Abstract
Background
Zika virus is an emerging pathogen of global importance. It has been responsible for recent outbreaks in the Americas and in the Pacific region. This study assessed five different mosquito species from the temperate climatic zone in Australia and included Aedes albopictus as a potentially invasive species.
Methods
Mosquitoes were orally challenged by membrane feeding with Zika virus strain of Cambodia 2010 origin, belonging to the Asian clade. Virus infection and dissemination were assessed by quantitative PCR on midgut and carcass after dissection. Transmission was assessed by determination of cytopathogenic effect of saliva (CPE) on Vero cells, followed by determination of 50% tissue culture infectious dose (TCID50) for CPE positive samples. Additionally, the presence of Wolbachia endosymbiont infection was assessed by qPCR and standard PCR.
Results
Culex mosquitoes were found unable to present Zika virus in saliva, as demonstrated by molecular as well as virological methods. Aedes aegypti, was used as a positive control for Zika infection and showed a high level of virus infection, dissemination and transmission. Local Aedes species, Ae. notoscriptus and, to a lesser degree, Ae. camptorhynchus were found to expel virus in their saliva and contained viral nucleic acid within the midgut. Molecular assessment identified low or no dissemination for these species, possibly due to low virus loads. Ae. albopictus from Torres Strait islands origin was shown as an efficient vector. Cx quinquefasciatus was shown to harbour Wolbachia endosymbionts at high prevalence, whilst no Wolbachia was found in Cx annulirostris. The Australian Ae. albopictus population was shown to harbour Wolbachia at high frequency.
Conclusions
The risk of local Aedes species triggering large Zika epidemics in the southern parts of Australia is low. The potentially invasive Ae. albopictus showed high prevalence of virus in the saliva and constitutes a potential threat if this mosquito species becomes established in mainland Australia. Complete risk analysis of Zika transmission in the temperate zone would require an assessment of the impact of temperature on Zika virus replication within local and invasive mosquito species.
Keywords: Zika virus, Vector competence, Aedes aegypti, Aedes albopictus, Culex quinquefasciatus, Aedes notoscriptus, Australia, Invasive
Background
Zika virus was first isolated in Uganda in 1947 from a febrile rhesus monkey. Aedes mosquitoes, and primarily Aedes (Stegomyia) africanus, was suspected as the main sylvatic vector following direct isolation of Zika virus in 1956 [1]. Subsequent vector competence studies in East [2] and West Africa [3] demonstrated that other members of the Stegomyia subgenus showed vector competence such as Aedes aegypti, an important vector in Yellow Fever transmission. Aedes (St.) luteocephalus has also been incriminated in Nigeria [4], with several Zika virus isolations and a frequent contact with humans. Isolated human cases, serological surveys and virus isolations attested to virus circulation initially in Africa and later in Asia. Virus was isolated from Ae. aegypti in Malaysia [5] in 1966 and clinical cases were detected in Indonesia [6] in 1977-1978. However, the authors also suspected Aedes (St.) albopictus whose local presence and assumed role in rural dengue transmission made it another possible candidate. More recently, populations of Ae. aegypti [7] and Ae. albopictus [8] in Singapore have been shown efficacious vectors in the laboratory. Since 2007, Zika virus has successively invaded the Pacific region: Yap island in 2007 [9, 10], then French Polynesia and New Caledonia [11] in 2014. In Yap, the most abundantly collected Ae. (St.) hensilli, instead of Ae. aegypti was suspected of being responsible for the outbreak, and was shown experimentally capable of infection and dissemination [12]. In French Polynesia, the locally abundant Aedes (St.) polynesiensis was found not able to transmit Zika virus [13]. In 2014, Zika virus reached the Americas and Ae. aegypti was shown to be a vector, both by molecular detection in field-collected mosquitoes [14] and experimental infection [14–16]. The role of other mosquito species is still under question, especially for Culex quinquefasciatus, with conflicting results which either showed it as an efficient experimental vector [14, 17] or not [16, 18, 19].
With a lack of approved vaccines and antivirals, vector control is a key measure for decreasing the public health burden and risk for Zika virus. Identifying vectors and understanding the transmission mechanism is the first step in designing the best suited vector control policy. Despite the presence of Zika virus in the Pacific region (Micronesia and Polynesia), and that, early in the outbreak, travellers returning to Australia have been found to carry the virus [20], no local transmission has been reported. A general consensus identifies the vectors for Zika virus as the same species involved in dengue and chikungunya transmission. Accordingly the global and local risk for Zika virus transmission is set same as dengue and chikungunya risk [21, 22]. In Australia, Ae. albopictus is exotic to the mainland and with Ae. aegypti’s limited distribution, the dengue risk is limited to the Northern tropical regions. Therefore, local populations of Ae. aegypti and potential tropical vectors from Queensland, such as Ae. (Ochlerotatus) vigilax, Ae. (Rampamyia) notoscriptus, and Culex. quinquefasciatus, have been tested for Zika virus vector competence [18]. From this study, Ae. aegypti was confirmed as the main suspected vector species, with no other local major or associated species demonstrating virus transmission or considered to play any role. Much of Australia is out of the current dengue risk zone, however the temperate southern zones do harbour dense urban populations and can experience warm summer temperatures. The climatic conditions of these zones is close to those of Southern and Western parts of Europe [23]. Endemic mosquito-borne viruses have been shown to trigger outbreaks in Southern parts of Australia: flaviviruses like Kunjin virus and Murray Valley encephalitis virus, as well as alphaviruses like Ross River virus and Barmah Forest virus. Local species such as Aedes camptorhynchus, or populations of Aedes notoscriptus and Culex annulirostris have been implicated in the circulation of these viruses. Incursion of invasive species, such as Ae. albopictus has been detected in the state of Victoria and successfully controlled [24]. Culex quinquefasciatus has been well established in Victoria and can be captured during summer. Similar to European researchers who have begun to worry about local Zika transmission [25–27], in order to address the Zika virus transmission risk in the temperate region of Australia, we have performed vector competence experiments on those mosquito species most frequently captured in close association with human populations. As bacterial endosymbiont, Wolbachia, infection is known to potentially impact on virus infection in insects [28], we have complemented the competence assessment with Wolbachia infection screening for tested mosquito populations. To get a full view of the Zika risk assessment for temperate zones of Australia, we have included Ae. albopictus collected from the top northern Torres Strait Island as a potentially invasive species to mainland Australia.
Methods
Mosquito sampling
- Aedes (Och.) camptorhynchus samples were collected as larvae in coastal Victorian region of Gippsland (Wellington) (Fig. 1). Following 24 h duration transport to the lab, the larvae were reared in trays with fish food pellets (300 mg/ 100 larvae every 2-3 days) to adulthood. Once at imago stage, they were kept at 25 °C, 65% humidity and under 14:10 day:night photoperiod. Adult mosquitoes were fed 10% sugar solution and starved 24 h before oral virus challenge at 5-8 days old.
- Aedes (Ram.) notoscriptus samples were collected as larvae in the Bellarine (Geelong - Highton), and the Melbourne regions (Fig. 1). Conditions of rearing were similar to those of Ae. camptorhynchus.
- Aedes aegypti colony originated from Cairns, Queensland, Australia. They were reared as described above. The 6th, 7th and 11th generations were used for experimental viral challenge.
- Aedes albopictus colony was established from egg batches collected in Hammond Island in the Torres Strait island group, at the top north of Australia, in Dec. 2015. They were reared under the same conditions as described above. The 4th and 9th generations were used for experimental infections.
- Culex annulirostris were from a 50 years old colony which originated in Shepparton, Victoria. Rearing principles followed Mc Donald et al. [29]. This colony has been shown to transmit West Nile virus [30].
- Culex quinquefasciatus were from a colony established by our laboratory in 2011, from specimens collected in Geelong, Victoria, Australia. Conditions of rearing are similar to Culex annulirostris, except oviposition occurred in 10 mL cups instead of petri dishes. Samples used for experimental infection correspond to 30th generation.
Fig. 1.
Map showing the origin of the mosquito populations. The Brisbane zone, as cited in the reference numbered 20, is indicated
The experiments were performed under biosafety level 3 (BSL-3) conditions in the insectary at the Australian Animal Health Laboratory.
Viral strain
Cambodia 2010 (Genbank KU955593) [31] Zika virus strain was used for Ae. aegypti, Ae. albopictus, Ae. camptorhynchus, Ae. notoscriptus, Cx quinquefasciatus, and Cx annulirostris. It belongs to the Asian/Pacific/American clade [32] and was passaged once in C6/36 cells and twice in Vero cells before using for mosquito infections.
Oral challenge
Five to eight days old females were starved the day before being challenged with an infected blood meal (TCID50 105.6/mL) through membrane feeding using chicken blood and skin. Uninfected chicken blood and skin were provided by the Small Animal Facility (Australian Animal Health Laboratory) from chicken bred in the laboratory without any arboviral infection. The procedure was conducted with approval from AAHL Animal Ethics Committee. The blood was spiked with Zika virus just before mosquito blood-feeding. For control, media supernatant was added to the blood before feeding. After one hour, the mosquitoes were anaesthetised with CO2 and blood fed females were sorted and kept in a 200 mL cardboard cup at 27.5 °C, 65% humidity and 14:10 day:night photoperiod. The blood-fed specimens were kept for 14 day extrinsic incubation period with 10% sugar solution provided ad libitum.
Sample processing: Specimens were anaesthetised with CO2 and saliva was collected as previously described [33], with a slightly modified protocol. Briefly, after removing mosquito’s legs and wings, the proboscis was inserted into a micro-capillary tube containing Foetal Bovine Serum (FBS) and left in place for 20 mins. Micro tubes containing the mix of FBS and expelled saliva were individually stored at -80 °C before virological assessment. Mosquitoes were then dissected in saline phosphate buffer, separating for each individual the midgut, the head with the anterior half of the thorax, and the rest of the carcass, containing ovaries and remains of the exoskeleton.
Virus titre assessment: Saliva testing was performed in two steps: after adding 80 μL of culture media and centrifuging at 2000 g for 3 min, the cytopathogenic effect (CPE) was tested in duplicates on Vero cells (2 × 25 μL) at Day 5 post inoculation. For most samples showing CPE, the TCID50 was calculated from the remaining 30 μL using Vero cells as previously described [34].
Molecular testing: After homogenisation of mosquito tissues by bead beating, RNA was extracted using either MagMax (Thermo Fisher) or RNeasy RNA isolation kit (Qiagen, Australia) as manufacturer’s protocol. Ten microliters of RNA was used to prepare cDNA using random hexamers and Superscript-III reverse transcriptase (Thermo Fisher Scientific Inc. Australia) as manufacturer’s protocol. A SYBR Green two step real-time PCR assay was designed for detection of Zika viral partial coding sequence (107 bp) of non-structural protein 5 (NS5) (forward primer: 5′-GAACGAGGATCACTGGATGG-3′, reverse primer: 5′-CTCCTGGTATGCGACTCATC-3′). Screening was conducted using the SYBR™ PreMIX Ex Taq™ II (Takara-Bio Inc., China) and run on a QuantStudio™ 6 Flex Real Time PCR System (Applied Biosystems). Cycling conditions were as follows, 95 °C for 30 s, 40 cycles of 95 °C for 5 s, and 60 °C for 30s, before performing melting curve analysis. The theoretical melting point of a Zika positive sample was 85 °C. However the discriminant melting point with our positive control (Cambodia 2010 strain) was fixed to 80.5 + −0.5 °C. Dilution curve showed positive signal up to the fourth 10-fold dilution, indicative of a concentration of ~100 TCID50 / mL. Negative control was a cDNA preparation from Vero cell culture infected with Chikungunya virus. Samples were considered positive if both duplicates had melting temperatures within the positive control range a CT value lower than 40.
Wolbachia screening
The presence of Wolbachia was first assessed for all species except Ae. albopictus, using pools of 5 individual cDNA samples obtained from mosquito carcass of the same species. Screening was performed using a SYBR™ Green based quantitative PCR adaptation of the protocol from Mee et al. [35]. Positive pools were retested by conventional PCR, targeting the coding sequences for 16S ribosomal RNA and Wolbachia surface protein (wsp) [35].
In a second step, prevalence of Wolbachia infection was identified by testing several individuals of each mosquito population, including Ae. albopictus with the two conventional 16S and wsp PCR assays.
Statistical analysis
Zika infection rate was defined by the number of midguts found positive for viral nucleic acid by qPCR. Similarly, the dissemination rate was calculated by the number of carcasses found positive by qPCR for viral nucleic. Transmission rate was defined by the number of saliva samples showing CPE in Vero cells over the number tested. These different rates were compared by Fisher exact two-tailed test. Average CT and TCID50 values were respectively compared by Kruskall-Wallis two-tailed tests.
Results
Vector competence of six mosquito species for transmission of Zika virus have been assessed.
Our results confirm Ae. aegypti as the most efficient vector, with a high rate of midgut infection and dissemination and high virus prevalence and loads in saliva (Table 1).
Table 1.
Infection, dissemination and transmission rate: calculated from prevalence of viral nucleic acid presence in the dissected midguts and carcasses and prevalence of CPE by saliva samples
| Species | Infection rate by qPCR positive midguts (%) | Dissemination rate by qPCR positive carcasses (%) | Transmission rate by CPE (%) |
|---|---|---|---|
| Aedes aegypti | 40/48 (83%) | 39/47 (83%) | 33/38 (87%) |
| Aedes notoscriptus | 12/35 (34.3%) | 2/59 (3.4%) | 24/57 (42.1%) |
| Aedes camptorhynchus | 5/18 (27.8%) | 5/40 (12.5%) | 5/37 (13.5%) |
| Aedes albopictus | 19/26 (73.1% | 19/26 (73.1%) | 20/26 (76.9%) |
| Culex annulirostris | 0/32 (0%) | 0/32 (0%) | 0/32 (0%) |
| Culex quinquefasciatus | 0/20 (0%) | 0/20 (0%) | 0/17 (0%) |
Ae. albopictus from Australia (Torres Strait Island) was also tested for Zika virus competence and showed high prevalence (>75%) of virus in the saliva at day 14. The TCID50 of virus in the saliva of Ae. aegypti was found to be significantly higher than Ae. albopictus (p < 0.0001) and Ae. notoscriptus (p = 0.0002) (Fig. 2). There was no statistical difference between Ae. albopictus and Ae. notoscriptus TCID50 averages. The CT values for midguts and carcass were significantly lower with Ae. aegypti (Fig. 3b).
Fig. 2.
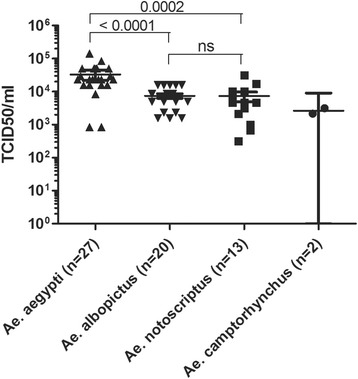
Quantitation of viral load in CPE positive saliva samples. Mosquito samples producing CPE and given a TCID50 value were plotted, by species with number of tested samples. Horizontal bars are means with 95%CI. P-values of two-tailed Mann Whitney tests are presented (ns = not significant). For Ae. camptorhynchus, tests are not applicable due to the low number of values
Fig. 3.
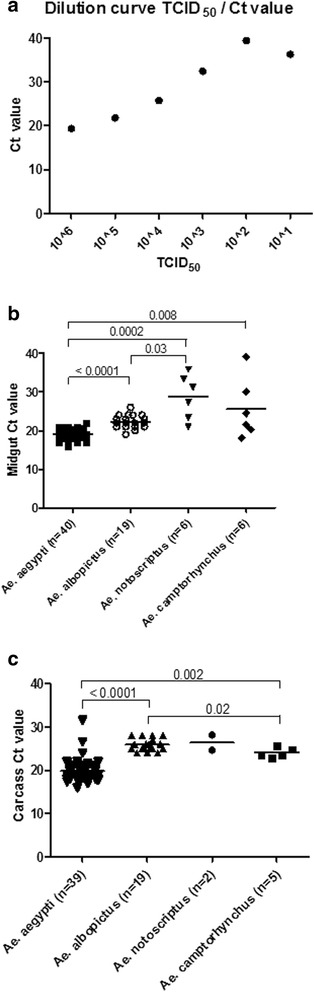
Viral RNA determination in mosquito organs. Real-time RT-PCR was performed on RNA collected from mosquito midgut (b) or carcass (c) using ZIKV NS5 primers. Horizontal bars as mean with 95% Confidence Interval. For comparison, a dilution curve for TCID50 is given in (a), where real-time RT-PCR was performed on RNA collected from 10-fold dilutions of Zika virus of known titre. The average Ct value of 3 samples of freshly blood-fed Ae. camptorhynchus whole bodies, including the midgut is 30.03; it is equivalent to the viral nucleic acid loading input before any replication, approximatively 10^3.5 of TCID50. Significant p values are denoted on the graph
The results show that two local temperate Aedinae, Ae. notoscriptus and Ae. camptorhynchus, could be infected by Zika virus and deliver virus in their saliva (Table 1). The molecular screening shows moderate dissemination rates (carcass infection, respectively 3 and 12%), much lower than the infection rates (midgut, respectively 34 and 28%) for both species. We found virus in the saliva of Ae. notoscriptus (42%) and Ae. camptorhynchus (13.5%) by assessing the cytopathogenic effect of the saliva on Vero cells. The prevalence of virus in the saliva was lower than that of Ae. aegypti (87%) (Table 1). To confirm the specificity of the CPE in the saliva as presence of Zika virus, we tested 18 CPE positive wells, corresponding to 12 different mosquito samples (3 Ae. aegypti and 9 Ae. notoscriptus, randomly chosen), by qPCR. All samples were positive for the presence of Zika virus RNA, with low CT values (average 16.06, with no difference between the two species) (data not shown).
We did not find any evidence of virus in the saliva of the samples of Cx quinquefasciatus, and Cx annulirostris. In addition, no midgut or carcass sample of any Culex species tested positive for viral nucleic acid 14 days post-infection using the Cambodia strain (Table 1).
We have also screened the tested populations for Wolbachia presence using molecular assays. Culex quinquefasciatus were shown to harbour Wolbachia, with 90% prevalence (Table 2), but not Cx annulirostris. Wolbachia infection was detected by molecular assay in all of the four Aedes species (Table 2). Aedes albopictus was the only species found with 100% infection rate, whilst Ae. aegypti, Ae. notoscriptus and Ae. camptorhynchus showed very low prevalence. Ae. camptorhynchus showed a positive result by qPCR, however this was not confirmed by conventional PCRs targeting 16S and wsp. Sequencing and blast analysis of the amplicons from both standard PCRs confirmed the presences of Wolbachia in Ae. aegypti, Cx quinquefasciatus and Cx pipiens gp.
Table 2.
Wolbachia infection prevalence for the tested mosquito species
| Pool of 5 mosquitoes | Individual | |||
|---|---|---|---|---|
| Species | qPCR (CT value) | 16S PCR | Wsp PCR | Prevalence PCR 16S positive |
| Aedes aegypti | + (15.7) | + | + | 1/14 |
| Aedes albopictus | NA | + | NA | 14/14 |
| Aedes notoscriptus | − | − | − | 1/15 |
| Aedes camptorhynchus | + (30.8) | − | − | 0/15 |
| Culex quinquefasciatus | + (14.3) | + | + | 9/10 |
| Culex annulirostris | − | − | − | 0/15 |
Discussion
Culex species
Understanding the importance of Culex species, especially Cx quinquefasciatus, in Zika virus transmission, with conflicting published data, is crucial for the implementation of vector control policies. Several studies addressing this matter have concluded that Culex species may not serve as vectors of Zika virus [16, 18, 19], however two studies found Cx quinquefasciatus as competent [14, 17]. Our study did not find any viral RNA or infectious virus in the samples of Cx quinquefasciatus. These results confirmed previous experiments performed using tropical Australian Culex quinquefasciatus [18], despite the potential differences in genetic background between populations. Culex quinquefasciatus was shown to harbour Wolbachia endosymbionts, with prevalence of 90% (Table 2). Despite the absence of an obvious relationship between Zika vector capacity and Wolbachia natural infection in our results, the screening for associated endosymbionts may be useful in the explanation of discordant results in the different Culex quinquefasciatus vector competence studies. Despite being sometimes a major vector for several arboviruses in Australia [36, 37, 38], Cx annulirostris was also not found to transmit Zika virus, at 14 days after oral challenge with Cambodia Zika virus strain. The Cx annulirostris colony used in this study has a long history and originated in Victoria [29]. Given its origin and the probability of major genetic bottleneck and drift, it may represent a very different population than the natural tropical populations tested by Hall-Mendelin et al. [18]. Additionally, this species has been shown to be composed of several cryptic lineages in Australia [39]. Our study, along with Hall-Mendelin et al. [18], have used Cx annulirostris and Cx quinquefasciatus from two different zones of Australia for Zika virus vector competence, with negative results obtained regardless of their source location. This places these species in a safe status conferring to no Zika virus transmission, thereby reducing the need for specific vector control.
Aedes aegypti
Contrary to the results obtained from Culex species, the data confirm Ae. aegypti as the most efficient vector with a high prevalence of midgut infection and dissemination rate detected by molecular screening and high virus prevalence and loads in the saliva. This species is confirmed of prime epidemiological importance and the Australian population, originating from Queensland, is considered as a major vector [18].
Local temperate Australian Aedes
The results of this study demonstrate that two local Aedinae of the temperate zone, Ae. notoscriptus and Ae. camptorhynchus, can be infected by Zika virus and deliver infectious virus in their saliva. Similar to the results of Hall-Mendelin et al. [18] for Ae. notoscriptus and Aedes vigilax (a saltmarsh species belonging to the same subgenus as Ae. camptorhynchus), our results showed moderate infection rates and low dissemination rate for these Aedes species. However, in contrast to Hall-Mendelin et al., our results showed presence of virus in the saliva of these mosquitoes’ Victorian populations: 42% and 13.5% of Ae. notoscriptus and Ae. camptorhynchus, respectively. The prevalence of virus in the saliva was lower than that of Ae. aegypti (87%) (Table 1). Hall-Medellin et al. reported viral RNA prevalence in saliva at 27% for Ae. aegypti at D14. Our results are closer to the 100% transmission results reported by Li et al. [7] who, as we did for saliva, used virological methods (CPE) rather than molecular. Our qPCR molecular test was assessed for relative sensitivity by a dilution curve which showed positive results down to 10−4-fold dilution of the initial cDNA from a TCID50 106/ml stock solution, being inconsistent at the 10−5 and negative at the 10−6 dilution (Fig. 3a). As a further insight of the viral dynamics within the mosquitoes, we tested three whole samples of freshly blood-fed Ae. camptorhynchus which gave an average CT value of 30.03 (SD 1.12), as a relative measure of the initial virus load.
The specimens of the population of this mid-sized species were among the larger of tested samples of the different species. The quantity of ingested virus was probably among the higher. The average CT value for Ae. camptorhynchus midgut samples is lower than this threshold, indicating a probable local replication after the blood meal. For Ae. notoscriptus, the midgut average CT values are similar to the viral input average represented by the whole freshly blood-fed Ae. camptorhynchus specimens (Fig. 3b). However, when positive, the carcass of Ae. notoscriptus shows lower CT values, indicative of replication (Fig. 3c). Our molecular test could have missed some specimens with low level virus load, either in the midgut or in the carcass. The virological method of virus detection by CPE could be a more robust and reliable method and the most informative indicator of transmission.
Interestingly, following Cornet et al.’s observation, Duschinka et al. [3, 14] proposed a dynamic model of infection in which a transient window time would be best for transmission. This means that mosquitoes would first tolerate virus replication, then would clear them and would not be able to transmit afterwards. Although this is not the current paradigm for virus vector transmission, a recent study [40] has shown that dengue virus would disappear from the saliva of aged Ae. aegypti, potentially supporting this temporal transient infection model, even with the “true” vectors.
Beyond the different methodological approaches, the different results between the vector competence assessments performed on two different populations of Ae. notoscriptus, from either tropical or temperate zones, could be linked to the vector’s differences in genetic background. Indeed, Endersby et al. [41] hypothesised that Ae. notoscriptus could be a complex of cryptic species. Also, screening for Wolbachia by conventional PCR on individual carcass (including ovaries) cDNA shows a very low prevalence of Wolbachia for the temperate zone population of Ae. notoscriptus and absence of positive molecular signal on pools of 5 cDNAs by qPCR. In contrast, a high prevalence of Wolbachia natural infection has previously been described in tropical Ae. notoscriptus populations either from a colony or field samples collected in Brisbane [42], with this being the same origin as the tropical population tested for Zika virus vector competence [18]. Although natural Wolbachia infection did not influence the dengue virus competence of infected Ae. notoscriptus populations [42], our study cannot dismiss the possibility of a potential protective effect of natural Wolbachia infection on Ae. notoscriptus for Zika virus infection and could justify further research. It is to be noted that, despite the distance, Ae. notoscriptus has been described as potentially invasive for USA [43]. Aedes notoscriptus from Brisbane has been shown to transmit yellow fever virus better than Ae. aegypti [44], however Ae. notoscriptus of unknown origin failed to develop infections with the same virus [45]. Aedes notoscriptus from Brisbane area has been previously shown as a poor vector for the four serotypes of dengue virus [46]. Indeed, populations of this mosquito in its southern distribution range, e.g. the state of Victoria, have not been documented for flavivirus vector competence. No dengue outbreak or documented secondary cases of dengue have been recorded in this region, in the absence of the known dengue vector Ae. aegypti. The contact between human populations and Ae. notoscriptus has been documented in Queensland but not in temperate regions. Aedes notoscriptus has been shown to bite humans 19% of the time for blood meals in residential areas of Brisbane [47]. Its dispersal and survival rates in the same area [48] are similar to Ae. aegypti and identify it as a potential vector in urban areas. Although it is known to be a backyard pest in the southern zones, much of its biology remains to be well defined in order to properly assess risk for transmission. The case of Ae. camptorhynchus is quite different: its ecology is restricted to coastal areas, or around inland brackish zones [49], with a lower abundance in urban areas [50], with local exceptions in suburb areas around Melbourne, Victoria (SL, pers. Comm.). It has been shown to be an efficient experimental vector for the Alphavirus, Ross River virus [51], and epidemiologically important in the natural transmission of this virus [37]. This includes the possibility of vertical transmission for Ross River and Sindbis viruses [52]. Except this current study, its status as vector for flavivirus is unknown. Similar to Ae. notoscriptus, but to a lesser degree, Ae. camptorhynchus showed intermediate levels of vector competence for Zika virus. Both are probably not primary vectors, meaning they may be unable to sustain large outbreaks on their own, unlike Ae. aegypti in tropical areas, however they could be potential candidates to trigger few secondary cases and reinforce outbreaks in the presence of primary vectors. Aedes notoscriptus is known as a backyard pest, due to its abundance and taste for human blood. Aedes camptorhynchus has been shown to be responsible for Ross River virus outbreaks, here too displaying a close proximity with humans. However, a big gap in our knowledge remains: the experiments were performed at 27.5 °C. Whilst the state of Victoria can experience very high temperatures and heat waves, on average, summer temperatures are much lower than 25 °C. Performing vector competence experiments at lower temperatures would be useful in establishing a proper risk assessment for local transmission of Zika virus, both by local and potentially invasive species.
Aedes albopictus
Our results demonstrated that Ae. albopictus, from Australian Torres Strait island, is a competent vector for Zika virus transmission. This is concordant with Wong et al. [8], who tested Ae. albopictus from Singapore and used the dissection of salivary glands and virological method. Beside the methodological approach, with dissection of salivary gland instead of saliva collection, the main differences between the studies are the temperature of rearing (29 °C versus 27.5 °C) and the Zika virus strain (Uganda 1947 strain belonging to the African clade, versus the Cambodia 2010 strain belonging to the Asian clade). Aedes albopictus is considered as an invasive species with a large threat potential [53] at a global level, including Europe [27], Africa [54] and Australia. However, variation in results of experimental challenge of vector competence may exist, and could influence greatly the risk model of Zika transmission by Ae. albopictus [25]. Studies have reported low (3%) level of experimental transmission, using either molecular or virology method for virus detection in the saliva of infected individuals of European [26] or American [15] Ae. albopictus populations. With our results, this demonstrates that distant populations of Ae albopictus could show large difference of vector competence. Consequently the risk analysis for this species cannot be carried across different regions, even within comparable climate [23] (Italy and South-Eastern Australia for instance) and needs assessment of local populations of vectors. Interestingly, the tested Aedes albopictus population coming from Torres Trait Islands seems to harbour Wolbachia endosymbionts. The results do not show them protected from being infected and transmitting Zika virus. However, a more quantitative experimental assessment could answer this question more fully.
Conclusion
The experimental assessment of vector competence for Australian mosquitoes focusing on the temperate region shows different degrees of risk. Culex species are devoid of proof of potential vector competence for Zika virus. Conversely, the potential invader for inland Australia, Ae. albopictus, is shown to be a competent vector, with a potential threat at a similar level as found in Ae. albopictus population in Singapore and very different from European populations. Two native Aedes species show an intermediate level of vector competence, whereby they are probably unable to sustain large outbreaks themselves, but are potential candidates to trigger some secondary cases. A gap in our knowledge exists in lower temperature assessment of vector competence to better mimic local climatic conditions.
Acknowledgments
- Thanks to the Victorian Department of Health and Human services for help in collection of Aedes camptorhynchus and Aedes notoscriptus mosquito larvae through the Victorian Arbovirus Disease Control program: Barry Curtain (Wellington Shire Council), Lyndon Ray, Rachel Evans and Melanie Renton (City of Greater Geelong) and Nicole Clift (Department of Agriculture and Water Resources).
- Thanks to Ary Hoffmann and Jason K Axford (Bio21, University of Melbourne) for Aedes aegypti colony.
- Thanks to Chris Freebairn for collection of eggs of Aedes albopictus and transport to the laboratory under import permit number 2016-01-22120617.
- Thanks to Stephen Doggett and Merilyn Geary, (Westmead Hospital, New South Wales, Australia) for the Culex annulirostris colony.
- Thanks to Rhonda Voysey for helping in establishing the colony of Culex quinquefasciatus in Geelong and initial genotyping for Culex pipiens group.
- Thanks to Robert B Tesh and Nikolaos Vasilakis (University of Texas Medical Branch, Galveston, USA) and valuable help of Anne-Sophie Brocard, for sending Zika strains under Australian Quarantine import permit 0000340837. Thanks to Assoc/Prof Allison Imrie from the School of Pathology and Laboratory Medicine, University of Western Australia for sending the PRVABC-59 strain.
- Thanks to Shunin Shi for help during the first sets of infections.
- Thanks for David Williams (AAHL, Geelong) and Rebecca Feldman (Department of Health & Human Services, Victoria) for linking with the National Arbovirus and Malaria Advisory Committee (NAMAC).
- Thanks to Dr. Finn Romanes, Deputy Chief Officer (Department of Health & Human Services, Victoria) for discussion on results.
- Thanks to Paul de Barro for valuable comments on risk analysis of our results.
- Finally thanks to Peter Walker (CSIRO Geelong, University of Queensland) having enthusiastically pushed forward for early work on Zika virus vector competence.
Funding
Funding was obtained from CSIRO for laboratory work and Victorian Department of Health for mosquito collection.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
JBD co-designed the study, performed experimental infection and dissection, analysed data and wrote the initial draft; PTM performed RNA extraction and qPCR; SEL co-designed the study, coordinated mosquito collection in the field and amended the draft; RV designed and optimized the Zika qPCR; LT prepared virus culture and amended the draft; PNP co-designed the study, performed experiment and participated to the drafting. All authors have read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable
Ethics approval
Animal ethics committee approval was obtained for collection of blood from chicken.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- cDNA
complementary Desoxyribonucleic Acid
- CO2
Carbon dioxide
- CPE
Cytopathogenic effect
- CT value
Cycle threshold value
- FBS
Foetal Bovine Serum
- PCR
Polymerase Chain Reaction
- qPCR
real time Polymerase Chain Reaction
- RNA
Ribonucleic Acid
- TCID50
50% tissue culture infectious dose
- Wsp
Wolbachia surface protein
Contributor Information
Jean-Bernard Duchemin, Email: Jean-Bernard.Duchemin@csiro.au.
Peter T. Mee, Email: Peter.Mee@csiro.au
Stacey E. Lynch, Email: stacey.lynch@ecodev.vic.gov.au
Ravikiran Vedururu, Email: Ravikiran.Vedururu@csiro.au.
Lee Trinidad, Email: Lee.Trinidad@csiro.au.
Prasad Paradkar, Email: Prasad.Paradkar@csiro.au.
References
- 1.Weinbren MP, Williams MC. Zika virus: further isolations in the Zika area, and some studies on the strains isolated. Trans R Soc Trop Med Hyg. 1958;52:263–268. doi: 10.1016/0035-9203(58)90085-3. [DOI] [PubMed] [Google Scholar]
- 2.Boorman JP, Porterfield JS. A simple technique for infection of mosquitoes with viruses; transmission of Zika virus. Trans R Soc Trop Med Hyg. 1956;50:238–242. doi: 10.1016/0035-9203(56)90029-3. [DOI] [PubMed] [Google Scholar]
- 3.Cornet M, Robin Y, Adam C, Valade M, Calvo MA. Transmission expérimentale comparée du virus amaril et du virus Zika chez Aedes aegypti L. (S) Cah ORSTOM, sér Etat méd et Parasitol. 1979;17:47–53. [Google Scholar]
- 4.Lee VH, Moore DL. Vectors of the 1969 yellow fever epidemic on the Jos plateau, Nigeria. Bull World Health Organ. 1972;46:669–673. [PMC free article] [PubMed] [Google Scholar]
- 5.Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am J Trop Med Hyg. 1969;18:411–415. doi: 10.4269/ajtmh.1969.18.411. [DOI] [PubMed] [Google Scholar]
- 6.Olson JG, Ksiazek TG, Suhandiman, Triwibowo Zika virus, a cause of fever in central java, Indonesia. Trans R Soc Trop Med Hyg. 1981;75:389–393. doi: 10.1016/0035-9203(81)90100-0. [DOI] [PubMed] [Google Scholar]
- 7.Li MI, Wong PS, Ng LC, Tan CH. Oral susceptibility of Singapore Aedes (Stegomyia) aegypti (Linnaeus) to Zika virus. PLoS Negl Trop Dis. 2012;6:e1792. doi: 10.1371/journal.pntd.0001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong PS, Li MZ, Chong CS, Ng LC, Tan CH. Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Negl Trop Dis. 2013;7:e2348. doi: 10.1371/journal.pntd.0002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR:. Genetic and serologic properties of Zika virus associated with an epidemic, yap state, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–9. [DOI] [PMC free article] [PubMed]
- 10.Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, et al: Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–43. [DOI] [PubMed]
- 11.Dupont-Rouzeyrol M, O’Connor O, Calvez E, Daurès M, John M, Grangeon JP, Gourinat AC: Co-infection with Zika and dengue viruses in 2 patients, New Caledonia, 2014. Emerg Infect Dis. 2015;21:381–2. [DOI] [PMC free article] [PubMed]
- 12.Ledermann JP, Guillaumot L, Yug L, Saweyog SC, Tided M, Machieng P, Pretrick M, Marfel M, Griggs A, Bel M, et al: Aedes hensilli as a potential vector of Chikungunya and Zika viruses. PLoS Negl Trop Dis. 2014;8:e3188. [DOI] [PMC free article] [PubMed]
- 13.Richard V, Paoaafaite T, Cao-Lormeau VM. Vector competence of French Polynesian Aedes aegypti and Aedes Polynesiensis for Zika virus. PLoS Negl Trop Dis. 2016;10:e0005024. doi: 10.1371/journal.pntd.0005024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duschinka RD, Guedes DRD, Paiva MHS, Donato MMA, Barbosa PP, Krokovsky L, dos S. Rocha SW, Saraiva KLA, Crespo MM, Barbosa RMR, et al: Zika virus replication in the mosquito Culex quinquefasciatus in Brazil. 2016. https://doi.org/10.1101/073197 [DOI] [PMC free article] [PubMed]
- 15.Chouin-Carneiro T, Vega-Rua A, Vazeille M, Yebakima A, Girod R, Goindin D, Dupont-Rouzeyrol M, Lourenço-de-Oliveira R, Failloux AB: Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus. PLoS Negl Trop Dis. 2016;10:e0004543. [DOI] [PMC free article] [PubMed]
- 16.Fernandes RS, Campos SS, Ferreira-de-Brito A, Miranda RMd, Barbosa da Silva KA, Castro MGd, Raphae LMS, Brasil P, Failloux AB, Bonaldo MC, Lourenço-de-Oliveira R. Culex quinquefasciatus from Rio de Janeiro Is Not Competent to Transmit the Local Zika Virus. PLoS Negl Trop Dis. 2016;10(9):e0004993. [DOI] [PMC free article] [PubMed]
- 17.Guo XX, Li CX, Deng YQ, Xing D, Liu QM, Wu Q, Sun AJ, Dong YD, Cao WC, Qin CF, Zhao TY: Culex pipiens quinquefasciatus: a potential vector to transmit Zika virus. Emerging Microbes & Infections. 2016;5:e102. [DOI] [PMC free article] [PubMed]
- 18.Hall-Mendelin S, Pyke AT, Moore PR, Mackay IM, McMahon JL, Ritchie SA, Taylor CT, Moore FAJ, AF vdH: Assessment of local mosquito species incriminates Aedes aegypti as the potential vector of Zika virus in Australia. PLoS Negl Trop Dis. 2016;10:e0004959. [DOI] [PMC free article] [PubMed]
- 19.Amraoui F, Atyame-Nten C, Vega-Rúa A, Lourenço-de-Oliveira R, Vazeille M, Failloux AB. Culex mosquitoes are experimentally unable to transmit Zika virus. Euro Surveill. 2016;21(35). [DOI] [PMC free article] [PubMed]
- 20.Pyke AT, Daly MT, Cameron JN, Moore PR, Taylor CT, Hewitson GR, Humphreys JL, R. G: Imported Zika virus infection from the Cook Islands into Australia, 2014. PLOS Currents Outbreaks. 2014;6:1–8. [DOI] [PMC free article] [PubMed]
- 21.Gyawali N, Bradbury RS, AW. Taylor-Robinson AW The global spread of Zika virus: is public and media concern justified in regions currently unaffected? Infect Dis Poverty. 2016;5:37. [DOI] [PMC free article] [PubMed]
- 22.Nah K, Mizumoto K, Miyamatsu Y, Yasuda Y, Kinoshita R, Nishiura H. Estimating risks of importation and local transmission of Zika virus infection. PeerJ. 2016;4:e1904. doi: 10.7717/peerj.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peel MC, Finlayson BL, McMahon TA. Updated world map of the Köppen-Geiger climate classification. Hydrol Earth Syst Sci. 2007;11:1633–1644. doi: 10.5194/hess-11-1633-2007. [DOI] [Google Scholar]
- 24.Knope KE, Kurucz N, Doggett SL, Muller M, Johansen CA, Feldman R, Hobby M, Bennett S, Sly A, Lynch S, et al: Arboviral diseases and malaria in Australia, 2012-13: annual report of the National Arbovirus and malaria advisory committee. Commun Dis Intell Q Rep. 2016;40:E17–47. [PubMed]
- 25.Guzzetta G, Poletti P, Montarsi F, Baldacchino F, Capelli G, Rizzoli A, Rosà R, Merler S: Assessing the potential risk of .Zika virus epidemics in temperate areas with established Aedes albopictus populations. Euro Surveill. 2016;21:30199. [DOI] [PubMed]
- 26.Di Luca M, Severini F, Toma L, Boccolini D, Romi R, Remoli ME, Sabbatucci M, Rizzo C, Venturi G, Rezza G, Fortuna C: Experimental studies of susceptibility of Italian Aedes albopictus to Zika virus. Euro Surveill. 2016;21:30223. [DOI] [PubMed]
- 27.Jupille H, Seixas G, Mousson L, Sousa CA, Failloux A-B. Zika virus, a new threat for Europe? PLoS Negl Trop Dis. 2016;10:e0004901. doi: 10.1371/journal.pntd.0004901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedges L, Brownlie J, O'Neill S, Johnson K. Wolbachia and virus protection in insects. Science. 2008;322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- 29.McDonald G, Smith R, Shelden G. Laboratory rearing of Culex annulirostris Skuse (Diptera, Culicidae) J Aust ent Soc. 1977;16:353–358. doi: 10.1111/j.1440-6055.1977.tb00117.x. [DOI] [Google Scholar]
- 30.Paradkar PN, Duchemin JB, Rodriguez-Andres J, Trinidad L, Walker PJ. Cullin4 is pro-viral during West Nile virus infection of Culex mosquitoes. PLoS Pathog. 2015;11:e1005143. doi: 10.1371/journal.ppat.1005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ladner JT, Wiley MR, Prieto K, Yasuda CY, Nagle E, Kasper MR, Reyes D, Vasilakis N, Heang V, Weaver SC, et al: Complete genome sequences of five Zika virus isolates. Genome Announc. 2016;4:e00377–16. [DOI] [PMC free article] [PubMed]
- 32.Lanciotti RS, Lambert AJ, Holodniy M, Saavedra S, Signor LD. Phylogeny of Zika virus in Western hemisphere, 2015. Emerg Infect Dis. 2016;22:933–935. doi: 10.3201/eid2205.160065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson SL, Richards SL, Smartt CT. A simple method for determining arbovirus transmission in mosquitoes. J Am Mosq Control Assoc. 2010;26:108–111. doi: 10.2987/09-5935.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoint. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 35.Mee PT, Weeks AR, Walker PJ, Hoffmann AA, Duchemin JB. Detection of low-level Cardinium and Wolbachia infections in Culicoides. Appl Environ Microbiol. 2015;81:6177–6188. doi: 10.1128/AEM.01239-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell R. A review of the status and significance of the species within the Culex pipiens group in Australia. J Am Mosq Control Assoc. 2012;28:24–27. doi: 10.2987/8756-971X-28.4s.24. [DOI] [PubMed] [Google Scholar]
- 37.Russell RC. Ross River virus: ecology and distribution. Annu Rev Entomol. 2002;47:1–31. doi: 10.1146/annurev.ento.47.091201.145100. [DOI] [PubMed] [Google Scholar]
- 38.Jansen CC, Webb CE, Northill JA, Ritchie SA, Russell RC, Van Den Hurk AF. Vector competence of Australian mosquito species for a north American strain of West Nile virus. Vector-Borne and Zoonotic Diseases. 2008;8:805–811. doi: 10.1089/vbz.2008.0037. [DOI] [PubMed] [Google Scholar]
- 39.Hemmerter S, Šlapeta J, Beebe N. Resolving genetic diversity in Australasian Culex mosquitoes: incongruence between the mitochondrial cytochrome c oxidase I and nuclear acetylcholine esterase 2. Mol Phylogenet Evol. 2009;50:317–325. doi: 10.1016/j.ympev.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 40.Ye YH, Chenoweth SF, Carrasco AM, Allen SL, Frentiu FD, van den Hurk AF, Beebe NW, EA. M: Evolutionary potential of the extrinsic incubation period of dengue virus in Aedes aegypti. Evolution. 2016;70:2459–69. [DOI] [PubMed]
- 41.Endersby NM, White VL, Chan J, Hurst T, Rašic G, Miller A, AA H: Evidence of cryptic genetic lineages within Aedes notoscriptus (Skuse). Infect Genet Evol. 2013;18:191–201. [DOI] [PubMed]
- 42.Skelton E, Rances E, Frentiu FD, Kusmintarsih ES, Iturbe-Ormaetxe I, Caragata EP EP, Woolfit M, O’Neill SL: A native Wolbachia Endosymbiont does not limit dengue virus infection in the mosquito Aedes notoscriptus (Diptera: Culicidae). J Med Entomol. 2016;53:401–8. [DOI] [PMC free article] [PubMed]
- 43.Peterson AT, Campbell LP. Global potential distribution of the mosquito Aedes notoscriptus, a new alien species in the United States. Journal of Vector Ecology. 2015;40:191–194. doi: 10.1111/jvec.12151. [DOI] [PubMed] [Google Scholar]
- 44.van den Hurk AF, McElroy K, Pyke AT, McGee CE, Hall-Mendelin S, Day A, Ryan PA, Ritchie SA, Vanlandingham DL, Higgs S: Vector competence of Australian mosquitoes for yellow fever virus. Am J Trop Med Hyg. 2011;85:446–51. [DOI] [PMC free article] [PubMed]
- 45.Russell R, Mukwaya L, Lule M. Laboratory studies on the transmission of yellow fever virus by Aedes (Finlaya) notoscriptus (Dipt., Culicidae) Aust J Exp Biol Med Sci. 1977;55:649–651. doi: 10.1038/icb.1977.64. [DOI] [PubMed] [Google Scholar]
- 46.Watson TM, Kay BH. Vector competence of Aedes notoscriptus (Diptera: Culicidae) for Barmah Forest virus and of Aedes aegypti (Diptera: Culicidae) for dengue 1-4 viruses in Queensland, Australia. J Med Entomol. 1999;36:508–514. doi: 10.1093/jmedent/36.4.508. [DOI] [PubMed] [Google Scholar]
- 47.Kay BH, Boyd AM, Ryan PA, Hall RA. Mosquito feeding patterns and natural infection of vertebrates with Ross River and Barmah Forest viruses in Brisbane, Australia. Am J Trop Med Hyg. 2007;76:417–423. [PubMed] [Google Scholar]
- 48.Watson T, Saul A, Kay B. Aedes notoscriptus (Diptera: Culicidae) survival and dispersal estimated by mark-release-recapture in Brisbane, Queensland, Australia. J Med Entomol. 2000;37:380–384. doi: 10.1093/jmedent/37.3.380. [DOI] [PubMed] [Google Scholar]
- 49.Dobrotworsky N. The mosquitoes of Victoria. Carlton: Melbourne University Press; 1965. [Google Scholar]
- 50.Johnston E, Weinstein P, Slaney D, Flies AS, Fricker S, Williams C. Mosquito communities with trap height and urban-rural gradient in Adelaide, South Australia: implications for disease vector surveillance. Journal of Vector Ecology. 2014;39:48–55. doi: 10.1111/j.1948-7134.2014.12069.x. [DOI] [PubMed] [Google Scholar]
- 51.Ballard JW, Marshall ID. An investigation of the potential of Aedes camptorhynchus (Thom.) as a vector of Ross River virus. Aust J Exp Biol Med Sci. 1986;64:197–200. doi: 10.1038/icb.1986.21. [DOI] [PubMed] [Google Scholar]
- 52.Dhileepan K, Azuolas JK, Gibson CA. Evidence of vertical transmission of Ross River and Sindbis viruses (Togaviridae: Alphavirus) by mosquitoes (Diptera: Culicidae) in southeastern Australia. J Med Entomol. 1996;33:180–182. doi: 10.1093/jmedent/33.1.180. [DOI] [PubMed] [Google Scholar]
- 53.Gardner LM, Chen N, Sarkar S. Global risk of Zika virus depends critically on vector status of Aedes albopictus. Lancet Infect Dis. 2016;16:522–523. doi: 10.1016/S1473-3099(16)00176-6. [DOI] [PubMed] [Google Scholar]
- 54.Grard G, Caron M, Mombo IM, Nkoghe D, Ondo SM, Jiolle D, Fontenille D, Paupy C, Leroy EM: Zika virus in Gabon (Central Africa) – 2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8:e2681. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


