Abstract
Tobacco smoking is the world’s leading cause of premature death and disability. Global targets to reduce premature deaths by 25% by 2025 will require a substantial increase in the number of smokers making a quit attempt, and a significant improvement in the success rates of those attempts in low, middle and high income countries. In many countries the only place where the majority of smokers can access support to quit is primary care. There is strong evidence of cost-effective interventions in primary care yet many opportunities to put these into practice are missed. This paper revises the approach proposed by the International Primary Care Respiratory Group published in 2008 in this journal to reflect important new evidence and the global variation in primary-care experience and knowledge of smoking cessation. Specific for primary care, that advocates for a holistic, bio-psycho-social approach to most problems, the starting point is to approach tobacco dependence as an eminently treatable condition. We offer a hierarchy of interventions depending on time and available resources. We present an equitable approach to behavioural and drug interventions. This includes an update to the evidence on behaviour change, gender difference, comparative information on numbers needed to treat, drug safety and availability of drugs, including the relatively cheap drug cytisine, and a summary of new approaches such as harm reduction. This paper also extends the guidance on special populations such as people with long-term conditions including tuberculosis, human immunodeficiency virus, cardiovascular disease and respiratory disease, pregnant women, children and adolescents, and people with serious mental illness. We use expert clinical opinion where the research evidence is insufficient or inconclusive. The paper describes trends in the use of waterpipes and cannabis smoking and offers guidance to primary-care clinicians on what to do faced with uncertain evidence. Throughout, it recognises that clinical decisions should be tailored to the individual’s circumstances and attitudes and be influenced by the availability and affordability of drugs and specialist services. Finally it argues that the role of the International Primary Care Respiratory Group is to improve the confidence as well as the competence of primary care and, therefore, makes recommendations about clinical education and evaluation. We also advocate for an update to the WHO Model List of Essential Medicines to optimise each primary-care intervention. This International Primary Care Respiratory Group statement has been endorsed by the Member Organisations of World Organization of Family Doctors Europe.
Background: the tobacco epidemic
This paper provides an update to the original paper IPCRG Consensus statement: Tackling the smoking epidemic—practical guidance for primary care of 2008 published in this journal.1 Tobacco dependence is a deadly epidemic killing up to half its users.2 There are currently one billion smokers in the world and 80% live in low and middle income countries.3 Tobacco use is the leading cause of premature death globally due to the many diseases, which are attributed wholly or partially to smoking resulting in nearly six million deaths a year.4, 5 This annual total is higher than the combined mortality from malaria, tuberculosis and human immunodeficiency virus (HIV).6 Based on current trends, tobacco is expected to be responsible for 10% of global deaths, or eight million deaths a year by 2030, 80% in low and middle income countries: this includes a 9% decline in high income, but a doubling in low and middle income to 6.8 million.7 This is creating growing health inequalities at national and global levels due to the strong association of tobacco use with socio-economic deprivation.
There is a global commitment to tackle the epidemic. In 2011, the UN General Assembly adopted a political declaration that committed member states to the prevention and control of non-communicable diseases (NCDs). This included a target of a 25% reduction in premature mortality from NCDs by 2025 (“25 × 25”) and targets for nine risk factors, including a 30% relative reduction in smoking prevalence.8 This is anticipated to bring most health and economic benefit to low and middle income countries and included modelling of smoking attributable diseases such as cardiovascular diseases (CVDs), chronic respiratory diseases, tuberculosis, cancer, diabetes and respiratory infection.9 However, the latest modelling suggests a more ambitious target of a 50% reduction in smoking prevalence relative to 2010 levels would be the most feasible way to ensure the 25 × 25 target is reached.9
Tobacco control policies have already been very successful, lending force to the UN’s specialised agency, the World Health Organisation’s (WHO), Framework Convention on Tobacco Control (FCTC).10, 11 However, while public health measures such as raising taxes on cigarettes, plain packaging and advertising controls will prevent many from taking up smoking and encourage some to quit, many of those who are most tobacco dependent will not be able to quit without treatment from a health-care professional. Therefore, the FCTC includes a specific article on treating tobacco dependence, Article 14, which argues that cessation support is essential, and will also have a synergistic effect on the other control measures. The WHO has also called specifically for smoking cessation to be integrated into primary health care, as it is seen as the most suitable health setting for providing advice and support on smoking cessation globally. Yet only 15% of the world’s population has access to appropriate cessation support.9, 12
This statement is about treatment and not about prevention. However, we recognise that primary care has a role to play in prevention in measures such as:
public advocacy for the rigorous uptake of FCTC wherever the opportunity arises;
acting as role models by not smoking, maintaining smokefree premises, and placing no smoking posters in prominent places in their offices;
application of family medicine principles to identify and coach other members of the family who may be at risk of taking up smoking if there is a family member who smokes;
recording smoking status so that it is possible to monitor the impact of the interventions.
However, with regard to the FCTC, the most important duty is article 14—to identify smokers and treat them, with whatever resources are available, taking note of new research findings.
Individuals gain significant health benefits by quitting smoking (Table 1).13 Helping smokers quit is one of the most cost-effective interventions available to clinicians.14 Where smokers are aware of the dangers, most want to quit.1, 2 However, in countries such as China, that consumes 30% of the world’s tobacco, there is less awareness of the dangers and many less affluent people who remain more dependent smokers do not yet have intention to quit.15 Nicotine is addictive; tobacco dependence is recognised by WHO as a chronic relapsing condition16–18 that normally begins in childhood or adolescence.19, 20 Success rates are low for unaided attempts to quit.21 Clinicians should apply the same principles to tobacco dependence as to other chronic relapsing conditions. This includes offering patients accurate and timely diagnosis, evidence-based treatment that aims to co-produce outcomes with the patient using behavioural and pharmacological support, and regular review, anticipating the likelihood of relapse and, therefore, offering a system for repeated support to quit.
Table 1.
Health benefits of smoking cessations
| Time since quitting | Beneficial health changes that take place |
|---|---|
| 24 h | Lungs start to clear out mucus and other smoking debris |
| 48 h | Carbon monoxide will be eliminated from the body. Ability to taste and smell is greatly improved |
| 72 h | Breathing becomes easier. Bronchial tubes begin to relax and energy levels increase |
| 2–12 weeks | Circulation improves |
| 3–9 months | Coughs, wheezing and breathing problems improve as lung function is increased by up to 10% |
| 1 year | Risk of a heart attack falls to about half that of a smoker |
| 10 years | Risk of lung cancer falls to half that of a smoker |
| 15 years | Risk of heart attack falls to the same as someone who has never smoked |
From: McEwen, A., McRobbie, H., West, R. and Hajek, P. (2006) Manual for Smoking
Cessation: a guide for counsellors and practitioners. Oxford: Blackwell
Introduction
The health benefits of smoking cessation, and the efficacy and cost-effectiveness of medical treatment for tobacco dependence, are well established.22
For pharmacological therapies, it has been estimated that one person will successfully quit (achieve 6-month abstinence) for every 6–23 people treated.23 Given that approximately half of all long-term smokers will die of a smoking-related illness,24 smoking cessation is a highly effective preventive strategy, compared with other widely used primary prevention interventions, such as the use of statins, and, therefore, deserves to be made a priority by clinicians and policy-makers. This comparison can be demonstrated by numbers needed to treat (NNT) to achieve an intervention effect (Table 2). When it comes to treatment of tobacco dependence, the NNT to achieve one long-term quitter is low. Absolute quit rates data can differ between trials due to a number of variables, e.g., the definition of cessation, the socio-economic status of the population included and level of follow-up.22 To obtain one long-term quitter by using brief advice (<5 min) the NNT is 40,23 although the NNT can be as low as 10 when medication is combined with behavioural support.24 The NNT to achieve abstinence can be translated into NNT to prevent premature death if we take into account that for every two smokers who stops long-term, one would otherwise die from a smoking-related disease.1, 24 This figure is very low compared to other interventions such as the use of statins to prevent death (NNT 107), the primary prevention of MI or stroke death over one year (NNT 700)5, 25 or the 10-year prevention for cervical cancer screening (NNT 1140)26 (see Table 2).
Table 2.
Comparison of number needed to treat (NNT) to prevent one death. Smoking cessation medication is usually used for 3–6 months, while statins or antihypertensive medication might be used throughout life
| NNT comparison | ||
|---|---|---|
| Intervention | Outcome | NNT |
| Smoking cessation behavioural support plus | ||
| - NRT | Long-term quitter/premature death | 23/46 |
| - Bupropion | Long-term quitter/premature death | 18/36 |
| - Varenicline | Long-term quitter /premature death | 10/20 |
| Statins as primary prevention | Prevent one death over 5 years | 107 |
| Antihypertensive treatment for mild hypertension | Prevent one stroke or MI death over 1 year | 700 |
| Screening for cervical cancer | Prevent one death over 10 years | 1140 |
Health professionals such as family doctors working in primary care can play a vital role in helping their patients quit smoking. In 2008, the International Primary Care Respiratory Group (IPCRG) published the first Consensus Statement on practical smoking cessation guidance for primary care.1 The IPCRG is working closely with the WHO on the prevention and treatment of chronic lung disease as part of its NCD programme and it is important we offer practical guidance to support the WHO’s stated ambition for the primary-care diagnosis and treatment of tobacco dependence (http://www.who.int/tobacco/publications/building_capacity/training_package/treatingtobaccodependence/en/). A recent review indicates that brief advice to stop from a health-care worker, automated text messaging, and cytisine are globally affordable interventions.27 The next step is to review the essential medicines list produced by the WHO Model List of Essential Medicines28 and to increase the smoking cessation products that are included.
Opportunities for primary care
Primary health-care encounters represent frequent and important opportunities to identify tobacco use, provide advice and help people to quit.29, 30 The majority of smokers with a family physician see them at least once a year.31, 32 Many people consider their family physician as a key influence and source of advice about smoking.33, 34 However, despite the opportunity, and the effectiveness of interventions to help patients quit, many smokers do not receive support.35 There are many stated barriers to intervene including time constraints, confidence in raising the issue, competence, lack of training and resources, health professionals’ beliefs that brief advice is unlikely to be effective or that patients are not motivated to quit, and reluctance to jeopardise the doctor–patient relationship by giving unwelcome advice. 36–42
In reality, however, patient satisfaction with the consultation is generally higher when smoking is addressed.43, 44 There are many short and long-term beneficial health changes to motivate patients to quit (Table 1).
In addition health-care professionals’ own smoking behaviour and their attitude towards smokers and their motivation to help people quit is important, particularly in relation to the most dependent people from low socio-economic backgrounds.44
Since tobacco smoking is addictive, causes substantial morbidity and mortality and as a result causes huge costs for the health-care system, we believe it is our clinical responsibility to use every opportunity to prompt and help smokers to quit in an evidence-based way.
Guidance
Primary-care health professionals have an important role to play in triggering quit attempts and assisting with those quit attempts. Effective smoking cessation interventions in primary care are based on an awareness of which strategies have been shown to work (Table 3), and on making the most of available resources.
Table 3.
Adapting Very Brief Advice on Smoking to your context
| Intervention | Rationale | Considerations |
|---|---|---|
| Establish smoking status (ASK): “Do you smoke or use a waterpipe?” | Knowledge of smoking status is a prerequisite to any intervention | How will smoking status be recorded and how will you ensure that smoking status is recorded for all patients? |
| Advise that the best thing that the patient can do for their current and future health is to stop smoking (ADVISE) “Smoking tobacco leads to lung disease, cancers, heart disease and an early death. Stopping smoking is the single most important thing that you can do to improve your health.” | In some countries, knowledge about the harmful effects of smoking is high and so there is no need to advise smokers that smoking is harmful. Additionally, a meta-analysis revealed that offering advice without the offer of support did not prompt quit attempts. Where cessation services (face-to-face or quitlines) you can simply advise on the best way of quitting: “The best way of quitting is with a combination of behavioural support and medication. We have a local, friendly stop smoking service who are experts in this and I can refer you if you’d like?” | Does this element need to include more information on the harmful effects of smoking and the benefits of cessation? Are resources needed to supplement the advice? The advice will be dependent upon what support is available |
| Act on patients' response to advice (ACT) by either: (a) facilitating referral to cessation services, or alternative support (e.g., prescribing or referring to a pharmacy or doctor with appropriate recommendations); (b) making a note in their medical records that advice has been delivered if they do not want to quit | Behavioural support (from a trained specialist practitioner or primary care professional) is the most effective method of supporting a quit attempt, but quit lines or self-help materials can be used. Recording that advice has been delivered is a prompt to health professionals that they need to deliver it again at the next appropriate contact with the patient | If referral for support is not available, can support be offered within your practice? What medications are available? Are written support materials or other resources (e.g., websites) available? |
Key recommendations are summarised in Table 4.
Table 4.
Key recommendations
| Recommendation | Gradea |
|---|---|
| Make your practice ‘‘smoke free’’ by banning smoking on the premises, displaying information on smoking cessation in the waiting room, asking every patient about smoking status, and promoting smoking cessation services | B |
| Opportunistically provide brief, clear advice to quit whenever appropriate (doctors) and offer available assistance with any quit attempt | A |
| Train practice nurses and other staff to encourage smokers to quit and offer assistance | C |
| Recommend a local telephone counselling service (‘‘quit line’’), where available, to all smokers who indicate interest in quitting | A |
| Consider prescribing drug treatment for tobacco dependence (e.g., nicotine replacement therapy, bupropion, varenicline) to people who smoke 10 or more cigarettes per day, after consideration of contraindications and comorbidity | A |
| Tailor your approach to smoking cessation advice or treatment to the individual’s degree of readiness to quit | D |
| Use a non-judgemental communication style | C |
| Use motivational interviewing techniquesb to help people understand their own attitudes to smoking and quitting, make their own decisions and solve problems encountered during a quit attempt | B |
| Provide or arrange intensive behavioural counselling, where resources permit | A |
a Recommendations graded according to the Scottish Intercollegiate Guidelines Network system (described at http://www.bmj.com/cgi/content/full/323/7308/334 accessed January 2008)
b Effective when provided by trained counsellors
Use every opportunity to promote smoke-free living
Consider whether your practice can implement some or all of these strategies to raise awareness of the benefits of quitting and offer support.45 Become a ‘‘no-smoking’’ practice; institute a smoking ban on practice premises for staff as well as visitors. Place posters and smoking cessation literature in the waiting area. Set up systems to prompt you to ask each patient about smoking; routinely record smoking status in medical records, and review it at least yearly. A combination of these strategies, promoting use of effective medication and use of support services (such as telephone counselling services: ‘‘quit lines’’) can more than double quit rates among your patients, compared with no intervention.45–47
Opportunistically provide clear, personalised, non-judgemental advice to quit, and offer appropriate and available help to quit. For every 100 people who receive brief advice from a primary-care doctor to quit smoking, up to three extra people will succeed in quitting for at least 6 months than if no advice is given.7, 9 Approximately 40% of smokers make some attempt to quit after such advice.7 This shows that intervention really can make a difference (Fig. 1). We support the every contact count model (https://www.nice.org.uk/sharedlearning/making-every-contact-count-implementing-nice-behaviour-change-guidance), which requires every health-care professional in the system in contact with a smoker to have the competence and confidence to be able to help someone quit. More people see primary-care providers in a year, than any other health-care professional, and, therefore, primary care needs to get it right. Where people with respiratory conditions are managed in secondary care, smoking cessation should be the first line treatment for all smokers. Where primary care is the main caregiver, but refers to secondary care for an episode of care, for example from surgeons, anaesthetists and midwives then collaboration is essential as outcomes in surgery and maternity will improve with smoking cessation.
Fig. 1.
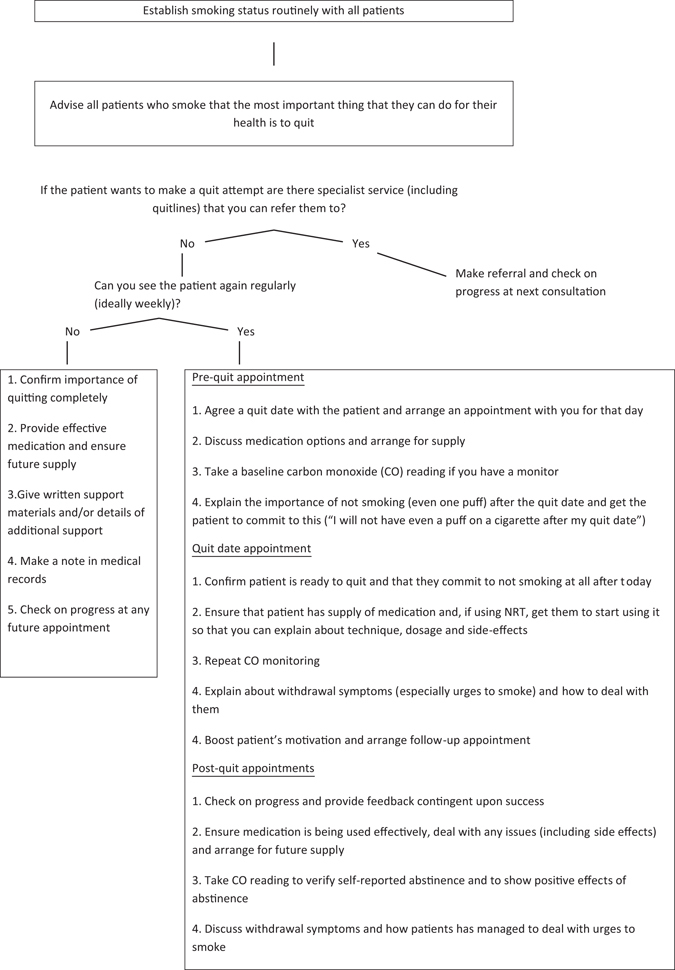
Deciding what smoking cessation interventions you can deliver
Support smokers who want to quit
Whenever smokers indicate they would like to quit, offer your support.45, 48–54 Provide reliable verbal and written information and offer consultations to help them to plan the quit attempt. Refer people to quit lines, where available, as this will be of additional benefit. Services that actively call people back for follow-up counselling are more effective than those providing only counselling on demand.46–51
Prescribe drug treatment for tobacco dependence as indicated (Table 5). Pharmacotherapy, particularly when accompanied by behavioural support, significantly improves long-term quit rates compared with no treatment or placebo.52
Table 5.
Pharmacotherapy for nicotine dependence
| Medicationa: |
| Any patient smoking more than 10 cigarettes a day or who smoke within 30 -60 minutes of waking will suffer from withdrawal symptoms and should be offered pharmacological support once they set a quit date. Remember to offer psychological support during the first 3 months of the cessation attempt |
| Nicotine replacement therapy (NRT) |
| NRT should not be combined with smoking. Its main effect is to reduce abstinence and help the patient through the first couple of months of craving. Most patients use too low doses for too short a time. They should use a dose that takes away abstinence symptoms. Most people need a full dose for 2–3 months, then they might gradually reduce the use over some months. Added success has been shown if NRT is started 14 days prior to quit date |
| Dosage: It is often wise to combine two different NRTs—a patch to cover most of the day and gum or other types of NRT (e.g. spray) for craving situations during daytime |
| Patch: Comes in 14 mg/24 h or 10 mg/16 h for light smokers or in 21 mg/24 h—15 mg/16 h for more heavy smokers. Some patients need more than one patch a day to keep the symptoms low |
| Side effects: Skin rash, allergy, insomnia, wild dreams |
| Gum, inhalers, lozenges, sublingual tablets: To be administered every 1–2 h for relief of symptoms while awake. Since nicotine is absorbed through the mucosa in the mouth it is important to instruct the patient in the use of gum carefully. Chew a few times on the gum then “park” it in the mouth |
| Side effects: local-sore dry mouth, dyspepsia, nausea, headache, jaw ache. Often dose dependent |
| Contraindication: Pregnancy (in some countries) |
| Varenicline (©Champix, ©Chantix) |
| Varenicline is a nicotinic receptor partial agonist. In addition to blocking the receptor it also stimulates it thus reducing abstinence. It is the first drug designed for smoking cessation. Results are promising with quit rates up to 44% in some studies |
| Dosage: Start 1 week before quit date: 0.5 mg for 3 days, 0.5 mg bid for 4 days, then 1 mg bid from quit date for 12 weeks |
| Side effects: nausea and headache. There is no danger of seizures. Risk of psychiatric side effects is the same as for other smoking cessation medications |
| Contraindication: Pregnancy |
| Bupropion (©Zyban) |
| Bupropion is the first medication proven to reduce the craving |
| Dosage: twice daily starting with one tablet a day for a week two weeks prior to quit date, then regularly 150 mg bid from quit date for 7–12 weeks |
| Adverse effects: insomnia, headache, dry mouth, dizziness, anxiety |
| Contraindications: Seizures, pregnancy, major depression, schizophrenia, drugs for treating depression or schizophrenia |
| Other medication: |
| Other drugs have shown to be effective in smoking cessation but are not licenced for use. The cost of these drugs are often low and should be considered in case cost is a limiting factor |
| Nortryptilyn (©Noritren) is cheap and has also been shown to be effective, but adverse effects that include sedation, dry mouth, lightheadedness and risks of cardiac arrhythmia in patients with CHD limit its application. It should thus be a second line agent |
| Cystisine (©Tabex) has a mechanism of action like Varenicline, binding to the nicotinic receptor. It has been used for smoking cessation in eastern European countries and has received increasing interest due to its low cost. Side effects include stomach-ache, dry mouth, dyspepsia and nausea |
| Which drug to advice? |
| Previous experience, availability, cost and patient’s preference will often guide the choice of medication. In a cost-effectiveness study from a high-income country, both NRT, Varenicline and Bupropion were shown to be cost-effective compared to placebob. Varenicline was show to be most cost-effective |
a Adapted from: http://www.theipcrg.org/display/TreatP/Helping+patients+quit+smoking%3A+Desktop+Helper+No.+4+-+2nd+Edition
b Hagen G., Wisløff T., Klemp M. Cost-effectiveness of varenicline, bupropion and nicotine replacement therapy for smoking cessation. Rapport fra Kunnskapssenteret nr. 10—2010. ISBN 978-82-8121-341-8 ISSN 1890-1298
Pharmacotherapy should be offered to people who smoke 10 or more cigarettes per day53–55 or who smoke within 30–60 min of waking, after consideration of contraindications and comorbidity. Selection of pharmacotherapy is based on clinical suitability, availability and patient choice.55
A motivational, non-judgemental style during consultations is more likely to engage patients.56–59 Take the role of an interested, empathising partner who asks questions that explore the smoker’s determination and ability to quit. Motivational interviewing acknowledges that the patient, not the doctor, is responsible for changing behaviour. Expired air carbon monoxide monitoring can validate self-reported quits but also act as a strong motivational tool.60 Nowadays carbon monoxide monitoring is not only used for research purposes, but also for clinical and this kind of feedback may considerably facilitate smoking cessation in primary care.61
Make the most of available time—Very Brief Advice on Smoking (VBA)
The aim of smoking cessation interventions is first of all to trigger or prompt quit attempts, and then to assist with a quit attempt. There is a role for every primary health-care professional in prompting a quit attempt by delivering VBA. This involves establishing smoking status (ASK); advising on the benefits of cessation and/or making the offer of help to quit (ADVISE); acting on the patients response and making a referral/providing support to quit as appropriate (ACT) [Table 3]. VBA is a short evidence-based and effective intervention that does not require assessment of readiness to quit (as the US 5 A’s approach does) and which combines the Assist and Arrange elements.62 The key elements are providing VBA opportunistically to all patients and offering whatever assistance is available to help prompt and support quit attempts. If a general practitioner diagnosed someone with hypertension, would he or she wait until they were ready to be treated, or start to talk to them about a treatment plan ?
Effective strategies can be incorporated into routine primary-care encounters, even where available consultation time is limited (Fig. 1). In addition, before health workers even mention smoking, a practice can already have sent patients a strong visual “quit smoking” message through posters and literature in the waiting room63 (Table 4).
“Asking” should be accompanied by an appropriate entry in the medical notes: tobacco dependency should be part of past medical history: consider it a “vital sign.”64
Harm reduction
For people who do not want, or who feel unable, to stop smoking, it could be beneficial to try to reduce harm from continued tobacco use. Options include reducing the amount of tobacco used; cutting down only or prior to stopping smoking (“cutting down to quit”) and smoking reduction and temporary abstinence from smoking.65 There is some evidence that smokers who regularly engage in temporary abstinence with the use of NRT are more likely to stop smoking in the future and there is evidence to support use of nicotine replacement prior to smoking cessation.66 Smokers who use NRT for smoking reduction are approximately twice as likely to progress to quitting than those who do not.67, 68
Electronic cigarettes
There is considerable debate about the place of electronic cigarettes (ECs) in smoking cessation and harm reduction and, as yet, only a limited number of good quality studies, especially on the subject of cessation. There are indications that suggest that on average e-cigarettes improve success rates, but evidence is still preliminary.69 The debate illustrates the importance of acknowledging and understanding the context for implementing all scientific evidence and primary-care practitioners should be aware of and be open to changes in both the context and the evidence. The context includes different national or regional public health approaches towards harm reduction,70 interpretation of current evidence by different medical associations, legal frameworks and licensing decisions. Most governments have not licensed e-cigarettes for smoking cessation. In some countries, the medicinal products listed in Table 5 are hard to access or unaffordable, but e-cigarettes are available.
Based on the concentrations of chemicals in e-cigarettes, vapour would be expected to be less harmful than smoking, though not free of risk.70 As the evidence accumulates recommendations may change but at present it is recommended that patients be advised to use methods of quitting with strong evidence for effectiveness where these are available. If patients ask about e-cigarettes, they should be informed that there is currently little hard evidence on the effectiveness of e-cigarettes in smoking cessation. They should also be told that while they are likely to be substantially safer than smoking, they probably do carry some risk and long-term use should be avoided if possible. On the basis of current evidence, they should also be told that use of an e-cigarette may impede their chances of quitting smoking at a later date. E-cigarettes should never be advised as a life-style choice. The family doctor has to realise that he is not only treating the adult smoker but also the smokers’ family and for adolescents observing the use of e-cigarettes by parents may be a gateway to ultimately smoking.
Groups that needs special attention
Pregnant women
Smoking cessation interventions are particularly important during pregnancy as tobacco smoking in pregnancy remains one of the few preventable factors associated with stillbirth, complications in pregnancy, low birthweight, preterm birth71, 72 and has serious long-term health implications for women and infants including the development of asthma.73 Post-partum follow-up reduces relapse rates.
While smoking in pregnancy is decreasing in high-income countries, worldwide the prevalence of tobacco smoking and smokeless tobacco use among women is increasing and the WHO characterises this rise of tobacco use in young women in low-income, high-population countries as one of the most ominous developments of the tobacco epidemic.74 The use of carbon monoxide screening or monitoring can be an effective non-judgemental way of identifying maternal exposure to tobacco smoke that might not otherwise be discussed.75, 76
Nicotine replacement therapy may be appropriate, subject to local prescribing regulations, given that the dose of nicotine is lower than the dose from cigarettes. Single dose NRT will typically give less than half the amount of nicotine delivered via smoking, but without carbon monoxide and other toxins.77 An observational study has suggested combination NRT may be more effective than single form NRT (patch or rapid acting form) in pregnant women.78 This finding may be related to faster metabolism of nicotine in pregnancy.67 Using NRT is clearly better than smoking cigarettes and, therefore, most national smoking cessation guidelines weigh up the benefits as stronger than the risks and, therefore, recommend using some form of NRT during pregnancy. If used, oral forms are usually preferred due to lower total dose of nicotine. Bupropion and varenicline are not recommended in pregnancy.79 Breastfeeding mothers can use NRT since nicotine levels in the infant from NRT while breastfeeding are low and are unlikely to be harmful. Advise that they use it after breastfeeding, and if they relapse, similarly advise them to smoke immediately after the feed to allow more time for the toxins to leave the breast milk before the next feed.80–82
Children and adolescents
Approximately 80% of smokers begin smoking during their teenage years. Nicotine dependence develops very rapidly in teenagers. Among teenagers, 10% do so within 2 days of inhaling from a cigarette for the first time, and 50% by the time they are smoking seven cigarettes per month.83
In general, teenagers care more about the immediate benefits to their appearance, current well-being and financial status than about future health gains. Therefore, it is useful to emphasise the following benefits of quitting in addition to long-term health: better physical appearance including teeth, avoiding bad breath, cost savings (e.g., calculate the amount spent per year on cigarettes) and better sexual performance.
Sociocultural background and socioeconomic status
Cultural issues will differ between primary-care settings, depending on the country and its cultural norms, the sociocultural backgrounds of health professionals, and the socioeconomic status of their patients. Independent of desire to quit, groups who are socioeconomically deprived face additional challenges and may need more support to quit and avoid relapse. In Europe, for instance, quit rates seen over the past 10 years have been higher and uptake lower among groups with higher socioeconomic and education status.84 Those groups that have not yet benefited from the trend towards a smoke-free lifestyle stand to benefit most from well-directed support from their primary-care health professionals. Governments should take a firm stand in this regard, not being led by considerations of losing tax revenues by reduced sales of cigarettes.
Patients with chronic diseases
It is important to target this large population of smokers for smoking cessation support given the role that smoking plays in causing or exacerbating many long-term conditions. Patients who continue to smoke despite suffering from a smoking-related condition are likely to be more dependent and thus require more support.
Respiratory diseases
Smoking cessation is the key intervention for slowing progression of chronic obstructive pulmonary disease (COPD). There is a clear relationship between continued smoking and progression of COPD.85 Smoking in patients with COPD is associated with a faster decline in lung function and an increase in symptoms, as well as an increased risk for respiratory tract infection and hospitalisation86 and they show higher levels of depression and cigarette dependence.87 In people with asthma, smoking further impairs lung function, increases symptoms and exacerbations and impairs the effectiveness of treatment and quality of life.88 Successful cessation support strategies that are specific to patients with COPD include the use of pharmacotherapy, explaining the relationship between smoking and COPD and using spirometric results, CO monitors and “lung age” to increase motivation to quit.89
Cardiovascular diseases
For patients with CVD smoking cessation is a key intervention.90 In people with coronary heart disease, smoking cessation is associated with a 36% reduction in mortality compared to those who continue to smoke. Many people admitted to hospital with CVD attempt to quit but it has been shown that follow-up in the community is important for sustained cessation.91
Cancers
Continued smoking after a diagnosis of lung cancer is associated with worse quality of life and all clinical outcomes, including survival. People with successfully treated cancers who continue to smoke are at increased risk of a second cancer. Many studies report that smoking cessation after a diagnosis of lung cancer is associated with health improvements. There is good evidence that interventions in this group are effective and health-care personel must remember to offer both medication and psychosocial support for these patients.92
Human immunodeficiency virus
Evidence suggests the prevalence of smoking among HIV infected people is higher than in the general population. Smoking in HIV infected people is associated with an increase in the occurrence of several communicable and NCDs. CVD risk is increased twofold and all-cause mortality is higher in HIV infected smokers compared to those who do not smoke. In one study, the risk of bacterial pneumonia decreased by 27% following smoking cessation.93 Smokers with HIV who receive and take antiretroviral therapy now lose more life years to smoking than they do to HIV itself.94, 95 The majority of smokers living with HIV report being interested in quitting, and a significant proportion have made recent quit attempts. Based on this evidence, every HIV care programme should include a service to screen for tobacco dependence and support smoking cessation for clients.
Tuberculosis
Evidence exists that smoking greatly increases the risk of tuberculosis (TB).96 Surveys have shown that the prevalence of smoking among confirmed and presumptive TB patients is higher than in the general population.97, 98 Continued smoking during TB treatment is also associated with worse TB treatment outcomes (treatment failure and death).99, 100 There is good evidence that smoking cessation works for TB patients: abstinence rates greater than 50% can be achieved at the end of TB treatment with simple interventions such as brief advice.101–103
Patients living with mental illness
Smoking is common among people diagnosed with psychiatric disorders and smoking rates are substantially higher than the general population.104 Depression is especially related to smoking.105 Patients with major mental illness die prematurely compared with people with non-major mental illness diagnoses. The causes are mostly due to an increase of smoking related diseases including heart disease, cancer, cerebrovascular, respiratory, and lung diseases.106
Therefore, smoking cessation is essential for people with mental illness. This goal is difficult because many health professionals are reluctant to tackle the problem, as they are afraid that quitting may worsen patients’ mental health. The fact is that smoking cessation actually improves mental health107 and should be recommended for all people with mental illness. Although mood problems are clearly different from psychotic disorders there are no indications that these patients should be treated differently in smoking cessation. Importantly, people with serious mental illness are almost as likely as the general population to want to stop smoking.108 There is also good evidence of efficacy of treatment in this group of patients.109 People with mental illness should be offered the same pharmacotherapy as the general population, although with closer monitoring110 of possible interaction between cessation medication and antipsychotic medication (for more information on drug interactions with tobacco smoke, please have a look at the fact sheet: Drug Interactions With Tobacco Smoke https://smokingcessationleadership.ucsf.edu/sites/smokingcessationleadership.ucsf.edu/files/Drug-Interactions-with-Tobacco-Smoke.pdf).
The doses of antipsychotic medication may need to be adjusted after smoking cessation.111
In meta analyses of varenicline, the most effective treatment in smoking cessation,112 there was no evidence of increased risk of suicide or attempted suicide, suicidal ideation, depression or death in varenicline users compared with placebo users.113 A recent large study examining safety and efficacy of varenicline, bupropion and nicotine patch in smokers with and without psychiatric disorders found no increase in neuropsychiatric adverse effects attributable to these medicines compared to placebo.114
In conclusion, smokers with chronic mental illness can quit smoking with minimal effects on psychiatric symptoms using standard cessation approaches, but with closer monitoring from experienced health professionals, and may need more quit attempts.115, 116 Their benefits in terms of years of life gained are more significant than the general population given their current premature mortality from cardio-respiratory conditions largely due to smoking.
People using waterpipes—shisha
Waterpipe tobacco smoking (also known as shisha, hookah, or narghile smoking) is now a common form of tobacco use among adolescents worldwide, especially in the Middle East and Eastern Europe,117 and prevalence is rising in adults.118
Due to the mistaken perception of reduced harm associated with waterpipe tobacco use,119 asking specifically about waterpipe tobacco use in a consultation may uncover a significant tobacco history that would otherwise be missed.120 A recent Cochrane review concluded that waterpipe cessation interventions are sparse.121 However, given a shared nicotine and adverse health outcome component, interventions for cigarette cessation could be a useful consideration for waterpipe tobacco users.121
People using cannabis and tobacco—joints
Fifty percent of cannabis users also smoke tobacco and this impairs successful withdrawal from cannabis.122 Tobacco smoking precedes cannabis use but the reverse gateway effect has also been described.123 Quitting tobacco can help people withdraw from other substance misuse so there appears to be a case for added value by helping people and designing services that assist with dual use. It has been suggested that the absence of an ICD code for cannabis withdrawal disorder may impact on the availability of pathways and services for this group. It, therefore, may fall to the general practitioner to support the cannabis and tobacco user in a quit attempt utilising what is available locally for each problem separately.
While there is limited evidence for what constitutes effective treatment, there is increasing concern about the impact of smoking cannabis and tobacco in ‘‘joints’’ and its impact on respiratory health.124 Studies suggest that simultaneously quitting both tobacco and cannabis may yield benefits at both the psychological and neurobiological level.125 One study involved a twelve-week program comprising computer-assisted delivery of Motivational Enhancement Therapy, Cognitive Behavioral Therapy, and Contingency Management, i.e., abstinence-based incentives for cannabis use disorder (CUD).122 In addition, participants were encouraged to complete an optional tobacco intervention consisting of nicotine-replacement therapy and computer-assisted delivery of a behavioural treatment tailored for tobacco and cannabis users. Simultaneously targeting tobacco during treatment for CUD did not negatively impact cannabis outcomes. Participation in the tobacco intervention was high, but cessation outcomes were poor suggesting that alternative strategies might be needed to more effectively prompt quit attempts and enhance quit rates. In terms of pharmacotherapy, a Cochrane systematic review has not found evidence for drugs that are used in other addiction therapy such as bupropion but that cannabinoid replacement therapy studies suggest a potential hope.126 Currently, however, it appears the best available evidence supports a tobacco intervention with pharmacotherapy in combination with a dual behavioural intervention.
Need for gender-specific interventions
Our knowledge on gender shows that women need special attention as they are more predisposed to suffer the adverse consequences of tobacco smoking and need gender-specific counselling when quitting. Women develop COPD at an earlier age and with a greater degree of lung function impairment for a given amount of tobacco exposure.127 Although the benefit is clear, women have more difficulties in stopping smoking128, 129 and among quit attempters, women had 31% lower odds of successfully quitting.130
Another important factor that should be taken in consideration is the menstrual phase. Better treatment outcomes can be achieved by scheduling quit dates to coincide with the follicular phase of the menstrual cycle in female smokers.131 Nicotine and cotinine metabolism is faster in women than in men and is faster in women taking oral contraceptives compared with those who are not;132 there may be a need for higher doses of NRT in women. Regarding pharmacological approaches, NRT may have less beneficial effects.133, 134 Long-term maintenance of NRT treatment gains decrease more rapidly for women than men.125 By contrast, both buproprion, and varenicline135 are equally effective for men and women.
Conclusions
There are growing health inequalities at national and global levels, with a strong association between tobacco use and socio-economic deprivation. For example, in the UK it is estimated one million people could be raised out of poverty if they quit smoking.136 Global targets to reduce premature deaths by 25% by 2025 require a substantial increase in smokers making a quit attempt especially in low and middle income countries. Physicians (including GPs) readily prescribe statins or antihypertensive treatment while the efficacy to prevent death is much better with smoking cessation treatment.
We strongly advocate for all primary-care professionals to avoid assumptions and to Ask (and record), Advise and Act. Act as if smoking—tobacco dependence—is a chronic relapsing-remitting disorder with highly effective and cost effective treatments, deliverable in primary care.
All can make an effective contribution in encouraging and helping existing adult smokers to quit and encouraging teenagers not to begin smoking. Evidence-based smoking cessation strategies can be incorporated into any primary-care practice, tailored to practice style, the patient demographics and the time and resources available.
Accordingly, the IPCRG guidance is offered as a set of suggested strategies based on those that have proved effective in their original sociocultural settings. In the absence of supportive government policies, implementation of the guidance will be particularly challenging for individual primary-care health professionals.
Acknowledgements
We would like to thank the following persons for their valuable contributions to this manuscript: Dr Andy Mc Ewen of the National Centre of Smoking Cessation Training (NCSCT), London, UK and prof Robert West, University College London, UK.
Author contributions
All authors contributed to this paper. C.P.v.S., S.W., A.O. coordinated the writings and C.P. v.S. had the final responsibility for this paper.
Competing interests
The authors declare that they have no competing financial interests.
Footnotes
An erratum to this article is available at https://doi.org/10.1038/s41533-017-0048-4.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history: A correction to this article has been published and is linked from the HTML version of this article.
References
- 1.Schayck OCP, et al. IPCRG Consensus statement: tackling the smoking epidemic-practical guidance for primary care. Prim. Care Respir. J. 2008;17:185–193. doi: 10.3132/pcrj.2008.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Tobacco. Fact Sheet 339. http://www.who.int/mediacentre/factsheets/fs339/en/Accessed 31 January 2016.
- 3.Eriksen, M., Mackay, J., Neil Schluger, N. et al. Tobacco Atlas 5th edn, (American Cancer Society, 2015). www.tobaccoatlas.org. Accessed 1 February 2016.
- 4.World Health Organization WHO Report on the Global Tobacco Epidemic, 2008: The MPOWER package. Geneva: World Health Organization, 2008.
- 5.WHO Report on the Global Tobacco Epidemic, 2011: Warning about the dangers of tobacco. http://apps.who.int/iris/bitstream/10665/44616/1/9789240687813_eng.pdf. Accessed 1 February 2016.
- 6.WHO global report: mortality attributable to tobacco. World Health Organization 2011. http://apps.who.int/iris/bitstream/10665/44815/1/9789241564434_eng.pdf. Accessed 2 February 2016.
- 7.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.United Nations, General Assembly Political declaration of the high-level meeting of the general assembly on the prevention and control of non-communicable diseases, A/66/L.1 (16 September 2011). http://www.un.org/ga/search/view_doc.asp?symbol=A/66/L.1. Accessed 31 January 2016.
- 9.Kontis V, Mathers CD, Rehm J, et al. Contribution of six risk factors to achieving the 25x25 non-communicable disease mortality reduction target: a modelling study. Lancet. 2014;384:427–437. doi: 10.1016/S0140-6736(14)60616-4. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization WHO Framework Convention on Tobacco Control, (World Health Organization, 2003).
- 11.WHO. on the Global Tobacco Epidemic, 2015. http://www.who.int/tobacco/global_report/2015/report/en/ Accessed 1 February 2016.
- 12.Raw M, Mackay J, Reddy S. Lancet [Internet]. Elsevier Ltd. 2016. Time to take tobacco dependence treatment seriously; pp. 1–2. [DOI] [PubMed] [Google Scholar]
- 13.Action on Smoking and Health. ASH Fact Sheet on Stopping smoking: the benefits and aids to quitting. September 2014. www.ash.org.uk. Accessed 1 February 2016.
- 14.Papadakis S, Pipe A, Kelly S, Pritchard G, Wells GA. Strategies to improve the delivery of tobacco use treatment in primary care practice (Protocol) Cochrane Database Syst. Rev. 2015 [Google Scholar]
- 15.She J, Yang P, Hong Q, Bai C. Lung cancer in China. Chest. 2013;143:1117–1126. doi: 10.1378/chest.11-2948. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. International Statistical Classification of Diseases World Health Organization. International Statistical and Related Health Problems (10th Revision). (WHO, 1992).
- 17.Fiore, M. C., Jaen, C. R., Baker, T. B. et al. Treating Tobacco Use and Dependence: 2008 Update, (U.S. Department of Health and Human Services, U.S. Public Health Service, 2008).
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edn. (American Psychiatric Association, 2013).
- 19.Fact Sheet on Youth and Smoking. Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion. 2014. http://www.cdc.gov/tobacco/data_statistics/fact_sheets/youth_data/tobacco_use/accessed 31 January 2016.
- 20.Stanton, A., Grimshaw, G. (2013) Tobacco cessation interventions for young people. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No.: CD003289. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD003289.pub5/abstract. Accessed from 1 February 2016.
- 21.Baillie AJ, Mattick RP, Hall W. Quitting smoking: estimation by meta-analysis of the rate of unaided smoking cessation. Aust. J. Public Health. 1995;19:129–131. doi: 10.1111/j.1753-6405.1995.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 22.da Costa e Silva V., (eds). Tools for Advancing Tobacco Control in the XXIst Century: Policy Recommendations for Smoking Cessation and Treatment of Tobacco Dependence. Tools for Public Health, (World Health Organization, 2003).
- 23.Watts SA, Noble SL, Smith PO, Disco M. First-line pharmacotherapy for tobacco use and dependence. J. Am. Board. Fam. Pract. 2002;15:489–497. [PubMed] [Google Scholar]
- 24.Edwards R. The problem of tobacco smoking. BMJ. 2004;328:217–219. doi: 10.1136/bmj.328.7433.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbot NC, Stead LF, White AR, Barnes J. Hypnotherapy for smoking cessation. Cochrane Database Syst. Rev. 1998 doi: 10.1002/14651858.CD001008. [DOI] [PubMed] [Google Scholar]
- 26.West R, McNeill A, Raw M. Smoking cessation guidelines for health professionals: an update. Thorax. 2000;55:987–999. doi: 10.1136/thorax.55.12.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.West R, Raw M, McNeill A, et al. Health-care interventions to promote and assist tobacco cessation: a review of efficacy, effectiveness and affordability for use in national guideline development. Addiction. 2015;110:1388–1403. doi: 10.1111/add.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker N, et al. Getting cytisine licensed for use world‐wide: a call to action. Addiction. 2016;111:1895–1898. doi: 10.1111/add.13464. [DOI] [PubMed] [Google Scholar]
- 29.Royal College of General Practitioners. The 2022 GP. Compendium of Evidence. Royal College of General Practitioners, London. 2013. http://www.rcgp.org.uk/~/media/Files/Policy/A-Z-policy/The-2022-GP-Compendium-of-Evidence.ashx. Accessed 10 February 2016.
- 30.Tønnesen H, Nielsen PR, Lauritzen JB, et al. Smoking and alcohol intervention before surgery: evidence for best practice. Br. J. Anaesth. 2009;102:297–306. doi: 10.1093/bja/aen401. [DOI] [PubMed] [Google Scholar]
- 31.Zwar NA, Richmond RL. Role of the general practitioner in smoking cessation. Drug Alcohol Rev. 2006;25:21–26. doi: 10.1080/09595230500459487. [DOI] [PubMed] [Google Scholar]
- 32.Jorm LR, Shepherd LC, Rogers KD, Blyth FM. Smoking and use of primary care services: findings from a population-based cohort study linked with administrative claims data. BMC Health Serv. Res. 2012;18:263. doi: 10.1186/1472-6963-12-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richmond R, Kehoe L, Heather N, Wodak A, Webster I. General practitioners’ promotion of healthy life styles: what patients think. Aust. N. Z. J. Pub. Health. 1996;20:195–200. doi: 10.1111/j.1753-6405.1996.tb01818.x. [DOI] [PubMed] [Google Scholar]
- 34.Duaso M, Cheung P. Health promotion and lifestyle advice in general practice: what do patients think? J. Adv. Nurs. 2002;39:472–9. doi: 10.1046/j.1365-2648.2002.02312.x. [DOI] [PubMed] [Google Scholar]
- 35.Abi-Fadel F, Gorga J, Fahmy S. Smoking cessation counselling: who does best pulmonologists or GPs? Prim. Care. Respir. J. 2013;22:17–18. doi: 10.4104/pcrj.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coleman T, Wilson A. Anti-smoking advice in general practice consultations: general practitioners’ attitudes, reported practice and preconceived problems. Br. J. Gen. Pract. 1996;46:87–91. [PMC free article] [PubMed] [Google Scholar]
- 37.Bauman A, Mant A, Middleton L, et al. Do general practitioners promote health? A needs assessment. Med. J. Aust. 1989;151:262–269. [PubMed] [Google Scholar]
- 38.Twardella D, Brenner H. Lack of training as a central barrier to the promotion of smoking cessation: a survey among general practitioners in Germany. Eur. J. Public Health. 2005;15:140–145. doi: 10.1093/eurpub/cki123. [DOI] [PubMed] [Google Scholar]
- 39.Edwards D, Freeman T, Litt J, Roche A. GPs’ confidence in and barriers to implementing smoking cessation activities: Compared to dentists, dental hygienists and pharmacists. Aust. J. Primary Health. 2006;12:117–125. doi: 10.1071/PY06054. [DOI] [Google Scholar]
- 40.Richmond RL, Anderson P. Research in general practice for smokers and drinkers. Part 3: Dissemination of interventions. Addiction. 1994;89:49–62. doi: 10.1111/j.1360-0443.1994.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 41.Young JM, Ward JE. Implementing guidelines for smoking cessation advice in Australian general practice: opinions, current practices, readiness to change and perceived barriers. Fam. Pract. 2001;18:14–20. doi: 10.1093/fampra/18.1.14. [DOI] [PubMed] [Google Scholar]
- 42.Barzilai D, Goodwin M, Stange K. Does health habit counseling affect patient satisfaction? Prev. Med. 2001;33:595–599. doi: 10.1006/pmed.2001.0931. [DOI] [PubMed] [Google Scholar]
- 43.Sciamanna C, Novak S, Houston T, Gramling R, Marcus B. Visit satisfaction and tailored health behavior communications in primary care. Am. J. Prev. Med. 2004;26:426–430. doi: 10.1016/j.amepre.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Dirven JAM, Moser A, Tange HJ, Muris JWM, van Schayck OCP. An innovative COPD early detection programme in general practice: evaluating barriers to implementation. NPJ Prim. Care Respir. Med. [Internet] 2014;24:14055. doi: 10.1038/npjpcrm.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zwar N., et al. Smoking Cessation Guidelines for Australian General Practice (Commonwealth of Australia, 2006). Available from. doi:10.1038/npjpcrm.2014.55 [PubMed]
- 46.Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst. Rev. 2007 doi: 10.1002/14651858.CD006103.pub2. [DOI] [PubMed] [Google Scholar]
- 47.World Health Organization (WHO). European Partnership Project to Reduce Tobacco Dependence. WHO evidence based recommendations on the treatment of tobacco dependence. Copenhagen: WHO, 2001.
- 48.Gailly J. Arrêter de Fumer / Stoppen met Roken. 2005. (http://www.ssmg.be/new/files/RBP_Tabac.pdf and http://www.wvvh.be/Page.aspx?id=945, accessed December 2007).
- 49.Chavannes NH, et al. NHG-Standaard Stoppen met roken. Huisarts. Wet. 2007;50:306–314. doi: 10.1007/BF03085152. [DOI] [Google Scholar]
- 50.Sosial- og helsedirektoratet, Røykeavvenning i primærhelsetjenesten. June 2004 (http://www.shdir.no/vp/multimedia/archive/00001/IS-1171_1860a.pdf. Accessed December 2016).
- 51.Torrecilla García, M., Plaza Martin, M. D., Ruano García, R. Consejo medico e intervención minima sitematizada. En: Manual de Prevención y Tratamiento del Tabaquismo. Capítulo IV. Barrueco Ferrero M et al. 2006.
- 52.Barchilón Cohen, V., Morán Rodríguez, A., Espigares Jiménez, M., et al. Tabaquismo. Abordaje en Atención Primaria. Sociedad Andaluza de Medicina Familiar y Comunitaria (SAMFYC). (http://www.cica.es/~samfyc-gr Accessed December 2007).
- 53.Raw M, et al. Europe evidence based recommendations on the treatment of tobacco dependence. Tob. Control. 2002;11:44–46. doi: 10.1136/tc.11.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aveyard P, West R. Managing smoking cessation. BMJ. 2007;335:37–41. doi: 10.1136/bmj.39252.591806.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zwar N., Richmond R., Borland R., et al. Smoking Cessation Pharmacotherapy: an Update for Health Professionals (Royal Australian College of General Practitioners, 2007).
- 56.Miller W. R., Rollnick S. Motivational Interviewing, Preparing People to Change Addictive Behavior (The Guildford Press, 1991).
- 57.Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br. J. Gen. Pract. 2005;55:305–312. [PMC free article] [PubMed] [Google Scholar]
- 58.Rollnick S, et al. Consultations about changing behaviour. BMJ. 2005;331:961–963. doi: 10.1136/bmj.331.7522.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soria R, Legido A, Escolano C, López Yeste A, Montoya J. A randomised controlled trial of motivational interviewing for smoking cessation. Br. J. Gen. Pract. 2006;56:768–774. [PMC free article] [PubMed] [Google Scholar]
- 60.West R, Michie S. Carbon monoxide verified 4-week quit rates in the English stop smoking services before versus after establishment of the National centre for smoking cessation and training. Smok. Br. 2013;1:1–5. [Google Scholar]
- 61.Bell, R., et al. Evaluation of a complex healthcare intervention to increase smoking cessation in pregnant women: interrupted time series analysis with economic evaluation. Tob. Control.(2017) Feb 10:tobaccocontrol-2016. [DOI] [PMC free article] [PubMed]
- 62.Aveyard P, Begh R, Parsons A, West R. Brief opportunistic smoking cessation interventions: a systematic review and meta‐analysis to compare advice to quit and offer of assistance. Addiction. 2012;107:1066–1073. doi: 10.1111/j.1360-0443.2011.03770.x. [DOI] [PubMed] [Google Scholar]
- 63.Persson LG, Lindström U. A waiting room inquiry about smoking resulted in more smoking cessation referrals. Unexpected many smokers quit smoking. Lakartidningen. 2006;104:2427–2430. [PubMed] [Google Scholar]
- 64.Fiore MC. The new vital sign: assessing and documenting smoking status. JAMA. 1991;266:3183–3184. doi: 10.1001/jama.1991.03470220099036. [DOI] [PubMed] [Google Scholar]
- 65.NICE guidelines [PH45] smoking: Harm Reduction. Published date: June 2013.
- 66.Schiffman S, Ferguson SG. Nicotine patch therapy prior to quiting smoking: a metaanalysis. Addiction. 2008;103:557–563. doi: 10.1111/j.1360-0443.2008.02138.x. [DOI] [PubMed] [Google Scholar]
- 67.Stead, L. F., Lancaster, T. Interventions to reduce harm from continued tobacco use. The Cochrane Library (2007). [DOI] [PubMed]
- 68.The Royal Australian College of General Practitioners Supporting smoking cessation: a guide for health professionals ISBN 978-0-86906-331-6 Published December 2011. Updated June 2012.
- 69.Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir. Med. 2016;4:116–128. doi: 10.1016/S2213-2600(15)00521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Green SH, Bayer R, Fairchild AL. Evidence, policy, and E- cigarettes- Will England reframe the debate? N. Engl. J. Med. 2016;374:1301–1303. doi: 10.1056/NEJMp1601154. [DOI] [PubMed] [Google Scholar]
- 71.Coleman, T., Chamberlain, C., Davey, M. A., Cooper, S. E., Leonardi-Bee, J.Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst. Rev. (2015), Issue 12 [DOI] [PubMed]
- 72.Dechanet C, et al. Effects of cigarette smoking on reproduction. Hum. Reprod. Update. 2011;17:76–95. doi: 10.1093/humupd/dmq033. [DOI] [PubMed] [Google Scholar]
- 73.Den Dekker, H. T., Der Voort, A. M. M. S., De Jongste, J. C. Tobacco smoke exposure, airway resistance, and asthma in school-age children. Chest., 148, 607–617 (2015). [DOI] [PubMed]
- 74.Salihu HM, Wilson RE. Epidemiology of prenatal smoking and perinatal outcomes. Early Hum. Dev. 2007;83:713–720. doi: 10.1016/j.earlhumdev.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 75.Shahab L, West R, McNeill A. A randomized, controlled trial of adding expired carbon monoxide feedback tob rief stop smoking advice: evaluation of cognitive and behavioral effects. Health Psychol. 2011;30:49–57. doi: 10.1037/a0021821. [DOI] [PubMed] [Google Scholar]
- 76.Shipton D, et al. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ. 2009;339:b4347. doi: 10.1136/bmj.b4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst. Rev. 2012;9:CD010078. doi: 10.1002/14651858.CD010078. [DOI] [PubMed] [Google Scholar]
- 78.Brose LS, McEwen A, West R. Association between nicotine replacement therapy use in pregnancy and smoking cessation. Drug Alcohol Depend. 2013;132:660–664. doi: 10.1016/j.drugalcdep.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 79.Zwar N., Richmond R., Borland R.et al. Supporting Smoking Cessation: a Guide for Health Professionals (The Royal Australian College of General Practitioners, 2011). Available at www.racgp.org.au/download/documents/Guidelines/smoking-cessation.pdf.
- 80.Dempsey DA, Benowitz NL. Risks and benefits of nicotine to aid smoking cessation in pregnancy. Drug Saf. 2001;24:277–322. doi: 10.2165/00002018-200124040-00005. [DOI] [PubMed] [Google Scholar]
- 81.Kendzor DE, Businelle MS, Costello TJ, et al. Breast feeding is associated with postpartum smoking abstinence among women who quit smoking due to pregnancy. Nicotine Tob. Res. 2010;12:983–988. doi: 10.1093/ntr/ntq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.DiFranza JR, Savageau JA, Fletcher K, et al. Susceptibility to nicotine dependence: the development and assessment of Nicotine dependence in youth study. Pediatrics. 2007;120:e974–e983. doi: 10.1542/peds.2007-0027. [DOI] [PubMed] [Google Scholar]
- 83.Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016. Available from: http://goldcopd.org/. [DOI] [PubMed]
- 84.Gielkens-Sijstermans CM, et al. Reduction of smoking in Dutch adolescents over the past decade and its health gains: a repeated cross-sectional study. Eur. J. Public Health. 2010;20:146–150. doi: 10.1093/eurpub/ckp115. [DOI] [PubMed] [Google Scholar]
- 85.Stockley RA, Mannino D, Barnes PJ. Burden and pathogenesis of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2009;6:524–526. doi: 10.1513/pats.200904-016DS. [DOI] [PubMed] [Google Scholar]
- 86.Gritz ER, Vidrine DJ, Fingeret MC. Smoking cessation a critical component of medical management in chronic disease populations. Am. J. Prev. Med. 2007;33:S414–S422. doi: 10.1016/j.amepre.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 87.van Eerd EA, et al. Do we need tailored smoking cessation interventions for smokers with COPD? A comparative study of smokers with and without COPD regarding factors associated with tobacco smoking. Respiration. 2015;90:211–219. doi: 10.1159/000398816. [DOI] [PubMed] [Google Scholar]
- 88.Van Schayck OC, Haughney J, Aubier M, et al. Do asthmatic smokers benefit as much as non-smokers on budesonide/formoterol maintenance and reliever therapy? Respir. Med. 2012;106:189–196. doi: 10.1016/j.rmed.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 89.Jiménez-Ruiz, C. A., Andreas, S., Lewis, K. E. et al. Statement on Smoking Cessation in COPD and other Pulmonary Diseases and in Smokers with Comorbidities Who Find it Difficult to Quit. ERS doi:10.1183/09031936.00092614. Published 1 July 2015. [DOI] [PubMed]
- 90.Critchley, J. A., Capewell, S.Smoking cessation for the secondary prevention of coronary heart disease. Cochrane Database Syst Rev (2004), Issue 1. Art. no. CD003041. [DOI] [PubMed]
- 91.Rigotti, N., Munafo, M. R., Stead, L. F. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst. Rev.(2007), Issue 3. Art. no. CD001837. [DOI] [PubMed]
- 92.Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 93.De P, Farley A, Lindson N, Aveyard P. Systematic review and meta-analysis: influence of smoking cessation on incidence of pneumonia in HIV. BMC Med. 2013;11:1. doi: 10.1186/1741-7015-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lifson AR, et al. Smoking-related health risks among persons with HIV in the strategies for management of antiretroviral therapy clinical trial. Am. J. Public Health. 2010;100:1896–1903. doi: 10.2105/AJPH.2009.188664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Crothers K, et al. Impact of cigarette smoking on mortality in HIV-positive and HIV-negative veterans. AIDS educ. Prev. 2009;21:40. doi: 10.1521/aeap.2009.21.3_supp.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bates MN, et al. Risk of tuberculosis from exposure to tobacco smoke: a systematic review and meta-analysis. Arch. Intern. Med. 2007;167:335–342. doi: 10.1001/archinte.167.4.335. [DOI] [PubMed] [Google Scholar]
- 97.Brunet L, et al. High prevalence of smoking among patients with suspected tuberculosis in South Africa. Eur. Respir. J. 2011;38:139–146. doi: 10.1183/09031936.00137710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kirenga BJ, et al. Tuberculosis risk factors among tuberculosis patients in Kampala, Uganda: implications for tuberculosis control. BMC Public Health. 2015;15:1. doi: 10.1186/s12889-015-1376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gajalakshmi V, Peto R, Kanaka TS, Jha P. Smoking and mortality from tuberculosis and other diseases in India: retrospective study of 43000 adult male deaths and 35000 controls. Lancet. 2003;362:507–515. doi: 10.1016/S0140-6736(03)14109-8. [DOI] [PubMed] [Google Scholar]
- 100.Jee SH, et al. Smoking and risk of tuberculosis incidence, mortality, and recurrence in South Korean men and women. Am. J. Epidemiol. 2009;170:1478–1485. doi: 10.1093/aje/kwp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Y, et al. A smoking cessation intervention among tuberculosis patients in rural China. Public Health Action. 2015;5:183–187. doi: 10.5588/pha.15.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bam TS, Aditama TY, Chiang CY, Rubaeah R, Suhaemi A. Smoking cessation and smokefree environments for tuberculosis patients in Indonesia-a cohort study. BMC Public Health. 2015;15:015–1972. doi: 10.1186/s12889-015-1972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Enarson DA, Slama K, Chiang CY. Providing and monitoring quality service for smoking cessation in tuberculosis care. Int. J. Tuberc. Lung Dis. 2007;11:838–47.. [PubMed] [Google Scholar]
- 104.Centers for Disease Control and Prevention (CDC) Vital signs: current cigarette smoking among adults aged ≥ 18 years with mental illness—United States, 2009–2011. MMWR Morb. Mortal. Wkly. Rep. 2013;62:81–87. [PMC free article] [PubMed] [Google Scholar]
- 105.Farrell M, et al. Substance misuse and psychiatric comorbidity: an overview of the OPCS National Psychiatric Morbidity Survey. Addict. Behav. 1998;23:909–918. doi: 10.1016/S0306-4603(98)00075-6. [DOI] [PubMed] [Google Scholar]
- 106.Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev. Chronic Dis. 2006;3:A42. [PMC free article] [PubMed] [Google Scholar]
- 107.Taylor G, et al. Change in mental health after smoking cessation: systematic review and meta-analysis. BMJ. 2015;350:h1109. doi: 10.1136/bmj.h432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Royal College of Physicians, Royal College of Psychiatrists. Smoking and Mental Health. RCP, 2013. Royal College of Psychiatrists Council Report CR178.
- 109.Banham L, Gilbody S. Smoking cessation in severe mental illness: what works? Addiction. 2010;105:1176–1189. doi: 10.1111/j.1360-0443.2010.02946.x. [DOI] [PubMed] [Google Scholar]
- 110.Zwar N., Richmond R., Borland R., et al. Supporting Smoking Cessation: A Guide for Health Professionals. The Royal Australian College of General Practitioners, 2014.
- 111.Gelenberg AJ. Smoking cessation in patients with psychiatric disorders. Prim. Care Companion J. Clin. Psychiatry. 2008;10:52–58. doi: 10.4088/PCC.v10n0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cahill K. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst. Rev. 2013 doi: 10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thomas KH, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109. doi: 10.1136/bmj.h1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Anthenelli R, Benowitz N, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES) Lancet. 2016;387:2507–2520. doi: 10.1016/S0140-6736(16)30272-0. [DOI] [PubMed] [Google Scholar]
- 115.Kotz D, et al. Cardiovascular and neuropsychiatric risks of varenicline: a retrospective cohort study. Lancet Respir. Med. 2015;3:761–768. doi: 10.1016/S2213-2600(15)00320-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tidey JW, Miller ME. Smoking cessation and reduction in people with chronic mental illness. BMJ. 2015;351:h4065. doi: 10.1136/bmj.h4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jawad M, Lee JT, Millett C. Waterpipe tobacco smoking prevalence and correlates in 25 Eastern Mediterranean and Eastern European countries: cross-sectional analysis of the Global Youth Tobacco Survey. Nicotine Tob. Res. 2015 doi: 10.1093/ntr/ntv101. [DOI] [PubMed] [Google Scholar]
- 118.Agaku IT, et al. Poly-tobacco use among adults in 44 countries during 2009-2012: evidence for an integrative and comprehensive approach in tobacco control. Drug Alcohol Depend. 2014;139:60–70. doi: 10.1016/j.drugalcdep.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 119.Akl EA, Jawad M, Lam WY, Obeid R, Irani J. Motives, beliefs and attitudes towards waterpipe tobacco smoking: a systematic review. Harm. Reduct. J. 2013;10:12. doi: 10.1186/1477-7517-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jawad M, Khaki H, Hamilton F. Shisha guidance for GPs: eliciting the hidden history. Br. J. Gen. Pract. 2012;62:66–67. doi: 10.3399/bjgp12X625030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maziak W, et al. Interventions for waterpipe smoking cessation. Cochrane Database Syst. Rev. 2015;7:CD005549. doi: 10.1002/14651858.CD005549.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee DC, et al. Outcomes from a computer-assisted intervention simultaneously targeting cannabis and tobacco use. Drug Alcohol Depend. 2015;155:134–140. doi: 10.1016/j.drugalcdep.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Becker J, Schaub MP, Gmel G, Haug S. Cannabis use and other predictors of the onset of daily cigarette use in young men: what matters most? Results from a longitudinal study. BMC Public Health. 2015;15:843. doi: 10.1186/s12889-015-2194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Biehl JR, Burnham EL. Cannabis smoking in 2015: A concern for lung health? Chest doi: 2015 doi: 10.1378/chest.15-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rabin RA, George TP. A review of co-morbid tobacco and cannabis use disorders: possible mechanisms to explain high rates of co-use. Am. J. Addict. 2015;24:105–116. doi: 10.1111/ajad.12186. [DOI] [PubMed] [Google Scholar]
- 126.Marshall K, Gowing L, Ali R, Le Foll B. Pharmacotherapies for cannabis dependence. Cochrane Database Syst. Rev. 2014;12:CD008940. doi: 10.1002/14651858.CD008940.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chapman KR. Chronic obstructive pulmonary disease: are women more susceptible than men? Clin. Chest Med. 2004;25:331–341. doi: 10.1016/j.ccm.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 128.Rahmanian SD, Diaz PT, Wewers ME. Tobacco use and cessation among women: research and treatment-related issues. J. Womens Health (Larchmt). 2011;20:349–357. doi: 10.1089/jwh.2010.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Scharf D, Shiffman S. Are there gender differences in smoking cessation, with and without bupropion? Pooled- and meta-analyses of clinical trials of Bupropion SR. Addiction. 2004;99:1462–1469. doi: 10.1111/j.1360-0443.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- 130.Smith PH, et al. Gender differences in medication use and cigarette smoking cessation: results from the international tobacco control four country survey. Nicotine Tob. Res. 2015;17:463–472. doi: 10.1093/ntr/ntu212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Franklin TR, Ehrman R, Lynch KG, et al. Menstrual cycle phase at quit date predicts smoking status in an NRT treatment trial: a retrospective analysis. J Womens Health. 2008;17:287–292. doi: 10.1089/jwh.2007.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P., 3rd Female sex and oral contraceptive use accelerate nicotine metabolism. Clin. Pharmacol. Ther. 2006;79:480–488. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 133.Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob. Res. 2008;10:1245–1250. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- 134.Cepeda-Benito A, Reynoso JT, Erath S. Meta-analysis of the efficacy of nicotine replacement therapy for smoking cessation: differences between men and women. J. Consult. Clin. Psychol. 2004;72:712–722. doi: 10.1037/0022-006X.72.4.712. [DOI] [PubMed] [Google Scholar]
- 135.GonzalesD., et al. Varenicline Phase 3 Study Group. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 296, 47–55 (2006). [DOI] [PubMed]
- 136.Action on Smoking and Health (ASH). http://www.ash.org.uk/current-policy-issues/health-inequalities/health-inequalities-resource-pack.


