayama Y, Zhang JC, Ren Q, Hashimoto K. Peripheral interleukin-6 promotes resilience versus susceptibility to inescapable electric stress. Acta Neuropsychaitr. 2015;27:312–326. doi: 10.1017/neu.2015.36. [DOI] [PubMed] [Google Scholar]
- 73.Yang C, et al. Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice. Sci Rep. 2017;7:45942. doi: 10.1038/srep45942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kelly JR, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbiota axis: challenges for translation in psychiatry. Ann Epidemiol. 2016;26:366–372. doi: 10.1016/j.annepidem.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 75.Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20:145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bambico FR, et al. Neuroplasticity-dependent and -independent mechanisms of chronic deep brain stimulation in stressed rats. Transl Psychiatry. 2015;5:e674. doi: 10.1038/tp.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao X, Wang C, Cui WG, Ma Q, Zhou WH. Fisetin exerts antihyperalgesic effect in a mouse model of neuropathic pain: engagement of spinal serotonergic system. Sci Rep. 2015;5:9043. doi: 10.1038/srep09043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haj-Mirzaian A, et al. Evidence for the involvement of NMDA receptors in the antidepressant-like effect of nicotine in mouse forced swimming and tail suspension tests. Psychopharmacology (Berl) 2015;232:3551–3561. doi: 10.1007/s00213-015-4004-0. [DOI] [PubMed] [Google Scholar]
- 79.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 80.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 81.Bourquin AF, et al. Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain. 2006;122:14.e1–14. doi: 10.1016/j.pain.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 82.Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


