Abstract
Background
Despite National Surgical Quality Improvement guidelines to integrate frailty into surgical elder assessments, a quick, accurate, and simple frailty assessment tool suitable for busy clinical settings is still not available. Recently we have demonstrated that a simple upper-extremity function (UEF) test based on wearable sensors could identify frailty with high agreement with conventional assessments by testing 20-second repetitive elbow flexion and extension.
Objective
We examined whether UEF parameters are sensitive to predict adverse health outcomes in bedbound older adults admitted to hospital due to ground-level fall injuries.
Study Design
Frailty in 101 eligible older adults (Age: 79±9 years) admitted to trauma setting were assessed using UEF test at the time of admission. All participants were followed up for two months using phone calls and chart reviews. Measured health outcomes included: discharge disposition (favorable: discharge home or rehabilitation; and unfavorable: discharge to skilled nursing facility or death), hospital length of stay, 30-day readmission, 60-day readmission, and 30-day prospective falls. Multiple variate analyses were used to identify independent predictors of adverse health outcomes based on participants’ demographic (i.e. age, gender, and BMI) and UEF index/parameters.
Results
Based on the UEF frailty status, 53 (52%) of participants were frail and 48 (48%) were non-frail. Among all adverse health outcomes, age was only a significant predictor of 30-day prospective fall (p=0.023). On the other hand, UEF index was a significant predictor of all measured outcomes except length of stay (p<0.010). Among UEF parameters, those indicator of slowness, weakness, and exhaustion had highest effect size to predict unfavorable discharge disposition (p<0.010, effect size = 0.65 – 0.92).
Conclusion
Results of this study suggest that a 20-second upper-extremity test is practical for trauma setting and could be used as a quick measure for predicting adverse events and outcomes in bedbound patients post discharge. Assessing frailty using UEF may assist in objective triage, treat, and post discharge decision making in geriatric trauma patients.
Keywords: frailty, discharge disposition, readmission, inpatient care, trauma, fall incident, wearable technology, functional test, bedbound patients, inpatient triage
Introduction
Falls, and fall-related injuries, are a significant health and safety problem for older adults. Nearly 30% of community-dwellers over the age of 65 experience one or more falls every year, leading to significant risk for hospitalization, institutionalization, and even death [1]. Among older adults who are hospitalized for falls, only half survive, and half succumb to the trauma a year later [2]. Although age is strongly associated with adverse health outcomes due to traumatic falls, the response of an older adult to traumatic events, hospitalization, treatment and rehabilitation is highly variable due to the heterogeneity of aging [3]. To account for this variation, risk stratification using a measure of physiological function is required. Recently, measures of frailty have been used to predict low physiological functional reserve and high vulnerability to poor outcomes [4,5].
We previously developed an objective method for assessing frailty based on upper-extremity function (UEF), and validated this test among trauma patients with convergence to the trauma-specific frailty index (TSFI) [6,7]. The UEF test incorporates several frailty features. Within UEF, the strength of upper-extremity muscles in performing a rapid task can be measured, resembling the grip strength test. Also, the theorized biological mechanism is that sarcopenia/dynapenia, as a manifestation of physical frailty, would influence the entire muscular structure of the human body, including both lower and upper-extremities [4,8]. Therefore, speed of upper-extremity movement was measured as a surrogate of gait speed. The UEF assessment method integrates low cost sensors, the physical task (elbow flexion) is easily performed in under one minute, and the post-processing is performed in less than two minutes. Within the recent validation study among 101 trauma patients (≥ 65 years), the UEF index was developed inclusive of: speed of elbow flexion (slowness), elbow power (weakness), and speed reduction (exhaustion) in a repetitive 20-second elbow flexion test [7]. The purpose of the current prospective study was to assess the validity of the previously developed UEF index (based on TSFI) in predicting in-hospital and 30-day post-discharge health outcomes in 101 older adult patients (aged 65 and above) admitted for standing falls within a southwestern academic integrated health care system Level I trauma center. We hypothesized that the inhospital UEF index frailty score would be significantly associated with discharge disposition, readmission, as well as prospective falls.
Methods
Participants
Older adults with ground-level fall injuries were recruited (January 2014 – August 2015) from the Division of Trauma, Critical Care, and Emergency Surgery service at the University of Arizona. A fall was defined as an incident that participants unintentionally came to rest on a lower surface [9,10]. Inclusion criteria were: 1) aged 65 years and older, 2) ability to understand study instructions for performing repetitive elbow flexion, and 3) having had at least one ground-level fall causing an injury within the prior two weeks that led to hospital admission. Participants were excluded if they had significant upper-extremity disorders in both arms (e.g., bilateral fractures or rheumatoid arthritis with elbow or shoulder involvement), or if they were in a physical condition (e.g., such as severe head injury and unconsciousness) that precluded them from performing the UEF test. Participants were not screened for cognition, since cognitive impairment is a common comorbidity among older adults and is associated with falls and adverse health outcomes [11–13]. The study was approved by the University of Arizona Institutional Review Board. Written informed consent was obtained by trained research coordinators according to the principles expressed in the Declaration of Helsinki [14] from all participants, or from their authorized healthcare power of attorney if a participant was clinically assessed as lacking capacity for informed consent.
Health outcome assessment
All participants were followed up for two months using monthly phone calls and chart reviews. Measured health outcomes included: discharge disposition (favorable: discharge home or rehabilitation; and unfavorable: discharge to skilled nursing facility or death), hospital length of stay, 30-day readmission, 60-day readmission, and 30-day prospective falls. Readmission and fall events were assessed from the day of discharge. Of note, all readmissions to the same center or other centers due to trauma and falls were recorded. Further, 30- and 60-day readmission and 30-day prospective fall events were confirmed by a relative or an authorized caregiver in case participants were not able to recall. Additionally comorbid conditions were recorded, included hypertension, stroke, blood dyscrasias, hypercholesterolemia, cirrhosis, chronic obstructive pulmonary disease, history of caner, use of immunosuppressive medications, autoimmune disorders, coronary artery disease, dementia, and chronic renal failure. Injury severity scale (ISS) and abbreviated injury scale (AIS) were also recorded to assess the severity of injury.
UEF assessment and index
Per our previously validated method [15], wearable sensor technology was used to measure forearm and upper-arm motion. A triaxial wearable gyroscope sensor (dynamic range: ±2000 deg/sec, sample frequency 100Hz, BioSensics LLC, Boston, MA, USA) was attached to the upper-arm near the biceps and one to the wrist using a band attached with Velcro, to estimate three-dimensional angular velocity of the upper-arm and forearm segments, and ultimately the elbow angular velocity. Each participant performed a ~20-second trial of elbow flexion, within which they repetitively flexed and extended their dominant elbow to full flexion and extension as quickly as possible in supine posture (bedbound position) while wearing the UEF system. Participants performed the elbow flexion task with their non-dominant side in the case of injury or attachments of vital sign monitoring equipment. Before the actual test, participants performed a short practice trial to become familiar with the protocol. The protocol was explained to participants and they were encouraged only once, before elbow flexion, to do the task as fast as possible (participants were not further encouraged during the task). All UEF assessments were performed by trained researchers (NT, MRH, and TOJ) during the participant’s hospital stay.
Several outcome measures representing kinematics and kinetics of elbow flexion were derived using angular velocity and anthropometric data (i.e., participants’ stature and body mass). Outcome measures included: 1) speed; 2) flexibility; 3) power; 4) rise time; 5) moment; 6) speed variability; 7) speed reduction; and 8) flexion number (see Table 1 for definitions) [15] . The UEF index represents the continuous frailty index and categorical frailty status (i.e., non-frail and frail) based on UEF parameters and has been validated using a modified Rockwood questionnaire, the trauma-specific frailty index (TSFI) [6]. Result from the validation study showed a high agreement (r=0.72, p<.0001) between TSFI score and UEF model; sensitivity and specificity for predicting the frailty status were 78% and 82%, respectively, compared to TSFI as the gold standard. Readers are referred to previous work [7,15] for more details regarding validation of UEF using a motion capture system, and for a detailed description of parameter calculations and the UEF index development. Briefly, the UEF index is a zero to one score (zero: minimum frailty symptoms and one: maximum frailty symptoms), and those with a score of ≤ 0.27 are considered non-frail (score of > 0.27 is frail). Of note, only speed, power, and speed reduction (reduction in angular velocity within 20 seconds) parameters were used to develop the UEF index, as they showed the best associations with TSFI. These parameters, respectively, represent “slowness”, “weakness”, and “exhaustion” frailty markers.
TABLE 1.
UEF parameter definitions. Among UEF parameters, speed, power, and speed reduction were used to develop the UEF index.
| PARAMETER | DEFINITION |
|---|---|
| Speed | Mean value of elbow angular velocity range (maximum minus minimum speed) |
| Flexibility | Mean value of elbow flexion range |
| Power | Mean value of product of the angular acceleration range and the range of angular velocity |
| Rise time | Mean value of time required to reach the maximum angular velocity |
| Moment | Mean value of maximum moments on elbow within each flexion/extension estimated from moment of inertia of forearm and hand, and elbow motion |
| Speed variability | Coefficient of variation (standard deviation divided by the mean) of angular velocity range |
| Speed reduction | Difference in angular velocity range between the last and the first five seconds of elbow flexion as a percentage of initial angular velocity range |
| Flexion number | Number of flexion/extensions during 20 seconds |
Statistical analysis
Between-group differences in demographic information and adverse health outcomes for frailty groups (i.e., non-frail: UEF index ≤ 0.27; frail: UEF index > 0.27) determined using analyses of variance (ANOVAs) and Cohen’s effect size was calculated [16]; statistical analyses were adjusted with age, gender, and body mass index (BMI) for adverse health outcome comparisons between frailty groups. Further, separate models of logistic regression (for nominal health outcomes) and ANOVA regression (for continuous health outcomes) were used to determine the association between the UEF index (as independent variables) with each of the health outcomes (as the dependent variable); all regressions were adjusted with age, gender, and BMI (total of five models). Statistical analyses were adjusted with age, gender, and BMI to account for possible influence of these parameters on health outcomes. For those adverse health outcomes that showed significant association with the UEF index, we performed further statistical analyses to determine the association between individual UEF parameters with each adverse health outcomes. For this purpose, UEF parameters were compared between two groups with positive and negative health outcomes using separate ANOVAs with age, gender, and BMI as covariates. To account for the effect of discharge disposition (favorable vs. unfavorable) on the results, additional logistic regression models were developed to associate UEF index with 30- and 60-day readmission and 30-day prospective fall, adjusting with age and discharge disposition. All analyses were performed using JMPTM (version 11; SAS Institute Inc., Cary, N.C., USA), with a significance level of p<0.05. Summary results are presented as means with standard deviation (SD) and standard errors (SE).
Results
Participants
One-hundred and one older adults were recruited for the current study. Based on the UEF frailty status, 53 (52%) of participants were frail and 48 (48%) were non-frail. Mean (SD) age and BMI of frail participants were 81 (9) years and 24.74 (4.84) kg/m2, corresponding values were 76 (9) years and 25.55 (4.40) kg/m2 for non-frail participants. Among demographic parameters, only age was significantly different between UEF defined non-frail and frail groups (p=0.004). Other sociodemographic information is reported in Table 2. Overall frail participants were more likely to have pre-existing comorbid conditions compared to non-frail patients. Table 3 provides the comparison of several pre-existing comorbidities between the groups. Blunt injuries were predominant mechanism of injury in our population. Frail participants were more likely to present with fall as mechanism compared to non-frail patients (p=0.02). Table 4 elaborates comparison of the injury pattern and severity.
Table 2.
Demographic information, health outcome measures for frailty groups defined by the UEF test. The asterisk symbol represents a significant between-group difference.
| Variable | Non-frail | Frail | P-value † | Lower CI | Upper CI | Effect size |
|---|---|---|---|---|---|---|
| Number, n (% of total) | 48 (48%) | 53 (52%) | - | - | - | - |
| UEF index (SD) | 0.18 (0.05) | 0.39 (0.10) | - | - | - | - |
| Male, n (% of the group) | 26 (54%) | 21 (40%) | 0.143 | −0.10 | 0.69 | - |
| Age, year (SD) | 76 (9) | 81 (9) | 0.004* | 0.88 | 4.39 | 0.56 |
| Stature, cm (SD) | 168.32 (9.41) | 166.48 (10.36) | 0.360 | −2.91 | 1.07 | 0.19 |
| Body mass, kg (SD) | 72.72 (15.14) | 68.84 (15.98) | 0.221 | −5.06 | 1.18 | 0.25 |
| Body mass index, kg/m2 (SD) | 25.55 (4.40) | 24.74 (4.84) | 0.388 | −1.33 | 0.52 | 0.18 |
| Unfavorable discharge disposition, n (% of the group) | 8 (17%) | 24 (45%) | 0.003* | 0.25 | 1.32 | - |
| Hospital length of stay, days (SD) | 4.44 (2.98) | 5.85 (5.31) | 0.200 | −0.33 | 1.54 | 0.33 |
| 30-day readmission, n (% of the group) | 10 (21%) | 19 (36%) | 0.136 | −0.85 | 0.11 | - |
| 60-day readmission, n (% of the group) | 15 (31%) | 28 (53%) | 0.097 | −0.80 | 0.07 | - |
| 30-day prospective fall, n (% of the group) | 5 (10%) | 16 (30%) | 0.049* | −1.14 | 0.04 | - |
P-values for between-group differences in adverse health outcome measures are adjusted for age, gender, and body mass index (BMI)
TABLE 3.
Comorbidity measures for frailty groups defined by the UEF test. The asterisk symbol represents a significant between-group difference.
| COMORBIDITIES | NON-FRAIL N = 48 | FRAIL N = 53 | P-VALUE † |
|---|---|---|---|
| Hypertension | 60% | 92% | <0.001* |
| Hyperlipidemia | 43% | 56% | 0.056 |
| Coronary artery disease | 25% | 46% | 0.001* |
| Stroke | 4% | 13% | 0.018* |
| Dementia | 23% | 45% | <0.001* |
| Cirrhosis | <1% | 1% | 0.436 |
| Chronic renal failure | 8% | 7% | 0.546 |
| Chronic obstructive disease | 3% | 18% | <0.001* |
TABLE 4.
Injury details for frailty groups defined by the UEF test. Median and interquartile range of injury scores. The asterisk symbol represents a significant between-group difference.
| VARIABLE | NON-FRAIL N = 48 | FRAIL N = 53 | P-VALUE † |
|---|---|---|---|
| Mechanism | |||
| Blunt | 96% | 96% | 0.608 |
| Fall | 53% | 68% | 0.020* |
| Motor vehicle collision | 40% | 26% | 0.022* |
| ISS | 10 [10–14] | 10 [9–14] | 0.220 |
| Head-AIS | 2 [2–3] | 2 [2–3] | 0.082 |
| Thorax-AIS | 3 [2–3] | 3 [2–3] | 0.550 |
| Abdomen-AIS | 2 [2-2] | 2 [1–2] | 0.124 |
| Extremity-AIS | 2 [2–3] | 2 [1–2] | 0.123 |
ISS: injury severity scale; AIS: abbreviated injury scale
Association between UEF and adverse health outcomes
As indicated in Table 2, the percentage of participants who have adverse prospective health outcomes following hospital stay is greater among the frail compared to the non-frail group defined by UEF; differences were significant for the rate of unfavorable discharge disposition and prospective fall within the 30-day follow up (p<0.049). Further, when adjusted for sociodemographic data, the UEF index was a significant predictor of all adverse health outcomes (p<0.010) except hospital length of stay (Table 5); specifically, the UEF index was a strong predictor of unfordable discharge disposition (p<0.001) and readmission (p<0.010). When logistic models were adjusted for discharge disposition (in addition to age), the UEF index remained significant predictor of 30- and 60-day readmission and 30-day prospective fall (p<0.03). Of importance, chronological age itself was significantly associated with a single adverse outcome, prospective falls (p=0.02, Table 5).
Table 5.
Association between the UEF index and prospective health outcomes. Independent variables for each regression analysis included the UEF index, age, gender, and body mass index. The asterisk symbol represents a significant independent association.
| Independent variable | Dependent variable | Parameter estimate † | Chi- square or t ratio | p-value | 95% CI lower | 95% CI upper |
|---|---|---|---|---|---|---|
| UEF index, (0–1) | Unfavorable discharge disposition | 9.23 | 14.58 | <0.001* | 4.84 | 14.40 |
| Age, year | 0.01 | 0.07 | 0.794 | −0.05 | 0.07 | |
| Gender, [female] | 0.15 | 0.31 | 0.578 | −0.38 | 0.68 | |
| BMI, kg/m2 | 0.04 | 0.45 | 0.498 | −0.07 | 0.15 | |
|
| ||||||
| UEF index, (0-1) | Hospital length of stay | 4.16 | 1.14 | 0.256 | −3.06 | 11.37 |
| Age, year | 0.02 | 0.41 | 0.681 | −0.08 | 0.12 | |
| Gender, [female] | −0.27 | −0.56 | 0.576 | −1.21 | 0.68 | |
| BMI, kg/m2 | −0.14 | −1.41 | 0.162 | −0.34 | 0.06 | |
|
| ||||||
| UEF index, (0–1) | 30-day readmission | 4.88 | 6.65 | 0.008* | 1.28 | 8.78 |
| Age, year | 0.01 | 0.06 | 0.808 | −0.05 | 0.06 | |
| Gender, [female] | −0.32 | 1.71 | 0.186 | −0.82 | 0.15 | |
| BMI, kg/m2 | −0.01 | 0.01 | 0.939 | −0.11 | 0.10 | |
|
| ||||||
| UEF index, (0–1) | 60-day readmission | 4.44 | 6.11 | 0.010* | 1.04 | 8.14 |
| Age, year | 0.02 | 0.92 | 0.345 | −0.02 | 0.07 | |
| Gender, [female] | −0.21 | 0.84 | 0.356 | −0.67 | 0.23 | |
| BMI, kg/m2 | 0.01 | 0.01 | 0.935 | −0.09 | 0.10 | |
|
| ||||||
| UEF index, (0–1) | 30-day prospective fall | 5.55 | 6.23 | 0.010* | 1.31 | 10.14 |
| Age, year | 0.07 | 4.47 | 0.023* | 0.01 | 0.14 | |
| Gender, [female] | −0.56 | 3.42 | 0.056 | −1.18 | 0.01 | |
| BMI, kg/m2 | −0.11 | 2.39 | 0.103 | −0.25 | 0.02 | |
Parameter estimates for nominal health outcomes are presented for log odds of adverse event presence/absence
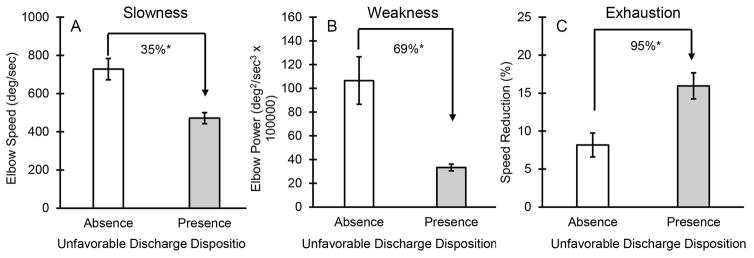
ANOVA analyses revealed that all three UEF index parameters (i.e., speed, power, and speed reduction), were significantly different between individuals who had unfavorable discharge disposition (p<0.010, effect size = 0.65 - 0.92, Figure 1). ANOVA results for other adverse health outcome measures, including 30- and 60-day readmission and 30-day prospective falls, showed that only the speed reduction parameter was consistently worse among participants who had adverse health outcomes following hospitalization (p<0.03, effect size = 0.42–0.65).
Figure 1.
Differences in UEF parameters among those who had favorable and unfavorable discharge disposition following hospital stay. Significant differences are defined by asterisk symbols.
Discussion
UEF and frailty
Although several researchers have focused on identifying frailty, assessing frailty is still controversial [17]. Two common methods for defining frailty are: 1) the Fried categorical index based on a specific phenotype of five criteria (weight loss, exhaustion, low physical activity, grip strength, and walking speed); and 2) the Rockwood frailty index based on a count of 70 accumulated deficits, including presence and/or severity of diseases, ability in activities of daily living, and physical signs from the clinical and neurological examinations [4,5]. Among more than 2000 older adults (≥ 70 years), previous work showed that the two measures of Fried and Rockwood were moderately correlated (r = 0.65) [5].
The UEF test was initially developed and validated using the “gold standard” Fried index among community dwelling older adults. We subsequently validated the test based on TSFI among trauma patients, and we developed a trauma specific index. Since ~50% of trauma patients were not able to walk at the time of the measurements, the Fried test was not performed in the trauma setting [7]. Further, the UEF test validated in a separate study among trauma patients (in addition to community dwelling older adults) to account for possible differences in function due to injury severity and medications. Overall, worse UEF performance was observed among hospitalized participants compared to non-hospitalized community dwelling participants [15] when adjusted for age, gender, and BMI. For instance, speed was ~30% less and speed reduction was ~60% larger among trauma patients (p<0.02).
UEF and discharge disposition
As hypothesized, within the current sample of hospitalized older adults who had a ground-level fall, prospective post-discharge adverse health outcomes were significantly associated with frailty measured using the UEF index. Among all prospective adverse health outcomes, the strongest association was observed between UEF and unfavorable discharge disposition. Previous studies on trauma patients revealed that the Rockwood 50-item frailty index (which includes comorbidities, daily activities, health attitude, function, and nutrition factors) is a strong predictor of unfavorable discharge disposition, independent of age [18]. To date, no other study, to the best of our knowledge, has utilized admission frailty measures for predicting discharge disposition among ground-level or any type of trauma patients. In other clinical research, the Abbreviated Injury Score (AIS), the Injury Severity Score (ISS), and the Glasgow Coma Scale (GCS) have been commonly used as predictors of post-hospitalization outcomes among trauma patients with weak correlations (r<0.34) between these injury measures and prospective adverse health outcomes [19,20]. Of note, all previously implemented methods for assessing prospective adverse health outcome in trauma patients, including frailty and injury severity assessments, involve subjective questionnaires. The current study, to the best of our knowledge, is the first proposed method for objective physical frailty assessment among trauma geriatric patients.
Among older hospitalized adults with non-traumatic injuries, several researchers have reported significant association between frailty and discharge disposition after surgery [21–23]. Commonly implemented assessment within these studies include measure of activity of daily living (e.g., Katz score), comorbidity (e.g., Charlson), function (e.g., gait speed and timed-up-and-go), nutrition (e.g., albumin level), cognition (e.g., mini-cog test), geriatric syndromes (e.g., falls), as well as social factors. Among all these factors, functional measures of frailty have been shown to be more closely associated with the need for discharge institutionalization [22,23]. Performing common frailty function measures such as the gait and timed-up-and-go tests are, however, impossible for most bedbound patients with traumatic injury, and subjective questionnaires are often time-consuming. Accordingly, Goldstein et al. in a review of frailty measures in emergency medicine settings, highlighted that although the utility of measuring frailty is clear, a practical measure of frailty is lacking [24]. The current study focused on validating an objective, rapid method for prospective adverse health outcome evaluation, which is easily performed in the acute care setting.
UEF and readmission
Readmission rate is often considered a measure of quality of care. Due to the recent congressional enactment of a Medicare hospital readmissions reduction program, American hospitals are now penalized for 30-day readmissions [25]. Our findings show that while age was not significantly associated with readmission, frailty may, to some extent, predict 30-day and 60-day readmission among older trauma patients. Similar to discharge disposition assessments, there are a limited number of studies regarding readmission prediction among trauma patients. In a recent study, Housley et al. have used pre-injury medications and comorbidities to assess frailty in trauma patients [26]. Within a large sample of 879 trauma patients, they have demonstrated that frailty is a significantly stronger predictor of readmission than age [26].
Several studies have been published recently showing the association between frailty and readmission rates among non-trauma older adults, including heart and elective surgical patients. Among implemented approaches for assessing frailty, the Fried index [27–30] and Rockwood [31,32] were the most common tools. Overall, the Fried index, which involves functional measures in addition to subjective questionnaires, provides better prediction of readmission compared to the Rockwood score. Within the current study, a significant difference in UEF index was also observed for those who were readmitted to hospital and those who were not (p<0.010, effect size = 0.57 and 0.64 for 30- and 60-day readmission, respectively). Overall, current findings and previous literature suggest that frailty assessment may provide a promising tool for identifying older adults with a higher chance of readmission, among those in acute and outpatient care.
UEF and fall
The UEF index was associated with both prospective falls (current results) and retrospective falls within a prior year [7]. Although several previous studies have associated fall risk with lack of strength and function in lower-extremity muscles [33–37], current findings suggest that upper-extremity function may also related to higher rates of falls. This observation suggests that this lack of strength or function seen in older adults may be systematic and related to dynapenia and sarcopenia seen with aging, influencing both lower- and upper-extremity performance. This hypothesis is in agreement with previous studies that revealed association between upper-body and lower-extremity muscle strength with fall risk [34,35].
Predicting those who are at higher risk of falling would have important financial and healthcare benefits. In previous research, poor balance and fall risk have been related to lack of physiological reserve and frailty among older adults [4,37–39]. However, measuring frailty in those who are at higher risk of falling, especially older adults who are admitted for fall injuries, still requires further research. The use of new, objective technology may play a substantial role in enhancement of frailty assessment. Although our current findings require further confirmation in larger samples, they show promise for the practical implementation of wearable sensor technology and UEF for fall assessment in an outpatient setting.
Limitations
First, as with measurement limitations in gait-based frailty measures, upper-extremity disability or injury may limit measurements; we found this to be the case for some individuals who were excluded from the study because they were unable to perform the UEF task. That said, it is likely that this limitation would also apply to measuring grip strength, which would likewise limit measurements within the Fried index. Furthermore, due to upper-extremity attachment of vital sign monitoring equipment within the hospital setting, 32 (32%) participants were not able to perform the UEF test on their dominant arm; thus the assessment was performed using the non-dominant arm. However, this is likely to be less important, as our previous findings demonstrated similar prediction qualities for assessing frailty using either the dominant or the non-dominant arm [15].
Second, several screened individuals (35%) that were admitted to the hospital were not able to perform the UEF test due to severe head injuries or unconsciousness. Although several fall patients were not able to perform the UEF test, this limitation also co-exists for other common frailty measures such as Fried (gait-limited) or Rockwood (memory-limited).
Third, all consecutively admitted trauma patients with ground-level falls were screened for the study. As the purpose of the current study was to predict adverse health outcome in general geriatric trauma patients, participants were not stratified based on the type of treatments and medications. Also, information regarding physical therapy or occupation therapy evaluations and treatments during and after hospitalization were not available. However, only one trauma surgeon (BJ, who was blinded to frailty and health outcome results) was assigned to all recruited participants.
Fourth, due to time limitations for trauma patient visits, extensive subjective questionnaires including depression, comorbidity, and cognition were not collected within the current study. The purpose of the study was, however, to predict health outcomes using a general UEF test without considering the source of functional decline (either comorbidity or cognition). Of note, within our previous work we observed that cognitive impairment causes a reduction in UEF performance [40].
Lastly, our frailty measure was not able to predict hospital length of stay, probably due to other confounding variables that may influence hospitalization such as diagnostic related group (DRG) reimbursement, surgical processes and rehabilitation requirements. Also 60-day prospective fall was not considered as an outcome, since some data were missing.
Summary of findings and clinical implications
Health outcomes following traumatic fall events are widely variable due to heterogeneity of health conditions among older adults. Risk adjustment is crucial in order to determine the appropriate treatment, support, and discharge of geriatric trauma patients, identifying those who will benefit from intensive and expensive treatments, versus those who will best respond to palliation, and to better manage discharge to appropriate disposition. Within the current study, we developed an objective method for assessing frailty based on speed, strength, and exhaustion level of upper-extremity function. Using this approach, unfavorable discharge disposition to skilled nursing facility or death, readmission, and prospective falls were identified among older adults with ground-level fall events. Importantly, chronological age was not a significant predictor of unfavorable discharge disposition and readmission. Among functional parameters used for developing the UEF index, the level of upper-extremity exhaustion was the only parameter that was consistently different among all adverse health outcome measures following hospitalization. In conclusion, current results suggest that a simple UEF measure that can be performed at the bedside would allow clinicians to understand the level of frailty, to help identify older adults with higher risk of discharge complications and readmission.
Acknowledgments
We thank Bardiya Zangbar for data collection and clinical coordination. Partial support was provided by the National Institutes of Health/ National Institute of Aging (Award number: 1R44AG050338-0), National Institute for Biomedical Imaging and Bioengineering (award number 1R25EB012973), and the Flinn Foundation (award number 1907). The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsors.
Footnotes
Conflict of Interest: Upper Extremity Frailty meter is protected by a patent pending (US Patent 20,150,332,004). The patent is owned by University of Arizona, and Nima Toosizadeh, Jane Mohler, and Bijan Najafi are listed as co-inventors on this patent pending. No other conflict of interest was reported by other authors.
Authors contributions: BJ: Concept and design, clinical supervision, interpretation of data, obtaining funding; MRH: Patient recruitment, study coordination, acquisition of data, and data analysis; NT: Concept and design, study coordination, analysis and interpretation of data, preparation of manuscript, statistical analysis. TOJ: study coordination, acquisition of data; JM, Concept and design, interpretation of data; BN: Concept and design, study supervision, interpretation of data, obtaining funding. All authors contributed in critically revising the manuscript and have given final approval of the version to be published.
References
- 1.Liu-Ambrose T, Davis JC, Hsu CL, Gomez C, Vertes K, Marra C, Brasher PM, Dao E, Khan KM, Cook W. Action seniors!-secondary falls prevention in community-dwelling senior fallers: Study protocol for a randomized controlled trial. Trials. 2015;16:144–144. doi: 10.1186/s13063-015-0648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubenstein LZ. Falls in older people: Epidemiology, risk factors and strategies for prevention. Age and ageing. 2006;35:ii37–ii41. doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- 3.Rowe JW, Kahn RL. Human aging: Usual and successful. Science. 1987;237:143–149. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G. Frailty in older adults evidence for a phenotype. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2001;56:M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 5.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2007;62:738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 6.Joseph B, Pandit V, Zangbar B, Kulvatunyou N, Tang A, O'Keeffe T, Green DJ, Vercruysse G, Fain MJ, Friese RS. Validating trauma-specific frailty index for geriatric trauma patients: A prospective analysis. Journal of the American College of Surgeons. 2014;219:10–17. e11. doi: 10.1016/j.jamcollsurg.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Toosizadeh N, Joseph B, Heusser MR, Jokar TO, Mohler J, Phelan HA, Najafi B. Assessing upper-extremity motion: An innovative, objective method to identify frailty in older bedbound trauma patients. Journal of the American College of Surgeons. 2016 doi: 10.1016/j.jamcollsurg.2016.03.030. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manini TM, Clark BC. Dynapenia and aging: An update. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011:glr010. doi: 10.1093/gerona/glr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirelman A, Herman T, Brozgol M, Dorfman M, Sprecher E, Schweiger A, Giladi N, Hausdorff JM. Executive function and falls in older adults: New findings from a five-year prospective study link fall risk to cognition. PloS one. 2012;7:e40297. doi: 10.1371/journal.pone.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tinetti ME. Prevention of falls and fall injuries in elderly persons: A research agenda. Preventive medicine. 1994;23:756–762. doi: 10.1006/pmed.1994.1130. [DOI] [PubMed] [Google Scholar]
- 11.Sampson EL, Blanchard MR, Jones L, Tookman A, King M. Dementia in the acute hospital: Prospective cohort study of prevalence and mortality. The British Journal of Psychiatry. 2009;195:61–66. doi: 10.1192/bjp.bp.108.055335. [DOI] [PubMed] [Google Scholar]
- 12.Dodson JA, Truong T-TN, Towle VR, Kerins G, Chaudhry SI. Cognitive impairment in older adults with heart failure: Prevalence, documentation, and impact on outcomes. The American journal of medicine. 2013;126:120–126. doi: 10.1016/j.amjmed.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muir SW, Gopaul K, Odasso MMM. The role of cognitive impairment in fall risk among older adults: A systematic review and meta-analysis. Age and ageing. 2012;41:299–308. doi: 10.1093/ageing/afs012. [DOI] [PubMed] [Google Scholar]
- 14.Association WM. World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. Jama. 2013;310:2191. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 15.Toosizadeh N, Mohler J, Najafi B. Assessing upper extremity motion: An innovative method to identify frailty. Journal of the American Geriatrics Society. 2015;63:1181–1186. doi: 10.1111/jgs.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen J. Statistical power analysis for the behavioral sciences. New York: Academic Press; 1977. (revised ed.) [Google Scholar]
- 17.Fisher AL. Just what defines frailty? Journal of the American Geriatrics Society. 2005;53:2229–2230. doi: 10.1111/j.1532-5415.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- 18.Joseph B, Pandit V, Rhee P, Aziz H, Sadoun M, Wynne J, Tang A, Kulvatunyou N, O’Keeffe T, Fain MJ. Predicting hospital discharge disposition in geriatric trauma patients: Is frailty the answer? Journal of Trauma and Acute Care Surgery. 2014;76:196–200. doi: 10.1097/TA.0b013e3182a833ac. [DOI] [PubMed] [Google Scholar]
- 19.Cuthbert JP, Corrigan JD, Harrison-Felix C, Coronado V, Dijkers MP, Heinemann AW, Whiteneck GG. Factors that predict acute hospitalization discharge disposition for adults with moderate to severe traumatic brain injury. Archives of physical medicine and rehabilitation. 2011;92:721–730. e723. doi: 10.1016/j.apmr.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 20.Foreman BP, Caesar RR, Parks J, Madden C, Gentilello LM, Shafi S, Carlile MC, Harper CR, Diaz-Arrastia RR. Usefulness of the abbreviated injury score and the injury severity score in comparison to the glasgow coma scale in predicting outcome after traumatic brain injury. Journal of Trauma and Acute Care Surgery. 2007;62:946–950. doi: 10.1097/01.ta.0000229796.14717.3a. [DOI] [PubMed] [Google Scholar]
- 21.Robinson TN, Wallace JI, Wu DS, Wiktor A, Pointer LF, Pfister SM, Sharp TJ, Buckley MJ, Moss M. Accumulated frailty characteristics predict postoperative discharge institutionalization in the geriatric patient. Journal of the American College of Surgeons. 2011;213:37–42. doi: 10.1016/j.jamcollsurg.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DH, Buth KJ, Martin B-J, Yip AM, Hirsch GM. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010;121:973–978. doi: 10.1161/CIRCULATIONAHA.108.841437. [DOI] [PubMed] [Google Scholar]
- 23.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J. Frailty as a predictor of surgical outcomes in older patients. Journal of the American College of Surgeons. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein J, Andrew MK, Travers A. Frailty in older adults using pre-hospital care and the emergency department: A narrative review. Canadian Geriatrics Journal. 2012;15:16–22. doi: 10.5770/cgj.15.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hackbarth GM, Berenson R, Miller ME. Testimony on behalf of MedPAC. Mar 17, 2009. Report to the congress: Medicare payment policy. [Google Scholar]
- 26.Housley BC, Stawicki SP, Evans DC, Jones C. Comorbidity-polypharmacy score predicts readmission in older trauma patients. Journal of surgical research. 2015;199:237–243. doi: 10.1016/j.jss.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Dent E, Hoogendijk EO. Psychosocial factors modify the association of frailty with adverse outcomes: A prospective study of hospitalised older people. BMC geriatrics. 2014;14:1. doi: 10.1186/1471-2318-14-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McAdams-DeMarco MA, Law A, Salter ML, Chow E, Grams M, Walston J, Segev DL. Frailty and early hospital readmission after kidney transplantation. American journal of transplantation. 2013;13:2091–2095. doi: 10.1111/ajt.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang JMG, Govindan P, Grinnan JW, Liu M, Ali HM, Chakraborty A, Jain V, Ros RL, Hall EC, Berger JA. AMERICAN JOURNAL OF TRANSPLANTATION. Vol. 11. WILEY-BLACKWELL PUBLISHING, INC; COMMERCE PLACE, 350 MAIN ST, MALDEN 02148, MA USA: 2011. Frailty: An independent determinant of delayed-graft function in kidney transplant recipients; pp. 65–65. [Google Scholar]
- 30.Courtney-Brooks M, Tellawi AR, Scalici J, Duska LR, Jazaeri AA, Modesitt SC, Cantrell LA. Frailty: An outcome predictor for elderly gynecologic oncology patients. Gynecologic oncology. 2012;126:20–24. doi: 10.1016/j.ygyno.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 31.Conroy S, Dowsing T. The ability of frailty to predict outcomes in older people attending an acute medical unit. Acute medicine. 2012;12:74–76. [PubMed] [Google Scholar]
- 32.Hewitt J, Moug SJ, Middleton M, Chakrabarti M, Stechman MJ, McCarthy K, Collaboration OPSO. Prevalence of frailty and its association with mortality in general surgery. The American Journal of Surgery. 2015;209:254–259. doi: 10.1016/j.amjsurg.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 33.LaStayo PC, Ewy GA, Pierotti DD, Johns RK, Lindstedt S. The positive effects of negative work: Increased muscle strength and decreased fall risk in a frail elderly population. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2003;58:M419–M424. doi: 10.1093/gerona/58.5.m419. [DOI] [PubMed] [Google Scholar]
- 34.Pijnappels M, Reeves ND, van Dieën JH. Identification of elderly fallers by muscle strength measures. European journal of applied physiology. 2008;102:585–592. doi: 10.1007/s00421-007-0613-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle weakness and falls in older adults: A systematic review and meta-analysis. Journal of the American Geriatrics Society. 2004;52:1121–1129. doi: 10.1111/j.1532-5415.2004.52310.x. [DOI] [PubMed] [Google Scholar]
- 36.Fukagawa NK, Wolfson L, Judge J, Whipple R, King M. Strength is a major factor in balance, gait, and the occurrence of falls. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1995;50:64–67. doi: 10.1093/gerona/50a.special_issue.64. [DOI] [PubMed] [Google Scholar]
- 37.Toosizadeh N, Mohler J, Wendel C, Najafi B. Influences of frailty syndrome on open-loop and closed-loop postural control strategy. Gerontology. 2014;61:51–60. doi: 10.1159/000362549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis DH, Rockwood MR, Mitnitski AB, Rockwood K. Impairments in mobility and balance in relation to frailty. Archives of gerontology and geriatrics. 2011;53:79–83. doi: 10.1016/j.archger.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 39.Delbaere K, Crombez G, Vanderstraeten G, Willems T, Cambier D. Fear-related avoidance of activities, falls and physical frailty. A prospective community-based cohort study. Age and ageing. 2004;33:368–373. doi: 10.1093/ageing/afh106. [DOI] [PubMed] [Google Scholar]
- 40.Toosizadeh N, Najafi B, Reiman EM, Mager RM, Veldhuizen JK, O’Connor K, Zamrini E, Mohler J. Upper-extremity dual-task function: An innovative method to assess cognitive impairment in older adults. Frontiers in Aging Neuroscience. 2016;8 doi: 10.3389/fnagi.2016.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]