Abstract
Background
In recent years, highly frequent swine respiratory diseases have been caused by extraintestinal pathogenic Escherichia coli (ExPEC) in China. Due to this increase in ExPECs, this bacterial pathogen has become a threat to the development of the Chinese swine industry. To investigate ExPEC pathogenesis, we isolated a strain (named SLPE) from lesioned porcine lungs from Changchun in China, reported the draft genome and performed comparative genomic analyses.
Results
Based on the gross post-mortem examination, bacterial isolation, animal regression test and 16S rRNA gene sequence analysis, the pathogenic bacteria was identified as an ExPEC. The SLPE draft genome was 4.9 Mb with a G + C content of 51.7%. The phylogenomic comparison indicated that the SLPE strain belongs to the B1 monophyletic phylogroups and that its closest relative is Avian Pathogenic Escherichia coli (APEC) O78. However, the distribution diagram of the pan-genome virulence genes demonstrated significant differences between SLPE and APEC078. We also identified a capsular polysaccharide synthesis gene cluster (CPS) in the SLPE strain genomes using blastp.
Conclusions
We isolated the ExPEC (SLPE) from swine lungs in China, performed the whole genome sequencing and compared the sequence with other Escherichia coli (E. coli). The comparative genomic analysis revealed several genes including several virulence factors that are ExPEC strain-specific, such as fimbrial adhesins (papG II), ireA, pgtP, hlyF, the pix gene cluster and fecR for their further study. We found a CPS in the SLPE strain genomes for the first time, and this CPS is closely related to the CPS from Klebsiella pneumoniae.
Electronic supplementary material
The online version of this article (doi:10.1186/s12917-017-1093-5) contains supplementary material, which is available to authorized users.
Keywords: ExPEC, Pneumonia, Pigs, Genome
Background
According to genetic and clinical criteria, E. coli has been classified into commensal, intestinal pathogenic and ExPEC groups [1]. ExPEC has become a bacterial pathogen that threatens the health of humans and animals. These strains can cause a wide range of extraintestinal infections, including in the urinary tract, central nervous system, circulatory system and respiratory system [2]. In recent years, some reports have demonstrated that ExPEC has been frequently isolated from clinical samples in the swine industry in China [3], leading to significant economic losses.
To date, several E. coli isolates have had their whole genomes sequenced, including intestinal pathogenic E. coli and extraintestinal pathogenic E. coli. Based on the whole sequenced genomes, E. coli can be classified into four major phylogroups (A, B1, D1, D2 and B2). ExPEC are mainly distributed into phylogroups B2, D1, D2 and F, but occasionally into other phylogroups such as A and B1 depending on the virulence gene repertoire [4]. However, to date, little work has been conducted to characterize the ExPEC that caused fatal pneumonia at a pig farm in Changchun, China. In this paper, we isolated the ExPEC from swine lungs from China and describe the genome characteristics of the isolate.
Results
Gross post mortem examination and histological examination
The pig carcass was in fair nutritional condition. There was no evidence of any viruses or external parasites. Only the lungs had some congestion and bleeding. The stomach was devoid of contents. There was nothing remarkable about any of the other visceral systems. The histological preparations of the lungs showed the presence of inflammatory cells in the bronchioles and the surrounding alveoli (red arrow), and presence of some bleeding and edema (Fig. 1). Histological examinations of pneumonia-like presentation may be classified as bronchopneumonia.
Fig. 1.
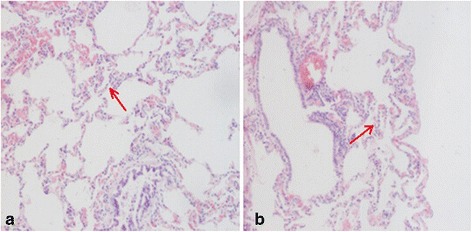
Histological preparation of swine lungs (haematoxylin and eosin). Note the presence of inflammatory cells in the bronchioles (a) and the surrounding alveoli (b)
Bacterial isolates and serotyping
Some colonies were obtained from the pig lung, in colonies, only one colony was selected, which contained two of the ExPEC virulence markers, iutA and papC, named SLPE. The SLPE isolate was positive for serogroup O149.
Experimental challenge studies
The SLPE isolate killed all of the mice at the 5 × 107 CFU, 5 × 106 CFU and 5 × 105 CFU challenge doses, but the surviving time of each group is different, the 5 × 107 CFU dose group only survived 6 to 8 h and the 5 × 106 CFU and 5 × 105 CFU dose group survived 12 to 18 h. All of the mice in the negative control group survived without any clinical signs. All tested were dissected and examined for lesion, the lungs had some bleeding and swelling, but there was nothing remarkable lesions in other tissues and organs. The SLPE presented as a highly virulent ExPEC strain in the mouse model. Chen et al., has isolated 315 ExPEC in china, only 2 isolated of these killed 5/5 mice at a challenge dose of 105 CFU [5].
Antimicrobial resistance
The MIC values for the 8 antimicrobial agents obtained from the examinations of the SLPE isolates are shown. Cefotaxime was found to be the most active compound in vitro (MIC < 0.03 μg/mL). The isolates was already resistant to the rest of the antimicrobials and displayed the following MIC values: (1) florfenicol, MIC = 128 μg/mL; (2) doxycycline, MIC = 128 μg/mL; (3) ciprofloxacin, MIC = 64 μg/mL; (4) tilmicosin, MIC = 512 μg/mL; (5) enrofloxacin, MIC = 128 μg/mL; (6) sulfamethoxazole, MIC>512 μg/mL and (7) amikacin, MIC = 128 μg/mL.
Genomic features
A total of 336 contigs from the genome were assembled onto 152 scaffolds. The predicted genome size was 4.9 Mb, with an N50 value of 106,661 for the assembly. Ombining the glimmer 3.02, Genemark and Z-Curve programs annotations gave 4806 genes with approximately 51.7% GC content, which is similar to other reported E. coli genomes. This Whole Genome Shotgun project has been deposited at DDBJ/EMBL/GenBank under the accession LJCG00000000.1. The SLPE genome harbour some resistance genes, including MarC, SoxR, MATE, ABC genes, tet, FloR and also carrie class Iintegrons. but the gene cassettes of Iintegrons genes, that conferred resistance to tetracycline, fluoroquinolone, aminoglycosides and so on.
Phylogenomic genome analysis of SLPE with other E. coli pathotypes
The phylogenomic tree was constructed to examine the SLPE strain in the context of the E. coli population structure. Figure 2 shows that the 43 E. coli strains were divided into five monophyletic phylogroups (A, B1, B2, D and E). The SLPE strain belongs to the B1 monophyletic phylogroup, and its closest relative is APEC 078.
Fig. 2.
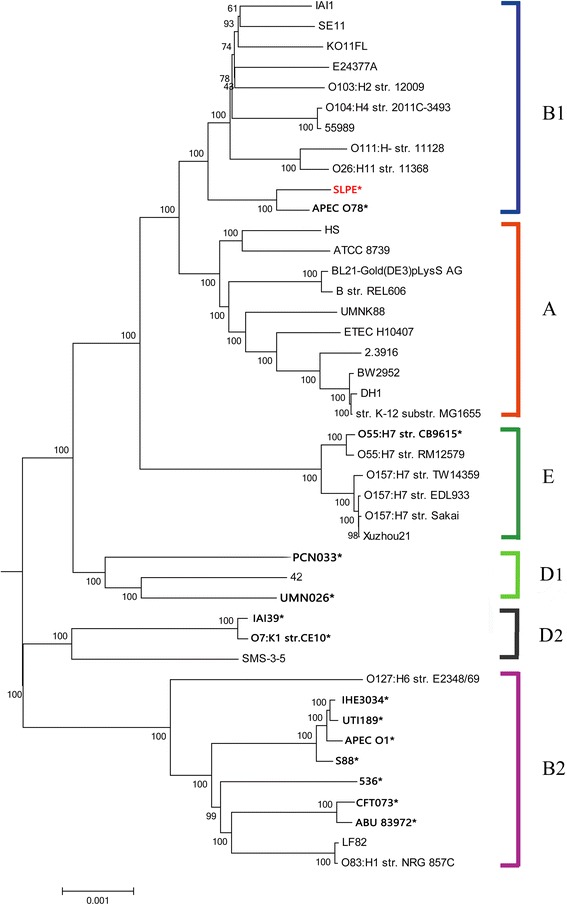
Phylogenomic tree of the 43 E. coli strains. Blastp, perl, mcl, clustalW and MEGA were used to reconstruct the phylogenomic tree. The phylogenomic tree of different E. coli based on the comparison of 2043 core genes. The 43 E. coli strains are divided into five monophyletic phylogroups (A, B1, B2, D and E). The SLPE strains are highlighted in blackbody. SLPE is the closest relative to APEC 078
Distribution diagram of the pan-genome virulence genes
The distribution of the ExPEC virulence factors were analysed among the 43 sequenced E. coli strains. Figure 2 shows that ExPEC-specific virulence factors were classified into the following six categories: (1) adhesins, (2) invasins, (3) toxins, (4) iron acquisition/transport systems, (5) polysialic acid synthesis and (6) other virulence genes. The 51 virulence genes from the 43 sequenced strains are shown in Fig. 3. The distribution diagram shows that even though there were significant differences between the SLPE and APEC078 strains, that these two strains are most closely related to one another. We also found that SLPE has more ExPEC-specific virulence factors, including several virulence factors that are ExPEC strain-specific, such as fimbrial adhesins (papG II), ireA, pgtP, hlyF, the pix gene cluster and fecR (Fig. 3).
Fig. 3.
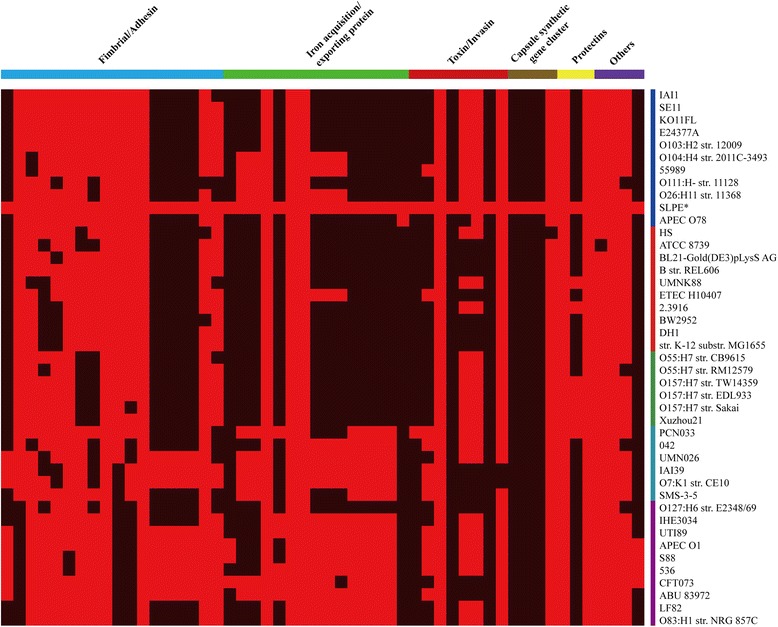
The distribution diagram of the pan-genome virulence genes among 43 E. coli strains. The right side of the vertical line shows E. coli strains that are consistent with the phylogenetic tree (Figure 2) with the following labels: (1) B1 monophyletic phylogroup, blue; (2) A monophyletic phylogroup, red; (3) E monophyletic phylogroup, green; (4) D monophyletic phylogroup, cyan and (5) B2 monophyletic phylogroup, purple. The SLPE strains are highlighted with the key symbol. The top shows the following six classified clusters: (1) fimbrial/adhesin, blue; (2) iron acquisition/exporting protein, green; (3) toxin/invasin, red; (4) capsule synthetic gene cluster, brown; (5) protectins, yellow and (6) others, purple. The red and black bodies show the distribution of the virulence genes among these strains. A red side implies that the virulence genes are present, while the black side implies that the genes are absent
Analysis of the capsular polysaccharide synthesis gene cluster
We found a capsular polysaccharide synthesis gene cluster (CPS) in the SLPE genomes using blastp. This CPS was closely related to the Klebsiella pneumonia CPS. The Klebsiella pneumonia CPS is closely related to Klebsiella pneumonia pathogenesis. The similarity and GC content of the 5 CPSs from Klebsiella pneumoniae genomes and 1 CPS from an E. coli strain were analysed. The results are shown in Fig. 4.
Fig. 4.
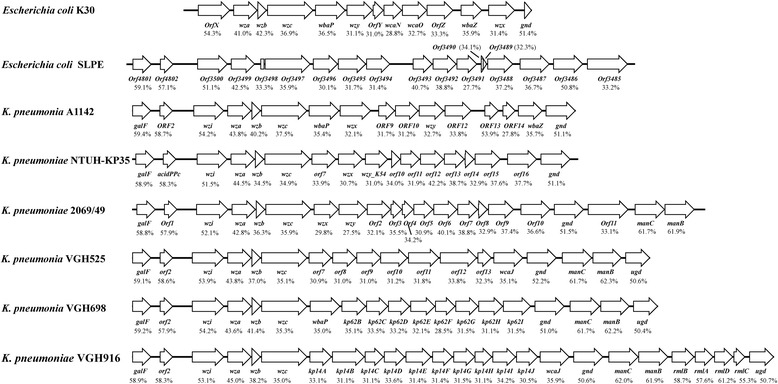
The genetic context for seven different types of capsular polysaccharide synthesis gene clusters. Shaded areas show conservative genes. Arrows represent the coding sequences and indicate the direction of transcription. The percentage under arrow represents the G/C content of the gene
Discussion
In this study, we isolated ExPEC isolates (ALPE) from pig farms and demonstrated that the SLPE isolates are pneumonia pathogens. Then, we performed genome sequencing and comparative genomic analysis with the SLPE isolates. Our analysis identified differences between SLPE and other ExPEC strains including various differences in the toxin and capsular polysaccharide synthesis gene clusters.
The phylogenomic comparison indicated that the SLPE strain belongs to the B1 monophyletic phylogroup, with its closest relative being APEC O78. APEC O78, an O78 strain, is an avian pathogenic E. coli isolated from the lungs of turkey that caused extensive animal and financial losses globally [6]. Although the SLPE and APEC O78 hosts are different, the strains have similar evolutionary relationships and both contain 4033 homologous genes. Now the question remains whether they have a common ancestor that is worthy of our attention.
ExPEC strains contain certain specific virulence traits that enable them to invade and colonize extraintestinal sites and cause a wide range of infections [7]. In this study, the distribution diagram of the pan-genome virulence genes showed that there were significant differences between the SLPE and APEC078, although they are most closely related to one another. We also found that SLPE has more ExPEC-specific virulence factors, including several virulence factors that are ExPEC strain-specific, such as fimbrial adhesins (papG II), ireA, pgtP, hlyF, the pix gene cluster and fecR. Of these genes, only papG II has been proven to have contact with urethral and avian pathogenicity [8]. These genes’ functions in pig ExPECs have yet to be explored.
In China, Liu et al., has reported the full genome characterization of ExPEC PCN033 strain isolated from pigs. The ExPEC virulence markers are different between PCN033 and SLPE, and the comparative genomic analysis results are different. SLPE was assigned to group B1 and clustered with APEC 078, while PCN033 was assigned to group D1 [9]. Furthermore, we found a capsular polysaccharide synthesis gene cluster (CPS) in the SLPE strain genomes, this CPS was closely related to the Klebsiella pneumonia CPS. The gene homology was approximately 96% (wzi, wza and wzc) and the homology of genes responsible for the serotype (wzx and wzy) was approximately 99%. Additionally, the GC content of the CPS was 51.7%, which is significantly lower than the full genome. These results suggest that the SLPE CPS may have been transferred from Klebsiella pneumoniae. Based on further analysis, the Klebsiella pneumonia CPS is the best characterized virulence factor in pneumonia and urinary tract infections [10, 11]. Functional analysis of the SLPE CPS will be forthcoming.
Conclusions
The SLPE isolated from swine lungs in China, was assigned to group B1 and clustered with APEC 078. The comparative genomic analysis revealed several genes for their further study. We found a CPS in the SLPE strain genomes, and this CPS is closely related to the CPS from Klebsiella pneumoniae. In conclusion, the mechanisms of ExPEC virulence still remain largely unknown. Studies on ExPEC pathogenesis will be enhanced by public access to high-quality genomic sequences.
Methods
Gross post-mortem examination
The swine industry was affected by pneumonia in the Changchun district of China in 2014. The pigs presented with pneumonia for 4–6 days prior to death. The autopsy performed after death included gross examination and observations of the lesions according to standard operating procedures. Lung tissue samples were quickly removed and fxed in formalin 10%. The diagonal section of samples was obtained and sectioned at 4 μm thickness, then the samples were stained using hematoxylin and eosin(H&E) stains, assessed using a light microscopy.
Bacterial isolation, culture conditions and serogroup
Bacteria were isolated from the surface of heat sterilized tissues, including lung, lymph, brain and heart blood samples.. All isolates were cultured on Mueller Hintom (MH), MacConkey, chocolate and 5% sheep’s blood agar plates. The isolates were identified by 16S rRNA gene sequence analysis and all isolates were identified in PCR for virulence marker genes of ExPEC (papA/papC, sfa/foc, afa/dra, kpsMTII and iutA) and ExPECs were defined as the isolates containing two or more virulence markers. Then, the serogroup was determined using antisera from the China Institute of Veterinary Drug Control (Beijing, China).
Experimental challenge studies
To test the pathogenicity of the porcine ExPEC strains, Experimental challenge studies was performed. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Jilin Agricultural University. A total of 20 BALB/c mice weighing 20(±2) g were randomly divided into 4 groups, every group contained 5 mice (purchased from the Animal Centre of Jilin University, Changchun, China) according to the institutional guidelines for the use of experimental animals (Laboratory animal─requirements of environment and housing facilities number GB 14925–2010). Approximately 5 × 107 CFU, 5 × 106 CFU and 5 × 105 CFU SLPE suspensions were prepared, and the bacterium were centrifuged and cleaned by physiological saline. Then, the strains were injected intraperitoneally into BALB/c mic, physiological saline was used as a negative control. Mice for health status were observed twice daily during 3 days. Deaths were recorded, and histological examination of dead mice lung was performed.
Antimicrobial resistance
Antimicrobial susceptibility testing was performed using the microplate dilution method [12]. The antimicrobial agents included cefotaxime, florfenicol, doxycycline, ciprofloxacin, tilmicosin, enrofloxacin, sulfamethoxazole and amikacin. The reference strain E. coli ATCC 25922 served as a standard for quality control. Each experiment was repeated four times.
DNA extraction
Total DNA was extracted using the Rapid Bacterial Genomic DNA Isolation Kit (Sangon Biotech, Shanghai, China) and stored at −80 °C until use. The DNA preparation was used for genome sequencing.
Genome sequencing and genome assembly
A whole genome shotgun library was produced with 1 μg of genomic DNA according to the instructions of the TruSeq™ DNA Sample Prep Kit-Set A (Illumina, USA) and the TruSeq PE Cluster Kit (Illumina, USA). The libraries were sequenced on an Illumina MiSeq platform at the Laboratory of Chinese National Human Genome Center (Shanghai, China). Afterward, the sequences were assembled using the SOAPdenovo software package [13, 14]. A total of 9,569,782 paired-end reads were assembled into 336 contigs.
Gene prediction and functional annotation
Coding sequences were predicted by combining the results obtained with the glimmer 3.02, Genemark and Z-Curve programs [15, 16]. Translational products of the coding sequences were annotated with the GenBank database. Putative functions of translation products were confirmed using the KEGG and Clusters of Orthologous Groups (COGs) databases [17, 18]. Putative genes of interest were identified with NCBI ORF finder and iterative BLASTn and BLASTp searches.
Phylogenomic comparison of SLPE with other E. coli pathotypes
Forty-three E. coli strain genomes were downloaded from the NCBI GenBank, fourteen strains was ExPEC, belong to different groups. The common genes were considered by the BLAST-like alignment tool (e-value = 1-e−10) and mcl [19]. All of the common genes were aligned by MUSCLE and concatenated together [20]. Then, clustalW and MEGA were used to reconstruct the phylogenomic tree. Genomes of Escherichia coli used for phylogenetic construction and core genome content used to construct phylogenetic tree were listed in Additional file 1: Table S1 and Additional file 2: Table S2.
The distribution diagram of pan-genome virulence genes
To examine whether SLPE strains harboured unique ExPEC-specific virulence factors, the pan-genome analysis of 51 virulence factors were performed, which are all positive in SLPE isolate [21]. ExPEC-specific virulence factors were classified into the following six categories: (1) adhesins, (2) invasins, (3) toxins, (4) iron acquisition/transport systems, (5) polysialic acid synthesis and (6) other virulence genes. All of the virulence factors were conducted with blastp and the virulence genes were clustered with PGAP. Then, a distribution diagram was created with the R program (http://www.r-project.org/). Six groups of genes that were used for heat map construction were listed in Additional file 1: Table S1 and Additional file 3: Table S3.
Analysis of capsular polysaccharide synthesis gene cluster
We found a CPS in the genome of the SLPE strain using blastp. The SLPE CPS was a close relative to the CPS from Klebsiella pneumoniae in the NCBI GenBank. The Klebsiella pneumoniae CPS is considered to be closely related to pathogenesis. Therefore, analysis of the CPS gene cluster between SLPE and other Klebsiella pneumoniae was important. Six CPSs from Klebsiella pneumoniae genomes and 1 CPS from an E. coli strain were downloaded from the NCBI GenBank. The GC content was calculated by GC content.pl and analysed with clc sequence viewer. Then, the similarity was analysed by mauve. CPS gene clusters were listed in Additional file 4: Table S4.
Additional files
Genomes of Escherichia coli used for phylogenetic construction. (DOC 135 kb)
Core genome content used to construct phylogenetic tree. (XLSX 18 kb)
Six groups of genes that were used for heat map construction. (DOCX 16 kb)
Comparison of CPS gene cluster of SLPE with closely related CPS gene clusters from other genomes. (DOCX 14 kb)
Acknowledgements
We thank Dr. LUAN Wei-min (Jilin Agricultural University, Changchun) for critical review of this manuscript.
Funding
This research was funded by National Key Research and Development Plan (Grant number: 2016YFD0501301), the Science and Technology Development Plan of Jilin Province (Grant number 20170520074JH), and the Science and Technology Development Plan of Jilin Province (Grant number 20150101109JC).
Availability of data and materials
This Whole Genome Shotgun project has been deposited at DDBJ/EMBL/GenBank under the accession LJCG00000000.1.
Authors’ contributions
LCK and XG contributed equally, there were involved in devising the experimental. HXM contributed to the analysis of the data. ZW, YHG and SML contributed to the experimental challenge studies and antimicrobial resistance. All authors read and approved the final manuscript and accepted the final version of this manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
BALB/c mice (20 +/− 2 g) were purchased from the Experimental Animal Holding of Jilin University (Jilin, China). Post-mortem examination of pigs were allowed by animal owners. The investigation conforms with the Guide for the Care and Use of experimental animals (Laboratory animal-requirements of environment and housing facilities number GB 14925–2010). The study protocol was approved by Ethics Committee of Jilin Agricultural University.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- APEC
Avian pathogenic Escherichia coli
- CPS
Capsular polysaccharide synthesis
- E. coli
Escherichia coli
- ExPEC
extraintestinal pathogenic Escherichia coli
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12917-017-1093-5) contains supplementary material, which is available to authorized users.
References
- 1.Smith JL, Fratamico PM, Gunther NW. Extraintestinal pathogenic Escherichia coli. Foodborne Pathog Dis. 2007;4(2):134–163. doi: 10.1089/fpd.2007.0087. [DOI] [PubMed] [Google Scholar]
- 2.Orskov F, Orskov I. Escherichia coli serotyping and disease in man and animals. Can J Microbiol. 1992;38(7):699–704. doi: 10.1139/m92-115. [DOI] [PubMed] [Google Scholar]
- 3.Liu C, Chen Z, Tan C, Liu W, Xu Z, Zhou R, et al. Immunogenic characterization of outer membrane porins OmpC and OmpF of porcine extraintestinal pathogenic Escherichia coli. FEMS Microbiol Lett. 2012;337(2):104–11. [DOI] [PubMed]
- 4.Ge XZ, Jiang J, Pan Z, Hu L, Wang SH, Wang HJ, et al. Comparative Genomic Analysis Shows That Avian Pathogenic Escherichia coli Isolate IMT5155 (O2: K1: H5; ST Complex 95, ST140) Shares Close Relationship with ST95 APEC O1: K1 and Human ExPEC O18: K1 Strains. PLoS One. 2014;9(11):e112048. [DOI] [PMC free article] [PubMed]
- 5.Tan C, Tang X, Zhang X, Ding Y, Zhao ZQ, Wu B, et al. Serotypes and virulence genes of extraintestinal pathogenic Escherichia coli isolates from diseased pigs in China. Vet J. 2012;192(3):483–8. [DOI] [PubMed]
- 6.Lamarche MG, Dozois CM, Daigle F, Caza M, Curtiss R 3rd, Dubreuil JD, et al. Inactivation of the pst system reduces the virulence of an avian pathogenic Escherichia coli O78 strain. Infect Immun. 2005;73(7):4138–45. [DOI] [PMC free article] [PubMed]
- 7.Orskov I, Orskov F. Escherichia coli in extra-intestinal infections. J Hyg (Lond) 1985;95(03):551–575. doi: 10.1017/S0022172400060678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norinder BS, Luthje P, Yadav M, Kadas L, Fang H, Nord CE, et al. Cellulose and PapG are important for Escherichia coli causing recurrent urinary tract infection in women. Infection. 2011;39(6):571–4. [DOI] [PubMed]
- 9.Liu C, Zheng H, Yang M, et al. Genome analysis and in vivo virulence of porcine extraintestinal pathogenic Escherichia coli strain PCN033. BMC Genomics. 2015;16(1):1–18. doi: 10.1186/1471-2164-16-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomas A, Lery L, Regueiro V, Perez-Gutierrez C, Martinez V, Moranta D, et al. Tournebized Re, Bengoecheaf JA. Functional Genomic Screen Identifies Klebsiella pneumoniae Factors Implicated in Blocking Nuclear Factor kappaB (NF-kappaB) Signaling. J Biol Chem. 2015;290(27):16678–97. [DOI] [PMC free article] [PubMed]
- 11.Arakawa Y, Wacharotayankun R, Nagatsuka T, Ito H, Kato N, Ohta M. Genomic organization of the Klebsiella pneumoniae cps region responsible for serotype K2 capsular polysaccharide synthesis in the virulent strain Chedid. J Bacteriol. 1995;177(7):1788–1796. doi: 10.1128/jb.177.7.1788-1796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute . ClSI document. Wayne: CLSI; 2013. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals. Approved Standard, 4th ed; pp. VET01–VETA4. [Google Scholar]
- 13.Li R, Zhu H, Ruan J, Qian W, Fang X, Shi Z, et al. De novo assembly of human genomes with massively parallel short read sequencing. Genome Res. 2010;20(2):265–72. [DOI] [PMC free article] [PubMed]
- 14.Li R, Li Y, Kristiansen K, Wang J. SOAP: short oligonucleotide alignment program. Bioinformatics. 2008;24(5):713–714. doi: 10.1093/bioinformatics/btn025. [DOI] [PubMed] [Google Scholar]
- 15.Delcher AL, Harmon D, Kasif S, White O, Salzberg SL. Improved microbial gene identification with GLIMMER. Nucleic Acids Res. 1999;27(23):4636–4641. doi: 10.1093/nar/27.23.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukashin AV, Borodovsky M. GeneMark.hmm: new solutions for gene finding. Nucleic Acids Res. 1998;26(4):1107–1115. doi: 10.1093/nar/26.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tatusov RL, Fedorova ND, Jackson JD, Jacobs AR, Kiryutin B, Koonin EV, et al. The COG database: an updated version includes eukaryotes. BMC Bioinformatics. 2003;4(1):41. [DOI] [PMC free article] [PubMed]
- 18.Kanehisa M, Goto S, Kawashima S, Okuno Y, Hattori M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004;32:D277–D280. doi: 10.1093/nar/gkh063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kent WJ. BLAT--the BLAST-like alignment tool. Genome Res. 2002;12(4):656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Logue CM, Doetkott C, Mangiamele P, Wannemuehler YM, Johnson TJ, Tivendale KA, et al. Genotypic and phenotypic traits that distinguish neonatal meningitis-associated Escherichia coli from fecal E. coli isolates of healthy human hosts. Appl Environ Microbiol. 2012;78(16):5824–30. [DOI] [PMC free article] [PubMed]
- 22.Chen Tan, Xibiao Tang, Xuan Zhang, Yi Ding, Zhanqin Zhao, Bin Wu, Xuwang Cai, Zhengfei Liu, Qigai He, Huanchun Chen. Serotypes and virulence genes of extraintestinal pathogenic Escherichia coli isolates from diseased pigs in China. The Veterinary Journal. 2012;192(3):483-488 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genomes of Escherichia coli used for phylogenetic construction. (DOC 135 kb)
Core genome content used to construct phylogenetic tree. (XLSX 18 kb)
Six groups of genes that were used for heat map construction. (DOCX 16 kb)
Comparison of CPS gene cluster of SLPE with closely related CPS gene clusters from other genomes. (DOCX 14 kb)
Data Availability Statement
This Whole Genome Shotgun project has been deposited at DDBJ/EMBL/GenBank under the accession LJCG00000000.1.


