Abstract
Objectives
Associations between parental occupational pesticide exposure and childhood acute lymphoblastic leukemia (ALL) vary across studies, likely due to different exposure assessment methodologies.
Methods
We assessed parental occupational pesticide exposure from the year before pregnancy to the child’s third year of life for 669 children diagnosed with ALL and 1,021controls. We conducted expert rating using task-based job modules (JM) to estimate exposure to pesticides among farmer workers, gardeners, agricultural packers, and pesticide applicators. We compared this method to (1) partial JM using job titles and a brief description, but without completing the task-based questionnaire, and (2) job exposure matrix (JEM) linking job titles to the International Standard Classifications of Occupation Codes. We used unconditional logistic regression to calculate odds ratios (OR) and 95% confidence intervals (95% CI) for ALL cancer risk and pesticide exposure adjusting for child’s sex, age, race/ethnicity and household income.
Results
Compared to complete JMs, partial JMs and JEM led to 3.1 % and 9.4 % of parents with pesticide exposure misclassified, respectively. Misclassification was similar in cases and controls. Using complete JMs, we observed an increased risk of ALL for paternal occupational exposure to any pesticides (OR=1.7; 95% CI = 1.2, 2.5), with higher risks reported for pesticides to treat nut crops (OR=4.5; 95% CI = 0.9, 23.0), and for children diagnosed before five years of age (OR=2.3; 95% CI: 1.3, 4.1). Exposure misclassification from JEM attenuated these associations by about 57%. Maternal occupational pesticide exposure before and after birth was not associated with ALL.
Conclusions
The risk of ALL was elevated in young children with paternal occupational pesticide exposure during the perinatal period, using more detailed occupational information for exposure classification.
Keywords: childhood leukemia, job-specific modules, occupational exposure, pesticides
1. INTRODUCTION
The evidence for an association between parental occupational pesticide exposure and childhood leukemia has been limited by weaknesses of previous research methods, especially exposure assessment (Jurewicz and Hanke 2006). Two large studies did not observe an association between parental occupational pesticide exposure and childhood leukemia, however exposure in these studies was based on job title alone and exposure prevalence was very low (McKinney et al. 2003; Rudant et al. 2007). In contrast, two large studies that assessed exposure using in-depth parental interviews similar to job modules are consistent with an increased risk of childhood leukemia associated with maternal and paternal occupational pesticide exposure, perhaps due to reduced exposure misclassification (Meinert et al. 2000; Monge et al. 2007). Specifically, a case-control study in Germany that used telephone interviews to obtain detailed information on pesticide exposure observed elevated risk of childhood leukemia associated with both paternal and maternal occupational pesticide exposure during pregnancy with odds ratios (OR) and 95% confidence intervals (CI) of 1.6 (1.1–2.3) and 3.6 (1.5–8.8) respectively (Meinert et al. 2000). A study in Costa Rica conducted using in-depth interviews to collect information on job tasks, a calendar with life events and a pesticide checklist observed an OR=1.5 (1.0–2.3) for fathers occupational exposure to any pesticides during the second trimester and OR=2.4 (1.0–5.9) for mothers exposure during pregnancy (Monge et al. 2007). The findings from meta-analyses of childhood leukemia and parental occupational pesticide exposure support the need to rely more on studies that clearly stipulate exposure to pesticides rather than those that assume pesticide exposure because of farm/agriculture employment (Van Maele-Fabry et al. 2010; Wigle et al. 2009; Bailey et al. 2014).
Job modules have been developed to improve the specificity of occupational exposure assessment by utilizing closed ended branching questions that are task based (Gerin and Siemiatycki 1991) and a calendar with important life events to assess exposure during critical time periods of development (Monge et al. 2004; Zahm et al. 2001). A comparison of job title as a surrogate for job modules to assess occupational lead exposure found good specificity (~0.9), but only moderate sensitivity (~0.5) (Bhatti et al. 2010). In studies where detailed job module type information is not available, bias analysis can be used to characterize the magnitude of misclassification error from using an exposure surrogate such as job title and quantitative models can be developed to correct point and interval estimates of health effects (Spiegelman 2010).
The California Childhood Leukemia Study (CCLS) is a large, population-based case control study. We developed task-based, job-specific modules in the CCLS to assess occupational exposures including pesticides (Reinier et al. 2004). Utilizing the relatively large number of cases and controls in the CCLS, our goals in this analysis are to evaluate misclassification of exposure from using job titles alone compared to job modules and to estimate risks of childhood ALL by age, ethnicity and leukemia subtype.
2. METHODS
2.1 Study population
We used data from the CCLS, a case-control study conducted in 35 California counties from 1995–2008. As described previously (Bartley et al. 2010; Metayer et al. 2013), participants from the study area were eligible if they were less than 15 years of age at the time of diagnosis (or referent date for the controls) and had a parent that spoke English or Spanish. Among eligible cases, 86% consented and participated in the study. One or two controls were randomly selected from California birth certificate files and individually matched to cases by child’s age, sex, race, and Latino ethnicity, and maternal race. About 87% of eligible controls were enrolled in the study and a review of the control selection methods indicates that socio-demographic and birth characteristics of participating and non-participating controls are similar in the CCLS (Ma et al. 2004). The study was approved by the University of California Committee for the Protection of Human Subjects, the California Health and Human Services Agency Committee for the Protection of Human Subjects, and the institutional review boards of all participating hospitals. Written informed consent was obtained from the parents of all participants.
The main CCLS in-person interview includes extensive time-specific exposure information collected from the parents (97% mothers), such as home use of pesticides, parental smoking, job titles for parental occupational histories, and mother and child’s residential histories. For each question, parents were asked about use/exposure at critical windows of exposure related to childhood leukemia: the year before birth and from birth to age three years (Daniels et al. 1997; Colt et al. 1998; Zahm et al. 1998; Anderson et al. 2000; Wigle et al. 2009; Van Maele-Fabry et al. 2010). During this initial interview, a complete occupational history (i.e., job title and duties, company name and type, dates of employment) was collected separately for each parent for any full and part time jobs reported for more than one month (paid or volunteered) from one year before the child’s birth until the child’s third birthday or diagnosis date (or reference date in control children), whichever came first. We also collected information on the parent’s current job or occupation and industry and their usual job and industry since 18 years of age. In the main interview, specific questions were asked regarding whether or not the parent worked regularly with pesticides, insecticides, fungicides, or herbicides along with whether the parent worked in common agricultural occupations (e.g. farm or ranch worker, gardener, groundskeeper, landscaper, garden nursery worker, etc.).
2.2 Pesticide exposure assessment using completed task-based job modules (complete JM)
Nineteen job modules were developed for the CCLS to obtain the detailed occupational information necessary to provide semi-quantitative estimates of parental occupational exposure including the timing (i.e., the year before pregnancy to the child’s third year of life or to diagnosis/reference date, whichever came first), frequency, duration and intensity of exposure (Reinier et al. 2004; Metayer et al. 2016). Four of the nineteen job modules were developed for occupations with potential pesticide exposure in our study population: farm or ranch worker; gardener, landscaper, nursery worker or groundskeeper; agricultural packer; and pesticide applicator. All interviews and study material were available in English and Spanish.
Based on the complete occupational history gathered in the main interview as described above, parents were assigned one of the pesticide-related job-modules, and pesticide exposure was determined from the detailed task-based questions (Table 1). If no pesticide related job modules were deemed necessary based on job history, the parents were considered unexposed. Assignment of all job-modules was independently reviewed for quality control. Job-module interviews were administered in person with the mothers and possibly the fathers. To increase fathers’ participation, phone interviews were proposed when in-person interviews were not feasible. No surrogate interviews were conducted (see detailed methods in Metayer et al. 2016). Of the 1690 participants included in this study from 2000 to 2008, 277 were assigned and completed pesticide related job modules based on their detailed job history. Job modules were coded blinded to the participants’ case-control status. For each time period, we asked participants about the crops that they worked with and specific tasks they performed. If the participant applied pesticides, we asked about the target pests and pesticide products or active ingredients that they applied and whether they mixed or loaded pesticides.
Table 1.
Information used to assess parental occupational pesticide exposure using job exposure matrix (JEM), and partial or complete job modules (JM).
| Data Collected | JEM | Partial JM | Complete JM |
|---|---|---|---|
| Job-title | X | X | X |
| What did the company make or do? | X | X | |
| What were your work duties? | X | X | |
| Description of tasks | X | ||
| Expert rating | X |
Abbreviations: JEM = job exposure matrix, high and moderate probability of exposures; JM = job modules.
2.3 Pesticide exposure assessment using assigned–but not completed– JM (partial JM)
As an alternate occupational exposure classification method, parents assigned pesticide-related JM based on full occupational history as described above (n=277), were considered occupationally exposed to pesticides during that time period, regardless of the information provided in the task-based JM (Table 1).
2.4 Pesticide exposure assessment using job exposure matrix (JEM) derived from job-titles only
We used pesticide exposure information from a previously created job exposure matrix; details are available elsewhere (Bailey et al. 2014). In brief, job titles (Table 1) were assigned to International Standard Classifications of Occupation Codes and assigned a category of exposure probability based on the proportion of jobs assessed by occupational exposure experts as having pesticide exposure from previous studies in Australia and Canada. We considered parents occupationally exposed to pesticides if there was a moderate or high probability of exposure.
2.5 Data analysis
We calculated exposure prevalence (number exposed/total population), misclassification (number incorrectly categorized for exposure/total population), specificity (number categorized as unexposed/number actually exposed) and sensitivity (number categorized as exposed/number actually exposed) using complete JM as the “gold standard”. We included mothers (669 cases and 1,021 controls) and fathers (615 cases and 951 controls) that completed a pesticide related job module if one was assigned (n=277). To evaluate the relationships between parental occupational pesticide exposure and ALL risk, we used unconditional logistic regression to calculate odds ratios (OR) and 95% confidence intervals (CI). Models included original matching variables (sex, age, ethnicity and mother’s race) and household income as covariates.
We used unconditional logistic regression to retain as many participants as possible in the analyses. We also ran models with paternal prenatal smoking and paternal occupational exposure to chlorinated solvents included as potential confounders based on previous analyses in this study (Metayer et al. 2013; Metayer et al. 2016). We evaluated the relationship for paternal and maternal occupational pesticide exposure during the year before child’s birth and from birth to the child’s third birthday. We estimated the effects of these exposures as categorical binary variables (exposed/unexposed) using job titles alone and using job-specific modules. We calculated the point estimates and confidence intervals stratified by the child’s age at diagnosis (< 5 or ≥ 5 years of age), child’s sex and separately for Latino and non-Latino children. We also calculated risk estimates for the most common subtypes of ALL. We ran models using conditional logistic regression for matched cases and controls adjusted for household income to compare the results to adjusted unconditional logistic regression models. We calculated the population attributable risks for childhood ALL related to parental occupational pesticide exposure using the prevalence of occupational pesticide exposure in our population to estimate the reduction in incidence of childhood ALL that would be expected if the whole population were unexposed.
3. RESULTS
3.1 Demographics of study population
Basic demographic characteristics of the ALL cases (n=669) and controls (n=1,021) included in the occupational pesticide exposure analyses are presented in Table 2. The majority of cases were less than five years of age at diagnosis (57%) and male (56%). Among the cases, 50% of the children were Latino and maternal race was 82% white, 4% black and 14% Asian or other. Annual household income was below $30,000 for 34% of cases and above $60,000 for 35% of cases. Controls were similar to cases for the matching variables child’s age and sex, but were less likely (p<0.05) to be Latino (46%). Controls also had higher annual household income (p<0.001) than cases with only 23% below $30,000 and 51% above $60,000.
Table 2.
Characteristics of childhood acute lymphocytic leukemia cases and controls included in the parental occupational pesticide analyses.
| Characteristic | Cases (n=669)
|
Controls (n=1021)
|
||
|---|---|---|---|---|
| N | % | N | % | |
| Age, yearsa | ||||
| 0–4 | 379 | 57 | 581 | 57 |
| 5–9 | 191 | 29 | 290 | 28 |
| 10–14 | 99 | 14 | 150 | 15 |
| Sexa | ||||
| Male | 375 | 56 | 585 | 57 |
| Female | 294 | 44 | 436 | 43 |
| Latinoa* | ||||
| Yes | 334 | 50 | 468 | 46 |
| No | 329 | 50 | 553 | 54 |
| Maternal racea | ||||
| White | 547 | 82 | 872 | 85 |
| Black | 27 | 4 | 32 | 3 |
| Asian or other | 92 | 14 | 117 | 12 |
| Annual household income, $** | ||||
| <30,000 | 226 | 34 | 236 | 23 |
| 30,000–59,999 | 208 | 31 | 268 | 26 |
| ≥60,000 | 235 | 35 | 517 | 51 |
Matching criteria.
Chi-square test for difference between cases and controls p < 0.05.
Chi-square test for difference between cases and controls p < 0.001.
3.2 Pesticide exposure classification
Parental occupational pesticide exposures during the year prior to the child’s birth and from birth to the child’s third birthday were highly concordant (~99%) for both mothers and fathers, therefore we present results combining exposure during either of these time periods to represent perinatal occupational pesticide exposure. The prevalence of perinatal paternal pesticide exposure was higher using the JEM (15.4%) compared to partial JM (11.2%) or complete JM (8.2%) (Table 3). Using complete JM, only 2.9% of mothers had perinatal occupational pesticide exposure. Compared to completed JM, misclassification of paternal perinatal pesticide exposure was 9.4% for the JEM and 3.1% for partial JM. Misclassification of exposure was non-differential with respect to case/control status. For paternal pesticide exposure, partial JM had better specificity (97%) than the JEM (90%). The sensitivity of the JEM was 87% for paternal and 63% for maternal pesticide exposure.
Table 3.
Comparison of parental perinatal occupational pesticide exposure assessment methods for cases and controls from the year before child’s birth through child’s 3rd year of life
| Exposure Method | Unexposed (%) | Exposed (%) | Misclassified (%) | Specificity (%) | Sensitivity (%) |
|---|---|---|---|---|---|
| Paternal (n=1566) | |||||
| Complete JM | 1438 (91.8%) | 128 (8.2%) | Reference | 100.0% | 100.0% |
| Partial JM | 1390 (88.8%) | 176 (11.2%) | 3.1% | 96.7% | 100.0% |
| JEM | 1325 (84.6%) | 241 (15.4%) | 9.4% | 90.1% | 86.7% |
|
| |||||
| Maternal (n=1690) | |||||
| Complete JM | 1641 (97.1%) | 49 (2.9%) | Reference | 100.0% | 100.0% |
| Partial JM | 1633 (96.6%) | 57 (3.4%) | 3.5% | 96.4% | 100.0% |
| JEM | 1582 (93.6%) | 108 (6.4%) | 2.6% | 98.4% | 63.2% |
Abbreviations: JEM = job exposure matrix, high and moderate probability of exposures; JM = job modules.
3.3 Risk of childhood ALL
The ORs and 95% CI for perinatal maternal occupational pesticide exposure using a JEM and JM are provided in Table 4. The risk of ALL was not associated with maternal occupational pesticide exposure. The OR was 1.3 (95% CI: 0.8, 2.4) using complete JMs for maternal pesticide exposure. Results were attenuated and similar using either a JEM with moderate or high probability of pesticide exposure or partial JMs with ORs = 0.9 for perinatal maternal occupational pesticide exposure. Few mothers applied pesticides (n=3) and we did not estimate the risk separately for mothers by individual job module or crop due to the low prevalence of maternal occupational pesticide exposure.
Table 4.
Maternal perinatal occupational pesticide exposure and acute lymphocytic leukemia: adjusteda odds ratios (OR) and 95% confidence intervals for three exposure assessment methods.
| Pesticides | Cases (n=669) | Controls (n=1,021) | OR (95% CI) |
|---|---|---|---|
| JEM | |||
| Unexposed | 622 (93.0%) | 960 (94.0%) | Reference |
| Exposed | 47 (7.0%) | 61 (6.0%) | 0.9 (0.6, 1.4) |
| Partial JM | |||
| Unexposed | 645 (96.4%) | 986 (96.8%) | Reference |
| Exposed | 24 (3.6%) | 33 (3.2%) | 0.9 (0.5, 1.5) |
| Complete JM | |||
| Unexposed | 644 (96.3%) | 997 (97.7%) | Reference |
| Exposed | 25 (3.7%) | 24 (2.3%) | 1.3 (0.8, 2.4) |
Abbreviations: JEM = job exposure matrix, high and moderate probability of exposure; JM = job modules.
Models adjusted for child’s sex, age, ethnicity, mother’s race and household income.
Table 5 presents risk estimates of ALL for perinatal paternal occupational pesticide exposure. The risk of ALL was significantly elevated among children whose fathers were exposed during the perinatal period based on complete JMs (OR=1.7; 95% CI: 1.2, 2.5). The relationship with perinatal paternal pesticide exposure was strongest in children diagnosed at four years of age or earlier (OR=2.3; 95% CI: 1.3, 4.1). Paternal pesticide application was fairly rare (3%) among our study population based on complete JM and the OR was similar for applying pesticides and for overall pesticide exposure (OR=1.6; 95%CI: 0.9, 2.9). The associations were similar among those that completed the farm/ranch worker JM (OR=1.7; 95% CI: 1.1, 2.8) and those that completed gardener/landscaper/nursery worker JM (OR=1.5; 95% CI: 0.7, 3.3). Risk estimates were attenuated, but still elevated, for paternal occupational pesticide based on partial JM with OR=1.5 (95% CI: 1.0, 2.0) and the JEM OR=1.3 (95% CI: 0.9, 1.7).
Table 5.
Paternal perinatal occupational pesticide exposure and acute lymphocytic leukemia: adjusteda odds ratios (OR) and 95% confidence intervals for three exposure assessment methods.
| Pesticides | Cases (n=615) | Controls (n=951) | OR (95% CI) |
|---|---|---|---|
| JEM | |||
| Unexposed | 498 (81.0%) | 827 (87.0%) | Reference |
| Exposed | 117 (19.0%) | 124 (13.0%) | 1.3 (0.9, 1.7) |
| Partial JM | |||
| Unexposed | 524 (85.2%) | 866 (91.1%) | Reference |
| Exposed | 91 (14.8%) | 85 (8.9%) | 1.5 (1.0, 2.0) |
| Complete JM | |||
| Unexposed | 544 (88.5%) | 894 (94.0%) | Reference |
| Exposed | 71 (11.5%) | 57 (6.0%) | 1.7 (1.2, 2.5) |
| Child diagnosed < 5 years of age | |||
| Unexposed | 318 (89.3%) | 525 (95.8%) | Reference |
| Exposed | 38 (10.7%) | 23 (4.2%) | 2.3 (1.3, 4.1) |
| Child diagnosed ≥ 5 years of age | |||
| Unexposed | 226 (87.3%) | 365 (91.5%) | Reference |
| Exposed | 33 (12.7%) | 34 (8.5%) | 1.3 (0.8, 2.3) |
| Applied pesticidesb | |||
| Unexposed | 590 (95.9%) | 929 (97.7%) | Reference |
| Exposed | 25 (4.1%) | 22 (2.3%) | 1.6 (0.9, 2.9) |
| Farm/ranch workerb | |||
| Unexposed | 522 (92.1%) | 850 (96.1%) | Reference |
| Exposed | 45 (7.9%) | 35 (3.9%) | 1.7 (1.1, 2.8) |
| Gardener, nursery workerb | |||
| Unexposed | 423 (97.6%) | 856 (98.6%) | Reference |
| Exposed | 13 (2.4%) | 12 (1.4%) | 1.5 (0.7, 3.3) |
Abbreviations: JEM = job exposure matrix, high and moderate probability of exposure; JM = job modules.
Models adjusted for child’s sex, age, ethnicity, mother’s race and household income.
Not mutually exclusive categories.
Figure 1 shows the OR and 95% CI for fathers that completed the farm/ranch worker JM based on the type of agriculture. There were few fathers for each type of agriculture resulting in imprecise risk estimates. The OR were highest for fathers exposed to pesticides while working with nut crops (OR=4.5; 95% CI: 0.9, 23.0) and animals (OR=2.5; 95% CI: 0.9, 6.7). Risk estimates were similar to the overall estimates (OR=1.4–1.7) for fathers exposed to pesticides working with fruits, vegetables and grains.
Figure 1.
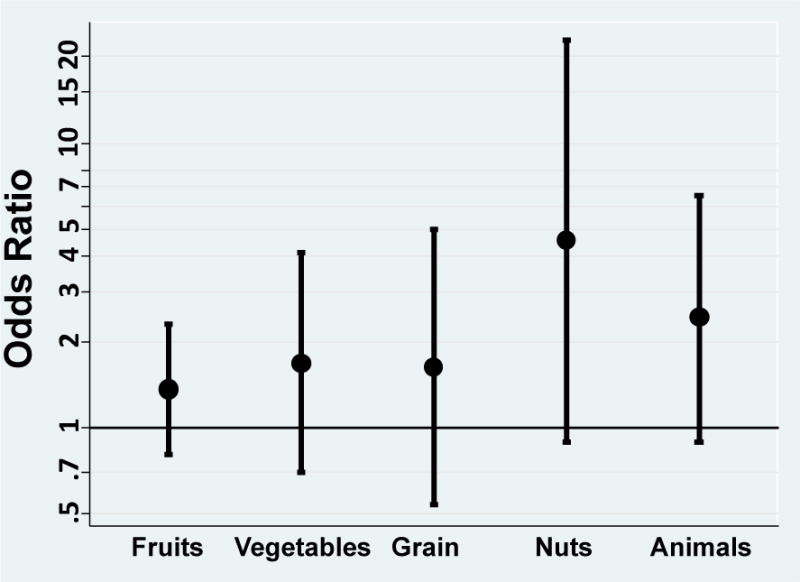
Adjusteda odds ratios (OR) and 95% confidence intervals for acute lymphocytic leukemia and perinatal paternal occupational pesticide exposure by type of agriculture.
a Models adjusted for child’s sex, age, ethnicity, mother’s race and household income.
Risk estimates for perinatal paternal pesticide exposure did not differ significantly by ALL subtype or cytogenetic class (Supplemental Figure 1). The risk estimate for paternal pesticide exposure among Latinos (OR=1.7; 95% CI: 1.1, 2.6) was nearly identical to the overall risk estimate (data not shown). Although point estimates were similar, the confidence intervals were very wide for risk estimates among non-Latinos because 87% of exposed fathers were Latino. Risk estimates for perinatal paternal pesticide exposure were similar for boys (OR=1.6; 95% CI: 1.0, 2.7) and girls (OR= 1.9; 95% CI: 1.1, 3.4) and when estimated using conditional logistic regression adjusting for annual household income (OR=1.6; 95% CI: 1.1, 2.4). Including previous exposures related to ALL in the risk models such as paternal smoking and occupational exposure to chlorinated solvents did not change the results. The overall population attributable risk estimate was 4% based on an exposure prevalence of 6% for paternal perinatal pesticide exposure among controls in our population. However, due to the much higher exposure prevalence among Latino fathers (10.9% for controls) the population attributable risk was 7.1% for Latino children.
4. DISCUSSION
We found that perinatal paternal occupational exposure to pesticides was associated with an approximately 70% increased risk of childhood ALL, based on expert occupational exposure rating and a large number of cases. We demonstrated that the use of a JEM with moderate and high probability of exposure instead of complete JM resulted in exposure misclassification, with the JEM over estimating exposure, and odds ratios were attenuated 57% for perinatal paternal pesticide exposure from 1.7 to 1.3. We did not observe a relationship between maternal perinatal occupational pesticide exposure and childhood ALL in our population, but there was a low prevalence of maternal exposure making our study underpowered.
Our findings are consistent with the largest pooled analyses of published and unpublished data from 12 childhood ALL studies participating in the Childhood Leukemia International Consortium (CLIC). This pooled analysis harmonized original occupational data and used a JEM to assess parental occupational pesticide exposure. No relationship was reported with maternal pesticide exposure (OR=1.0, 95% CI: 0.8, 1.3), but an increased risk (OR=1.2, 95% CI: 1.1, 1.4) was seen with paternal pesticide exposure (Bailey et al. 2014). Results from this pooled analysis were similar when combined with non-CLIC published studies. This is in contrast to smaller meta-analyses of published data on parental occupational pesticide exposure and childhood ALL reporting associations with maternal, but not paternal, exposure (Wigle et al. 2009; Van Maele-Fabry et al. 2010). These studies, however, suffered from heterogeneity in exposure assessment from published data and inability to conduct subgroup analyses specifically for childhood ALL.
A study conducted in Australia using job modules somewhat similar to ours did not observe a relationship with either paternal or maternal perinatal pesticide exposure (Glass et al. 2012). Null findings for maternal pesticide exposure are consistent, although based on very low prevalence of exposure. However, inconsistent findings for paternal exposure may be due to differential work habits in the two study populations in terms of type of pesticides and crops, and use of protective equipment. Details on the Australian study were not provided (Glass et al. 2012). Workers in our study were mostly Latinos. The most common tasks were harvesting and pruning and the most common crops were grapes and tomatoes. We observed large, but imprecise, odds ratios for paternal pesticide exposure when working with nut trees, a practice that releases large amounts of dust when harvesting (Faulkner et al. 2009), and animals indicating that future studies should evaluate specific pesticides and occupational exposures related to these production operations. A study in Costa Rica where up to 25% of case fathers and 4 % case mothers were exposed to pesticides at work (based on detailed task-based assessment) showed increased risks of childhood ALL associated with pesticide exposure in both parents (Monge et al. 2007).
We observed a stronger relationship between perinatal paternal pesticide exposure and ALL in children diagnosed at four years of age and younger, unlike the recent CLIC pooled analysis that found a stronger relationship with paternal pesticide exposure and ALL in children diagnosed at five years of age or older. Reasons for difference in risk estimates by age are unclear.
Job modules provide detailed exposure information including specific crops, tasks and pesticides. Compared to complete JM, JEM with moderate or high probability of exposure resulted in almost 10% of fathers being misclassified for occupational pesticide exposure and more than 50% attenuation of the odds ratio for childhood ALL. However, the cost and time required to administer JM is much higher than collecting job titles and using a JEM. In studies where detailed JM information is not available, bias analysis can be used to characterize the magnitude of misclassification error from using an exposure surrogate such as job title and quantitative models can be developed to correct point and interval estimates of health effects (Spiegelman 2010).
There were some limitations to our study. Due to the high concordance between prenatal and postnatal parental occupational pesticide exposure in our study population we were not able to assess these critical time windows of exposure separately. Our study, like previous studies of ALL, did not have sufficient power to investigate associations with individual pesticide active ingredients. Since it is not likely that the increased risk is associated with all pesticides, grouping all pesticides together contributes a type of exposure misclassification which is likely to attenuate the risk estimates. Although ultra-rapid case-ascertainment was used, information on prenatal exposure collected for older children may not be as accurate as for young children. Identifying and enrolling representative controls that are similar in income and ethnicity can be challenging and expensive for any case control studies that interview participants. However, socio-demographic characteristic were similar between participating and non-participating households. All controls had to be born in California to be eligible for selection, compared to 92% of leukemia cases. Excluding children born outside California from the control group such as immigrants likely from Latino descent may limit the generalizability of our findings. The study also had several strengths including the use of job-specific modules to assess occupational exposure and a comparison with exposure estimated using job titles and a JEM. The CCLS population used in this study had about twice as many cases of childhood leukemia as previous studies with comparable occupational pesticide exposure assessment methods (Monge et al. 2007; Glass et al. 2012).
5. CONCLUSIONS
We observed an increased risk of ALL, the most common cancer in children, associated with perinatal paternal, but not maternal, occupational pesticide exposure. Paternal occupational pesticide exposure represents a potentially preventable cause of childhood ALL that could reduce the overall incidence by an estimated 4%, and more than 7% among Latinos. Exposure assessment using job titles and a job exposure matrix instead of detailed job modules resulted in non-differential misclassification of exposure and attenuation of the risk estimates.
Supplementary Material
Supplemental Figure 1. Adjusteda odds ratios and 95% confidence intervals for perinatal paternal occupational pesticide exposure by acute lymphocytic leukemia subtype and cytogenetic class (n=number of cases).
aModels adjusted for child’s sex, age, ethnicity, mother’s race and household income. Definitions: numerical change=change in the number of chromosomes; hyperdiploid=subset of numerical change with more than diploid chromosomes; structural change=translocation, inversion or deletion on chromosomes; any deletion=subset of structural change with any chromosomal deletion.
Highlights.
Paternal occupational pesticide exposure increased the risk of ALL in offspring.
The relationship was stronger for children diagnosed before five years of age.
The relationship was attenuated when less detailed exposure information was used.
Perinatal maternal occupational pesticide exposure was not associated with ALL.
Acknowledgments
We thank the families for their participation. We also thank the clinical investigators at the following collaborating hospitals for help in recruiting patients: University of California Davis Medical Center (Dr. Jonathan Ducore), University of California San Francisco (Drs. Mignon Loh and Katherine Matthay), Children’s Hospital of Central California (Dr. Vonda Crouse), Lucile Packard Children’s Hospital (Dr. Gary Dahl), Children’s Hospital Oakland (Dr. James Feusner), Kaiser Permanente Roseville (former Sacramento) (Drs. Kent Jolly and Vincent Kiley), Kaiser Permanente Santa Clara (Drs. Carolyn Russo, Alan Wong and Denah Taggar), Kaiser Permanente San Francisco (Dr. Kenneth Leung) and Kaiser Permanente Oakland (Drs. Daniel Kronish and Stacy Month). Finally, we acknowledge late Professor Patricia Buffler, who was the Principal Investigator of the CCLS. We also thank the entire California Childhood Leukemia Study staff and the former UCB Survey Research Center for their effort and dedication.
Funding
National Institute of Environmental Health Sciences (Grant numbers R01ES009137, P42ES004705 and R03CA153048), US. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences. The study was approved by the University of California Committee for the Protection of Human Subjects, the California Health and Human Services Agency Committee for the Protection of Human Subjects, and the institutional review boards of all participating hospitals. Written informed consent was obtained from the parents of all participants.
Abbreviations
- ALL
acute lymphocytic leukemia
- CI
confidence interval
- JEM
job exposure matrix
- JM
job-specific module
- CCLS
California Childhood Leukemia Study
- OR
odds ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson LM, Diwan BA, Fear NT, Roman E. Critical windows of exposure for children’s health: cancer in human epidemiological studies and neoplasms in experimental animal models. Environ Health Perspect. 2000;108(Suppl 3):573–594. doi: 10.1289/ehp.00108s3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey HD, Fritschi L, Infante-Rivard C, Glass DC, Miligi L, Dockerty JD, et al. Parental occupational pesticide exposure and the risk of childhood leukemia in the offspring: findings from the childhood leukemia international consortium. Int J Cancer. 2014;135(9):2157–2172. doi: 10.1002/ijc.28854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley K, Metayer C, Selvin S, Ducore J, Buffler P. Diagnostic X-rays and risk of childhood leukaemia. Int J Epidemiol. 2010;39(6):1628–1637. doi: 10.1093/ije/dyq162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti P, Stewart PA, Linet MS, Blair A, Inskip PD, Rajaraman P. Comparison of occupational exposure assessment methods in a case-control study of lead, genetic susceptibility and risk of adult brain tumours. Occup Environ Med. 2011;68(1):4–9. doi: 10.1136/oem.2009.048132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colt JS, Blair A. Parental occupational exposures and risk of childhood cancer. Environ Health Perspect. 1998;106(Suppl 3):909–925. doi: 10.1289/ehp.98106909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels JL, Olshan AF, Savitz DA. Pesticides and childhood cancers. Environ Health Perspect. 1997;105(10):1068–1077. doi: 10.1289/ehp.971051068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner WB, Goodrich LB, Botlaguduru VS, Capareda SC, Parnell CB. Particulate matter emission factors for almond harvest as a function of harvester speed. J Air Waste Manag Assoc. 2009 Aug;59(8):943–9. doi: 10.3155/1047-3289.59.8.943. 2009. [DOI] [PubMed] [Google Scholar]
- Gerin M, Siemiatycki J. The occupational questionnaire in retrospective epidemiologic studies: recent approaches to community-based studies. Appl Occup Environ Hyg. 1991;6(6):495–9. [Google Scholar]
- Glass DC, Reid A, Bailey HD, Milne E, Fritschi L. Risk of childhood acute lymphoblastic leukaemia following parental occupational exposure to pesticides. Occup Environ Med. 2012;69(11):846–849. doi: 10.1136/oemed-2011-100250. [DOI] [PubMed] [Google Scholar]
- Jurewicz J, Hanke W. Exposure to pesticides and childhood cancer risk: has there been any progress in epidemiological studies? Int J Occup Med Environ Health. 2006;19(3):152–169. doi: 10.2478/v10001-006-0024-7. [DOI] [PubMed] [Google Scholar]
- Ma X, Buffler PA, Layefsky M, Does MB, Reynolds P. Control selection strategies in case-control studies of childhood diseases. Am J Epidemiol. 2004;159(10):915–921. doi: 10.1093/aje/kwh136. [DOI] [PubMed] [Google Scholar]
- McKinney PA, Fear NT, Stockton D, Investigators UKCCS Parental occupation at periconception: findings from the United Kingdom Childhood Cancer Study. Occup Environ Med. 2003;60(12):901–909. doi: 10.1136/oem.60.12.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinert R, Schuz J, Kaletsch U, Kaatsch P, Michaelis J. Leukemia and non-Hodgkin’s lymphoma in childhood and exposure to pesticides: results of a register-based case-control study in Germany. Am J Epidemiol. 2000;151(7):639–646. doi: 10.1093/oxfordjournals.aje.a010256. discussion 647–650. [DOI] [PubMed] [Google Scholar]
- Metayer C, Scelo G, Kang AY, Gunier RB, Reinier K, Lea S, et al. A task-based assessment of parental occupational exposure to organic solvents and other compounds and the risk of childhood leukemia in California. Environ Res. 2016;151:174–183. doi: 10.1016/j.envres.2016.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metayer C, Zhang L, Wiemels JL, Bartley K, Schiffman J, Ma X, et al. Tobacco smoke exposure and the risk of childhood acute lymphoblastic and myeloid leukemias by cytogenetic subtype. Cancer Epidemiol Biomarkers Prev. 2013;22(9):1600–1611. doi: 10.1158/1055-9965.EPI-13-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monge P, Wesseling C, Engel LS, Keifer M, Zuurbier M, Rojas M, et al. An icon-based interview for the assessment of occupational pesticide exposure in a case-control study of childhood leukemia. Int J Occup Environ Health. 2004;10(1):72–78. doi: 10.1179/oeh.2004.10.1.72. [DOI] [PubMed] [Google Scholar]
- Monge P, Wesseling C, Guardado J, Lundberg I, Ahlbom A, Cantor KP, et al. Parental occupational exposure to pesticides and the risk of childhood leukemia in Costa Rica. Scandinavian J Work Environ Health. 2007;33(4):293–303. doi: 10.5271/sjweh.1146. [DOI] [PubMed] [Google Scholar]
- Reinier K, Hammond SK, Buffler PA, Gunier RB, Lea CS, Quinlan P, et al. Development and evaluation of parental occupational exposure questionnaires for a childhood leukemia study. Scandinavian J Work Environ Health. 2004;30(6):450–458. doi: 10.5271/sjweh.834. [DOI] [PubMed] [Google Scholar]
- Rudant J, Menegaux F, Leverger G, Baruchel A, Nelken B, Bertrand Y, et al. Household exposure to pesticides and risk of childhood hematopoietic malignancies: The ESCALE study (SFCE) Environ Health Perspect. 2007;115(12):1787–1793. doi: 10.1289/ehp.10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman D. Approaches to uncertainty in exposure assessment in environmental epidemiology. Ann Rev Pub Health. 2010;31:149–163. doi: 10.1146/annurev.publhealth.012809.103720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Maele-Fabry G, Lantin AC, Hoet P, Lison D. Childhood leukaemia and parental occupational exposure to pesticides: a systematic review and meta-analysis. Cancer Causes Control. 2010;21(6):787–809. doi: 10.1007/s10552-010-9516-7. [DOI] [PubMed] [Google Scholar]
- Wigle DT, Turner MC, Krewski D. A systematic review and meta-analysis of childhood leukemia and parental occupational pesticide exposure. Environ Health Perspect. 2009;117(10):1505–1513. doi: 10.1289/ehp.0900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm SH, Colt JS, Engel LS, Keifer MC, Alvarado AJ, Burau K, et al. Development of a life events/icon calendar questionnaire to ascertain occupational histories and other characteristics of migrant farmworkers. Am J Ind Med. 2001;40(5):490–501. doi: 10.1002/ajim.1117. [DOI] [PubMed] [Google Scholar]
- Zahm SH, Ward MH. Pesticides and childhood cancer. Environ Health Perspect. 1998;106(Suppl 3):893–908. doi: 10.1289/ehp.98106893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Adjusteda odds ratios and 95% confidence intervals for perinatal paternal occupational pesticide exposure by acute lymphocytic leukemia subtype and cytogenetic class (n=number of cases).
aModels adjusted for child’s sex, age, ethnicity, mother’s race and household income. Definitions: numerical change=change in the number of chromosomes; hyperdiploid=subset of numerical change with more than diploid chromosomes; structural change=translocation, inversion or deletion on chromosomes; any deletion=subset of structural change with any chromosomal deletion.


