Abstract
Although it is challenging for individuals with cocaine addiction to achieve abstinence, the greatest difficulty is avoiding relapse to drug taking, which is often triggered by cues associated with prior cocaine use. This vulnerability to relapse persists for long periods (months to years) after abstinence is achieved. Here I discuss rodent studies of cue-induced cocaine craving during abstinence, with a focus on neuronal plasticity in the reward circuitry that maintains high levels of craving. Such work has the potential to identify new therapeutic targets and further our understanding of experience-dependent plasticity in the adult brain under normal circumstances and in the context of addiction.
Introduction
Neuroimaging studies of individuals with cocaine addiction and in non-human primate models of cocaine addiction suggest that the persistence of vulnerability to relapse, even after a long period of abstinence, is associated with drug-induced alterations in neuronal activity and structure in the limbic and frontal cortical circuitry; however, little is known about the underlying cellular mechanisms or the extent to which such adaptations may change as abstinence progresses1–3. Rodent models of cocaine addiction provide a way to characterize such adaptations in greater detail as the time-course of drug-induced effects can be determined by comparing multiple experimental groups that have a defined experience of the drug, and a defined duration of abstinence. Furthermore, rodent models offer the ability to study plasticity during abstinence at multiple levels, including neural circuits, synaptic transmission, and molecular changes. Most importantly, rodent models that involve cocaine self-administration, the gold standard in the field, provide a way to assess relationships between cocaine-induced neuroadaptations and the level of cocaine craving.
In this Review, I will discuss rodent studies of cocaine craving during abstinence, many of which have focused on the progressive intensification (incubation) of cue-induced cocaine craving that occurs as the duration of abstinence increases. I will describe adaptations that occur in critical regions of the reward circuitry during this process (FIG. 1), focusing on changes in synaptic transmission. The nucleus accumbens (NAc) will be emphasized because many pathways that regulate motivated behavior converge upon this structure. In most of the studies considered here, the level of analysis was a particular brain region or one of its major phenotypically defined cell types. I note, however, that there is growing evidence that small, functionally-linked subsets of neurons within a particular region – neuronal ensembles – contribute to behavioral changes in animal models of addiction4.
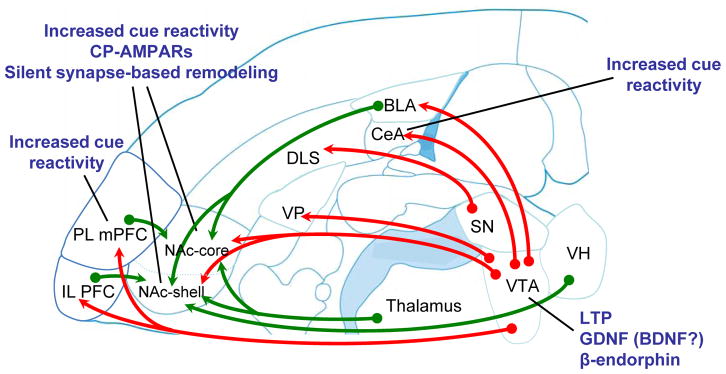
Figure 1. Reward pathways and their roles in incubation of cue-induced cocaine craving.
Glutamate inputs to medium spiny neurons (MSNs) of the nucleus accumbens (NAc) influence the behavioural response to rewards and the cues that predict them. MSNs receive convergent inputs from multiple brain regions, and these inputs interact synaptically61, 62. Inputs from the medial prefrontal cortex (mPFC) provide executive control (important for behavioural flexibility, response inhibition and salience attribution), basolateral amygdala (BLA) inputs relay conditioned associations and affective drive, the hippocampus (Hipp) supplies spatial and contextual information, and the thalamic inputs presumably convey input about arousal and direct attention to behaviourally significant events. The central nucleus of the amygdala (CeA) does not project directly to the NAc, but CeA (and BLA) can influence NAc and other regions indirectly through connections with the midbrain (not shown) as well as other routes (see Box 4). Note that different anatomical substrates underlie incubation of cue-induced cocaine craving versus reinstatement of drug seeking after extinction or even cocaine seeking upon return to the drug self-administration context60, 157. Furthermore, differences exist between the neuronal pathways implicated in incubation of craving for cocaine versus other drugs of abuse12, 15. DLS, dorsolateral striatum; IL, infralimbic; PL, prelimbic; Sub Nigra, substantia nigra. Red lines, dopamine pathways. Blue lines, glutamate pathways. Grey boxes, adaptations associated with incubation.
Rodent models of cue-induced craving
Rodent models of cocaine addiction are quite diverse5. In some models, cocaine is administered by the experimenter (termed non-contingent drug administration). These models do not enable assessment of drug craving, although they do provide other information. For example, the cocaine-conditioned place preference paradigm measures the rewarding effects of cocaine, while behavioural sensitization can provide insight into enduring effects of the drug that are related to locomotor activation and incentive-motivation.
By contrast, studies of drug self-administration (that is, those which involve contingent drug administration) enable the measurement of voluntary drug taking and drug seeking, from which the degree of drug craving can be inferred. In such studies, rodents are trained to perform an operant response (poking their nose in a hole or pressing a lever) to deliver an intravenous infusion of cocaine. Training continues until the behavior is well established (the criteria for this vary in different studies). After the behavior is established, extinction training is performed to reduce drug seeking so that the tendency of the animal to relapse can be studied using a reinstatement test. This approach has provided a wealth of information about relapse mechanisms and potential therapeutic agents6. However, it is not well suited to understanding the plasticity that maintains cocaine craving during long periods of abstinence, because extinction training itself induces plasticity in the reward circuitry7, which is difficult to separate from that elicited by drug exposure and abstinence8. Furthermore, most humans with cocaine addiction do not undergo extinction training as part of their treatment.
An alternative approach to studying relapse, introduced many years ago but recently gaining prominence, is to measure drug seeking after days to months of abstinence from drug self-administration, without an intervening period of extinction training (BOX 1). In such studies, rodents learn to perform an operant response to deliver a drug infusion paired with a discrete cue such as a tone or light. After different periods of abstinence, the animals undergo a seeking test during which the previously learned operant response now delivers the discrete cue but no cocaine infusion. Responding under these conditions provides a measure of cue-induced drug seeking or craving (these terms will be used interchangeably in this Review when describing rodent models). As the duration of abstinence increases, so does the level of responding displayed by the animal during such tests. This phenomenon, termed incubation of drug craving, has been observed during forced abstinence from self-administration of many drugs of abuse (cocaine, methamphetamine, heroin, nicotine, and alcohol)9–12. It appears to be the motivational impact of the drug-associated discrete cue and the operant drug seeking that is strengthened as incubation occurs11. In addition, there may be links between incubation of craving and other behavioural characteristics related to addiction. Thus, rats predisposed to risky decision-making exhibit more robust incubation of cocaine craving13, whereas rats that have undergone incubation are less sensitive to punishment-induced suppression of cocaine-taking, indicating that cocaine-taking has become compulsive in these animals14. The incubation model mimics some common scenarios experienced by humans with addiction, and indeed the incubation of craving has been demonstrated in humans (BOX 1). Furthermore, this model has been employed in most mechanistic studies of cocaine seeking during abstinence.
BOX 1. Incubation of craving in rodent models and correlates in humans.
Incubation of drug craving during forced abstinence has observed in rodents after self-administration of cocaine, methamphetamine, heroin, nicotine, ethanol and sucrose11, 12. This model is relevant to situations in humans in which drug-taking is prevented by incarceration or hospitalization129, as evidenced by clinical studies of humans addicted to nicotine130, methamphetamine131, and alcohol132 (other drugs have yet to be studied in humans). Findings from studies using two new rodent models suggest that incubation may also occur when humans forego drug use to avoid punishment, or with the help of support systems offering alternative sources of reinforcement.
The first of these rodent models — a punishment-induced abstinence model — evolved from efforts to model compulsive drug use in rats through a number of measures including assessing their response to punishment133. In such studies, rats undergo an extensive period of drug self-administration training (the duration typically ranges from 3 weeks to 3 months) before entering a second phase during which operant responses are intermittently paired with a footshock. After limited-access cocaine self-administration, intermittent punishment or a punishment-associated cue can suppress cocaine seeking and taking. After extended-access cocaine self-administration, some rats exhibit resistance to punishment, as well as other behavioral changes that are indicative of compulsive cocaine seeking. This “addicted phenotype” is evident in the first days after punishment training and has been demonstrated 30 days later, suggesting that these behavioral changes contribute to persistent vulnerability to relapse120. If shock intensity is increased, even rats that have undergone extended-access self-administration will cease operant responding, that is, their resistance to punishment is overcome. This has been exploited to produce a period of punishment-induced abstinence during which incubation of cue-induced methamphetamine seeking can be demonstrated134. Interestingly, after incubation of craving in a typical forced abstinence model, rats are less sensitive to punishment-induced suppression of cocaine-taking14.
In the second new model — a “voluntary abstinence” model — rats learn to self-administer drug in the presence of one set of discrete cues, and to self-administer palatable food in the presence of different cues. This is followed by discrete choice sessions in which they must choose one option or the other. Rats choose food in the vast majority of sessions, resulting in a period of voluntary abstinence, during which incubation of methamphetamine craving can be demonstrated119.
The time-course of incubation of craving in rodents and its underlying neuronal mechanisms vary for different drugs of abuse12, 15. For cocaine, cue-induced seeking increases progressively over the first 1–2 months of forced abstinence, plateaus and then returns towards baseline levels, although after 6 months cue-induced craving remains higher than during the first days of withdrawal11. This persistence is remarkable considering that rats live only 2–3 years. In adult rats, incubation of cue-induced cocaine craving is most robust after extended-access cocaine self-administration11 and such regimens are therefore the best suited to studying this phenomenon, although incubation of craving can also be observed after limited-access cocaine self-administration (for examples, see REFs11, 16). Notably, few studies on the incubation of cocaine craving have used female rodents17–19, although there is some evidence of greater persistence of incubation in females17.
Nucleus accumbens
The majority (90–95%) of neurons in the NAc are GABAergic projection neurons termed medium spiny neurons (MSNs). They receive and integrate convergent glutamate inputs from cortical and limbic regions that are important for generating and regulating motivated behaviours, and project to motor-related regions that enable the execution of these behaviours20, 21 (FIG. 1). Through this connectivity, MSNs play a critical role in translating motivation into action21, 22. The NAc consists of core and shell subregions that show differences in structure, function and connectivity20, 23. MSNs in both subregions of NAc, as well as in the adjacent dorsal striatum, can also be distinguished based on whether they express the D1 dopamine receptor (D1R) or the D2 dopamine receptor (D2R), which in turn is related to their connectivity and their responses to cocaine (BOX 2).
BOX 2. Cocaine-induced adaptations in D1R-expressing MSNs compared with D2R-expressing MSNs.
In the dorsal striatum, D1R-expressing MSNs project directly to basal ganglia output nuclei whereas D2R-expressing MSN do so indirectly — these are termed the direct and indirect pathways, respectively135. In the NAc, however, D1R- and D2R-expressing NAc projections should not be equated with direct and indirect pathways. Although D1R-expressing NAc MSNs project to output nuclei (the substantia nigra and VTA), both D1R- and D2R-expressing NAc MSNs project to the ventral pallidum, which serves as both a relay station and an output nucleus136. Putting aside the issue of terminology, it is clear that some cocaine regimens differentially affect D1R- and D2R-expressing NAc MSNs and that the disruption of the normal balance of activity between the two types of neurons contributes to cocaine-induced behavioural changes137. However, in the case of extended-access cocaine regimens leading to incubation of cocaine craving, the available evidence does not support the idea that there is differential plasticity in D1R- and D2R-expressing MSNs in NAc core28, the region most closely linked to expression of incubation16, 24, 25, 27, 31, or even in the dorsal striatum71. An important example is the elevation of CP-AMPAR levels that is observed in all NAc core MSNs after extended-access cocaine self-administration and >1 month of abstinence28.
I speculate that the duration of abstinence influences the degree of selectivity of effects on D1R- and D2R-expressing MSNs. During cocaine exposure and early abstinence, when MSNs are responding to the major pharmacological effect of cocaine, that is, the elevation of DA levels, it is to be expected that D1R- and D2R-expressing MSNs will respond differently to the drug owing to the different receptors that they express. By contrast, increased CP-AMPAR expression occurs weeks after the last cocaine exposure. During this period, it is likely that a cascade of events involving multiple brain regions ultimately influences the activity of glutamate afferents to NAc core MSNs. As these afferents do not selectively innervate D1R- or D2R-expressing MSNs, glutamate receptors on both MSN populations may experience similarly altered activity and therefore undergo similar activity-dependent plasticity. Interestingly, however, the incubation regimen that resulted in the upregulation of CP-AMPARs in all NAc core MSNs did so in only a portion of NAc shell MSNs51. This could reflect differences between D1R- and D2R-expressing shell MSNs55. On the other hand, a high-dose cocaine regimen leading to incubation of cocaine craving elicited CP-AMPAR elevation in D1R-expressing MSNs (postsynaptic to mPFC inputs) and D2R-expressing MSNs (postsynaptic to BLA inputs)19, adding to other evidence for pathway-specific plasticity in the NAc shell during abstinence53–55.
Activation of NAc core neurons during incubation
In rodents, the incubation of cocaine craving is associated with altered firing of NAc core neurons that encode cocaine-related information, that is, change their firing pattern within seconds of responding for cocaine or during the presentation of cues previously paired with cocaine 16, 24, 25. Relative to withdrawal day 1 (WD1; that is, 1 day after discontinuing cocaine self-administration), there was an increase on WD30 in the number of neurons in the NAc core (but not shell) responding during the resumption of cocaine self-administration, as well as an increase in the strength of their response16. Subsequently it was shown that there was an increase in the number of NAc core neurons responding to the presentation of cocaine cues, and the strength of those responses, on WD30 compared with WD124. Another study compared NAc neuronal firing during three periods: an extended-access cocaine self-administration regimen that leads to the escalation of cocaine intake (which mimics the tendency of human cocaine users to increase drug intake over time), subsequent abstinence (when incubation of cocaine craving occurred) and the post-abstinence re-establishment of cocaine taking and re-escalation of cocaine intake25. It was found that the NAc core was selectively involved in drug seeking and incubation, whereas the shell was selectively involved in drug taking and escalation. Specifically, the proportion of NAc core neurons that responded phasically to drug-reinforced lever presses or to cocaine cues did not change during drug self-administration, even when drug taking escalated, but increased during abstinence and remained increased, even after drug taking was re-established and then escalated. The magnitude of incubation correlated with the increase in the number of NAc core neurons that coded cocaine-seeking responses25. By contrast, escalation of cocaine intake correlated with a decrease in firing of NAc shell neurons; firing of the NAc shell neurons normalized during abstinence, but decreased again during re-escalation. Interestingly, these changes occurred exclusively in neurons of the NAc shell that did not code for cocaine-related information25.
Together, these results show that incubation of cocaine craving is accompanied by increased recruitment of NAc core neurons that fire in a manner that is time-locked to cue-induced cocaine-seeking16, 24, 25. A likely cellular underpinning of this is the strengthening of excitatory synapses onto NAc core MSNs due to synaptic incorporation of high conductance Ca2+-permeable AMPA receptors (CP-AMPARs), as discussed in the next section. MSN recruitment could also be facilitated by an increase in their intrinsic membrane excitability, which would promote action potential firing. Supporting this, NAc core MSN show increased intrinsic excitability shortly after discontinuing non-contingent cocaine26; however, their excitability has not been assessed after extended-access cocaine self-administration and abstinence (BOX 3). It has been proposed that increased intrinsic excitability in the NAc core may lead to increased excitatory synaptic strength23, therefore these candidate mechanisms are not mutually exclusive.
BOX 3. Baseline metabolic activity and excitability in the reward pathway during abstinence.
Early neuroimaging studies in humans found that rates of cerebral metabolism in the frontal cortex of chronic cocaine users were significantly decreased under baseline conditions, and that this decrease persisted for 3–4 months of abstinence. Other studies of such individuals found decreased performance on behavioral tasks requiring activity in the PFC, as well as deficits in its structural integrity. When cocaine cues were presented, however, chronic cocaine users exhibited heightened activation in the PFC that correlated with craving. Together, these alterations in PFC function may contribute to hallmarks of addiction, namely, impaired inhibitory control over behavior and heightened attribution of salience to the drug and drug-cues compared to non-drug reinforcers2, 138. Subsequent neuroimaging studies in monkeys defined the spatial and temporal progression of cocaine’s effects on resting metabolic activity in the forebrain139, 140. After 5 sessions of cocaine self-administration, cocaine-induced hypoactivity was observed in ventromedial PFC and ventral striatum (including the NAc). After 100 sessions, the area of hypoactivity expanded to include additional cortical and striatal regions as well as other areas not previously affected (such as the amygdala, hippocampus, temporal and parietal cortex). Decreased activity in NAc and dorsal striatum persisted after 1 month of abstinence (other regions were not studied)141.
As discussed in the main text, in rodents, the mPFC, NAc and CeA all show increased responsiveness to cocaine cues after prolonged abstinence. A study of basal cerebral blood flow in rats on WD10 after 33 sessions of extended-access cocaine self-administration found that there were decreases in baseline activity in the NAc core, the mPFC and other regions142. Together, these findings suggest that increased cue reactivity may occur against a background of depressed resting activity, as demonstrated for humans. Electrophysiological assessments of baseline activity and excitability have also been performed. In studies of the rat mPFC conducted shortly after discontinuing non-contingent or self-administered cocaine, depressed baseline activity and excitability have been found in this region143–145, and one study has linked decreased excitability to cocaine seeking under punishment contingencies82. Electrophysiological studies in the NAc have provided more mixed results that are likely owing to variability in cocaine regimens. In the NAc, intrinsic excitability is decreased in the shell and increased in the core on WD1–3 after non-contingent cocaine exposure23, 26, 146. Decreased excitability in the NAc shell persisted on WD21 after non-contingent but not self-administered cocaine; the core was not examined in this study147. After prolonged abstinence from extended-access cocaine self-administration, a subpopulation of CeA neurons showed increased intrinsic excitability93. Additional studies comparing multiple brain regions after the same regimen142 would help to further define abstinence-related plasticity at the circuit level.
NAc core CP-AMPARs mediate incubated craving
GluA2-containing Ca2+-impermeable AMPARs (CI-AMPARs) are responsible for 90–95% of the evoked excitatory post-synaptic current (EPSC) in the NAc core of adult drug-naïve rats and during the first weeks of abstinence from extended-access cocaine self-administration; however, between WD25 and WD35, there is a marked increase in synaptic levels of CP-AMPARs that persists until at least WD9027, 28. These are primarily homomers of GluA1 subunits27 although a role for other types of CP-AMPARs cannot be ruled out (see REF 29 and Supplementary information S1 (BOX)). The discovery that CP-AMPAR levels increase after incubation of craving was initially surprising, because all prior studies of adult rats subjected to non-contingent cocaine exposure and 2–3 weeks of withdrawal had demonstrated upregulation of CI-AMPARs in the NAc core (see Supplementary information S4 (table)). A comprehensive summary of studies on AMPAR levels and subtypes in the NAc after cocaine self-administration is also provided (see Supplementary information S3 (table)).
Once CP-AMPAR levels in the NAc core are elevated after incubation, the responsiveness of MSNs to glutamate drive is strengthened30 (presumably owing to the higher conductance of CP-AMPARs compared to CI-AMPARs), and blocking CP-AMPARs27 or removing them from NAc core synapses31 reduces the incubated portion of cue-induced cocaine seeking in rats. These results indicate that synaptic incorporation of CP-AMPARs enhances MSN activation in response to glutamate released by cocaine-paired cues and thereby augments the drug seeking response (FIG. 2, top). Activation of the central nucleus of the amygdala (CeA) and ventral medial prefrontal cortex (mPFC) is also required for incubated cocaine seeking after 3–4 weeks of abstinence32, 33. Thus, coordinated transmission within the reward circuitry is required for expression of incubation after prolonged abstinence, with strengthened AMPAR transmission in the NAc core playing a critical role in translating motivational signals into strengthened motor output. At earlier withdrawal times, incubation must depend upon other mechanisms and brain regions (BOX 4).
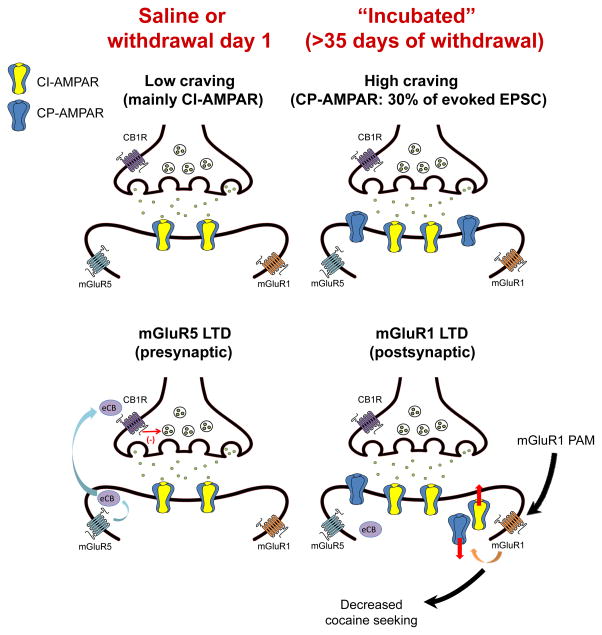
Figure 2. Ca2+-permeable AMPARs in NAc core synapses are required for expression of incubated cocaine craving.
a| In the nucleus accumbens (NAc) core of rats that undergo extended-access cocaine self-administration and prolonged withdrawal (>35 days), CP-AMPAR levels increase markedly and activation of these receptors is required for the expression of incubated cocaine seeking27, 31. We propose that this reflects the ability of high-conductance CP-AMPARs to augment MSN responsiveness to glutamate drive30, enabling MSNs to respond more robustly when a cocaine cue is presented. Some CP-AMPARs are inserted into previously silent synapses53, 54 b| Group I mGluR-mediated synaptic depression is altered in the NAc core of rats that show incubation of cocaine craving. In control rats, this synaptic depression depends upon mGluR5 stimulation, endocannabinoid formation, and activation of presynaptic CB1Rs. After prolonged withdrawal and incubation of craving, this mechanism is disabled39 and an mGluR1-mediated synaptic depression is observed that is expressed postsynaptically via CP-AMPAR removal31, 39. By eliciting this mGluR1-mediated synaptic depression, systemic or intra-NAc administration of mGluR1 positive allosteric modulators (PAMs) can reduce incubated cue-induced cocaine seeking31. Similarly, mGluR1 activation removes CP-AMPARs from VTA synapses of cocaine-exposed animals97, 98. Although mGluR1-mediated synaptic depression is most obvious when CP-AMPAR levels are high, we speculate that it operates at a low level under normal conditions and that loss of mGluR1 tone during abstinence helps account for CP-AMPAR accumulation. Supporting this notion, decreased mGluR1 surface expression in the NAc core precedes and enables CP-AMPAR accumulation and incubation of craving31.
BOX 4. Time-line of the involvement of different brain regions in the incubation of cocaine craving.
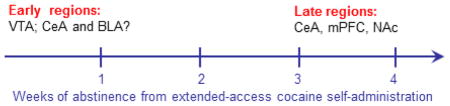
Incubation of cue-induced cocaine seeking begins during the first week of abstinence10, 11. Cocaine-induced adaptations in the VTA have been shown to play a causal role in early phases of incubation, namely LTP onto VTA DA neurons56 and elevated GDNF (and perhaps BDNF) signalling12, 50, 100, 101 (see the figure). Incubation is also dependent on the loss of an endogenous braking mechanism operating in the NAc core that occurs during the first month of abstinence; on WD1, the presentation of a cocaine cue releases β-endorphin in the NAc core, which suppresses cocaine seeking through activation of delta opioid receptors. By WD30, cue presentation no longer activates this protective mechanism148. After 3–4 weeks of withdrawal, activity in the CeA, mPFC and NAc is required for the expression of incubated cue-induced cocaine seeking27, 31–33, 53, 54, 89. The anatomical circuits through which adaptations in “early regions” lead to adaptations in “late regions” remain to be determined. It is likely that direct projections from the VTA to the “late regions” (FIG. 1) play a role in this progression. This is supported by evidence obtained over many decades that psychostimulant-induced adaptations in the VTA enable more persistent adaptations in downstream regions such as the NAc28. In addition, the observation that there is altered glutamate receptor expression in CeA and BLA on WD188 suggests that these regions may be involved in early stages of incubation, perhaps helping to trigger subsequent adaptations in the NAc and mPFC that are required for its expression during late withdrawal. The anatomical substrate for this hypothetical transition is unknown. It could depend on pathways connecting the amygdala to the NAc and mPFC, although the CeA, unlike the BLA, does not project directly to the NAc or the mPFC149. Alternatively, the progression could occur through direct (CeA) or indirect (BLA) connections with VTA DA neurons150.
In addition to strengthening excitatory drive to NAc MSN, increased synaptic levels of CP-AMPARs may change the requirements for the induction of plasticity in the NAc, as has been described previously in the VTA of cocaine-exposed rats34. At synapses where CP-AMPARs mediate excitatory transmission, an unusual form of LTP is observed which depends upon Ca2+ entry through CP-AMPARs and is independent of NMDARs; its induction requirements are the opposite to those of Hebbian NMDAR-dependent LTP35. Such an alteration in plasticity mechanisms in the NAc has the potential to affect future learning about rewards, including cocaine. Plasticity might be further altered by changes in NMDAR transmission in the NAc of rats after incubation of cocaine craving36 (see Supplementary information S1 (BOX)).
Multiple signaling pathways contribute to CP-AMPAR accumulation in the NAc core during incubation (Supplementary information S1 (BOX)). As in other brain regions37, 38, activation of metabotropic glutamate receptor 1 (mGluR1) negatively regulates CP-AMPAR levels in NAc core synapses39. A reduction in this negative regulation, owing to a time-dependent decrease in mGluR1 expression at the cell surface, precedes and enables CP-AMPAR accumulation and incubation of cocaine craving, although pharmacological stimulation of the remaining mGluR1 receptors is sufficient to reduce CP-AMPAR levels and thereby reduce cocaine craving31 (FIG. 2). Simultaneously, elevated GluA1 phosphorylation may promote synaptic insertion of homomeric GluA1 CP-AMPARs40. Interestingly, the mGluR5 and cannabinoid receptor 1 (CB1R)-dependent form of group I mGluR-mediated synaptic depression that is normally expressed by MSNs is disabled in the NAc after incubation of cocaine craving39, as has also been observed after some other types of cocaine exposure41 (see REF28 for a review). Interestingly, the maintenance of both elevated CP-AMPAR levels and altered group I mGluR plasticity after incubation of craving depends upon ongoing protein translation in the NAc42. Thus, incubation may share features with certain other disorders of the nervous system that involve long-term dysregulation of protein translation43, 44. There have been other reports of altered long-term potentiation (LTP) or long-term depression (LTD) during abstinence from cocaine self-administration (for examples see REFs45, 46; see REF28 for a review), but the relationship of such alterations to cocaine-seeking during abstinence or its incubation is unclear. Finally, brain-derived neurotrophic factor (BDNF) transmission in the NAc was originally considered a strong candidate for mediating incubation47 and CP-AMPAR elevation48, but recent work has demonstrated a relatively subtle role for BDNF that varies with NAc subregion and withdrawal time and does not appear to involve CP-AMPAR regulation49, 50.
In adult rats, all MSNs of the NAc core exhibit elevated CP-AMPAR levels after a month or more of abstinence from extended-access cocaine self-administration28, 51. This global plasticity might be expected to increase responses to all types of information processed by core MSNs, but instead it has been found that abstinence selectively alters responding to certain types of cocaine experiences16, 24, 25, 52. This can be explained by recalling that the detection, via whole-cell patch-clamp recording, of elevated CP-AMPAR levels in a MSN only establishes that CP-AMPARs have been added somewhere in its dendritic tree. CP-AMPAR insertion only into specific subsets of synapses could explain behavioural selectivity, because only certain afferents to the MSN – conveying specific types of information – would evoke a strengthened response. In fact, it is becoming clear from optogenetic studies that AMPARs on MSNs undergo distinct cocaine-induced plasticity depending on whether they are postsynaptic to glutamate inputs originating from mPFC, basolateral amygdala (BLA), or ventral hippocampus (VH)53–55. This hypothesis still leaves a conceptual gap between the AMPAR plasticity that is observed, and the evidence that cocaine-seeking and other responses to cocaine can be disrupted by inactivating neuronal ensembles that constitute only a small percentage of NAc neurons4.
CP-AMPARs are also added to NAc shell MSNs after prolonged abstinence from cocaine self-administration19, 51, 53–56, although more heterogeneity is apparent in the NAc shell; for example, a regimen that upregulates CP-AMPARs in all MSNs of the NAc core produces this effect in only a subset of MSNs in the NAc shell51 (see BOX 2). Most results indicate a correlation between CP-AMPAR expression in NAc shell and stronger cue-induced cocaine seeking53, 56 although this depends on the input pathway54.
Different roles of specific glutamate inputs to NAc MSNs
Optogenetic studies have helped elucidate the relationship between plasticity and behaviour by showing that abstinence from cocaine self-administration differentially affects specific glutamate pathways into the NAc. Three glutamatergic pathways that project to the NAc (running from prelimbic mPFC (PL) to NAc core, from infralimbic mPFC (IL) to NAc shell, and from BLA to NAc shell) undergo silent synapse formation after cocaine self-administration (as identified on WD1), followed by un-silencing via AMPAR incorporation during withdrawal (as identified on WD45)53, 54. However, in the BLA–NAc shell and IL–NAc shell pathways, un-silencing is mediated by CP-AMPAR insertion, whereas in the PL–NAc core pathway un-silencing is mediated by CI-AMPAR insertion. Removal of newly inserted CP-AMPARs by optogenetically-inducing LTD in the BLA–NAc shell pathway inhibited cocaine seeking on WD4553, suggesting that unsilencing promotes incubation. Similarly, un-silencing of the PL–NAc core pathway promoted incubation, whereas un-silencing of the IL–NAc shell pathway opposed incubation54, which is consistent with some results suggesting opposing effects of these mPFC subregions in other animal models of relapse (see below). However, importantly, although the silent synapse studies show that plasticity occurs between WD1 and WD45, they do not permit conclusions to be drawn about whether synaptic strength was altered on WD45 compared to the pre-cocaine baseline.
These and other results led Dong and Nestler57 to propose a neural rejuvenation hypothesis of addiction which states that cocaine-induced plasticity recapitulates events that are typically associated with development, including silent synapse formation and un-silencing. A similar hypothesis, focusing on glutamate receptor composition instead of silent synapses, has been suggested regarding cocaine-induced plasticity in the VTA58. An open question is how silent synapses form during withdrawal from cocaine —through the “birth” of new NMDAR-only synapses or through silencing of existing synapses through loss of AMPARs?
Investigation has begun into whether there is differential plasticity onto D1R- versus D2R MSNs during cocaine abstinence. After 1 month of withdrawal from a limited-access regimen, no plasticity was found in glutamate pathways terminating onto D2R MSNs of the NAc shell, while varied plasticity was observed in pathways terminating onto D1R MSNs: elevation of CP-AMPAR levels postsynaptic to IL inputs, increase in the AMPA/NMDA ratio (but no evidence for elevated CP-AMPAR levels) postsynaptic to VH inputs, and no alterations postsynaptic to BLA inputs55. An optogenetic LTD protocol that normalized both the VH-D1R MSN shell and the IL-D1R MSN shell pathways reduced cue-induced cocaine seeking to the level observed in saline controls, an effect which persisted after 1 week. Selective normalization of the VH-D1R MSN pathway or the IL-D1R MSN pathway reduced the magnitude of seeking and response discrimination, respectively55. Several points must be kept in mind when interpreting these results. First, the role of D2R MSNs in the cocaine seeking observed in this study remains to be determined because, although no cocaine-induced plasticity was observed for D2R MSNs, the optogenetic LTD protocols used to reverse the strengthening of pathways onto D1R MSNs (and thereby assess its behavioural significance) also reduced synaptic strength onto D2R MSNs. Second, the effect of this cocaine regimen might be quite different in the NAc core (BOX 2). Finally, this study used a limited-access cocaine regimen that was not demonstrated to elicit incubation of craving55. The experimental results may reflect a transitional state that, if cocaine exposure had been more robust or abstinence more protracted, would have led to different AMPAR plasticity and affected a wider population of MSNs, including D2R MSNs. Supporting this, utilizing a higher dose of cocaine (which led to incubation of craving) elicited elevation of CP-AMPAR levels in IL-D1R MSN shell and BLA-D2R MSN shell pathways, although the behavioral consequences of the CP-AMPAR plasticity were not tested19. Future studies should investigate the BLA-NAc core pathway, as this BLA output is most strongly implicated in cue-induced cocaine seeking59, 60.
Incubated cocaine seeking requires coordinated inputs to NAc
In conclusion, cue-induced cocaine seeking after about 1 month of abstinence is markedly reduced by any one of the following manipulations: Reducing CP-AMPAR transmission in the NAc core27, 31, weakening the BLA-NAc shell pathway53, weakening the PL-NAc core pathway54, or simultaneous weakening of IL-D1R MSN shell and VH-D1R MSN shell pathways55. This suggests that simultaneous activity in all of these pathways may be required for a high level of cocaine seeking. This can be understood by considering that the brain regions in question participate in a richly interconnected network59. It makes sense that coordinated activity across the network is required to sustain strong cocaine seeking. More specifically, it is important to recall that a single MSN often receives convergent glutamate inputs from BLA, mPFC, hippocampus, and other regions, and activation of more than one input to an MSN increases the probability of action potential firing; in addition, activation of one input often modulates or “gates” the response to other afferents61, 62. Based on these considerations, it is logical to expect that the activation of multiple glutamate pathways onto an MSN will yield the greatest response. Furthermore, as specific pathways are strengthened or weakened during incubation, the gating mechanisms that tune MSN responsiveness are expected to be disrupted (see REF63 for an example after non-contingent drug exposure).
Dorsal striatum
The dorsal striatum is implicated in cue- and context-induced reinstatement after extinction training6, 60 and in responding for cocaine under a second-order schedule of reinforcement64. In initial studies of its role in abstinence models, rats self-administered cocaine under limited-access conditions in the absence of discrete cues; after two weeks of forced abstinence, they were returned to the drug self-administration context for cocaine seeking tests. Reversible inactivation of the dorsolateral striatum (DLS), as well as ventral tegmental area (VTA) and substantia nigra, impaired cocaine seeking under these conditions, whereas inactivation of NAc core, dorsomedial PFC or BLA had no effect65, 66. As these studies used a regimen that is not expected to produce incubation of cocaine craving (see REF67 for negative results obtained with a similar regimen) and cocaine self-administration was not paired with cues, these results do not contradict evidence summarized above indicating that NAc core and mPFC are required for the expression of incubated cue-induced cocaine craving. In a subsequent study, the authors trained different groups of rats under limited- and extended-access cocaine self-administration regimens and assessed responding upon return to the drug self-administration context on withdrawal days 1, 14, and 60. Inactivation of the DLS decreased cocaine seeking in the tests on all withdrawal days and independently of the cocaine access conditions, suggesting a general role of the DLS in cocaine seeking after forced abstinence that does not depend upon the duration of abstinence67. It should be noted that clear incubation of craving was not observed in this study, despite the use of an extended-access regimen, perhaps because multiple seeking tests in the same animals resulted in competition between extinction of responding and incubation of responding67. Interestingly, reversible inactivation of a different portion of the DLS during the first week of abstinence selectively reduced punished cocaine seeking, whereas inactivation of a more ventral region (midlateral striatum) also reduced un-punished seeking68.
It is well-established that non-selective reversible inactivation (for example REF67) and selective pharmacological receptor blockade in a given brain area often lead to different or even opposite results69. In fact, after limited-access cocaine self-administration and 21 days of abstinence, infusion of an mGluR5 negative allosteric modulator into the NAc core, but not the DLS, decreased cocaine seeking upon return to the drug self-administration context70. In DLS tissue harvested on WD21 (without a prior test), mGluR5 surface expression was reduced and mGluR5-mediated LTD was eliminated70. In the only study of the dorsal striatum and incubation of methamphetamine craving, blockade of D1Rs in this brain region decreased incubated cue-induced methamphetamine seeking on WD30 but had no effect on methamphetamine seeking on WD171.
Medial prefrontal cortex
The prefrontal cortex is critical for working memory, decision making, and behavioural flexibility. Individuals with cocaine addiction show deficits in these behaviours, as well as structural and functional deficits in frontal cortical regions that correlate with compulsive cocaine craving2, prompting extensive studies of analogous regions in the rodent brain. Most such abstinence-related work has been performed in the mPFC, so here I will focus on this region.
The rodent mPFC can be subdivided into the PL, IL and anterior cingulate cortex (ACC) regions. Alternatively, it can be subdivided into dorsal mPFC (ACC and dorsal PL) and ventral mPFC (ventral PL and the IL)72. As discussed above, the glutamatergic PL–NAc core pathway promotes incubation16, 24, 54, whereas the IL–NAc shell pathway opposes it54. Consistent with these findings, an electrophysiological study in awake rats found that some neurons in both the IL and PL regions encode cocaine-associated stimuli and cocaine-seeking behaviour on WD1 but that the proportion of PL neurons encoding such information is nearly doubled on WD30, after incubation of craving has occurred73. However, another study found that on WD1, a cocaine cue failed to activate either the ventral mPFC or dorsal mPFC, whereas on WD30, the cue activated both regions but produced much stronger activation in the ventral mPFC (ERK phosphorylation was the measure of neuronal activation); furthermore, reversible inactivation of the ventral mPFC, but not the dorsal mPFC, prevented the expression of incubation on WD3033. It is possible that inactivation of the ventral mPFC in this study affected neurons of the PL mPFC that promote incubated craving through their projections to the NAc core. Alternatively, inactivation of the ventral mPFC may have altered activity in a pathway that does not terminate in the NAc, but nevertheless influences incubation. Interestingly, inactivation of the ventral mPFC does not prevent expression of incubation of methamphetamine craving74.
Together these studies show that some mPFC neurons, including PL mPFC neurons, are more reactive to cues after incubation of cocaine craving. This may be driven in part by enhanced cue-induced glutamate release in the mPFC, as observed during a seeking test on WD30 compared to WD3, combined with a decrease in cue-induced DA release75. Postsynaptic mechanisms may also contribute. On WD7, PKA phosphorylation of GluA1 and CREB was increased in the dorsal mPFC and intra-mPFC infusion of a PKA inhibitor reversed this effect and decreased cue-induced cocaine seeking76. These latter results suggest that strengthened AMPAR transmission in the mPFC may contribute to incubated cocaine seeking, given that PKA phosphorylation of GluA1 primes AMPAR for synaptic insertion in PFC neurons77. Decreased 5-hydroxytryptamine receptor 2C (5-HT2C) receptor availability and responsivity in the mPFC also enhances cue reactivity and thereby contributes to incubation78. Additional plasticity is apparent in the ventral mPFC in response to the presentation of cocaine-related cues. In rats that have undergone incubation of cocaine craving, cue presentation increases the relative amount of phosphorylated PKCε79 and decreases group I mGluR levels in the ventral mPFC80. Both effects contribute to maintaining high levels of cocaine seeking during abstinence, perhaps by impairing extinction of responding to the cue79, 80. Cue-induced ERK phosphorylation in ventral mPFC also correlates with expression of incubation of cocaine craving33.
Although synaptic transmission in the mPFC after forced abstinence has not been studied directly, two studies have recorded mPFC pyramidal neurons shortly after discontinuing long-term self-administration training (>6 weeks); one study found an increased AMPA/NMDA ratio that may be owing to impaired mGluR2/3-mediatedLTD81 and the other observed a decrease in intrinsic excitability (see BOX 3), the magnitude of which was correlated with resistance to punishment-induced suppression of cocaine seeking82, a measure of compulsive cocaine seeking (see BOX 1). It will be important to determine how these adaptations evolve during abstinence, and to examine other subregions of the PFC that differentially modulate compulsive cocaine seeking in similar animal models83.
Amygdala
The amygdala consists of a heterogeneous group of inter-connected nuclei that mediate emotional learning, particularly that related to cues that predict fear or reward. This learning involves activity-dependent recruitment of principal neurons to functional networks that can be segregated according to valence, that is, cells responding to reward cues do not respond to fear cues84. The BLA and CeA have been extensively studied in addiction models. The excitatory BLA–NAc projection is necessary for motivated behavior driven by cues associated with reward59, 60, 85 and, in humans, these are the two brain regions most strongly activated by drug-associated cues86. The CeA, a major output nucleus for amygdala projections to the brain stem and hypothalamus, works with the BLA in some aspects of reward processing but there are also functional dissociations between these regions.85 The CeA, and in particular corticotrophin releasing factor (CRF) transmission within the CeA, is critical for the aversive state that occurs during drug withdrawal87.
An initial study found that glutamate receptor levels were increased in both CeA and BLA after extended-access cocaine self-administration, with more pronounced changes observed in BLA on WD1 and in CeA on WD3088, suggesting that both subregions may have a role in the early phases of incubation (BOX 4). Subsequently it was found that neural activity (inferred from the level of ERK phosphorylation) is increased in the CeA after a cue-induced seeking test on WD30 but not WD1, and pharmacological reduction of ERK phosphorylation in the CeA decreased cocaine seeking on WD30, whereas NMDA infusion into the CeA increased ERK phosphorylation and cocaine seeking on WD132. Furthermore, intra-CeA administration of an mGluR2/3 agonist, expected to decrease glutamate release, reduced incubated cocaine seeking on WD21 without affecting drug seeking on WD389. These findings suggest that CeA neurons become more responsive to cocaine cues over the first month of withdrawal and that their activation during a test is required for expression of incubated craving. However, this conclusion is based on the use of ERK phosphorylation as a measure of neuronal activation. Results obtained using a more standard method (reversible inactivation) indicate that the CeA, but not other regions tested, is required for the expression of incubated methamphetamine craving74 suggesting that CeA may be critical for a core process common to both methamphetamine and cocaine incubation.
Based on evidence that Pavlovian-to-Instrumental Transfer (PIT) is sensitive to CeA but not BLA manipulations90, paralleling results from studies of incubation32, 89, it is possible that incubation depends upon a withdrawal-dependent augmentation of the ability of the CeA to impart motivational potency to psychostimulant cues and thereby increase operant responding74. As noted at the beginning of this section, LTP in the amygdala promotes recruitment of neurons into functional networks related to reward responding84, and several studies, taken together, have found adaptations during forced abstinence from cocaine self-administration that would be expected to facilitate LTP in the CeA91–93. Facilitated LTP might increase the proportion of CeA neurons that code information about cocaine-related cues and thereby promote incubation of cocaine craving.
More work is required to understand the role of the BLA in the incubation of cocaine craving. As discussed above, weakening of the BLA–NAc shell pathway eliminated the expression of incubation of cocaine craving53, and some activation of BLA principal neurons during the cue-induced seeking test is required to account for this observation. However, manipulations that decreased cocaine seeking on WD30 when performed in the CeA (that is, inhibition of ERK phosphorylation or administration of an mGluR2/3 agonist) did not affect cocaine seeking when performed in the BLA32, 89. Two considerations help reconcile these observations. First, ERK phosphorylation is not an ideal readout of neuronal activation. Second, mGluR2/3 stimulation might have less impact if activation of BLA neurons during a seeking test occurs via disinhibition (a mechanism that contributes to activation of BLA principal neurons in a model of conditioned fear94).
The ventral tegmental area and dopamine
Although all the major neuromodulatory transmitters – dopamine (DA), serotonin and noradrenaline– probably contribute to cocaine craving during abstinence, the focus of this Review is on synaptic plasticity, and DA has been best studied from this perspective.
Plasticity in the VTA during abstinence
DA projections originating in the ventral tegmental area (VTA) are critical for reward learning mechanisms and are a major target of drugs of abuse. One of the earliest responses to cocaine, and other addictive drugs, is the rapid induction of NMDAR-dependent LTP at excitatory synapses onto VTA DA neurons, mediated by synaptic insertion of CP-AMPARs28, 95, 96. The functional significance of this LTP has been studied mainly in the behavioral sensitization model28. However, in mice in which the gene for NR1 (an obligatory subunit of NMDARs) is deleted in DA neurons during adulthood, cocaine self-administration fails to produce the robust cocaine seeking or elevated NAc CP-AMPAR levels observed on WD35–37 in wild-type mice, suggesting that VTA plasticity enables incubation of craving56 (BOX 4). This finding adds to extensive literature showing that NMDAR-dependent plasticity in the VTA is required for persistent cocaine-induced adaptations in downstream regions28. Conversely, mGluR1 exerts an inhibitory influence on CP-AMPAR levels in VTA synapses, thereby limiting cocaine-induced synaptic potentiation37, 56, 97, 98. This relationship between mGluR1 and CP-AMPARs extends to other brain regions, including the NAc37, 56, 97, 98. Notably, cocaine-induced LTP in the VTA lasts only a few days after single or repeated non-contingent cocaine injections, but persists for at least 3 months after cocaine self-administration56, 99. This suggests that, during prolonged abstinence, animals that have self-administered cocaine remain in a state where activation of DA pathways (for example, by cues that predict cocaine) will be stronger than normal, perhaps promoting plasticity and learning in dopaminergic targets28.
In addition to plasticity of DA transmission, activity of endogenous glial-derived neurotrophic factor (GDNF) in the VTA during the first two weeks of cocaine withdrawal is essential for the incubation of cocaine craving, and cocaine seeking in early withdrawal can be enhanced by delivery of exogenous GDNF to the VTA 100 (BOX 4). Delivery of exogenous BDNF into the VTA also potentiates cue-induced cocaine seeking during the first month of withdrawal101, which may be linked to the ability of BDNF to enhance susceptibility to LTP induction in VTA DA neurons102, but a role for endogenous BDNF in the VTA in the incubation of craving has not been tested.
DA transmission in NAc during incubation
To date the role of DA in cue-induced cocaine seeking after drug self-administration and forced abstinence has not been evaluated, but it would be of interest to determine how adaptations in DA and glutamate transmission converge to regulate responsiveness of NAc MSNs and thereby cocaine seeking. There is evidence that cocaine cues increase DA release and thereby facilitate craving; however there is also evidence that negative affective states associated with decreased tonic DA transmission and increased excitation of NAc neurons can facilitate drug seeking103–105, and that these negative affective states may be linked to incubation of craving. For example, pairing saccharin, a normally rewarding substance, with a delay to cocaine self-administration renders saccharin aversive to rats, and the magnitude of this reward devaluation intensifies with incubation of cocaine craving104, 106. These findings may be related to the emergence of a negative affective state in human cocaine users that is thought to contribute to craving and relapse87.
Interestingly, as rats become more responsive to cue-induced cocaine seeking following abstinence from cocaine self-administration11, the relationship between NAc DA transmission and subsequent cue-reward learning is disrupted107. These findings emphasize that relationships between neurotransmission and behavior elucidated in naive animals or during drug-taking cannot necessarily be extrapolated to the abstinent state.
Abstinence alters DA receptors in striatal areas
Positron emission tomography (PET) imaging studies in humans that abuse cocaine have found reduced D2R levels in many striatal regions, including the NAc, that are evident in early withdrawal as well as after 3–4 months of detoxification; the reduced D2R levels correlate with decreased frontal cortical metabolism, which also persists into abstinence108. These changes are proposed to contribute to compulsive drug seeking and greater salience of drug reward compared to non-drug rewards108. A decrease in striatal D2R levels that persists into withdrawal has also been found in rhesus monkeys with extensive cocaine self-administration experience109, 110. Because monkeys can be studied before and after drug exposure, unlike humans, this work has confirmed evidence in humans that low D2R availability makes an individual more vulnerable to the reinforcing properties of cocaine but that continued exposure to cocaine further decreases D2R levels111. Decreased striatal D2R levels that persist into abstinence have also been found in rats. For example, in NAc tissue from the same rats used to demonstrate increased CP-AMPARs after incubation of cocaine craving, we found decreased D2R surface expression on both WD1 and WD45 112. In rats, like humans and primates, there is an association between low D2R levels and high impulsivity, a trait that is both a risk factor for, and a consequence of, drug abuse113, 114.
Less is known about D1R and D3R expression in humans that use cocaine. However, the role of the D3R in rat models of cocaine addiction has been studied extensively and based on this work the D3R has been proposed as a therapeutic target115. During abstinence, D3R antagonists may be effective in reducing cocaine craving, based on several studies showing that the D3R is upregulated in the NAc during abstinence (for example, REF112), and that this upregulation is linked to increased motivation for cocaine116. Both rat and monkey studies have found transient increases in D1R expression in the NAc, particularly in the shell, after discontinuing cocaine self-administration (for example, see REFs110, 112, 117), although different results are found depending on the cocaine regimen118.
Strategies that may prolong abstinence
Studies of abstinence-related plasticity in rodents have identified potential therapeutic strategies for prolonging abstinence. Most of these are pharmacological approaches, although behavioural interventions may also hold promise (BOX 5). One pharmacological approach is to decrease glutamate transmission by stimulating presynaptic mGluR2/3 receptors. For example, systemic or CeA injections of an mGluR2/3 agonist reduced incubated cue-induced cocaine seeking after 21 days but not 1 day of forced abstinence89 in the rat and similar results were reported recently for incubation of methamphetamine seeking during voluntary abstinence119 (BOX 1). Importantly, in a subset of rats that developed behavioural changes resembling human addiction following long-term cocaine self-administration120, systemic delivery of an mGluR2/3 agonist also reduced cue-induced cocaine seeking on WD30121. Other work supports the potential utility of mGluR1 PAMs, which reduce CP-AMPAR levels in the NAc of incubated rats and thereby reduce cue-induced cocaine craving31, 39 (FIG. 2) while also opposing cocaine action in the VTA56, 97, 98. Although incubation and CP-AMPAR levels recover within days of discontinuing mGluR1 PAM administration31, these drugs could nevertheless provide abstinent cocaine users with temporary protection against relapse. Their utility may extend to methamphetamine addiction, as mGluR1 PAMs also reduce incubated methamphetamine craving (unpublished observations; Andrew F. Scheyer, Jessica A. Loweth, Daniel T. Christian, Rana Rabei, and Marina E. Wolf).
BOX 5. Behavioural interventions that may reduce cocaine craving during abstinence.
Recent studies have explored non-pharmacological strategies for decreasing cocaine craving during abstinence. Stress is known to contribute to drug craving and relapse in animal models of addiction and in recovering drug users151, 152. Two rodent studies have evaluated the ability of environmental enrichment (EE) to reduce a potential stress-like state during withdrawal and thereby reduce incubation of cocaine craving. In a comparison of rats that were singly housed or maintained in EE conditions during 21 withdrawal days, it was found that EE depressed seeking in early and late withdrawal, but did not abolish incubation153. Different results were found using a more robust regimen and longer withdrawal period, which may provide a stronger “incubation signal”154. By switching rats between EE and normal housing during abstinence, this study found that EE prevented development of incubation and suppressed established incubation. However, once EE was discontinued, incubation recovered rapidly. Similarly, repeated restraint stress in early withdrawal accelerated incubation of cocaine craving, but incubation ultimately plateaued at the same level in stressed and control rats155. Together, these results suggest that stress or EE during withdrawal serve as accelerators or brakes on incubation but do not perturb the underlying mechanisms that drive it. However, this does not detract from the potential therapeutic utility of EE during periods of withdrawal, when individuals with drug addiction are most vulnerable to relapse. Sleep-based therapeutic interventions may also be useful in prolonging abstinence. A recent study demonstrated that normalizing cocaine-induced sleep disruptions attenuates incubation of cocaine craving and CP-AMPAR accumulation in the NAc shell156.
As reviewed recently115, 5-HT1B activation during stable self-administration in rats increases cocaine intake and motivation for cocaine through actions in the NAc shell, whereas the same treatments decrease motivation for cocaine and cocaine seeking after 21 days of abstinence, as well as after extinction training. Thus, 5-HT1B agonists may help abstinent users avoid relapse. 5-HT2C agonists and 5-HT2A antagonists may also be useful in this regard and it is particularly interesting that both receptors also regulate impulsivity, a critical factor in the propensity to relapse, as well as cocaine seeking122. For example, rats showing high impulsivity also exhibit greater cue-induced cocaine seeking during abstinence, and both are inversely related to the level of 5-HT2C receptor function in the mPFC123. Indeed, a decrease in membrane levels of 5-HT2C in the mPFC may contribute to incubation of craving, as well as explain the decreased ability of 5-HT2C agonists to reduce cocaine seeking after prolonged abstinence78. Other work, reviewed here and elsewhere115, suggests that D3R antagonists may reverse a withdrawal-dependent increase in motivation for cocaine mediated by upregulation of D3Rs in the NAc. Furthermore, although behavioural correlates were not examined, stimulating D1R in NAc shell reversed alterations in excitatory transmission observed during abstinence from limited-access cocaine self-administration124.
Summary
Cocaine self-administration experiments in rodents have identified several brain regions and mechanisms that underlie the persistence of cocaine craving after prolonged abstinence. Most such studies have focused on the incubation of cue-induced cocaine craving during forced abstinence. Early adaptations in the VTA, and perhaps amygdala, are necessary for the onset of incubation. After 3–4 weeks of forced abstinence, activation of a network including the NAc, mPFC and CeA is required for the expression of incubation. Related to this, neurons in these three regions undergo adaptations that render them more reactive to cocaine cues. This is significant because the ability of cocaine cues to activate similar regions of the human brain, assessed in neuroimaging studies of humans that use cocaine, is associated with addiction severity and risk of relapse125. The underlying cellular mechanisms of heightened cue reactivity in rodents during abstinence from cocaine have been most thoroughly explored in the NAc, where excitatory transmission onto MSNs is strengthened through changes in AMPAR subunit composition and silent synapse-based remodeling. Similar studies of synaptic transmission in the mPFC, CeA, BLA and VH during prolonged abstinence are needed (for example, REF126), as are studies relating changes in synaptic transmission to underlying molecular mechanisms and to structural plasticity. The role of epigenetic mechanisms in incubation of craving represents another important frontier71, 127, 128 (see Supplementary information S2 (BOX)). A particularly challenging problem is to understand the anatomical circuitry through which early neuroadaptations (for example, in the VTA) enable later adaptations (in the NAc, mPFC and CeA). Incorporating the dorsal striatum into this network is another important goal. Although it seems likely that the neuroadaptations underlying cocaine incubation will be similar regardless of whether abstinence is forced, punishment-induced or voluntary, this remains to be explored. The links between abstinence and impulsivity13, 123 or compulsivity14 also deserve more attention. Although much remains to be learned, work performed using abstinence models has already identified some strategies to reduce craving and prolong abstinence. Studies of persistent cocaine craving also have broader implications for plasticity mechanisms. The best studied forms of synaptic plasticity occur in response to very brief changes in synaptic activity (seconds to minutes for LTP or LTD, 1 to 2 days for synaptic scaling), and thus cannot fully account for the slowly developing plasticity that occurs over weeks-months of drug exposure and abstinence. Through the investigation of plasticity in abstinence models, we will advance our understanding of the rich repertoire of experience-dependent plasticity mechanisms that can be engaged in the adult brain.
Supplementary Material
Key Points.
Vulnerability to relapse that persists even after prolonged abstinence is a major problem in treating cocaine addiction. Mechanisms underlying this persistent vulnerability can be studied using rodent models of cue-induced cocaine craving during abstinence from cocaine self-administration.
Cue-induced cocaine craving in rodents progressively intensifies (incubates) over the first month of abstinence and remains high for months. Incubation of craving also occurs in human drug users.
Incubation of cocaine craving depends upon an evolving series of neuroadaptations in the reward circuitry. Early adaptations in the ventral tegmental area and perhaps the amygdala lead to more persistent changes in the nucleus accumbens, medial prefrontal cortex and central nucleus of the amygdala that increase reactivity of neurons in these regions to cocaine cues and are ultimately required for the expression of incubated craving.
Increased reactivity of these regions of the rodent brain to cocaine cues presented during abstinence is significant because neuroimaging studies in human cocaine users have found that heightened cue reactivity in related brain regions is associated with addiction severity and risk of relapse.
The relationship between cocaine craving and synaptic transmission has been most thoroughly studied in the nucleus accumbens, where abstinence is associated with changes in AMPAR subunit composition and silent synapse-based remodeling. Strengthening of excitatory inputs onto neurons of the core subregion is particularly important for maintenance of incubated craving after prolonged abstinence.
Dopamine transmission is altered during abstinence owing to plasticity within the VTA and changes in dopamine receptor expression in dopaminergic projection areas, but many questions remain about the role of dopamine transmission in modulating synaptic plasticity and behavior during abstinence.
Potential therapeutic strategies to prolong abstinence, identified through rodent studies, include the use of mGluR2/3 agonists, mGluR1 positive allosteric modulators, serotonin receptor ligands (5-HT1B agonists, 5-HT2C agonists, 5-HT2A antagonists), D3 dopamine receptor antagonists, environmental enrichment, and interventions to normalize sleep patterns.
Acknowledgments
This review includes work from my laboratory supported by NIH grants DA009621 and DA015835. I thank the members of my laboratory, Dr. Yavin Shaham, Dr. Regina Carelli and Dr. Karen Szumlinski for helpful discussions.
Glossary
- Addiction
A chronic disease that involves cycles of drug use, abstinence and relapse. It is characterized by compulsive drug seeking and use, despite harmful consequences.
- Relapse
Resumption of drug-taking in human drug users following a period of abstinence. Triggers for relapse include certain emotional states, stress, and exposure to cues or environments associated with prior drug use.
- Incubation
A time-dependent increase in cue-induced drug seeking that is observed in laboratory animals tested after different periods of abstinence from drug self-administration; incubation can also be revealed when animals are returned to the self-administration context in the absence of the discrete cue.
- Non-contingent drug administration
A type of drug administration regimen whereby the drug is administered by the experimenter to a laboratory animal.
- Drug craving
An affective state in humans that may lead to drug taking. In rodents, craving is inferred from the observed drug seeking operant response.
- Drug self-administration
An experimental paradigm during which animals are trained to perform an operant response (such as a lever press or nose poke) to receive an intravenous drug infusion that is often paired with a discrete cue (such as a tone or light) or a specific context.
- Contingent drug administration
A type of drug administration regimen whereby a laboratory animal performs an operant response to obtain the drug.
- Drug seeking
Behaviour that is assessed, following drug self-administration regimens in laboratory animals, by measuring the number of operant responses performed by an animal under conditions in which the operant responses (that previously delivered the drug) are no longer drug reinforced.
- Extinction training
A procedure carried out after stable self-administration is established—during which animals undergo repeated sessions during which the operant response no longer results in drug delivery—that leads to cessation of drug seeking.
- Reinstatement test
An experimental procedure designed to model relapse in laboratory animals, performed following drug self-administration regimens and a period of extinction training. During the reinstatement test, exposure to a drug-associated cue or context, a stressor, or non-contingent drug injection leads to resumption of drug seeking.
- Seeking test
An experimental procedure to assess drug craving and relapse in laboratory animals, performed at different abstinence periods after cessation of drug self-administration regimens (but without a period of extinction training). During a cue-induced seeking test, operant responses lead to presentation of the discrete cue previously paired with drug infusions but not to drug delivery.
- Extended-access cocaine self-administration
Regimens that use longer daily sessions of self-administration (for example 6 hours per session for 10–20 days) or shorter daily sessions (for example 2 hours) for 1–2 months. Such regimens produce behavioural changes that are thought to model compulsive drug seeking in addicts.
- Limited-access cocaine self-administration
Regimens during which cocaine is available for 1–2 hours per session for 1–2 weeks; they are not thought to model compulsive drug seeking in addicts.
- Withdrawal day (WD)
A term used to describe the duration of abstinence in experimental models.
- Ca2+-permeable AMPARs (CP-AMPARs)
AMPARs that lack the GluA2 subunit (or that contain unedited GluA2) and therefore exhibit permeability to Ca2+. Compared to CI-AMPARs, they exhibit larger single channel conductance and faster kinetics, and inward rectification owing to voltage-dependent block by intracellular polyamines.
- Ca2+-impermeable AMPARs (CI-AMPARs)
AMPARs that contain an edited GluA2 subunit and are therefore impermeable to Ca2+. Most AMPARs in the brain are of this type.
- Silent synapses
Synapses that contain NMDA receptors but not AMPA receptors and that are therefore silent at the hyperpolarized membrane potentials at which synaptic transmission is typically initiated.
- Second-order schedule of reinforcement
A procedure designed to model ‘real world’ seeking of drugs by humans, which is highly driven by cues and only sporadically results in drug delivery, during which rats respond to a cocaine-associated cue for a prolonged period to obtain an infusion of cocaine.
- Reversible inactivation
An experimental procedure during which drugs that inhibit neuronal activity are infused into a particular brain region of an experimental animal prior to a behavioral test in order to evaluate the requirement for that brain region in the expression of the behavior.
- Pavlovian-to-instrumental transfer (PIT)
The ability of a Pavlovian cue, previously paired with a positive reinforcer, to enhance operant (instrumental) responding for the same reinforcer when presented unexpectedly (independent of the operant response).
Biography
Dr. Marina Wolf is Professor and Chair of the Department of Neuroscience at the Chicago Medical School, Rosalind Franklin University of Medicine and Science. She has been a pioneer in studying the role of neuronal plasticity in drug addiction. Dr. Wolf received her Ph.D. in Pharmacology from Yale University, trained as a Postdoctoral Fellow at the Center for Cell Biology at Sinai Hospital of Detroit, and held her first faculty position at Wayne State University. Dr. Wolf has been supported by the National Institute on Drug Abuse since 1992.
Footnotes
Competing interest statement: I have no competing interests as defined by Nature Publishing Group, or other interests that might be perceived to influence the interpretation of the article.
References
- 1.O’Brien CP. A range of research-based pharmacotherapies for addiction. Science. 1997;278:66–70. doi: 10.1126/science.278.5335.66. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanlon CA, Beveridge TJ, Porrino LJ. Recovering from cocaine: insights from clinical and preclinical investigations. Neurosci Biobehav Rev. 2013;37:2037–2046. doi: 10.1016/j.neubiorev.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz FC, et al. New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat Rev Neurosci. 2013;14:743–754. doi: 10.1038/nrn3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venniro M, Caprioli D, Shaham Y. Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res. 2016;224:25–52. doi: 10.1016/bs.pbr.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 2013;229:453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Self DW, Choi KH, Simmons D, Walker JR, Smagula CS. Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn Mem. 2004;11:648–657. doi: 10.1101/lm.81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neisewander JL, et al. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Pickens CL, et al. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferland J-MN, WC Risk-preferring rats make worse decisions and show increased incubation of craving after cocaine self-administration. Addict Biol. 2016 doi: 10.1111/adb.12388. (in press) [DOI] [PubMed] [Google Scholar]
- 14.Gancarz-Kausch AM, Adank DN, Dietz DM. Prolonged withdrawal following cocaine self-administration increases resistance to punishment in a cocaine binge. Sci Rep. 2014;4:6876. doi: 10.1038/srep06876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Caprioli D, Marchant NJ. Recent updates on incubation of drug craving: a mini-review. Addict Biol. 2015;20:872–876. doi: 10.1111/adb.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollander JA, Carelli RM. Abstinence from cocaine self-administration heightens neural encoding of goal-directed behaviors in the accumbens. Neuropsychopharmacology. 2005;30:1464–1474. doi: 10.1038/sj.npp.1300748. [DOI] [PubMed] [Google Scholar]
- 17.Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology (Berl) 2008;198:63–75. doi: 10.1007/s00213-008-1089-8. [DOI] [PubMed] [Google Scholar]
- 18.Zlebnik NE, Carroll ME. Prevention of the incubation of cocaine seeking by aerobic exercise in female rats. Psychopharmacology (Berl) 2015;232:3507–3513. doi: 10.1007/s00213-015-3999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terrier J, Luscher C, Pascoli V. Cell-Type Specific Insertion of GluA2-Lacking AMPARs with Cocaine Exposure Leading to Sensitization, Cue-Induced Seeking, and Incubation of Craving. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct Funct. 2008;213:17–27. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 23.Kourrich S, Calu DJ, Bonci A. Intrinsic plasticity: an emerging player in addiction. Nat Rev Neurosci. 2015;16:173–184. doi: 10.1038/nrn3877. [DOI] [PubMed] [Google Scholar]
- 24.Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guillem K, Ahmed SH, Peoples LL. Escalation of cocaine intake and incubation of cocaine seeking are correlated with dissociable neuronal processes in different accumbens subregions. Biol Psychiatry. 2014;76:31–39. doi: 10.1016/j.biopsych.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 26.Kourrich S, Thomas MJ. Similar neurons, opposite adaptations: psychostimulant experience differentially alters firing properties in accumbens core versus shell. J Neurosci. 2009;29:12275–12283. doi: 10.1523/JNEUROSCI.3028-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conrad KL, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci. 2012;5:72. doi: 10.3389/fnmol.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt HD, et al. ADAR2-dependent GluA2 editing regulates cocaine seeking. Mol Psychiatry. 2015;20:1460–1466. doi: 10.1038/mp.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purgianto A, et al. Different adaptations in AMPA receptor transmission in the nucleus accumbens after short vs long access cocaine self-administration regimens. Neuropsychopharmacology. 2013;38:1789–1797. doi: 10.1038/npp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loweth JA, et al. Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nat Neurosci. 2014;17:73–80. doi: 10.1038/nn.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu L, et al. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- 33.Koya E, et al. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56(Suppl 1):177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mameli M, Bellone C, Brown MT, Luscher C. Cocaine inverts rules for synaptic plasticity of glutamate transmission in the ventral tegmental area. Nat Neurosci. 2011;14:414–416. doi: 10.1038/nn.2763. [DOI] [PubMed] [Google Scholar]
- 35.Kullmann DM, Lamsa K. Roles of distinct glutamate receptors in induction of anti-Hebbian long-term potentiation. J Physiol. 2008;586:1481–1486. doi: 10.1113/jphysiol.2007.148064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrario CR, Goussakov I, Stutzmann GE, Wolf ME. Withdrawal from cocaine self-administration alters NMDA receptor-mediated Ca2+ entry in nucleus accumbens dendritic spines. PLoS One. 2012;7:e40898. doi: 10.1371/journal.pone.0040898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellone C, Luscher C. mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur J Neurosci. 2005;21:1280–1288. doi: 10.1111/j.1460-9568.2005.03979.x. [DOI] [PubMed] [Google Scholar]
- 38.Loweth JA, Tseng KY, Wolf ME. Using metabotropic glutamate receptors to modulate cocaine’s synaptic and behavioral effects: mGluR1 finds a niche. Curr Opin Neurobiol. 2013;23:500–506. doi: 10.1016/j.conb.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCutcheon JE, et al. Group I mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C-dependent mechanism. J Neurosci. 2011;31:14536–14541. doi: 10.1523/JNEUROSCI.3625-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrario CR, et al. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca2+-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 2011;61:1141–1151. doi: 10.1016/j.neuropharm.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fourgeaud L, et al. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci. 2004;24:6939–6945. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scheyer AF, Wolf ME, Tseng KY. A protein synthesis-dependent mechanism sustains calcium-permeable AMPA receptor transmission in nucleus accumbens synapses during withdrawal from cocaine self-administration. J Neurosci. 2014;34:3095–3100. doi: 10.1523/JNEUROSCI.4940-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhakar AL, Dolen G, Bear MF. The pathophysiology of fragile X (and what it teaches us about synapses) Annu Rev Neurosci. 2012;35:417–443. doi: 10.1146/annurev-neuro-060909-153138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monteggia LM, Zarate C., Jr Antidepressant actions of ketamine: from molecular mechanisms to clinical practice. Curr Opin Neurobiol. 2015;30:139–143. doi: 10.1016/j.conb.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin M, Chen BT, Hopf FW, Bowers MS, Bonci A. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat Neurosci. 2006;9:868–869. doi: 10.1038/nn1713. [DOI] [PubMed] [Google Scholar]
- 46.Knackstedt LA, et al. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010;30:7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grimm JW, et al. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X, Wolf ME. Brain-derived neurotrophic factor rapidly increases AMPA receptor surface expression in rat nucleus accumbens. Eur J Neurosci. 2011;34:190–198. doi: 10.1111/j.1460-9568.2011.07754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, et al. Different roles of BDNF in nucleus accumbens core versus shell during the incubation of cue-induced cocaine craving and its long-term maintenance. J Neurosci. 2013;33:1130–1142. doi: 10.1523/JNEUROSCI.3082-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X, Wolf ME. Multiple faces of BDNF in cocaine addiction. Behav Brain Res. 2015;279:240–254. doi: 10.1016/j.bbr.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31:5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cameron CM, Carelli RM. Cocaine abstinence alters nucleus accumbens firing dynamics during goal-directed behaviors for cocaine and sucrose. Eur J Neurosci. 2012;35:940–951. doi: 10.1111/j.1460-9568.2012.08024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee BR, et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16:1644–1651. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma YY, et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83:1453–1467. doi: 10.1016/j.neuron.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pascoli V, et al. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014;509:459–464. doi: 10.1038/nature13257. [DOI] [PubMed] [Google Scholar]
- 56.Mameli M, et al. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- 57.Dong Y, Nestler EJ. The neural rejuvenation hypothesis of cocaine addiction. Trends Pharmacol Sci. 2014;35:374–383. doi: 10.1016/j.tips.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bellone C, Luscher C. Drug-evoked plasticity: do addictive drugs reopen a critical period of postnatal synaptic development? Front Mol Neurosci. 2012;5:75. doi: 10.3389/fnmol.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 60.Feltenstein MW, See RE. Systems level neuroplasticity in drug addiction. Cold Spring Harb Perspect Med. 2013;3:a011916. doi: 10.1101/cshperspect.a011916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Finch DM. Neurophysiology of converging synaptic inputs from the rat prefrontal cortex, amygdala, midline thalamus, and hippocampal formation onto single neurons of the caudate/putamen and nucleus accumbens. Hippocampus. 1996;6:495–512. doi: 10.1002/(SICI)1098-1063(1996)6:5<495::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 63.Brady AM, Glick SD, O’Donnell P. Selective disruption of nucleus accumbens gating mechanisms in rats behaviorally sensitized to methamphetamine. J Neurosci. 2005;25:6687–6695. doi: 10.1523/JNEUROSCI.0643-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berl) 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- 67.Pacchioni AM, Gabriele A, See RE. Dorsal striatum mediation of cocaine-seeking after withdrawal from short or long daily access cocaine self-administration in rats. Behav Brain Res. 2011;218:296–300. doi: 10.1016/j.bbr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jonkman S, Pelloux Y, Everitt BJ. Differential roles of the dorsolateral and midlateral striatum in punished cocaine seeking. J Neurosci. 2012;32:4645–4650. doi: 10.1523/JNEUROSCI.0348-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 70.Knackstedt LA, Trantham-Davidson HL, Schwendt M. The role of ventral and dorsal striatum mGluR5 in relapse to cocaine-seeking and extinction learning. Addict Biol. 2014;19:87–101. doi: 10.1111/adb.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li X, et al. Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos-expressing dorsal striatal neurons. J Neurosci. 2015;35:8232–8244. doi: 10.1523/JNEUROSCI.1022-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 73.West EA, Saddoris MP, Kerfoot EC, Carelli RM. Prelimbic and infralimbic cortical regions differentially encode cocaine-associated stimuli and cocaine-seeking before and following abstinence. Eur J Neurosci. 2014;39:1891–1902. doi: 10.1111/ejn.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li X, Zeric T, Kambhampati S, Bossert JM, Shaham Y. The central amygdala nucleus is critical for incubation of methamphetamine craving. Neuropsychopharmacology. 2015;40:1297–1306. doi: 10.1038/npp.2014.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shin CB, et al. Incubation of cocaine-craving relates to glutamate over-flow within ventromedial prefrontal cortex. Neuropharmacology. 2015;102:103–110. doi: 10.1016/j.neuropharm.2015.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun WL, et al. Relapse to cocaine-seeking after abstinence is regulated by cAMP-dependent protein kinase A in the prefrontal cortex. Addict Biol. 2014;19:77–86. doi: 10.1111/adb.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Swinford-Jackson SE, Anastasio NC, Fox RG, Stutz SJ, Cunningham KA. Incubation of cocaine cue reactivity associates with neuroadaptations in the cortical serotonin (5-HT) 5-HT receptor (5-HTR) system. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miller BW, et al. Cocaine craving during protracted withdrawal requires PKCepsilon priming within vmPFC. Addict Biol. 2016 doi: 10.1111/adb.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ben-Shahar O, et al. Deficits in ventromedial prefrontal cortex group 1 metabotropic glutamate receptor function mediate resistance to extinction during protracted withdrawal from an extensive history of cocaine self-administration. J Neurosci. 2013;33:495–506a. doi: 10.1523/JNEUROSCI.3710-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kasanetz F, et al. Prefrontal synaptic markers of cocaine addiction-like behavior in rats. Mol Psychiatry. 2013;18:729–737. doi: 10.1038/mp.2012.59. [DOI] [PubMed] [Google Scholar]
- 82.Chen BT, et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- 83.Pelloux Y, Murray JE, Everitt BJ. Differential roles of the prefrontal cortical subregions and basolateral amygdala in compulsive cocaine seeking and relapse after voluntary abstinence in rats. Eur J Neurosci. 2013;38:3018–3026. doi: 10.1111/ejn.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci. 2003;985:233–250. [PubMed] [Google Scholar]
- 86.Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2011;70:785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koob GF, et al. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76(Pt B):370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu L, Dempsey J, Shaham Y, Hope BT. Differential long-term neuroadaptations of glutamate receptors in the basolateral and central amygdala after withdrawal from cocaine self-administration in rats. J Neurochem. 2005;94:161–168. doi: 10.1111/j.1471-4159.2005.03178.x. [DOI] [PubMed] [Google Scholar]
- 89.Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 90.Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J Neurosci. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]
- 92.Pollandt S, et al. Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur J Neurosci. 2006;24:1733–1743. doi: 10.1111/j.1460-9568.2006.05049.x. [DOI] [PubMed] [Google Scholar]
- 93.Chen B, et al. Cocaine-induced membrane adaptation in the central nucleus of amygdala. Neuropsychopharmacology. 2013;38:2240–2248. doi: 10.1038/npp.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wolff SB, et al. Amygdala interneuron subtypes control fear learning through disinhibition. Nature. 2014;509:453–458. doi: 10.1038/nature13258. [DOI] [PubMed] [Google Scholar]
- 95.Luscher C. Cocaine-evoked synaptic plasticity of excitatory transmission in the ventral tegmental area. Cold Spring Harb Perspect Med. 2013;3:a012013. doi: 10.1101/cshperspect.a012013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pignatelli M, Bonci A. Role of Dopamine Neurons in Reward and Aversion: A Synaptic Plasticity Perspective. Neuron. 2015;86:1145–1157. doi: 10.1016/j.neuron.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 97.Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- 98.Mameli M, Balland B, Lujan R, Luscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- 99.Chen BT, et al. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu L, et al. Role of ventral tegmental area glial cell line-derived neurotrophic factor in incubation of cocaine craving. Biol Psychiatry. 2009;66:137–145. doi: 10.1016/j.biopsych.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pu L, Liu QS, Poo MM. BDNF-dependent synaptic sensitization in midbrain dopamine neurons after cocaine withdrawal. Nat Neurosci. 2006;9:605–607. doi: 10.1038/nn1687. [DOI] [PubMed] [Google Scholar]
- 103.Willuhn I, Wanat MJ, Clark JJ, Phillips PE. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Curr Top Behav Neurosci. 2010;3:29–71. doi: 10.1007/7854_2009_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carelli RM, West EA. When a good taste turns bad: Neural mechanisms underlying the emergence of negative affect and associated natural reward devaluation by cocaine. Neuropharmacology. 2014;76(Pt B):360–369. doi: 10.1016/j.neuropharm.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Calu D, Nasser H, Shaham Y. Unexpected results on the role of nucleus accumbens dopamine in stress-induced relapse. Biol Psychiatry. 2015;77:848–849. doi: 10.1016/j.biopsych.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behav Neurosci. 2002;116:321–333. [PubMed] [Google Scholar]
- 107.Saddoris MP, Wang X, Sugam JA, Carelli RM. Cocaine Self-Administration Experience Induces Pathological Phasic Accumbens Dopamine Signals and Abnormal Incentive Behaviors in Drug-Abstinent Rats. J Neurosci. 2016;36:235–250. doi: 10.1523/JNEUROSCI.3468-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nader MA, et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- 110.Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Abstinence from chronic cocaine self-administration alters striatal dopamine systems in rhesus monkeys. Neuropsychopharmacology. 2009;34:1162–1171. doi: 10.1038/npp.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gould RW, Duke AN, Nader MA. PET studies in nonhuman primate models of cocaine abuse: translational research related to vulnerability and neuroadaptations. Neuropharmacology. 2014;84:138–151. doi: 10.1016/j.neuropharm.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Conrad KL, Ford K, Marinelli M, Wolf ME. Dopamine receptor expression and distribution dynamically change in the rat nucleus accumbens after withdrawal from cocaine self-administration. Neuroscience. 2010;169:182–194. doi: 10.1016/j.neuroscience.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Everitt BJ. Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories--indications for novel treatments of addiction. Eur J Neurosci. 2014;40:2163–2182. doi: 10.1111/ejn.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jentsch JD, et al. Dissecting impulsivity and its relationships to drug addictions. Ann N Y Acad Sci. 2014;1327:1–26. doi: 10.1111/nyas.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Neisewander JL, Cheung TH, Pentkowski NS. Dopamine D3 and 5-HT1B receptor dysregulation as a result of psychostimulant intake and forced abstinence: Implications for medications development. Neuropharmacology. 2014;76(Pt B):301–319. doi: 10.1016/j.neuropharm.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Neisewander JL, et al. Increases in dopamine D3 receptor binding in rats receiving a cocaine challenge at various time points after cocaine self-administration: implications for cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1479–1487. doi: 10.1038/sj.npp.1300456. [DOI] [PubMed] [Google Scholar]
- 117.Ben-Shahar O, et al. Changes in levels of D1, D2, or NMDA receptors during withdrawal from brief or extended daily access to IV cocaine. Brain Res. 2007;1131:220–228. doi: 10.1016/j.brainres.2006.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moore RJ, Vinsant SL, Nader MA, Porrino LJ, Friedman DP. Effect of cocaine self-administration on striatal dopamine D1 receptors in rhesus monkeys. Synapse. 1998;28:1–9. doi: 10.1002/(SICI)1098-2396(199801)28:1<1::AID-SYN1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 119.Caprioli D, et al. Effect of the Novel Positive Allosteric Modulator of Metabotropic Glutamate Receptor 2 AZD8529 on Incubation of Methamphetamine Craving After Prolonged Voluntary Abstinence in a Rat Model. Biol Psychiatry. 2015;78:463–473. doi: 10.1016/j.biopsych.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 121.Cannella N, et al. The mGluR2/3 agonist LY379268 induced anti-reinstatement effects in rats exhibiting addiction-like behavior. Neuropsychopharmacology. 2013;38:2048–2056. doi: 10.1038/npp.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Howell LL, Cunningham KA. Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev. 2015;67:176–197. doi: 10.1124/pr.114.009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Anastasio NC, et al. Functional status of the serotonin 5-HT2C receptor (5-HT2CR) drives interlocked phenotypes that precipitate relapse-like behaviors in cocaine dependence. Neuropsychopharmacology. 2014;39:370–382. doi: 10.1038/npp.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ortinski PI, Vassoler FM, Carlson GC, Pierce RC. Temporally dependent changes in cocaine-induced synaptic plasticity in the nucleus accumbens shell are reversed by D1-like dopamine receptor stimulation. Neuropsychopharmacology. 2012;37:1671–1682. doi: 10.1038/npp.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Keralapurath MM, Briggs SB, Wagner JJ. Cocaine self-administration induces changes in synaptic transmission and plasticity in ventral hippocampus. Addict Biol. 2015 doi: 10.1111/adb.12345. [DOI] [PubMed] [Google Scholar]
- 127.Freeman WM, et al. Persistent alterations in mesolimbic gene expression with abstinence from cocaine self-administration. Neuropsychopharmacology. 2008;33:1807–1817. doi: 10.1038/sj.npp.1301577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Massart R, et al. Role of DNA methylation in the nucleus accumbens in incubation of cocaine craving. J Neurosci. 2015;35:8042–8058. doi: 10.1523/JNEUROSCI.3053-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Reichel CM, Bevins RA. Forced abstinence model of relapse to study pharmacological treatments of substance use disorder. Curr Drug Abuse Rev. 2009;2:184–194. doi: 10.2174/1874473710902020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bedi G, et al. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry. 2011;69:708–711. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang G, et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;8:e68791. doi: 10.1371/journal.pone.0068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li P, et al. Incubation of alcohol craving during abstinence in patients with alcohol dependence. Addict Biol. 2015;20:513–522. doi: 10.1111/adb.12140. [DOI] [PubMed] [Google Scholar]
- 133.Ahmed SH. The science of making drug-addicted animals. Neuroscience. 2012;211:107–125. doi: 10.1016/j.neuroscience.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 134.Krasnova IN, et al. Incubation of methamphetamine and palatable food craving after punishment-induced abstinence. Neuropsychopharmacology. 2014;39:2008–2016. doi: 10.1038/npp.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kupchik YM, et al. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci. 2015;18:1230–1232. doi: 10.1038/nn.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Smith RJ, Lobo MK, Spencer S, Kalivas PW. Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways) Curr Opin Neurobiol. 2013;23:546–552. doi: 10.1016/j.conb.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Beveridge TJ, Smith HR, Daunais JB, Nader MA, Porrino LJ. Chronic cocaine self-administration is associated with altered functional activity in the temporal lobes of non human primates. Eur J Neurosci. 2006;23:3109–3118. doi: 10.1111/j.1460-9568.2006.04788.x. [DOI] [PubMed] [Google Scholar]
- 141.Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gozzi A, et al. Neuroimaging evidence of altered fronto-cortical and striatal function after prolonged cocaine self-administration in the rat. Neuropsychopharmacology. 2011;36:2431–2440. doi: 10.1038/npp.2011.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hammer RP, Jr, Pires WS, Markou A, Koob GF. Withdrawal following cocaine self-administration decreases regional cerebral metabolic rate in critical brain reward regions. Synapse. 1993;14:73–80. doi: 10.1002/syn.890140110. [DOI] [PubMed] [Google Scholar]
- 144.Macey DJ, Rice WN, Freedland CS, Whitlow CT, Porrino LJ. Patterns of functional activity associated with cocaine self-administration in the rat change over time. Psychopharmacology (Berl) 2004;172:384–392. doi: 10.1007/s00213-003-1676-7. [DOI] [PubMed] [Google Scholar]
- 145.Sun W, Rebec GV. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J Neurosci. 2006;26:8004–8008. doi: 10.1523/JNEUROSCI.1413-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mu P, et al. Exposure to cocaine dynamically regulates the intrinsic membrane excitability of nucleus accumbens neurons. J Neurosci. 2010;30:3689–3699. doi: 10.1523/JNEUROSCI.4063-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dikshtein Y, et al. beta-endorphin via the delta opioid receptor is a major factor in the incubation of cocaine craving. Neuropsychopharmacology. 2013;38:2508–2514. doi: 10.1038/npp.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Reppucci CJ, Petrovich GD. Organization of connections between the amygdala, medial prefrontal cortex, and lateral hypothalamus: a single and double retrograde tracing study in rats. Brain Struct Funct. 2015 doi: 10.1007/s00429-015-1081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Phillips AG, Ahn S, Howland JG. Amygdalar control of the mesocorticolimbic dopamine system: parallel pathways to motivated behavior. Neurosci Biobehav Rev. 2003;27:543–554. doi: 10.1016/j.neubiorev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 151.Sinha R, Shaham Y, Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology (Berl) 2011;218:69–82. doi: 10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mantsch JR, Baker DA, Funk D, Le AD, Shaham Y. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology. 2016;41:335–356. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Thiel KJ, et al. Environmental enrichment counters cocaine abstinence-induced stress and brain reactivity to cocaine cues but fails to prevent the incubation effect. Addict Biol. 2012;17:365–377. doi: 10.1111/j.1369-1600.2011.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chauvet C, Goldberg SR, Jaber M, Solinas M. Effects of environmental enrichment on the incubation of cocaine craving. Neuropharmacology. 2012;63:635–641. doi: 10.1016/j.neuropharm.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Loweth JA, Glynn RM, Rosenkranz JA, Wolf ME. Chronic stress exposure during early withdrawal from extended access cocaine self-administration facilitates incubation of cue-induced cocaine craving. Soc Neurosci Abstr. 2015;41:315–320. [Google Scholar]
- 156.Chen B, et al. Sleep regulates incubation of cocaine craving. J Neurosci. 2015;35:13300–13310. doi: 10.1523/JNEUROSCI.1065-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Marchant NJ, Kaganovsky K, Shaham Y, Bossert JM. Role of corticostriatal circuits in context-induced reinstatement of drug seeking. Brain Res. 2015;1628:219–232. doi: 10.1016/j.brainres.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.