Abstract
Objective
Parallel measures of OA pathogenesis across species can help researchers evaluate OA models relative to the human condition. Toward this need, our group recently developed a magnetic nanoparticle-based technology, termed magnetic capture, to analyze biomarkers within a rat knee. The objectives of this study were two-fold: 1) directly compare magnetic capture to lavage, and 2) assess CTXII levels in the rat medial meniscus transection (MMT) model of knee OA.
Design
MMT surgery was performed in 30 male Lewis rats (3 months, 250 g). Using either lavage or magnetic capture, CTXII was assessed in the OA-affected and contralateral knee at 1 week (n=6 per group) or 4 weeks (n=8 per group) after surgery.
Results
While lavage detected elevated CTXII concentrations in the OA-affected knee at 1 week (p=0.002), magnetic capture detected elevated CTXII levels in the OA-affected knee at 4 weeks (p=0.016). While magnetic capture did not detect significant elevation of CTXII at week 1, five of the six rats evaluated with magnetic capture had higher CTXII levels in the OA-affected joint relative to the contralateral limb. Moreover, with magnetic capture, CTXII levels increased from 1 week to 4 weeks, corresponding to histological damage. CTXII concentrations evaluated via lavage were relatively constant across timepoints.
Conclusions
Magnetic capture and lavage evaluate CTXII in different ways: Magnetic capture measures total CTXII in the joint, while lavage measures CTXII concentration. Our data indicate that magnetic capture may be advantageous at later time points, where CTXII concentrations can be diluted by OA-associated effusions.
Keywords: Osteoarthritis, biomarker, magnetic capture, lavage, rat MMT model, CTX-II
Introduction
Parallel measures of OA pathogenesis across species can help to identify problems in the OA translational paradigm, such as failures of OA models to replicate the human condition [1]. Promising serum and urine biomarkers can be detected in multiple species [2,3]; however, these markers are not joint specific, are likely dilute, and may be confounded by other pathologies. Since biomarker production begins in the joint, synovial fluid biomarkers have greater potential to describe the local disease environment. While researchers have used lavage to assess synovial fluid biomarkers in rodents [4–6], this procedure often dilutes biomarkers below the level of detection [7]. As such, evaluation of synovial fluid biomarkers in rodents has significant technical challenges.
To address these challenges, our group developed a magnetic nanoparticle-based technology to collect biomarkers from synovial fluid, termed magnetic capture [8,9]. Previously, magnetic capture was demonstrated through in vitro tests and a proof-of-concept study in the rat monoiodoacetate (MIA) model [9]. While this work demonstrates improved sensitivity over published lavage data [10], direct comparison of magnetic capture and lavage has not yet been conducted. Finally, the MIA model involves aggressive joint destruction that does not parallel the human condition [11]. As such, our objectives were two-fold: 1) compare magnetic capture to lavage, and 2) detect elevated intra-articular levels of c-telopeptide of collagen type II (CTXII) in the rat medial meniscus transection (MMT) model of knee OA. CTXII was selected as a representative OA biomarker due to its common use, association to proteolytic cleavage of collagen type II, and correlation to the progression of OA [2,3].
Methods
Animal work was conducted under IACUC-approved protocols at the University of Florida.
Study Design
A flowchart is provided in Supplemental Figure 1. Thirty-two male Lewis rats (3 months, 250 g) were obtained from Charles River Laboratories and acclimated for 2 weeks. MMT surgery was performed in the right knee followed by euthanasia at 1 week (n=16) or 4 weeks (n=16). At both timepoints, CTXII was assessed in the right and left knees immediately after euthanasia using either lavage or magnetic capture. Due to an inability to aspirate lavage fluid (dry tap), only 6 MMT knees at 1 week, 6 contralateral knees at 1 week, and 7 contralateral knees at 4 weeks were analyzed using lavage. For magnetic capture, one animal was humanely euthanized during the MMT surgery and 1 probe was mislabeled, resulting in only 6 MMT and 6 contralateral samples for magnetic capture at 1 week.
MMT Surgery
Under isoflurane anesthesia (3–5% sleepbox induction maintained at 2% via mask inhalation), fur was removed and skin was prepared using povidone-iodine and alcohol in triplicate. A midline skin incision was made along the right knee, followed by transection of the medial collateral ligament. The knee was placed in a valgus alignment, and a complete radial meniscus transection was performed with an 11 blade scalpel. 4–0 polyethylene suture was used to close internal fascia and musculature, and wound clips (9 mm) or 4–0 nylon suture were used to close the skin. Clips or suture were removed 14 days after surgery. Perioperative buprenorphine (0.03 mg/kg) was administered twice per day out to 48 hrs post-operation.
Euthanasia
Animals were humanely euthanized by exsanguination under deep anesthesia. Collected blood was placed in serum-separating containers, centrifuged at 1300 G for 10 minutes, with isolated serum stored 4°C for 24 hrs prior to urea and CTXII assessment.
Lavage
A 29 gauge insulin syringe was directed behind the patella and along the femoral groove. Sterile saline (50 μl) was injected, needle removed, and the knee was fully flexed 10 times. A clean 29 gauge insulin syringe was then placed along the same path, and back pressure was used to collect as much fluid as possible. Lavage samples were analyzed for CTXII and urea, with CTXII concentration calculated as follows [12]:
| Eq. (1) |
Magnetic capture
Magnetic capture was performed as described previously [9]. Briefly, anti-CTXII antibody was obtained from the ImmunoDiagnostic Systems Cartilaps kit (Copenhagen, Denmark). This monoclonal antibody (mAb F4601) is highly specific for the EKGPDP sequence and is characterized in detail [10,15]. At 1 week, biotinylated anti-CTXII was conjugated to the surface of 1 μm polymeric particles containing superparamagnetic iron oxide via recombinant streptavidin covalently coupled to the particle surface (Dynabeads MyOne™ Streptavidin C1, Life Technologies Cat. #65001). At 4 weeks, anti-CTXII antibodies were conjugated to carboxylic groups on the surface of polystyrene particles (780 nm diameter) using 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) cross-linker (Corpuscular, Inc., Cat. #107319-02). The ability of particles to bind and recover CTXII was assessed prior to use, as described in [9]. The change in particles between timepoints was due to commercial availability.
At each timepoint, anti-CTXII particles were injected into the rat knee (70 ng of conjugated antibody in 10 μL saline) and allowed to equilibrate with CTXII for at least 2 hrs. Then, using a magnetic probe, a portion of the particles was collected from the joint, washed, and treated to release CTXII (see [9]). Samples were then analyzed for CTXII using ELISA (kit AC-08F1, ImmunoDiagnostic Systems, Copenhagen, Denmark) and for the amount of collected particles via absorbance (450 nm) or fluorescence (530/590 nm). Total CTXII in the knee was the calculated as follows [9]:
| Eq. (2) |
Detailed characterization of magnetic capture including antibody-particle crosslinking, required equilibration time, CTXII release, and the minimum amount of conjugated antibody needed to deplete CTXII are described in prior work [9].
Urea Quantification
At 1 week, urea was quantified using Urea kit MAK006 from Sigma-Aldrich (MilliporeSigma, St. Louis, MO). At 4 weeks, urea was quantified using the Urea Nitrogen Reagent Set (Pointe Scientific, Inc, Canton, MI, USA). Again, the change in urea measurement was due to commercial availability.
Histology
After lavage and magnetic capture, knees were dissected, fixed in 10% neutral buffered formalin for 48 hrs, decalcified in Cal-Ex for 1–2 weeks, then embedded in paraffin via vacuum infiltration. Knees were sectioned frontally at 10 μm, with at least one section acquired every 100 μm between the anterior and posterior horn of the medial meniscus. Slides were stained with toluidine blue, and the section representing the most severe cartilage loss in each knee was graded by 3 blinded graders using the OARSI histopathology scheme [13].
Statistics
Differences between groups in CTXII were assessed via a 1-way nonparametric Kruskal Wallis ANOVA with a protected Mann-Whitney U post-hoc test. Differences between groups in histological scores were assessed via a 2-way ANOVA with a Tukey’s HSD post-hoc test (Statistica 13)
Results
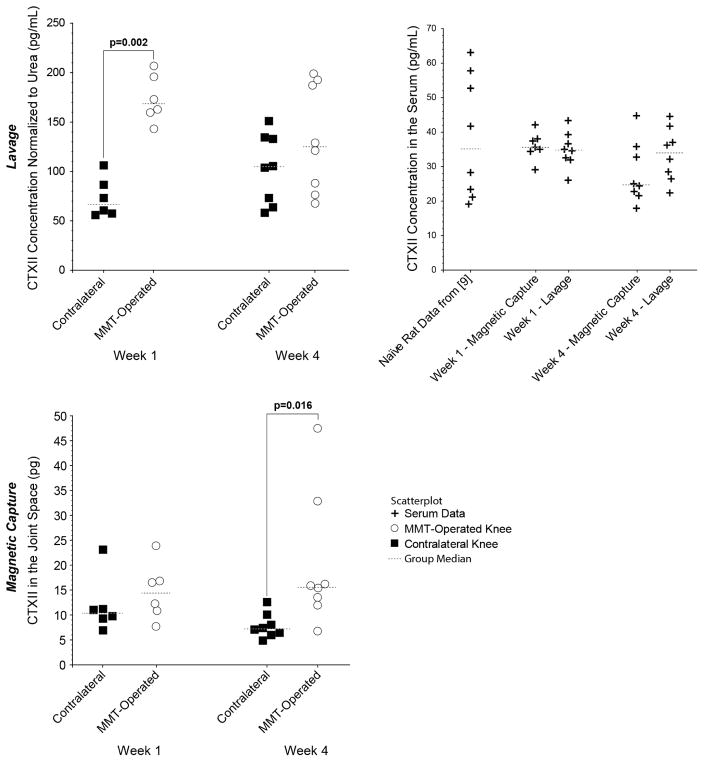
CTXII levels in serum, MMT-operated knee, and contralateral kneee are shown in Figure 1. Note, lavage measures CTXII concentration while magnetic capture quantifies total CTXII in the knee.
Figure 1. CTXII in OA-affected and contralateral joints as measured by magnetic capture and lavage.
In lavage, CTXII concentration was elevated in MMT-operated knees relative to contralateral knees at 1 week (p=0.002), but lavage did not identify significant changes in CTXII at week 4 (p=1.000). In magnetic capture, no significant difference was observed at 1 week (p=1.000), but a significant increase was found in the OA-affected joint relative to the contralateral joint at 4 weeks (p = 0.016). Serum did not show changes in CTXII levels relative to historical values from rats of a similar age from our group [9]. While magnetic capture did not detect significant elevation of CTXII at week 1, five of the six rats assessed via magnetic capture had higher CTXII levels in the OA-affected joint relative to the contralateral limb. Moreover, one point in the contralateral knee in the magnetic capture group in Week 1 was identified as a potential outlier, well outside the 1.5x interquartile range fence. No differences were observed in CTXII levels between 1 week and 4 week in either the lavage or magnetic capture cohorts. Statistical differences between groups in CTXII were assessed via a 1-way nonparametric Kruskal Wallis ANOVA with a protected Mann-Whitney U post-hoc test (a statistical test of the differences between medians). Data are presented as a scatterplot, with a line indicating the median value.
In lavage, CTXII was elevated in MMT-operated knees relative to contralateral knees at 1 week (p=0.002), but no difference was identified at 4 weeks (p=1.000). Average CTXII levels in the OA knee went down from 173.7 ± 24.5 pg/ml on week 1 to 132.6 ± 45.3 pg/ml on week 4 (95% confidence intervals), though this change was not statistically significant (p=0.845).
In magnetic capture, no difference was identified at 1 week (p=1.000), but CTXII was elevated in the OA-affected joint relative to contralateral knees at 4 weeks (p=0.016). While magnetic capture did not detect significant elevation of CTXII at week 1, five of the six rats had higher CTXII levels in the OA-affected joint relative to the contralateral limb. Moreover, one datum in the contralateral knee was marked as a potential outlier outside of the 1.5x interquartile range fence. In magnetic capture, average CTXII levels in the OA knee went up from 14.7 ± 6.0 pg on week 1 to 20.0 ± 11.2 pg on week 4 (95% confidence intervals), though this change was not statistically significant (p=1.000).
In all magnetic capture samples, CTXII levels were measurable by ELISA. In other words, samples lost in the magnetic capture cohort were unrelated to the magnetic capture procedure itself. One animal was lost during surgery (Day 0); thus, magnetic capture was not conducted. Two other samples were lost due to mislabeling a collection probe (human error), and samples collected from MMT-operated and contralateral knees could not be identified (though CTXII was measurable in both). Conversely, dry taps occurred in 5 of 32 knees undergoing the lavage procedure (Supplemental Figure 1).
Histology confirmed OA damage due to the MMT surgery (Supplemental Table 1). As expected, animals assigned to the lavage and magnetic capture had similar OA severity. The exception was osteophyte size at week 4, which was higher in the lavage cohort (p=0.0003). However, lavage failed to show evidence of CTXII elevation at this time point, while magnetic capture did; hence, differences in magnetic capture and lavage results are unlikely to be driven by differences in OA severity.
Discussion
Lavage detected CTXII changes at 1 week, but not 4 weeks. This is surprising given clinical evidence that correlates CTXII with OA progression in humans [2,3]. Our animals may have developed some OA in contralateral joints at 4 weeks, which may explain the near doubling of CTXII in the contralateral limb of lavage animals between 1 and 4 weeks. However, histology did not identify significant cartilage damage in contralateral joints, and despite an identical surgery, CTXII does not follow a similar trend in magnetic capture. Alternatively, effusions may form by 4 weeks, which could lower intra-articular CTXII concentrations due to excess fluid in the joint. Since lavage measures concentration rather than total CTXII in the joint, lavage may be unable to correct for joint swelling. This hypothesis is indirectly supported by a decrease in CTXII concentration from week 1 to week 4 in MMT-operated knees, though this change was not statistically significant.
Unlike lavage, magnetic capture detected elevated CTXII at 4 weeks, but not at 1 week. Looking at individual rats, 8 of 8 animals at 4 weeks and 5 of 6 animals at 1 week had elevated CTXII levels in the MMT-operated knee relative to contralateral knees. Thus, failure to identify elevated CTXII via magnetic capture at 1 week may be due to an outlier, though it is important to note, we could not eliminate this datum through scientific justification. Furthermore, the average CTXII in the MMT-operated knee increased from week 1 to week 4, although this difference in not statistically significant. As such, a larger sample size may be required to detect progressive increase of CTXII biomarker in rodents.
One advantage of magnetic capture is equilibration of capture antibodies with the target biomarker in the joint, essentially moving the incubation step of an ELISA into the intra-articular space. This step allows particles to passively diffuse and bind biomarker prior to recovery. As such, magnetic capture can concentrate CTXII in synovial fluid, serum, or urine samples [9]. Conversely, lavage dilutes the biomarker, and while this dilution does not fatally jeopardize lavage per se, dilution along with potential for urea and biomarker to mix at different rates may add uncontrolled experimental error.
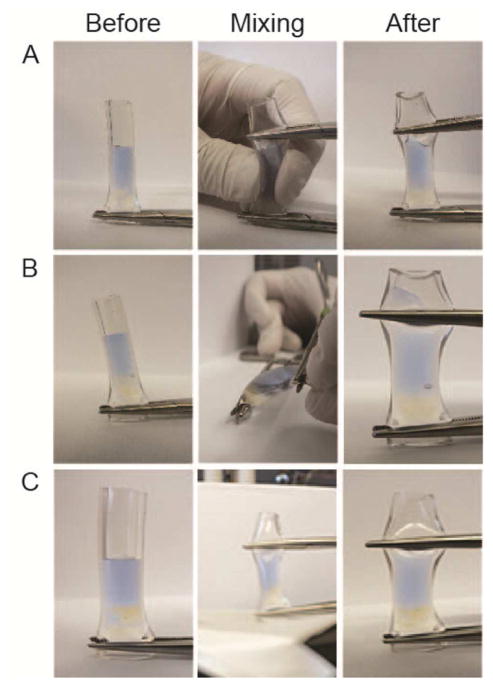
Interestingly, 15 pg of CTXII in a rat knee containing 50 μL of synovial fluid corresponds to a concentration of 300 pg/mL, approximately double the CTXII concentration measured via lavage. This discrepancy may be due to poor mixing of saline and synovial fluid (demonstrated in Figure 2). Alternatively, magnetic particles may penetrate joint tissues, bind excess CTXII, and then pull CTXII into the synovial fluid during magnetic collection, thereby raising CTXII levels measured via magnetic capture.
Figure 2. Mixing of saline and synovial fluid is challenging.
A demonstrative bench top experiment with a silicone tube imitating a joint illustrates the challenges of mixing saline and synovial fluid. Here, saline containing Evan’s blue dye is injected into a tube containing bovine synovial fluid (clear). The tube is closed from both sides with hemostats and is then squeezed or flexed ten times. Panels A and B show that squeezing and flexing could not appropriately mix the saline with synovial fluid. Panel C demonstrates that aggressive mixing using 10 secs of vortexing also fails to mix the two fluids.
To be clear, we are not recommending magnetic capture for all species. In humans and large animals, direct aspiration of synovial fluid and assessment of synovial fluid volume are possible [14], allowing total biomarker in the joint to be estimated. Neither direct aspiration nor measurement of synovial fluid volume are possible in the rat; however, total biomarker in the joint can still be achieved with magnetic capture.
In conclusion, our data demonstrate CTXII assessment in the rat MMT model using magnetic capture. Future work will develop magnetic capture protocols for additional OA biomarkers.
Supplementary Material
A total of thirty-two male Lewis rats underwent an MMT surgery. The first cohort (n=16) was euthanized one week after surgery and the second cohort (n=16) was euthanized four weeks post operation. At both time points, the CTX II levels were assessed in both the ipsilateral and contralateral limbs using either lavage or magnetic capture immediately after euthanasia. Due to an inability to aspirate lavage fluid (dry tap), only 6 MMT knees at 1 week, 6 contralateral knees at 1 week, and 7 contralateral knees at 4 weeks could be analyzed for CTXII in the lavage group. For magnetic capture, one animal was humanely euthanized due to unintended complications from the MMT surgery, and 1 probe was mislabeled (human error), resulting in only 6 MMT and 6 contralateral samples analyzed in the 1 week cohort.
Differences between groups in histological scores were assessed via a 2-way ANOVA with a Tukey’s HSD post-hoc test. Data are presented as mean ± 95% confidence intervals with associated p-values from the Tukey’s HSD post-hoc test.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01AR068424, R21AR064402, and R00AR057426 grants. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study was also supported by a University of Florida Research Opportunity Seed Fund.
Footnotes
Role of the Funding Source
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the University of Florida.
Author Contributions
E. G. Yarmola, Y. Y. Shah, J. Dobson, and K. D. Allen conceived the experiment. H. E. Kloefkorn performed the rodent surgeries and performed the histological analyses. E.G. Yarmola performed magnetic capture with technical assistance from H. E. Kloefkorn and Y. Y. Shah, and analyzed the data. Lavage was performed by K. D. Allen with technical assistance from H. E. Kloefkorn and E. G. Yarmola. All biochemical assays were conducted by E. G. Yarmola or Y. Y. Shah. Statistical analysis was performed by K. D. Allen. All authors participated in drafting the article or in its critical revision for intellectual content, and all authors have approved the final version.
Competing Interests
E. G. Yarmola, J. Dobson, and K. D. Allen have a pending patent application on the magnetic capture technology.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mobasheri A, Henrotin Y. Biomarkers of (osteo)arthritis. Biomarkers. 2015;20:513–8. doi: 10.3109/1354750X.2016.1140930. http://dx.doi.org/10.3109/1354750X.2016.1140930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kraus VB, Blanco FJ, Englund M, Henrotin Y, Lohmander LS, Losina E, et al. OARSI Clinical Trials Recommendations: Soluble biomarker assessments in clinical trials in osteoarthritis. Osteoarthritis Cartilage. 2015;23:686–97. doi: 10.1016/j.joca.2015.03.002. http://dx.doi.org/10.1016/j.joca.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lotz M, Martel-Pelletier J, Christiansen C, Brandi ML, Bruyere O, Chapurlat R, et al. Value of biomarkers in osteoarthritis: current status and perspectives. Ann Rheum Dis. 2013;72:1756–63. doi: 10.1136/annrheumdis-2013-203726. http://dx.doi.org/10.1136/annrheumdis-2013-203726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barton NJ, Stevens DA, Hughes JP, Rossi AG, Chessell IP, Reeve AJ, et al. Demonstration of a novel technique to quantitatively assess inflammatory mediators and cells in rat knee joints. J Inflamm (Lond) 2007;4:13. doi: 10.1186/1476-9255-4-13. http://dx.doi.org/10.1186/1476-9255-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel L, Sun W, Glasson SS, Morris EA, Flannery CR, Chockalingam PS. Tenascin-C induces inflammatory mediators and matrix degradation in osteoarthritic cartilage. BMC Musculoskelet Disord. 2011;12:164. doi: 10.1186/1471-2474-12-164. http://dx.doi.org/10.1186/1471-2474-12-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swearingen CA, Chambers MG, Lin C, Marimuthu J, Rito CJ, Carter QL, et al. A short-term pharmacodynamic model for monitoring aggrecanase activity: injection of monosodium iodoacetate (MIA) in rats and assessment of aggrecan neoepitope release in synovial fluid using novel ELISAs. Osteoarthritis Cartilage. 2010;18:1159–66. doi: 10.1016/j.joca.2010.02.019. http://dx.doi.org/10.1016/j.joca.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Allen KD, Mata BA, Gabr MA, Huebner JL, Adams SB, Jr, Kraus VB, et al. Kinematic and dynamic gait compensations resulting from knee instability in a rat model of osteoarthritis. Arthritis Res Ther. 2012;14:R78. doi: 10.1186/ar3801. http://dx.doi.org/10.1186/ar3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garraud A, Velez C, Shah Y, Garraud N, Kozissnik B, Yarmola EG, et al. Investigation of the Capture of Magnetic Particles From High-Viscosity Fluids Using Permanent Magnets. IEEE Trans Biomed Eng. 2016;63:372–378. doi: 10.1109/TBME.2015.2458783. http://dx.doi.org/10.1109/TBME.2015.2458783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yarmola EG, Shah Y, Arnold DP, Dobson J, Allen KD. Magnetic Capture of a Molecular Biomarker from Synovial Fluid in a Rat Model of Knee Osteoarthritis. Ann Biomed Eng. 2016;44:1159–1169. doi: 10.1007/s10439-015-1371-y. http://dx.doi.org/10.1007/s10439-015-1371-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oestergaard S, Chouinard L, Doyle N, Karsdal MA, Smith SY, Qvist P, et al. The utility of measuring C-terminal telopeptides of collagen type II (CTXII) in serum and synovial fluid samples for estimation of articular cartilage status in experimental models of destructive joint diseases. Osteoarthritis Cartilage. 2006;14:670–679. doi: 10.1016/j.joca.2006.01.004. http://dx.doi.org/10.1016/j.joca.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Barve RA, Minnerly JC, Weiss DJ, Meyer DM, Aguiar DJ, Sullivan PM, et al. Transcriptional profiling and pathway analysis of monosodium iodoacetate-induced experimental osteoarthritis in rats: relevance to human disease. Osteoarthritis Cartilage. 2007;15:1190–1198. doi: 10.1016/j.joca.2007.03.014. http://dx.doi.org/10.1016/j.joca.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Kraus VB, Huebner JL, Fink C, King JB, Brown S, Vail TP, et al. Urea as a passive transport marker for arthritis biomarker studies. Arthritis Rheum. 2002;46:420–7. doi: 10.1002/art.10124. http://dx.doi.org/10.1002/art.10124. [DOI] [PubMed] [Google Scholar]
- 13.Gerwin N, Bendele AM, Glasson S, Carlson CS. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis and Cartilage. 2010;18(Suppl 3):S24–34. doi: 10.1016/j.joca.2010.05.030. http://dx.doi.org/10.1016/j.joca.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 14.Ostergaard M, Stoltenberg M, Henriksen O, Lorenzen I. The accuracy of MRI-determined synovial membrane and joint effusion volumes in arthritis. A comparison of pre- and post-aspiration volumes. Scand J Rheumatol. 1995;24:305–11. doi: 10.3109/03009749509095168. [DOI] [PubMed] [Google Scholar]
- 15.Christgau S, Garnero P, Fledelius C, Moniz C, Ensig M, Gineyts E, et al. Collagen type II C-telopeptide fragments as an index of cartilage degradation. Bone. 2001;29:209–15. doi: 10.1016/s8756-3282(01)00504-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A total of thirty-two male Lewis rats underwent an MMT surgery. The first cohort (n=16) was euthanized one week after surgery and the second cohort (n=16) was euthanized four weeks post operation. At both time points, the CTX II levels were assessed in both the ipsilateral and contralateral limbs using either lavage or magnetic capture immediately after euthanasia. Due to an inability to aspirate lavage fluid (dry tap), only 6 MMT knees at 1 week, 6 contralateral knees at 1 week, and 7 contralateral knees at 4 weeks could be analyzed for CTXII in the lavage group. For magnetic capture, one animal was humanely euthanized due to unintended complications from the MMT surgery, and 1 probe was mislabeled (human error), resulting in only 6 MMT and 6 contralateral samples analyzed in the 1 week cohort.
Differences between groups in histological scores were assessed via a 2-way ANOVA with a Tukey’s HSD post-hoc test. Data are presented as mean ± 95% confidence intervals with associated p-values from the Tukey’s HSD post-hoc test.