Abstract
We showed by a laboratory experiment that four different Campylobacter jejuni strains are able to infect the protozoan Acanthamoeba polyphaga. C. jejuni cells survived for longer periods when cocultured with amoebae than when grown in culture alone. The infecting C. jejuni cells aggregated in amoebic vacuoles, in which they were seen to be actively moving. Furthermore, a resuscitation of bacterial cultures that were previously negative in culturability tests was observed after reinoculation into fresh amoeba cultures. After spontaneous rupture of the amoebae, C. jejuni could be detected by microscopy and culturability tests. Our results indicate that amoebae may serve as a nonvertebrate reservoir for C. jejuni in the environment.
The enteropathogen Campylobacter jejuni has a major effect on human health. Studies have identified contaminated food, primarily undercooked chicken meat, and unchlorinated water or unpasteurized milk as important risk factors (6). Still, the transmission modes in a large proportion of cases remain unsolved, and the epidemiological pathways have not been fully elucidated, even for the contained environments of poultry houses.
For the present study, we investigated a potentially important aspect of C. jejuni epidemiology, namely, the role of protozoan vectors. Protozoa are nearly ubiquitous in aquatic environments, and they feed on microorganisms, including bacteria. Acanthamoeba protozoa, which were used for this study, are well adapted to hostile environments, such as elevated temperatures, chlorination, and various disinfectants (1, 10), by their ability to encystate and may therefore potentially provide shelter for less tolerant bacteria. By evolving mechanisms to survive within amoebae, bacteria not only may escape the threat of being preyed upon but may also benefit from protection from conditions occurring outside the protozoan host. Interactions between protozoa and bacteria have been established for several bacterial pathogens (reviewed in reference 4). Perhaps the most famous example is Legionella pneumophila, which can utilize Acanthamoeba for protection and growth, thereby increasing its range and survival in unfavorable environments (19). Also, Vibrio cholerae, the agent responsible for human cholera, and Listeria monocytogenes have, among other bacterial species, been found to infect and multiply within amoebae (12, 21).
To our knowledge, there are only a few published accounts regarding interactions between Campylobacter spp. and protozoa. One significant study showed that coliform bacteria and bacterial pathogens, including a strain of C. jejuni, can obtain some protection from free chlorine residuals by growing in amoebic or ciliate cultures (11). Most of the tested bacteria had a >50-fold increase in resistance to free chlorine when grown in cocultures with protozoa compared to that when they were grown in pure cultures. For C. jejuni, the 99% inactivation level was reached after approximately 1 h in cocultures, in contrast to <1 min for pure cultures (11). Furthermore, it has become established that Helicobacter pylori can also infect amoebae (23). Since this genus and Campylobacter are two morphologically and taxonomically closely related genera—actually, the former was recognized as a distinct genus only in the early 1990s (reviewed in reference 14)—intensified research on Campylobacter-protozoan interactions is warranted.
Using the laboratory model presented here, we successfully infected Acanthamoeba polyphaga amoeba with C. jejuni cells at four different temperatures ranging from 4 to 30°C. Motile C. jejuni cells aggregated in amoebic vacuoles, and intracellular bacteria survived longer than extracellular bacteria that were located in the medium. The cocultivation of amoebae and C. jejuni at 37°C resulted in the rupture of the amoeba cells, and living C. jejuni cells could thereafter be detected both by microscopy and by culturability tests. This phenomenon was also seen when cultures that were previously negative in culturability tests were reinoculated into fresh amoeba cultures. Using these initial findings, we designed a study to investigate these phenomena quantitatively.
MATERIALS AND METHODS
Experimental setup.
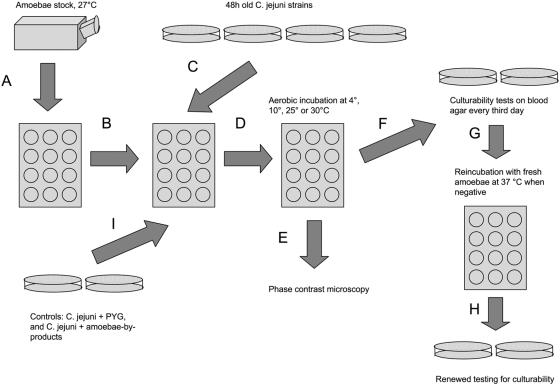
The general setup of the experiment was as follows (Fig. 1). A. polyphaga trophozoites (strain Linc Ap-1) were grown for 48 h in 12-well culture plates at 27°C in peptone-yeast-glucose broth (PYG medium) (19) to allow the amoebae to attach to the surfaces of the wells (Fig. 1A). Before the amoebae were challenged with C. jejuni, the medium in each well was gently removed, with care taken not to disturb the attached amoebae in the bottom of the wells, and replaced with fresh PYG medium (Fig. 1B). The amoebae were challenged with a C. jejuni strain isolated from a human patient (Fig. 1C). Each of the bacterial strains had been grown on conventional blood agar plates in a microaerobic environment (85% N2, 10% CO2, 5% O2) at 42°C for 48 h. The bacterial cells were harvested and suspended in fresh PYG medium, and the resulting cell concentration for each sample was determined optically in a Bürker chamber. After dilution of the samples, the infection of amoebae was performed by adding 100 μl of bacteria in PYG solution to the culture plate wells, resulting in a cell ratio of amoebae to C. jejuni of approximately 1:100. After infection, the cocultures of amoebae and C. jejuni were incubated aerobically at four different temperatures, 4, 10, 25, and 30°C (Fig. 1D).
FIG. 1.
Experimental setup (see text for details).
To determine if the C. jejuni cells had entered the amoebae, we examined the cocultures at regular intervals throughout the study by phase-contrast microscopy (Fig. 1E). Ten microliters of each sample was investigated at a magnification of ×1,000 in a wet mount. Occurrences of live bacteria with a Campylobacter-like morphology within and outside amoebae were noted, and digital photos and films were captured with a JVC GR-D70E digital video camera for selected cultures.
Culturability experiment.
The initial findings enabled us to perform a two-step test of culturability. For the first step, a 100-μl sample was taken from each coculture (and also from the different sets of controls) (see below), plated out on blood agar, and then cultivated in a microaerobic environment at 42°C for 48 h for the detection of C. jejuni colony growth (Fig. 1F). If this test was culture positive for C. jejuni, it was repeated every third day until the culturability of C. jejuni cells in the coculture was negative in three consecutive tests, at which time the second step started. Utilizing the fact that fresh amoeba cultures seemed to resuscitate C. jejuni cells in previously culture-negative cocultures, we took a 100-μl sample of each Campylobacter-negative coculture from step one and transferred it to a fresh amoeba culture. These new cultures were incubated aerobically at 37°C for 48 h (Fig. 1G). A 10-μl sample of each 48 h-culture was placed on microscopy glass and investigated at a magnification of ×1,000 for the presence of live bacteria with a Campylobacter-like morphology and for the occurrence of ruptured amoeba cell walls. In all cases in which ruptured amoebae were seen, a renewed culturability test was performed on blood agar under microaerobic conditions at 42°C for 48 h (Fig. 1H). The second step of the survival experiment was repeated for each culture every third day until the occurrence of three consecutive negative culturability tests.
Three sets of control treatments were used for the experiments (Fig. 1I), including C. jejuni in PYG medium without amoebae, amoebae without C. jejuni, and C. jejuni in an amoebic byproduct medium (a PYG medium in which amoebae had been grown for 7 days at 27°C and then removed by centrifugation at 5,000 × g for 5 min). This medium was filtered (0.22-μm-pore-size filter) before use.
We used six replicates for each experimental combination, giving a total of 96 cultures at the start of the experiment (cocultures plus three controls, with six cultures each at 4, 10, 25, and 30°C). All cultures were started on the same day, and for each culturability test we noted the amount of growth on blood agar plates according to the following scale: rich growth, >100 colonies; medium growth, 11 to 100 colonies; weak growth, 1 to 10 colonies.
Fluorescent in situ hybridization.
To visualize intracellular bacterial cells, we performed fluorescent in situ hybridization experiments. For these experiments, 50 μl of a paraformaldehyde-fixed coculture was added to polylysine-coated glass slides and allowed to air dry. The slides were washed with phosphate-buffered saline and MQ water (high-quality deionized water) to remove the fixative and were later dehydrated with 50, 70, and 99% ethanol. Each slide was incubated with 20 μl of hybridization solution (0.9 M NaCl, 20 mM Tris-HCl [pH 7.4], 35% formamide, 0.01% sodium dodecyl sulfate) containing 50 ng of the EUB338 universal bacterial probe (3) at room temperature in a moisturized incubation chamber for 90 min. After being washed for 15 min in washing buffer (70 mM NaCl, 20 mM Tris-HCl [pH 7.4], 5 mM EDTA, 0.01% sodium dodecyl sulfate), the slides were again air dried and visualized by epifluorescence microscopy, and digital pictures were taken.
Wild bird strains.
We also tested whether three wild bird strains of C. jejuni had the capacity to infect A. polyphaga by using the infection model described above. These strains were from a mallard (Anas platyrhyncos), a dunlin (Calidris alpina), and a blackbird (Turdus merula). These strains were tested for survival at 4, 10, 25, and 30°C, but no quantitative data were recorded.
RESULTS
Amoeba-Campylobacter interactions.
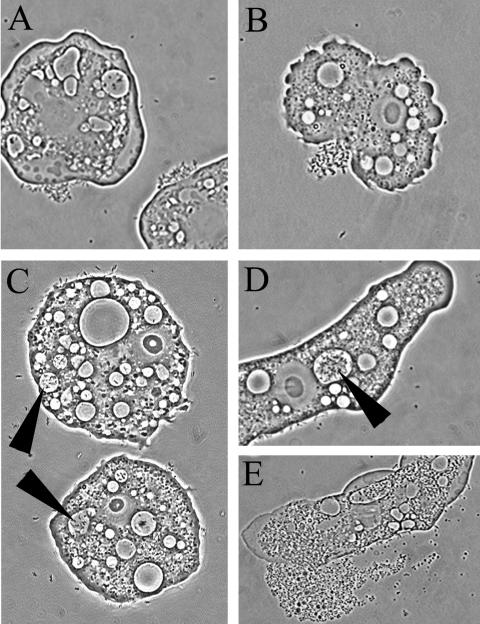
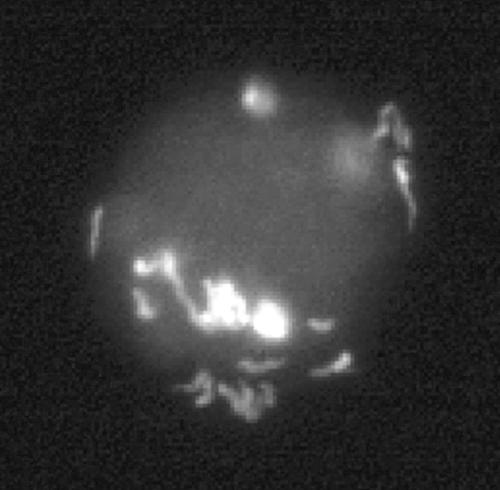
By using a phase-contrast microscope, we observed interactions between C. jejuni cells and amoebae in experimental cocultures at all tested temperatures. At the initial phase of the experiment, just after the inoculation of C. jejuni into amoebic cultures, motile C. jejuni cells could be seen both unattached and close to the cell walls of the amoebae. The bacteria that occurred in close proximity to amoebae showed strong tendencies to gather at certain sites of the amoeba cell walls, resulting in large aggregations of C. jejuni cells at these sites (Fig. 2A and B). In contrast to the fast-moving C. jejuni cells in the medium, these cells seemed to remain attached to the amoeba cell walls at a fixed position but exhibited some flagellar movement (live pictures are available at http://www.zooeco.org/zooeco/rizee/gal/amoeba-camp-A.asp). Already 1 h after inoculation, motile bacteria with the typical C. jejuni curved-rod morphology could be seen inside some of the living amoebae. These invading bacteria were confined to amoebic vacuoles (Fig. 2C and D). Bacterial cells could not be seen in the amoebic cytosol, nor were they found in the vacuoles of amoeba cultures grown without C. jejuni. The cells within the amoebae were highly motile and could be seen moving inside the vacuoles (live pictures are available at http://www.zooeco.org/zooeco/rizee/gal/amoeba-camp-C.asp). The existence of intracellular bacteria in the amoebae was also evident in pictures from fluorescent in situ hybridization experiments (Fig. 3).
FIG. 2.
Early in the infection model, C. jejuni cells aggregated at certain positions on A. polyphaga cell walls (A and B), and after some time, live bacterial cells were observed in amoebic vacuoles (C and D). Subculturing of culture-negative samples together with fresh amoebae at 37°C resulted in lysis of the amoebae, after which live C. jejuni cells could be retrieved (E).
FIG. 3.
In situ hybridization with a fluorescent EUB338 probe identified the presence of intact bacterial cells within amoebic vacuoles.
Culturability and survival.
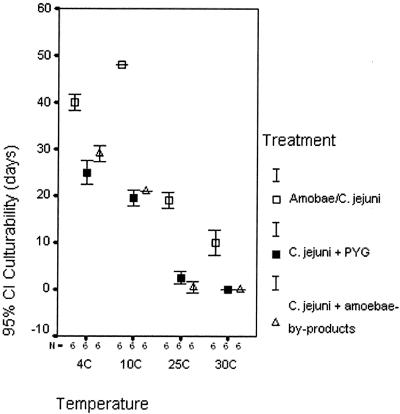
Motile C. jejuni cells were detected in the medium by microscopy at the start of the experiment and then in the first-step culturability test up to 48 days after inoculation (Table 1). Culturability was retained longest in the amoeba-C. jejuni cocultures compared to the controls (C. jejuni plus PYG medium and C. jejuni plus amoeba byproduct medium). For all treatments, there were decreasing trends in the longevity of retained culturability with increasing temperatures (Fig. 4). For each test temperature, we calculated differences in culturability for step one between the three treatments by Kruskal-Wallis nonparametric tests (Table 1), treating each quantitative level separately (e.g., how long rich growth was retained in one treatment compared to the two others). These differences were generally statistically significant, showing that, on average, amoeba-C. jejuni cocultures had both longer survival and richer growth in step one of the culturability test.
TABLE 1.
Quantification of C. jejuni culturability after different treatments (culturability test step one)
| Original temp (°C) | Growth | No. of days (SD) of culturability with indicated treatmenta
|
Kruskal-Wallis test value
|
|||
|---|---|---|---|---|---|---|
| A | B | C | Chi square | P | ||
| 4 | Rich | 21.0 (0.0) | 15.0 (0.0) | 21.0 (0.0) | 17.00 | <0.001 |
| Medium | 27.0 (0.0) | 19.9 (1.5) | 24.0 (0.0) | 16.54 | <0.001 | |
| Weak | 40.0 (1.5) | 25.0 (2.4) | 29.0 (1.5) | 14.63 | 0.001 | |
| 10 | Rich | 15.0 (0.0) | 12.5 (1.2) | 15.0 (0.0) | 13.08 | 0.001 |
| Medium | 27.0 (0.0) | 15.0 (0.0) | 16.0 (1.5) | 14.88 | 0.001 | |
| Weak | 48.0 (0.0) | 19.5 (1.6) | 21.0 (0.0) | 14.73 | 0.001 | |
| 25 | Rich | 6.0 (0.0) | 0 | 0 | 17.00 | <0.001 |
| Medium | 11.0 (1.5) | 0 | 0 | 16.39 | <0.001 | |
| Weak | 19.0 (1.5) | 2.5 (1.2) | 0.5 (1.2) | 14.24 | 0.001 | |
| 30 | Rich | 0 | 0 | 0 | 0.00 | NSb |
| Medium | 6.0 (0.0) | 0 | 0 | 17.00 | <0.001 | |
| Weak | 10.0 (2.4) | 0 | 0 | 16.25 | <0.001 | |
Treatments: A, amoeba-C. jejuni coculture; B, C. jejuni plus PYG; C, C. jejuni plus amoeba byproducts.
NS, not significant.
FIG. 4.
Mean percentages of retained culturability of C. jejuni grown in different media and at different temperatures. CI, confidence interval.
When a culture had been negative three times in a row, we proceeded to step two of the culturability test. Each culture was reinoculated into a fresh amoeba culture and incubated at 37°C. Fifteen of 72 samples turned culture positive again when retested for culturability, all of which had rich growth (Table 2). Of these, 12 were from amoeba-C. jejuni cocultures, 1 was from a culture with a C. jejuni-plus-PYG medium treatment, and 2 were from C. jejuni-plus-amoeba-byproduct cultures. In all resuscitated samples, there was an abundance of amoebae with ruptured cell walls and large numbers of free-swimming bacteria with a Campylobacter-like morphology (as detected by phase-contrast microscopy), which were not seen in any of the nonresuscitated samples (Fig. 2E; live pictures showing the growth of C. jejuni are available at http://www.zooeco.org/zooeco/rizee/gal/amoeba-camp-B.asp).
TABLE 2.
Resuscitation of culture-negative samples through reincubation with fresh amoebae at 37°C
| Original temp (°C) | Treat- menta | No. resuscitated/ total no. | Range (days) of survival | Mean survival (days) | SD |
|---|---|---|---|---|---|
| 4 | A | 5/6 | 0-13 | 7.8 | 4.8 |
| B | 0/6 | ||||
| C | 0/6 | ||||
| 10 | A | 6/6 | 13-25 | 19.0 | 4.2 |
| B | 0/6 | ||||
| C | 2/6 | 0-7 | 2.3 | 3.6 | |
| 25 | A | 0/6 | |||
| B | 1/6 | 0-4 | 0.7 | 1.6 | |
| C | 0/6 | ||||
| 30 | A | 1/6 | 0-4 | 0.7 | 1.6 |
| B | 0/6 | ||||
| C | 0/6 |
Treatments: A, amoeba-C. jejuni coculture; B, C. jejuni plus PYG; C, C. jejuni plus amoeba byproducts.
At the lower temperatures, 4 and 10°C, amoeba-C. jejuni cocultures were resuscitated after 13 and 25 days, respectively. This gave mean survival times (measured as the mean of retained culturability for step one plus the mean for all samples in step two for each treatment or temperature) of 47.8 days for amoeba-C. jejuni cocultures grown at 4°C and 67.0 days for cultures grown at 10°C (Tables 1 and 2).
Wild bird strains.
The three C. jejuni strains from wild birds entered and survived within amoebae in an identical fashion to that by the human isolate described above. They were found to behave similarly to each other and differed slightly from the human isolate by not surviving as long in any of the different setups. They were seen to be resuscitated at 37°C in a similar way as the human isolate (data not shown).
DISCUSSION
The present study provides results that may have a large impact on our understanding of C. jejuni epidemiology. Several studies have shown the broad host range of this bacterium, from wild and domestic birds (2, 22) and insects (17) to mammals (e.g., see reference 20). Furthermore, isolation from or PCR detection in many water systems, including tap water, private wells, rivers, and seawaters (7, 9, 18), also indicates that C. jejuni is capable of surviving under a large spectrum of environmental conditions. On the other hand, laboratory experiments have shown the organism to be comparatively sensitive to environmental stresses such as disinfectants, desiccation at high temperatures, and oxygen (15). Taken together, these facts, including a large proportion of unexplained human cases (13), point to the existence of an as yet unknown environmental reservoir. Amoebae or other protozoa may constitute such a reservoir, with the ability to protect the bacterium from unfavorable environmental conditions. King and coworkers proposed that “…resistance to digestion by predatory protozoa was an evolutionary precursor of pathogenicity in bacteria and that today it is a mechanism for survival of fastidious bacteria in dilute and inhospitable aquatic environments” (11).
We have shown that four different C. jejuni strains isolated from different sources are capable of infecting A. polyphaga cells in vitro and are able to survive and multiply intracellularly. This conclusion is based on several observations. Firstly, live C. jejuni cells appeared in aggregations at certain positions on amoeba cell walls (Fig. 2A and B). Secondly, apparently live and motile bacterial cells exhibiting a C. jejuni morphology were seen inside amoebic vacuoles in all tested amoeba-C. jejuni cocultures (Fig. 2C and D), while no such structures could be observed in amoeba cultures without C. jejuni. Furthermore, the in situ hybridization technique identified aggregations of bacteria in infected amoebae (Fig. 3). Lastly, when culture-negative samples were reinoculated into fresh amoeba cultures and incubated at 37°C, bacterial growth was regained in several cases.
We noted intracellular C. jejuni bacteria at all tested temperatures, including temperatures that normally exist in natural waters, and these bacteria had improved longevity. This agrees with earlier findings of Campylobacter survival in water and biofilms (5) and increases the plausibility that infections of amoebae may also occur in nature. There are ample opportunities for C. jejuni and amoebae to interact in water environments and in biofilms, as Acanthamoeba spp. and other amoebae are present at high densities in both natural and manmade water systems.
C. jejuni can occur in two different morphological forms, the normal spiral rod form and a smaller condensed coccoid form. A transition between the two states usually takes place in old, starved, or stressed cultures (16). The latter form is nonculturable with conventional methods and does not grow or divide, but it retains metabolic activity and is usually denoted as being in a viable but nonculturable (VBNC) state (8, 16). There is controversy regarding the nature of VBNC cells: do they represent a dormant state adapted to outlast short adverse environmental conditions or are they degenerating or dying cells that are unable to be resuscitated? Several studies have investigated the potential of resuscitating VBNC cells by using animal models, but with mixed results. Firstly, there may be strain-specific differences in the capacity of cells to enter the VBNC state and, likewise, in the ability to convert to a normal morphology again. Secondly, it is hard to know with absolute certainty that resuscitation is not observed as a consequence of the presence of a few unnoted residual cells in the medium. At present, we cannot separate these two alternatives for the resuscitation of C. jejuni after reinoculation into fresh amoeba cultures. However, amoebae may play a role in the transmission of campylobacters as a transient vector for resuscitating nonculturable campylobacters, either by internalizing bacterial cells prior to the ingestion of contaminated water by the animal host or as an event taking place in the gut lumen of the host. These possibilities may be tested by the use of cofeeding experiments in an animal model.
To conclude, we have for the first time empirically shown that C. jejuni can infect A. polyphaga cells in vitro. Bacterial cells were alive and motile within the amoebic vacuoles and survived longer while inside the vacuoles than extracellular bacteria. Further studies are needed to validate whether this infection route can also occur outside the laboratory setting and if the C. jejuni cells that survive in amoebae are capable of infecting vertebrate hosts.
Acknowledgments
The amoebae strain was kindly provided by Bernard La Scola, Université de la Méditerranée, Marseille, France. Ingvar Eliasson, Kalmar County Hospital, Kalmar, Sweden, is acknowledged for his logistic support. The wild bird strains were collected by the staff at Ottenby Bird Observatory, Ottenby, Sweden.
This work was supported financially by the Health Research Council of Southeast Sweden (FORSS) and by The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS) (2003-1146).
REFERENCES
- 1.Ahearn, D. G., and M. M. Gabriel. 1997. Contact lenses, disinfectants and acanthamoebae keratitis. Adv. Appl. Microbiol. 43:35-56. [DOI] [PubMed] [Google Scholar]
- 2.Alterkruse, S. F., N. J. Stern, P. J. Fields, and D. L. Swerdlow. 1999. Campylobacter jejuni—an emerging foodborne pathogen. Emerg. Infect. Dis. 5:28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann, R. I., L. Krumholz, and D. A. Stahl. 1990. Fluorescent oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J. Bacteriol. 172:762-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker, J., and R. W. Brown. 1994. Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology 140:1253-1259. [DOI] [PubMed] [Google Scholar]
- 5.Buswell, C. M., Y. M. Herlihy, L. M. Lawrence, J. T. M. McGuiggan, P. D. Marsh, C. W. Keevil, and S. A. Leach. 1998. Extended survival and persistence of Campylobacter spp. in water and aquatic biofilms and their detection by immunofluorescent-antibody and -rRNA staining. Appl. Environ. Microbiol. 64:733-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman, C. R., J. Neimann, H. C. Wegener, and R. V. Tauxe. 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations, p. 121-138. In I. Nachamkin and M. J. Blaser, Campylobacter 2000. ASM Press, Washington, D.C.
- 7.Hänninen, M. L., H. Haajanen, T. Pummi, K. Wermundsen, M. L. Katila, H. Sarkkinen, I. Miettinen, and H. Rautelin. 2003. Detection and typing of Campylobacter jejuni and Campylobacter coli and analysis of indicator organisms in three waterborne outbreaks in Finland. Appl. Environ. Microbiol. 69:1391-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hazeleger, W. C., J. D. Janse, P. M. F. J. Koenrad, R. R. Beumer, F. M. Rombouts, and T. Abee. 1995. Temperature-dependent membrane fatty acid and cell physiology changes in coccoid forms of Campylobacter jejuni. Appl. Environ. Microbiol. 61:2713-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones, K., M. Betaieb, and D. R. Telford. 1990. Thermophilic campylobacters in surface waters around Lancaster, UK: negative correlation with campylobacter infections in the community. J. Appl. Bacteriol. 69:758-764. [DOI] [PubMed] [Google Scholar]
- 10.Kilvington, S., and J. Prie. 1990. Survival of Legionella pneumophila within cysts of Acanthamoeba polyphaga following chlorine exposure. J. Appl. Bacteriol. 68:519-525. [DOI] [PubMed] [Google Scholar]
- 11.King, C. H., E. B. Shotts, Jr., R. E. Wooley, and K. G. Porter. 1988. Survival of coliforms and bacterial pathogens within protozoa during chlorination. Appl. Environ. Microbiol. 54:3023-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ly, T. M., and H. E. Muller. 1990. Ingested Listeria monocytogenes survive and multiply in protozoa. J. Med. Microbiol. 33:51-54. [DOI] [PubMed] [Google Scholar]
- 13.Neal, K. R., and R. C. Slack. 1997. Diabetes mellitus, anti-secretory drugs and other risk factors for campylobacter gastro-enteritis in adults: a case-control study. Epidemiol. Infect. 119:307-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.On, S. L. 2001. Taxonomy of Campylobacter, Arcobacter, Helicobacter and related bacteria: current status, future prospects and immediate concerns. Symp. Ser. Soc. Appl. Microbiol. 30:1S-15S. [DOI] [PubMed] [Google Scholar]
- 15.Park, S. F. 2002. The physiology of Campylobacter species and its relevance to their role as foodborne pathogens. Int. J. Food Microbiol. 74:177-188. [DOI] [PubMed] [Google Scholar]
- 16.Rollins, D. M., and R. R. Colwell. 1986. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl. Environ. Microbiol. 52:531-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosef, O., and G. Kapperud. 1983. House flies (Musca domestica) as possible vectors of Campylobacter fetus subsp. jejuni. Appl. Environ. Microbiol. 45:381-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosef, O., G. Rettedal, and L. Lageide. 2001. Thermophilic campylobacters in surface water: a potential risk of campylobacteriosis. Int. J. Environ. Health Res. 11:321-327. [DOI] [PubMed] [Google Scholar]
- 19.Rowbotham, T. J. 1980. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 33:1179-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanley, K. N., J. S. Wallace, J. E. Currie, P. J. Diggle, and K. Jones. 1998. The seasonal variation of thermophilic campylobacters in beef cattle, dairy cattle and calves. J. Appl. Microbiol. 85:472-480. [DOI] [PubMed] [Google Scholar]
- 21.Thom, S., D. Warhurst, and B. S. Drasar. 1992. Association of Vibrio cholerae with fresh water amoebae. J. Med. Microbiol. 36:303-306. [DOI] [PubMed] [Google Scholar]
- 22.Waldenström, J., T. Broman, I. Carlsson, D. Hasselquist, R. P. Achterberg, J. A. Wagenaar, and B. Olsen. 2002. Prevalence of Campylobacter jejuni, Campylobacter lari, and Campylobacter coli in different ecological guilds and taxa of migrating birds. Appl. Environ. Microbiol. 68:5911-5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winiecka-Krusnell, J., K. Wreiber, A. von Euler, L. Engstrand, and E. Linder. 2002. Free-living amoebae promote growth and survival of Helicobacter pylori. Scand. J. Infect. Dis. 34:253-256. [DOI] [PubMed] [Google Scholar]