Abstract
Harmful algal blooms cause serious problems worldwide due to large quantities of cyanotoxins produced by cyanobacteria in eutrophic water. In this study, a new compound named 2-(3, 4-dihydroxy-2-methoxyphenyl)-1, 3-benzodioxole-5-carbaldehyde (Compound 1), together with one known compound, 3, 4-dihydroxybenzalacetone (DBL), was purified from Phellinus noxius HN-1 (CCTCC M 2016242). Compound 1 and DBL displayed activity against the cyanobacteria Microcystis aeruginosa with a half maximal effective concentration of 21 and 5 μg/mL, respectively. Scanning electron and transmission electron microscopic observations showed that the compounds caused serious damage and significant lysis to M. aeruginosa cells. qRT-PCR assay indicated that compound 1 and DBL exposure up-regulated the expression of gene mcyB and down-regulated the expression of genes ftsZ, psbA1, and glmS in M. aeruginosa. This study provides the first evidence of bactericidal activity of a new compound and DBL. In summary, our results suggest that compound 1 and DBL might be developed as naturally-based biocontrol agents.
Introduction
Harmful algal blooms (HABs) are considerable problems worldwide because of large quantities of toxins produced by cyanobacteria in eutrophic water1–4. It causes economic losses to aquaculture industry, water pollution, and harms to wildlife and human health5, 6. However, traditional control strategies usually induce the secondary pollution due to the release of phytotoxins6–8.
A growing body of evidence suggests that biological products can control harmful algae such as Microcystis aeruginosa 9. Therefore, finding new bioactive compounds by screening microbial secondary metabolites has become increasingly important. Algicidal bacteria, such as Alteromonas, Bacillus, Cytophaga, Micrococcus, Pseudomonas, and Vibrio, are algal species-specific in most cases and their abundance has been found to increase during the decline of an algal bloom7, 10, 11. Those bacteria are inhibitory to harmful algae and affect their algal properties, such as toxin production12, 13. Previous studies have shown that those bacteria may produce extracellular algicidal substances, such as the β-cyano-L-alanine produced by Vibrio spp., 1-methyl-β-carboline and phenazine pigments from Pseudomonas spp., and lactones from Ruegeria pomeroyi 14–16. In addition, some plants chemicals such as ellagic acid, eugeniin17, ethyl-2-methylacetoacetate18, gallic acids, nonanoic acid, and pyrogallol, (+)-catechin19 that have anti-cyanobacteria activities. Two compounds isolated from the endophytic fungi Seimatosporium sp. and Microsphaeropsis sp. have powerful algicidal properties20. To the best of our knowledge, there have been few similar reports on algicidal substances isolated from fungi. Moreover, the inhibition mechanisms remain elusive even though some substances have been reported to control M. aeruginosa.
In the previous study, Phellinus noxius HN-1 stored in our lab21 was tested a variety of biological activities and could inhibit the growth of M. aeruginosa (unpublished). In this study, we purified a new compound and 3, 4-dihydroxybenzalacetone (DBL) from P. noxius HN-1. We evaluated the potential bactericidal properties of these two compounds, and the effects on cell morphology of M. aeruginosa. In order to dissect the inhibition mechanisms of the compounds, we conducted genes expression survey using the microcystin peptide synthesis gene mcyB, cell division gene ftsZ, photosynthesis gene psbA1, and peptidoglycan synthesis gene glmS.
Results
Purification of compounds
Compound 1 was isolated as a brown crystal. Its molecular formula was assigned as C15H12O6 from its high resolution-electron spin ionization-mass spectrometry (HR-ESI-MS) with a molecular ion at m/z 289.2638 [M + H]+ (see Supplementary Fig. S1), UV (MeOH) λmax (log ε) 207. The IR spectrum displayed the presence of, phenyl (1580 cm−1), methyl (2953 cm−1), methylene (2923 cm−1), aldehyde (1719 cm−1). The NMR data (Table 1) indicated ten degrees of unsaturation. The 1H NMR spectrum of compound 1 (Table 1 and see Supplementary Fig. S2) showed the presence of one oxygenated methine proton [δ 5.18 (1 H, s, H-2)], five aromatic protons [δ 7.28 (1 H, d, J = 1.6 Hz, H-4), δ 7.27 (1 H, d, J = 1.6 Hz, 6.6 Hz, H-6), δ 6.89 (1 H, d, J = 8.6 Hz, H-7), δ 6.82 (1 H, d, J = 8.2 Hz, H-5a), δ 6.71 (1 H, d, J = 9.8 Hz H-6a)], one aldehyde group [δ 9.65 (1 H, s, H-10)], and one methoxy group [δ 3.32 (3 H, s, H-11)]. The 13C NMR spectrum of compound 1 (see Supplementary Fig. S3) showed 15 carbon signals, including a methoxy group (CH3O-11, δC 49.8), one aldehyde group (CHO-10, δC 193.1), one methine group (C-2, δC 104.8), and 12 olefinic carbons (δC 115.8, 131.1, 126.4, 116.2, 147.1, 153.7, 146.6, 115.3, 146.1, 130.8, 114.8, and 119.4) ascribed for two phenyl groups. Its planar structure was unambiguously established by 1H-1H COSY (Fig. 1 and see Supplementary Fig. S4) correlations of H-6/H-7 and H-5a/H-6a as well as the HMBC (see Supplementary Fig. S5) correlations from CHO-10 to C-4, C-5 and C-6, from H-2 to C-1a, C-2a, C-6a, C-8 and C-9, from CH3O-11 to C-2a. Therefore, the structure of compound 1 was established as shown in Fig. 1 and named 2-(3, 4-dihydroxy-2-methoxyphenyl)-1, 3-benzodioxole-5-carbaldehyde (1).
Table 1.
1H (500 MHz) and 13C (125 MHz) NMR spectral data of compound 1 and Reference (δ, ppm and J, Hz, CD3OD).
| Compound 1 | Reference | |||
|---|---|---|---|---|
| 1H NMR | 13C NMR | 1H NMR | 13C NMR | |
| 2 | 5.18(s) | 104.8 | 5.21(s) | 105.3 |
| 4 | 7.28(d, J = 1.6 Hz) | 115.8 | 7.31(d, J = 2.0 Hz) | 115.9 |
| 5 | 131.1 | 131.3 | ||
| 6 | 7.27(d, J = 1.6 Hz, 6.6 Hz) | 126.4 | 7.31(dd, J = 2.0 Hz, 6.5 Hz) | 126.9 |
| 7 | 6.89(d, J = 8.6 Hz) | 116.2 | 6.91(d, J = 7.0 Hz) | 116.7 |
| 8 | 147.1 | 147.6 | ||
| 9 | 153.7 | 154.1 | ||
| 10 | 9.65(s) | 193.1 | 9.68(s) | 193.6 |
| 11 | 3.32(s) | 49.8 | ||
| 1a | 146.6 | 147.1 | ||
| 2a | 115.3 | 6.86(d, J = 1.5 Hz) | 115.3 | |
| 3a | 146.1 | 146.5 | ||
| 4a | 130.8 | 131.6 | ||
| 5a | 6.82(d, J = 8.2 Hz) | 114.8 | 6.75(d, J = 6.5 Hz) | 116.3 |
| 6a | 6.71(d, J = 9.8 Hz) | 119.4 | 6.73(dd, J = 1.5 Hz, 7.0 Hz) | 119.9 |
Reference Tagashira et al.35 reported.
Figure 1.
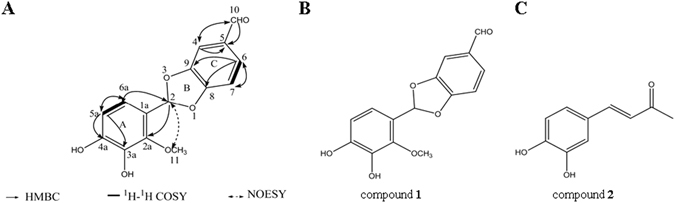
The structures of compound 1 and DBL. (A) Key HMBC, 1H-1H COSY, and NOESY correlations of compound 1, (B) The structure of compound 1, (C) The structure of DBL.
Compound 2 was elucidated as 3, 4-dihydroxybenzalacetone (DBL) (Fig. 2, see Supplementary Figs S6–10)22: yellow crystal (MeOH) ESI-MS m/z 179.2 [M + H]+. 1H NMR (500 MHz, CD3OD) (see Supplementary Fig. S8): δ 7.50 (1 H, d, J = 16.1 Hz, H-7), 7.10 (1 H, s, H-5), 6.97 (1 H, d, J = 8.2 Hz, H-8), 6.77 (1 H, d, J = 8.1 Hz, H-2), 6.53 (1 H, d, J = 16.2 Hz, H-6), 2.31 (3 H, s, CH3-1); 13C NMR (125 MHz, CD3OD) (see Supplementary Fig. S9): δ127.7 (C-1), 116.6 (C-2), 146.9 (C-3), 149.9 (C-4), 115.3 (C-5), 124.7 (C-6), 146.9 (C-7), 123.5 (C-8), 201.6 (C-9), 27.0 (C-10).
Figure 2.

Anticyanobacteria activities of compound 1 and DBL against M. aeruginosa. (A) The bactericidal efficiency of compound 1 and DBL with different concentrations (1 to 200 μg/mL) against M. aeruginosa cells were calculated by measuring the cell density for 72 h; (B) The chlorophyll a concentrations of M. aeruginosa exposed to compound 1 with different concentrates at 24, 48, 72 h; (C) The chlorophyll a concentrations of M. aeruginosa exposed to DBL with different concentrates at 24, 48, 72 h. Experiments were performed in triplicate. Error bars represent the SD.
Bactericidal activities of compound 1 and DBL
The experimental aim was to determine the inhibitory potency against the growth of M. aeruginosa by measuring the cell density after exposed to compound 1 and DBL for 72 h, (Fig. 2A). The two compounds exhibited bactericidal activities against an M. aeruginosa culture as the cell densities significantly decreased in comparison to that of the control. As the data shown in Fig. 2, DBL has more efficient anti-cyanobacterial activity against M. aeruginosa. The EC50,72h values of compound 1 and DBL were 20.6 and 5.1 μg/mL, respectively. The algicidal assay indicated that the anti-M. aeruginosa activities of two compounds increased with the dosage.
As shown in the Fig. 2, with the increase of concentration of DBL, the content of chlorophyll a was decreased gradually from 0.28 to 0.04 μg/mL at 72 h, which was 90.45% lower than that of the control. Compound 1 has little inhibitory effect on algae at low concentration, which was 0.37 μg/mL at 1 μg/mL, while the content of chlorophyll a was decreased to 0.07 μg/mL with the increasing concentration of compound 1 (200 μg/mL). According to the OD value, the EC50 values of DBL and compound 1 were 5.86 and 18.24 μg/mL, respectively, which were close to the cell density test results. Based on the above, we conclude that compound 1 and DBL can inhibit the growth of M. aeruginosa in a dose-dependant manner.
O2•− and electric conductivity assay
O2 •− in M. aeruginosa cells was induced largely by DBL (4 μg/mL) and the content of O2 •− increased from 0. 36 ± 0.001 μg/g3, which was higher than that of compound 1 with peak ratio of 0.36 ± 0.002 μg/g3. The content of O2 •− in cells exposed to DBL and compound 1 were maximum value of 0.40 ± 0.001 and 0.39 ± 0.001 μg/g3 at 72 h (Fig. 3A).
Figure 3.
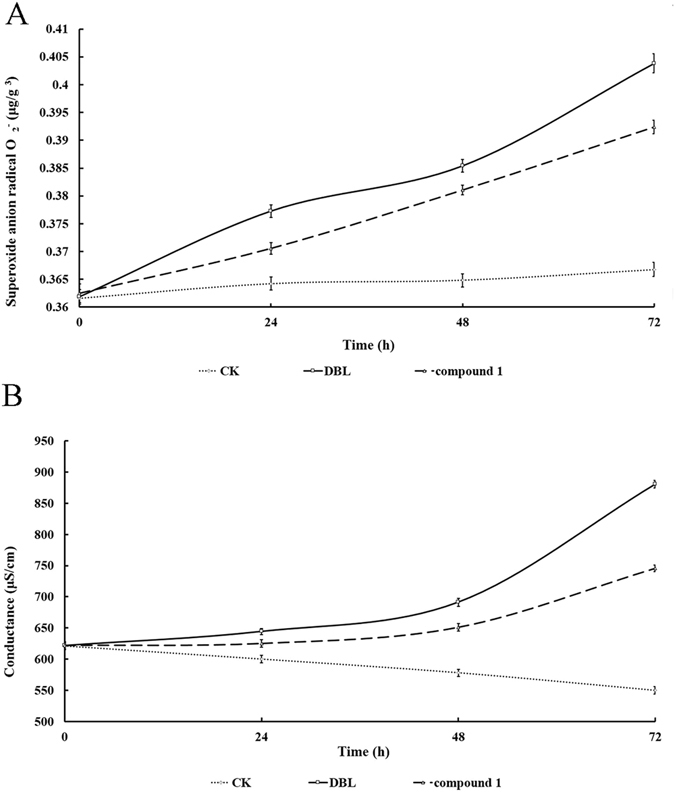
Influences of DBL and compound 1 (4 μg/mL) on O2 •− and EC of M. aeruginosa cells, respectively. All error bars indicate SD of the three replicates. (A) The two compounds effect on O2 •− of M. aeruginosa cells, (B) The two compounds effect on EC of M. aeruginosa cells.
Figure 3B shows effects of two compounds on the electric conductivity (EC) ratio. The EC ratio of DBL was 622 μS/cm initially and increased to 880 ± 6.03 μS/cm on 72 h, which was higher than that of compound 1 (745 ± 5.25 μS/cm). Compared with the control, DBL and compound 1 significantly effected on the EC ratios of M. aeruginosa.
Micro and ultrastructure changes of M. aeruginosa exposed to compound 1 and DBL
Our results demonstrated that compound 1 and DBL significantly affected the morphology of M. aeruginosa cells. Compared to the control cells (Fig. 4A), the morphological changes of the cells after exposure to 4 μg/mL of compound 1 and DBL were observed under SEM and TEM to evaluate the bactericidal mechanism of tested compounds on morphological micro and ultrastructures (Fig. 4B and C). The M. aeruginosa cells appeared to be normal shaped as plump, and round with smooth exteriors in the control (Fig. 4D). After exposure to compound 1 or DBL, majority of M. aeruginosa cells exhibited obvious changed in morphology and lost their integrity. Figure 4E and F show that the cytoplasm became notably condensed and plasmolysis occurred in the cells. The untreated cell had complete cell wall and a basic structure, including a nuclear area, vesicle, and other cell organelles (Fig. 4G), whereas the exposed cells were disrupted and lysed. The compounds severely damaged the cell-walls and caused cell disruption, collapsed, perforation and content lysis (Fig. 4H and I). DBL damage was more severe as loss of nuclear area and gas vesicle and is integration of cell architecture.
Figure 4.
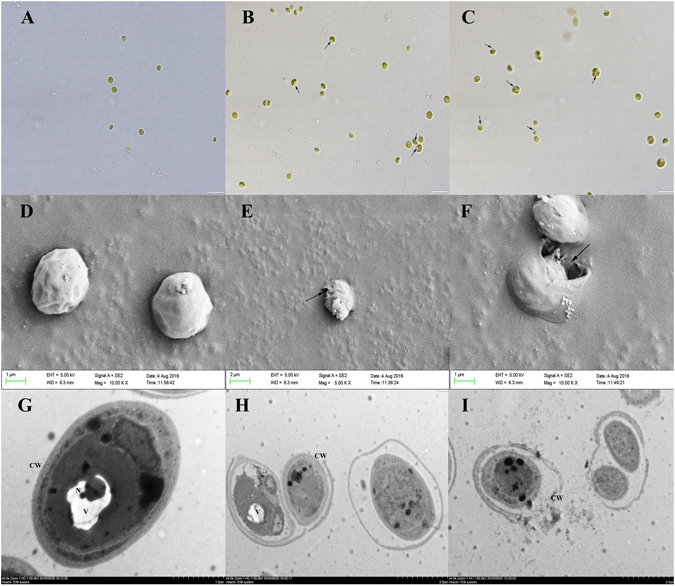
Microstructural and ultramicroscopic structure changes of M. aeruginosa exposure to 4 μg/mL compound 1 and DBL for 72 h. Light micrographs of Microcystis cells: (A) Normal M. aeruginosa cells, scale bar = 20 μm; (B) M. aeruginosa cells exposed to compound 1, scale bar = 20 μm; (C) M. aeruginosa cells exposed to DBL, the arrows in B and C pointed to the shrinkage and perforation of cell membrane, scale bar = 20 μm; SEM of Microcystis cells: (D) Control M. aeruginosa cells present normal shape, scale bar = 1 μm; (E) M. aeruginosa cells exposed to compound 1, scale bar = 1 μm; (F) M. aeruginosa cells exposed to DBL, scale bar = 0.5 μm; TEM of Microcystis cells: (G) Control M. aeruginosa cells present normal shape and structure, CW: cell wall, V: vesicles, N: nucleus, scale bar = 1.0 μm; (H) M. aeruginosa cells exposed to compound 1, scale bar = 2.0 μm (I) M. aeruginosa cells exposed to DBL, scale bar = 2.0 μm. Experiments were performed in triplicate.
Effects on transcription level of M. aeruginosa genes
Based on the experiments of microscopic observation and determinations of chlorophyll a, electrical conductivity and superoxide anion O2 •−, to further clarify the bactericidal mechanism on gene expression, we tested the key synthesis gene of chlorophyll a and related genes of cell membrane. The four targeted genes, including microcystin in several cyanobacterial generasynthesis genes mcyB 23, cell division gene ftsZ, photosynthesis gene psbA1, and peptidoglycan synthesis gene glmS, were chosen to analyze the effects of the compound 1 and DBL on gene transcription. We detected the transcriptional expression changes of these genes of M. aeruginosa exposed to the two compounds (Fig. 5). Compared to the control, ftsZ, glmS and psbA1 genes were slightly down-regulated after 24 h, while expression was reduced significantly after 48 h exposures to compound 1. The mcyB was up-regulated and then reduced. The qRT-PCR analysis demonstrated that DBL increased the transcriptional expressions of mcyB then decrease it. Consequently, a decrease in ftsZ gene, psbA1 gene, and glmS gene, were observed. The results suggested that DBL seriously influenced the transcription of genes in M. aeruginosa.
Figure 5.

qRT-PCR analysis of the expression of mcyB, ftsZ, psbA1 and glmS in M. aeruginosa exposed to compound 1 (A) and DBL (B) (4 μg/mL) for 24, 48, and 72 h. The values were normalized to the levels of 16 S rRNA, which is a housekeeping gene and an internal reference gene. The y axis values represent the mean expression ± the standard deviations (n = 3) relative to the control. Experiments were performed in triplicate.
Discussion
Previous studies have demonstrated that some microorganisms have powerful algicidal activity against harmful algal blooms11. However, only a few algicidal compounds were purified and identified, which included biosurfactants24, bacillamides25, 26, peptides27, proteins28, 1-methyl-β-carboline14, β-carbolines7, 29, and pigments30. These algaecides are natural chemicals and, therefore, may be environmentally friendly in controlling HABs31.
In this study, we isolated two compounds from P. noxius HN-1. Based on the 1H and 13C NMR spectra the structure of compound 1 is similar to that of the known compound 2-(3′,4′-dihydroxyphenyl)-1,3-benzodioxole-5-aldehyde isolated from Melissa officinalis 32, differing in a methoxy group is replaced by H at C-11 (δC 49.8). The known compound is 10-fold more active than ascorbic acid and is easily degraded into two molecules of protocatechualdehyde32. Accordingly a hypothesis is suggested that they probably have homogeneous activities.
DBL is a polyphenol derived from the medicinal fungus Chaga (Inonotus obliquus) in Japan, and is used as a folk medicine to treat cancers in Russia33, 34. DBL has growth-inhibitory effects35 and shows strong antioxidant activity in terms of both superoxide and hydroxyl radical scavenging activities34, suggesting the therapeutic effects of DBL. However, to our best knowledge, there is no report available on the bactericidal activity of DBL as a natural metabolite produced by P. noxius.
The present study is for the first time to show that compound 1 and DBL exhibit anti-cyanobacterial activities against M. aeruginosa with EC50 values of 20.6 and 5.1 μg/mL. The differential effects of the two compounds may be due to their structural differences. It is similar to other previously reported compounds. It was shown that the EC50 values of salcolin A and B isolated from Hordeum vulgare, were 6.0 and 9.6 μg/mL against M. aeruginosa 36. The antialgal allelochemical ethyl 2-methylacetoacetate was isolated from Phragmites communis and with the EC50 value of 0.65 μg/mL against M. aeruginosa 18. In addition, compound 1 weakened light result in inhibiting photosynthesis of M. Aeruginosa, and the EC50 value was close to the cell density test results.
The cell membrane is the target for many antimicrobial agents37, 38 and some electrolytes tend to leach out first, then large molecules such as DNA, RNA, and other materials leak out39. The release of intracellular components is a good indicator of membrane integrity38, 39. In recent studies, it was suggested that some compounds, which act as an environmental stress, can increase the production of O2 •− in cells40, 41. O2 •− is the precursor of active free radicals that have the potential for reacting with biological macromolecules inducing cell damage.
Exposure to compound 1 and DBL lead to increase of O2 •− contents in M. aeruginosa cells, which may induce lipid peroxidation, indicated the leakage and release of electrolytes, nucleic acids, and proteins from the cyanobacteria and contribute to the increase of EC. Compound 1 and DBL belong to phenolic compounds which are similar to phenolic acid compounds, therefore we infer that target of these two compounds might be the cell membrane. Other report indicated that antioxidant enzyme (superoxide dismutase) activities and specific activities of A. flos-aquae were enhanced at the beginning of ρ-hydroxybenzoic acid and ferulic acid oxidative stress conditions42.
Although some substances have been reported to control M. aeruginosa, their inhibition mechanism remain unknown. Previous studies suggested that those compounds destroy cell structure, cause oxidative damage, and affect algal photosynthesis and enzymatic activities6, 17, 43. Zhang et al.44 demonstrated that 2′-deoxyadenosine produced by Streptomyces jiujiangensis strain JXJ 0074T led to severe crumpling, collapse, and perforation of M. aeruginosa, and a reduction in chlorophyll content. Bacilysin, isolated from B. amyloliquefaciens FZB42, acts against cell walls and also has significant anti-cyanobacterial effects5. In the present study, the morphometric analysis at the microstructural and ultrastructural levels by SEM and TEM indicate that compound 1 and DBL primarily affected the cell wall and increase cell permeability, leading to the efflux of intracellular components and eventually cell lysis. Based on the O2 •− and EC contents assay, M. aeruginosa cell membrane was irreversibly damaged under the conditions of two compounds deoxidize stress.
To define the molecular bactericidal mechanism, the expression of microcystin peptide synthesis gene mcyB, cell division gene ftsZ, photosynthesis gene psbA1, and peptidoglycan synthesis gene glmS were analyzed by qRT-PCR. The expression abundance of these genes was reduced by compound 1 and DBL and the growth of M. aeruginosa was significantly suppressed. Our results are similar to the previous studies that also suggest that the transcript abundance of regulated genes were obviously reduced when M. aeruginosa under pyrogallol stress or algicidal bacterium stress45, 46. The ftsZ gene encodes cell division protein FtsZ, which is essential to the cyanobacterium Synechocystis sp. PCC 6803 survival47. Combined with cell wall breakage, the decrease in the expression of genes ftsZ and glmS, indicates that membrane damage may be the bactericidal mechanism for DBL in M. aeruginosa cells. Compound 1 had less effect on the cell membrane than DBL.
M. aeruginosa, a toxic cyanobacterium, can produce microcystins. Microcystin formation is catalyzed by a complex multifunctional enzyme containing peptide synthetase (mcyABC) and hybrid polyketide-peptide synthetase (mcyDE)48. After M. aeruginosa cells were stimulated by compound 1 and DBL, the mcyB expression increased, which might be related to increase of the microcystin content caused by release of microcystin from dead M. aeruginosa cells. Dziga et al. concluded that the expression of mcyB is up-regulated under exposure to pyrogallol because of the release of hepatotoxin from dead Microcystis cells49, which increase microcystin content. Zhang et al. have also proved that the transcription expression of the microcystin synthetase gene is affected by ginkgolic acid6.
The photosynthetic gene expression is possibly regulated at the transcriptional level50, 51. Some studies have indicated that the interruption of the electron transfer chain which affects photosynthetic processes, and oxidant damage may be the inhibitory mechanisms6, 45, 49, 52. It has been known that PS II was sensitive to the environment53. The reduced abundances of psbA1 in PS II implies that the repair rate does not keep up with the damage rate and that compound 1 and DBL stress would interfere with electron transport. The psbA1 gene, the possible target for compound 1, was significant and rapid downregulated than that of DBL. It may be another factor in the effect on M. aeruginosa growth. This result is similar to other compound such as amoxicillin and levofloxacin hydrochloride that decrease PS II activity in Synechocystis sp54, 55. Based on the qRT-PCR analysis, we suggest that the psbA1 gene is the potential binding site of compound 1 affecting algal photosynthesis. DBL multisite action, including releasing of microcystin, the cells membrane and cell structure damage, and reduction photosynthesis cause M. aeruginosa death. The morphological and molecular analysis results indicated that compound 1 and DBL might have different mechanisms against M. aeruginosa and we will study the protein expression changes in the future to clarify the bactericidal mechanism. In conclusion, compound 1 and DBL, isolated from P. noxius HN-1, show potent bactericidal activity and may be useful to mitigate harmful algal blooms in a synergistic manner.
Material and Methods
General experimental procedures
Thin-layer chromatography (TLC) was performed on silica gel GF254 (Qingdao Haiyang Chemical Co., Ltd, China) and column chromatography was performed with silica gel (60–80, 200–300 mesh, Qingdao Haiyang Chemical Co.). Sephadex LH-20 (Merck, Germany). The ESI-MS spectra were measured with a VG Auto-3000 Spectrometer, Sephadex LH-20 (Merck, Germany) and MS-C18 column (3.5 μm, 4.6 by 150 mm, Waters). Nuclear Magnetic Resonance (NMR) spectra were obtained on a Bruker AV-500 spectrometer with tetramethylsilane (TMS) as an internal standard. Infrared Spectroscopy (IR) spectra were recorded on a Nicolet 380 FT-IR instrument, as KBr pellets (Thermo, Pittsburgh, PA, USA). UV spectra were obtained on a Shimadzu UV-2550 spectrometer (Beckman, Brea, CA, USA).
Microorganisms
P. noxius strain HN-1 was isolated from brown root pathogens collected in Changjiang city, Hainan Province, China21, was cultured in potato dextrose agar (PDA) medium at 28 °C and stored in our lab (see Supplementary Fig. S1). The strain HN-1 was deposited in China Center for Type Culture Collection (CCTCC) (CCTCC M 2016242) (GenBank accession number KX592167).
Isolation and identification of the compounds
P. noxius strain HN-1 was cultured on PDA at 28 °C for 7 days. Two pieces of mycelial agar plugs (0.5 cm × 0.5 cm) were inoculated into 1 L Erlenmeyer flasks containing 400 mL potato dextrose broth (PDB). The cultivation was shaken at 120 r/min at 28 °C for 7 days, and then kept in still at 28 °C for 45 days. The culture broth (60 L) was filtered to give the filtrate and mycelia. The crude extract was reduced in vacuo to approximately 1 L and partitioned in succession between H2O and petroleum ether, ethyl acetate (EtOAc) and n-butyl alcohol56. The EtOAc extract (1.26 g) was separated on a silica gel column (200–300 mesh) with sequential gradient elution with 100% chloroform (CHCl3), a mixture of CHCl3/methanol (MeOH) (100:1, 50:1, 25:1, 15:1, 10:1, 5:1, 2:1, 1:1, v/v), and finally 100% MeOH into 10 fractions. The resulting fractions were combined according to TLC profiles on silica gel GF254 (Marine Chemical Industry Factory, Qingdao, China). Based on the bioassay, the fractions were tested for inhibition against M. aeruginosa. Fraction 5 (Fr.5) (180 mg) was submitted to chromatography and further separated via Sephadex LH-20 and on silica gel column with CHCl3/MeOH (10:1, v/v), yielding the compound 1 (21.57 mg). Fraction 4 (Fr.4) (110 mg) was submitted to chromatography on a silica gel column with CHCl3/MeOH (15:1, v/v) as an eluent and further separated by chromatography via Sephadex LH-20 column with ethanol as an eluent and on a silica gel column with CHCl3/MeOH (20:1, v/v), yielding DBL (15.78 mg).
M. aeruginosa culture
M. aeruginosa NIES-843 was purchased from the Freshwater Algae Culture Collection of the Institute of Hydrobiology (Wuhan, China) and cultured in sterilized BG11 medium at 25 ± 1 °C under a 12 h: 12 h (light: dark) cycle with 60 μmol photons m−2 s−1 5.
Bioassay
Compound 1 or DBL was added to the cultures of M. aeruginosa (1 × 107 cells/mL) with the final concentrations of 1, 2, 5, 10, 25, 50, 100, 200 μg/mL, and cultivated at 25 °C under 40 μmol photons/(m2 s) and a 12 h:12 h (light: dark) cycle. The bactericidal activities of compound 1 and DBL against M. aeruginosa were assayed according to the procedure described by Li et al.57. A control was tested using sterile water inoculation. The number of cells was observed under Olympus BX51 (Olympus, Japan).
M. aeruginosa samples (20 mL) were centrifuged at 3,500 × g for 20 min and then extracted in 90% acetone for 24 h at 4 °C, and the supernatant removed into a 10 mL volumetric flask and diluted with 90% acetone to 10 mL, after which the chlorophyll a concentrations were determined by using the following equation: chlorophyll a concentration (mg/m3) = [11.64 × (OD663 − OD750) − 2.16 × (OD645 − OD750) + 0.1 × (OD645 − OD750)] × 1/2.
The bactericidal activities of compound 1 and DBL were calculated by the following equation: bactericidal efficiencies (%) = (1 − treatment/control) × 100, where the treatment and control are cell densities of M. aeruginosa with and without compound 1 or DBL inoculation, respectively58. M. aeruginosa growth was monitored at 72 h. The EC50,72h values (i.e., median lethal concentration relative to the control) were obtained from the sigmoidal inhibition curves fitted by probit regression analysis (SPSS 19.0). To verify the reliability of the experimental results, all the experiments were carried out three times, and good repeatability was obtained.
The measurement of electric conductivity
Compound 1 or DBL was added to the cultures of M. aeruginosa (1 × 107 cells/mL) with the final concentration of 4 μg/mL for 72 h. The electric conductivity (EC) was analyzed using a portable conductivity meter (Cole-Parmer Instrument Company, USA). Five milliliters of each sample was taken from the culture flask and was immediately filtered with a 0.22 μm Millipore59. The supernatant was used for analysis.
Measurement Method for Superoxide Anion Radical
Compound 1 or DBL was added to the cultures of M. aeruginosa (1 × 107 cells/mL) with the final concentration of 4 μg/mL for 72 h. M. aeruginosa sample (20 mL) was centrifuged at 4000 × g for 20 min and then was homogenized with ice-cold phosphate buffered saline (PBS) (6 mL, 65 mM, pH 7.8), filtered with filter paper, and centrifuged at 5000 × g for 10 min at 4 °C. 2 mL supernatant was added to 1.5 mL PBS (65 mM, pH 7.8) and 0.5 mL hydroxylamine hydrochloride (10 mM), followed by incubation at 25 °C for 20 min. After that, 2 mL of the mixture was added to 2 mL sulfanilic acid (17 mM) and 2 mL α-naphthylamine (17 mM), incubated for 20 min at 25 °C. The samples were settled for 10 min at room temperature and was measured at 530 nm. O2 •− was determined by using the following equation: M (μg/g3) = 2 × Vt × n/(FW × VS), n is concentration of NO2 − (μg/mL), Vt is total volume, FW is weight of sample, VS is the crude enzyme extract volume59.
Cells microstructure and ultrastructural analysis
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) analysis were used to test the influence of compound 1 and DBL. M. aeruginosa was cultivated at 25 °C under 60 μmol photons/(m2 s) and a 12 h:12 h (light: dark) cycle. The cells were exposed to compound 1 and DBL with the final concentration of 4 μg/mL for 72 h, respectively. The fixed cells were collected by centrifugation, prefixed in 2.5% glutaraldehyde and washed three times in 0.1 M phosphate buffer for 10 min. Dehydration was done with a gradient series of ethanol. For SEM analysis samples, the cells were coated with gold, and examined with a Hitachi S-3000N SEM (Hitachi, Japan). For TEM analysis samples, the cells were postfixed in 1% osmium tetroxide for 1 h and dehydrated with a gradient series of ethanol. After dehydration, the samples were embedded in Epon 812 and sectioned with an ultramicrotome (LKB-V, Sweden). The sections were examined under a Hitachi H-600 TEM (Hitachi, Japan). Micrographs were taken at 10.0 kV5.
qRT-PCR Analysis
M. aeruginosa was exposed to 4 μg/mL compound 1, DBL or water as the control for 24 , 48 , 72 h. After incubation, the cells were collected by centrifuging at 10,000 rpm for 10 min at 4 °C. Total RNA was extracted with TRIzol reagent (Invitrogen, USA). cDNA was synthesized with the reverse transcriptase kit (TaKaRa Bio Inc, Dalian, China). qRT-PCR was performed with SYBR Premix Ex Taq (TaKaRa Bio) and an ABI 7500 Fast Real-Time PCR Detection System in a 20 μL volume. The conditions consisted of one cycle of 3 min at 95 °C followed by 40 cycles of 95 °C for 15 s, 56 °C for 30 s. Primers of target genes were listed in Table 2 and the 16 S rRNA gene was used as the internal reference for normalization.
Table 2.
Primers designed for qRT-PCR analysis.
| Gene name | Sequence (5′–3′) |
|---|---|
| 16 S rRNA | F: GGACGGGTGAGTAACGCGTA R: CCCATTGCGGAAAATTCCCC |
| glmS | F: TGTGCCTCCGATGTCAGT R: ATGAAGTGACGATAACCCT |
| psbA1 | F: GGTCAAGARGAAGAAACCTACAAT R: GTTGAAACCGTTGAGGTTGAA |
| mcyB | F: CCTACCGAGCGCTTGGG R: GAAAATCCCCTAAAGATTCCTGAGT |
| ftsZ | F: TCGCTGCTATTTCCTCGC R: TGACTTCTCCCTGCATTTTCT |
Reference Wu et al.5 reported.
Statistical analysis
All experiments were at least in triplicate. Statistical analyses were performed with SPSS 19.0. qRT-PCR data were analyzed by the 2−△△CT method. The mean value and standard deviation (SD) of the three replicates were calculated.
Electronic supplementary material
Acknowledgements
This study was supported by Special Project of Production Teaching andResearch combination from Hainan Province (CXY20140038), National Natural Science Foundation of China (31160359, 31360029), National Key Basic Research Plan of China (2011CB111612), National agricultural industrial technology system (ARS-34-GW8). We thank Dr. Haofu Dai (Key Laboratory of Biology and Genetic Resources of Tropical Crops, Ministry of Agriculture, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences) for helpful discussions, and Dr. Qing X. Li (University of Hawaii) and Mr Alexander Harkness (University of Minnesota) for kindly helping us with the English in the article.
Author Contributions
P.F.J. and W.G.M. conceived and designed the study, P.F.J. and H.N.W. performed the experiments and wrote the main manuscript text. W.B.L. and C.H.L. provided comments on the study. S.J.Z., F.C.Z. and W.G.M. reviewed and edited the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Pengfei Jin and Haonan Wang contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-03440-2
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen WM, Sheu FS, Sheu SY. Novel L-amino acid oxidase with algicidal activity against toxic cyanobacterium Microcystis aeruginosa synthesized by a bacterium Aquimarina sp. Enzyme Microb Technol. 2011;49:372–379. doi: 10.1016/j.enzmictec.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Dagnino D, de Meireles DA, de Almeida AJC. Growth of nutrient-replete Microcystis PCC 7806 cultures is inhibited by an extra cellular signal produced by chlorotic cultures. Environ Microbiol. 2006;8:30–36. doi: 10.1111/j.1462-2920.2005.00866.x. [DOI] [PubMed] [Google Scholar]
- 3.Guo L. Doing battle with the green monster of Taihu Lake. Science. 2007;317:1166–1166. doi: 10.1126/science.317.5842.1166. [DOI] [PubMed] [Google Scholar]
- 4.Zhang TT, Zheng CY, Hu W, Xu WW, Wang HF. The allelopathy and allelopathic mechanism of phenolic acids on toxic Microcystis aeruginosa. J Appl Phycol. 2010;22:71–77. doi: 10.1007/s10811-009-9429-6. [DOI] [Google Scholar]
- 5.Wu LM, et al. Bacilysin from Bacillus amyloliquefaciens FZB42 has specific bactericidal activity against harmful algal bloom species. Appl Environ Microbiol. 2014;80:7512–7520. doi: 10.1128/AEM.02605-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang C, Ling F, Yi YL, Zhang HY, Wang GX. Algicidal activity and potential mechanisms of ginkgolic acids isolated from Ginkgo biloba exocarp on Microcystis aeruginosa. J Appl Phycol. 2014;26:323–332. doi: 10.1007/s10811-013-0057-9. [DOI] [Google Scholar]
- 7.Alamri SA, Mohamed ZA. Selective inhibition of toxic cyanobacteria by β-carboline-containing bacterium Bacillus flexus isolated from Saudi freshwaters. Saudi J Biol Sci. 2013;20:357–363. doi: 10.1016/j.sjbs.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oh MY, Lee SB, Jin DH, Hong YK, Jin HJ. Isolation of algicidal compounds from the red alga Corallina pilulifera against red tidemicroalgae. J Appl Phycol. 2010;22:453–458. doi: 10.1007/s10811-009-9478-x. [DOI] [Google Scholar]
- 9.Park MH, Chung IM, Ahmad A, Kim BH, Hwang SJ. Growth inhibition of unicellular and colonial Microcystis strains (Cyanophyceae) by compounds isolated from rice (Oryza sativa) hulls Myung-Hwan Park. Aquat Botany. 2009;90:309–314. doi: 10.1016/j.aquabot.2008.11.007. [DOI] [Google Scholar]
- 10.Amaro AM, Fuentes MS, Ogalde SR, Venegas JA, Suarez-Isla BA. Identification and characterization of potentially algallyticmarine bacteria strongly associated with the toxic dinoflagellate. Alexandrium catenella. J Eukaryot Microbiol. 2005;52:191–200. doi: 10.1111/j.1550-7408.2005.00031.x. [DOI] [PubMed] [Google Scholar]
- 11.Lovejoy C, Bowman JP, Hallegraeff GM. Algicidal effects of anovel marine Pseudoalteromonas isolate (class Proteobacteria, gamma subdivision) on harmful algal bloom species of the genera Chattonella, Gymnodinium, and Heterosigma. Appl Environ Microbiol. 1998;64:2806–2813. doi: 10.1128/aem.64.8.2806-2813.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su JQ, et al. Isolation and characterization of a marine algicidal bacterium against the toxic dinoflagellate Alexandrium tamarense. Harmful Algae. 2007;6:799–810. doi: 10.1016/j.hal.2007.04.004. [DOI] [Google Scholar]
- 13.Uribe P, Espejo RT. Effect of associated bacteria on the growth and toxicity of Alexandrium catenella. Appl. Environ. Microbiol. 2003;69:659–662. doi: 10.1128/AEM.69.1.659-662.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kodani S, Imoto A, Mitsutani A, Murakami M. Isolation and identification of the antialgal compound, harmane (1-methyl-β-carboline), produced by the algicidal bacterium, Pseudomonas sp.K44-1. J Appl Phycol. 2002;14:109–114. doi: 10.1023/A:1019533414018. [DOI] [Google Scholar]
- 15.Riclea R, et al. Algicidal lactones from the marine Roseobacter clade bacterium Ruegeriapomeroyi. Beilstein J Org Chem. 2012;8:941–950. doi: 10.3762/bjoc.8.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshikawa K, et al. β-Cyanoalanine production by marine bacteria on cyanide-free medium and its specific inhibitory activity toward cyanobacteria. Appl Environ Microbiol. 2000;66:718–722. doi: 10.1128/AEM.66.2.718-722.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakai S, Yutaka I, Hosomi M, Murakami A. Myriophyllum spicatum-released allelopathic polyphenols inhibiting growth of blue-green algae Microcystis aeruginosa. Water Res. 2000;34:3026–3032. doi: 10.1016/S0043-1354(00)00039-7. [DOI] [Google Scholar]
- 18.Li FM, Hu HY. Isolation and characterization of a novel antialgal allelochemical from Phragmites communis. Appl Environ Microbiol. 2005;71:6545–6553. doi: 10.1128/AEM.71.11.6545-6553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakai S, Yamada S, Hosomi M. Anti-cyanobacterial fatty acids released from Myriophyllum spicatum. Hydrobiologia. 2005;543:71–78. doi: 10.1007/s10750-004-6822-7. [DOI] [Google Scholar]
- 20.Hussaina H, et al. Microsphaerol and Seimatorone: Two new compounds isolated from the endophytic fungi, Microsphaeropsis sp. and Seimatosporium sp. Chinese Chemical Letters. 2015;12:289–294. doi: 10.1002/cbdv.201400098. [DOI] [PubMed] [Google Scholar]
- 21.Liu WB, Wu GL. Biological characteristics of fungus Phellinus noxius causing brown root disease of Hevea brasiliensis. Chinese Journal of Tropical Crops. 2009;30:1835–1839. [Google Scholar]
- 22.Li, B. W., Zhang, D., Yang, L., Wang, M. Y. & Zhu, X. X. Chemical constituents of Osmundae Rhizoma. Nat Prod Res Dev. 24, 1214–1216, 1213 (2012).
- 23.Pearson LA, Neilan BA. The molecular genetics of cyanobacterial toxicity as a basis for monitoring water quality and public health risk. Curr. Opin. Biotech. 2008;19:281–288. doi: 10.1016/j.copbio.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Wang XL, et al. Algicidal activity of rhamnolipid biosurfactants produced by Pseudomonas aeruginosa. Harmful Algae. 2005;4:433–443. doi: 10.1016/j.hal.2004.06.001. [DOI] [Google Scholar]
- 25.Jeong JH, Jin HJ, Sohn CH, Suh KH, Hong YK. Algicidal activity of the seaweed Corallina pilulifera against red tide microalgae. J Appl Phycol. 2000;12:37–43. doi: 10.1023/A:1008139129057. [DOI] [Google Scholar]
- 26.Jeong SY, Ishida K, Ito Y, Okada S, Murakami M. Bacillamide, a novel algicide from the marine bacterium, Bacillus sp SY-1, against the harmful dinoflagellate, Cochlodinium polykrikoides. Tetrahedron Lett. 2003;44:8005–8007. doi: 10.1016/j.tetlet.2003.08.115. [DOI] [Google Scholar]
- 27.Park SC, Lee JK, Kim SW, Park Y. Selective algicidal action of peptides against harmful algal bloom species. PLoS One. 2011;6:27–34. doi: 10.1371/journal.pone.0026733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paul C, Pohnert G. Induction of protease release of the resistant diatom Chaetoceros didymus in response to lytic enzymes from analgicidal bacterium. PLoS One. 2013;8:1919–1930. doi: 10.1371/journal.pone.0057577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrier MD, Butler BR, Sr., Terlizzi DE, Lacouture RV. The effects of barley straw (Hordeum vulgare) on the growth of freshwater algae. Bioresour Technol. 2005;96:1788–1795. doi: 10.1016/j.biortech.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Nakashima T, et al. Producing mechanism of an algicidal compound against red tide phytoplankton in a marine bacterium gammaproteo bacterium. Appl Microbiol Biotechnol. 2006;73:684–690. doi: 10.1007/s00253-006-0507-2. [DOI] [PubMed] [Google Scholar]
- 31.Park MH, et al. Growth inhibition of bloom-forming cyanobacterium Microcystis aeruginosa by rice straw extract. Lett Appl Microbiol. 2006;43:307–312. doi: 10.1111/j.1472-765X.2006.01951.x. [DOI] [PubMed] [Google Scholar]
- 32.Tagashira M, Ohtake Y. A new antioxidative 1, 3-benzodioxole fom melissa off icinalis. Planta Med. 1998;64:558–558. doi: 10.1055/s-2006-957513. [DOI] [Google Scholar]
- 33.Nakajima Y, Sato Y, Konishi T. Antioxidant small phenolic ingredients in Inonotus obliquus (persoon) Pilat (Chaga) Chem Pharm Bull. 2007;55:1222–1226. doi: 10.1248/cpb.55.1222. [DOI] [PubMed] [Google Scholar]
- 34.Sung B, et al. Identification of a novel blocker of IκBα kinase activation that enhances apoptosis and inhibits proliferation and invasion by suppressing nuclear factor-κB. Mol Cancer Ther. 2008;7:191–201. doi: 10.1158/1535-7163.MCT-07-0406. [DOI] [PubMed] [Google Scholar]
- 35.Markaverich BM, Shoulars K, Alejandro MA. Nuclear type II[3H] estradiol binding site ligands: inhibition of ER-positive and ER-negative cell proliferation and c-Myc and cyclin D1 gene expression. Steroids. 2006;71:865–74. doi: 10.1016/j.steroids.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 36.Xiao X, et al. A pair of chiral flavonolignans as novel anti-cyanobacterial allelochemicals derived from barley straw (Hordeum vulgare): characterization and comparison of their anti-cyanobacterial activities. Environ Microbiol. 2014;16:1238–51. doi: 10.1111/1462-2920.12226. [DOI] [PubMed] [Google Scholar]
- 37.Denyer SP, Stewart GSAB. Mechanism of action of disinfectants. Int Biodeter Biodegr. 1998;41:261–268. doi: 10.1016/S0964-8305(98)00023-7. [DOI] [Google Scholar]
- 38.Sun XX, Choi JK, Kim EK. A preliminary study on the mechanism of harmful algal bloom mitigation by use of sophorolipid treatment. J Exp Mar Biol Ecol. 2004;304:35–49. doi: 10.1016/j.jembe.2003.11.020. [DOI] [Google Scholar]
- 39.Chen CZ, Cooper SL. Interactions between dendrimer biocides and bacterial membranes. Biomaterials. 2002;23:3359–3368. doi: 10.1016/S0142-9612(02)00036-4. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, T. T., He, M., Wu, A. P. & Nie, L. W. Allelopathic inhibition of ρ-hydroxybenzoic acid on Microcystis aeruginosa Kuetz with no toxicological effects on Cyprinus carpio Linnaeus. Acta Scientiae Circumstantiae28, 1887–1893 (2008). In Chinese with English abstract.
- 41.Vardi A, et al. Dinoflagellate–cyanobacterium communication may determine the composition of phytoplankton assemblage in a mesotrophic lake. Curr Biol. 2002;12:1767–1772. doi: 10.1016/S0960-9822(02)01217-4. [DOI] [PubMed] [Google Scholar]
- 42.Zhang TT, Wu AP, He M, Nie LW. The allelopathic inhibition and allelopathic mechanism of phenolic acids on algae in water bloom. China Environ Sci. 2007;27:472–476. [Google Scholar]
- 43.Qian HF, et al. Effects of glufosinate on antioxidant enzymes, subcellular structure, and gene expression in the unicellular green alga Chlorella vulgaris. Aquat Toxicol. 2008;88:301–307. doi: 10.1016/j.aquatox.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Zhang BH, et al. An antialgal compound produced by Streptomyces jiujiangensis JXJ 0074T. Appl Microbiol Biotechnol. 2015;99:7673–83. doi: 10.1007/s00253-015-6584-3. [DOI] [PubMed] [Google Scholar]
- 45.Shao JH, Wu ZX, Yu GL, Peng X, Li RH. Allelopathic mechanism of pyrogallol to Microcystis aeruginosa PCC7806 (cyanobacteria): from views of gene expression and antioxidant system. Chemosphere. 2009;75:924–928. doi: 10.1016/j.chemosphere.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 46.Shao JH, et al. Interactions between algicidal bacteria and the cyanobacterium Microcystis aeruginosa: lytic characteristics and physiological responses in the cyanobacteria. Int J Environ Sci Technol. 2014;11:469–476. doi: 10.1007/s13762-013-0205-4. [DOI] [Google Scholar]
- 47.Mazouni K, Domain F, Cassier-Chauvat C, Chauvat F. Molecular analysis of the key cytokinetic components of cyanobacteria: FtsZ, ZipN and MinCDE. Mol Microbiol. 2004;52:1145–1158. doi: 10.1111/j.1365-2958.2004.04042.x. [DOI] [PubMed] [Google Scholar]
- 48.Tillett D, et al. Structural organisation of microcystin biosynthesis in M. aeruginosa PCC7806: an integrated peptide-polyketide synthetase system. Chem Biol. 2000;7:753–764. doi: 10.1016/S1074-5521(00)00021-1. [DOI] [PubMed] [Google Scholar]
- 49.Dziga D, Suda M, Bialczyk J, Urszula CP, c Lechowski Z. The alteration of Microcystis aeruginosa biomass and dissolved microcystin-LR concentration following exposure to plant producing phenols. Environ Toxicol. 2007;22:341–346. doi: 10.1002/tox.20276. [DOI] [PubMed] [Google Scholar]
- 50.Golden SS. Light-responsive gene expression in cyanobacteria. J. Bacteriol. 1995;177:1651–1654. doi: 10.1128/jb.177.7.1651-1654.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Link G. Green life: control of chloroplast gene transcription. Bio Essays. 1996;18:465–471. [Google Scholar]
- 52.Gross EM, Meyer H, Schilling G. Release and ecological impact of algicidal hydrolysable polyphenols in Myriophyllum spicatum. Phytochemistry. 1996;41:133–138. doi: 10.1016/0031-9422(95)00598-6. [DOI] [Google Scholar]
- 53.Aro EM, Virgin I, Andersson B. Photoinhibition of photosystem.II. Inactivation, protein damage, and turnover. Biochim Biophys Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- 54.Pan XL, et al. Toxic effects of amoxicillin on the photosystem II of Synechocystis sp. characterized by a variety of in vivo chlorophyll fluorescence tests. Aquat Toxicol. 2008;89:207–13. doi: 10.1016/j.aquatox.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 55.Pan XL, et al. Effects of levofloxacin hydrochlordie on photosystem II activity and heterogeneity of Synechocystis sp. Chemosphere. 2009;77:413–418. doi: 10.1016/j.chemosphere.2009.06.051. [DOI] [PubMed] [Google Scholar]
- 56.Zuo WJ, Jin PF, Dong WH, Dai HF, Mei WL. Metabolites from the endophytic fungus HP-1 of Chinese eaglewood. Chin J Nat Med. 2014;12:0151–0153. doi: 10.1016/S1875-5364(14)60025-X. [DOI] [PubMed] [Google Scholar]
- 57.Li ZH, Geng MX, Yang H. Algicidal activity of Bacillus sp. Lzh-5 and its algicidal compounds against Microcystis aeruginosa. Appl Microbiol Biotechnol. 2015;99:981–990. doi: 10.1007/s00253-014-6043-6. [DOI] [PubMed] [Google Scholar]
- 58.Kim JD, Kim B, Lee CG. Alga-lytic of Pseudomonas fluorescens against the red tide causing marine alga Heterosigma akashiwo (Raphidophyceae) Biol Control. 2007;41:296–303. doi: 10.1016/j.biocontrol.2007.02.010. [DOI] [Google Scholar]
- 59.Zhang TT, Zheng CY, Hu W, Xu WW, Wang HF. The allelopathy and allelopathic mechanism of phenolic acids on toxic Microcystis aeruginosa. J Appl Phycol. 2010;22:71–77. doi: 10.1007/s10811-009-9429-6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


