Abstract
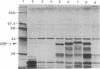
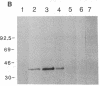
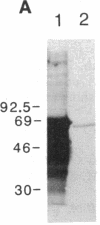
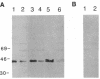
The LINE-1 (L1) family of interspersed DNA sequences found throughout the human genome (L1 Homo sapiens, L1Hs) includes active transposable elements. Current models for the mechanism of transposition involve reverse transcription of an RNA intermediate and utilization of element-encoded proteins. We report that an antiserum against the polypeptide encoded by the L1Hs 5' open reading frame (ORF1) detects, in human cells, an endogenous ORF1 protein as well as the ORF1 product of an appropriate transfecting recombinant vector. The endogenous polypeptide is most abundant in teratocarcinoma and choriocarcinoma cells, among those cell lines tested; it appears to be a single species of approximately 38 kDa. In contrast, RNAs synthesized in vitro from cDNAs representing full-length, polyadenylylated cytoplasmic L1Hs RNA yield, upon in vitro translation, ORF1 products of slightly different sizes. This is consistent with the fact that the various cDNAs are different and represent transcription of different genomic L1Hs elements. In vitro studies additionally suggest that translation of ORF1 is initiated at the first AUG codon. Finally, in no case was an ORF1-ORF2 fusion protein detected.
Full text
PDF


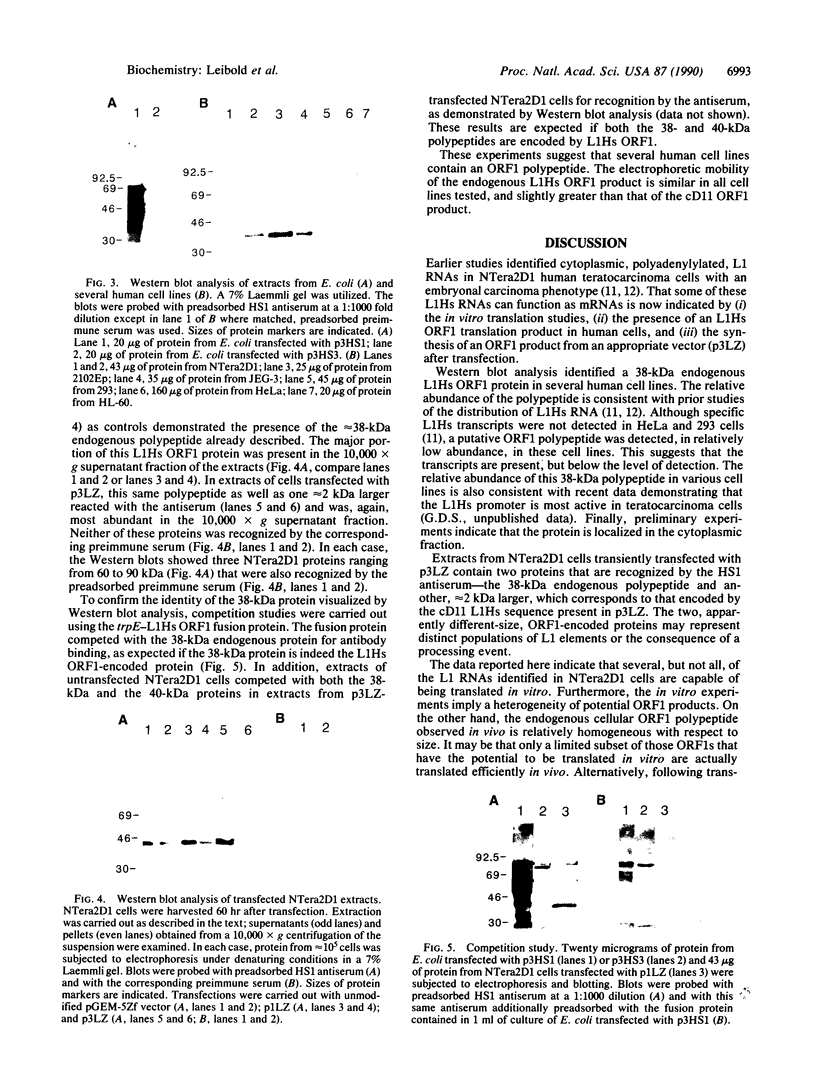

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abad P., Vaury C., Pélisson A., Chaboissier M. C., Busseau I., Bucheton A. A long interspersed repetitive element--the I factor of Drosophila teissieri--is able to transpose in different Drosophila species. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8887–8891. doi: 10.1073/pnas.86.22.8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. W., Bronson D. L., Benham F., Strickland S., Knowles B. B. A comparative study of eight cell lines derived from human testicular teratocarcinoma. Int J Cancer. 1980 Sep 15;26(3):269–280. doi: 10.1002/ijc.2910260304. [DOI] [PubMed] [Google Scholar]
- Andrews P. W., Damjanov I., Simon D., Banting G. S., Carlin C., Dracopoli N. C., Føgh J. Pluripotent embryonal carcinoma clones derived from the human teratocarcinoma cell line Tera-2. Differentiation in vivo and in vitro. Lab Invest. 1984 Feb;50(2):147–162. [PubMed] [Google Scholar]
- Boeke J. D., Corces V. G. Transcription and reverse transcription of retrotransposons. Annu Rev Microbiol. 1989;43:403–434. doi: 10.1146/annurev.mi.43.100189.002155. [DOI] [PubMed] [Google Scholar]
- Chang L. J., Pryciak P., Ganem D., Varmus H. E. Biosynthesis of the reverse transcriptase of hepatitis B viruses involves de novo translational initiation not ribosomal frameshifting. Nature. 1989 Jan 26;337(6205):364–368. doi: 10.1038/337364a0. [DOI] [PubMed] [Google Scholar]
- Darveau A., Pelletier J., Sonenberg N. Differential efficiencies of in vitro translation of mouse c-myc transcripts differing in the 5' untranslated region. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2315–2319. doi: 10.1073/pnas.82.8.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nocera P. P., Sakaki Y. LINEs: a superfamily of retrotransposable ubiquitous DNA elements. Trends Genet. 1990 Feb;6(2):29–30. doi: 10.1016/0168-9525(90)90051-7. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F., Johnson M. S., McClure M. A. Origins and evolutionary relationships of retroviruses. Q Rev Biol. 1989 Mar;64(1):1–30. doi: 10.1086/416128. [DOI] [PubMed] [Google Scholar]
- Gallagher R., Collins S., Trujillo J., McCredie K., Ahearn M., Tsai S., Metzgar R., Aulakh G., Ting R., Ruscetti F. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979 Sep;54(3):713–733. [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Wong C., Youssoufian H., Scott A. F., Phillips D. G., Antonarakis S. E. Haemophilia A resulting from de novo insertion of L1 sequences represents a novel mechanism for mutation in man. Nature. 1988 Mar 10;332(6160):164–166. doi: 10.1038/332164a0. [DOI] [PubMed] [Google Scholar]
- Kinsey J. A., Helber J. Isolation of a transposable element from Neurospora crassa. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1929–1933. doi: 10.1073/pnas.86.6.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Effects of intercistronic length on the efficiency of reinitiation by eucaryotic ribosomes. Mol Cell Biol. 1987 Oct;7(10):3438–3445. doi: 10.1128/mcb.7.10.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Morse B., Rotherg P. G., South V. J., Spandorfer J. M., Astrin S. M. Insertional mutagenesis of the myc locus by a LINE-1 sequence in a human breast carcinoma. Nature. 1988 May 5;333(6168):87–90. doi: 10.1038/333087a0. [DOI] [PubMed] [Google Scholar]
- Peabody D. S., Berg P. Termination-reinitiation occurs in the translation of mammalian cell mRNAs. Mol Cell Biol. 1986 Jul;6(7):2695–2703. doi: 10.1128/mcb.6.7.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard M. A., Dura J. M., Pélisson A., Bucheton A., Finnegan D. J. A cloned I-factor is fully functional in Drosophila melanogaster. Mol Gen Genet. 1988 Nov;214(3):533–540. doi: 10.1007/BF00330491. [DOI] [PubMed] [Google Scholar]
- Schlicht H. J., Radziwill G., Schaller H. Synthesis and encapsidation of duck hepatitis B virus reverse transcriptase do not require formation of core-polymerase fusion proteins. Cell. 1989 Jan 13;56(1):85–92. doi: 10.1016/0092-8674(89)90986-0. [DOI] [PubMed] [Google Scholar]
- Schultze M., Hohn T., Jiricny J. The reverse transcriptase gene of cauliflower mosaic virus is translated separately from the capsid gene. EMBO J. 1990 Apr;9(4):1177–1185. doi: 10.1002/j.1460-2075.1990.tb08225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A. F., Schmeckpeper B. J., Abdelrazik M., Comey C. T., O'Hara B., Rossiter J. P., Cooley T., Heath P., Smith K. D., Margolet L. Origin of the human L1 elements: proposed progenitor genes deduced from a consensus DNA sequence. Genomics. 1987 Oct;1(2):113–125. doi: 10.1016/0888-7543(87)90003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowronski J., Fanning T. G., Singer M. F. Unit-length line-1 transcripts in human teratocarcinoma cells. Mol Cell Biol. 1988 Apr;8(4):1385–1397. doi: 10.1128/mcb.8.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowronski J., Singer M. F. Expression of a cytoplasmic LINE-1 transcript is regulated in a human teratocarcinoma cell line. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6050–6054. doi: 10.1073/pnas.82.18.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanese N., Roth M., Goff S. P. Expression of enzymatically active reverse transcriptase in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4944–4948. doi: 10.1073/pnas.82.15.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Eickbush T. H. Similarity of reverse transcriptase-like sequences of viruses, transposable elements, and mitochondrial introns. Mol Biol Evol. 1988 Nov;5(6):675–690. doi: 10.1093/oxfordjournals.molbev.a040521. [DOI] [PubMed] [Google Scholar]