Abstract
Objective
Using a large US claims database (MarketScan®), we investigated the controversy surrounding the role of statins in Parkinson’s disease (PD).
Methods
We performed a retrospective case-control analysis. First, we identified 2,322 incident PD cases having a minimum of 2.5 y of continuous enrollment prior to earliest diagnosis code or prescription of antiparkinson medication. Then, 2,322 Controls were matched individually by age, gender, and a “follow-up window” to explore the relationship of statin use with incident PD.
Results
Statin usage was significantly associated with PD risk, with the strongest associations being for lipophilic (OR=1.58, p<0.0001) vs. hydrophilic (OR=1.19, p=0.25) statins, statins plus non-statins (OR=1.95, p<0.0001), and for the initial period after starting statins (<1 y OR=.82, 1–2.5 y OR =1.75, and ≥2.5 y OR =1.37; ptrend<0.0001).
Conclusion
Use of statin (especially lipophilics) was associated with higher risk of PD, and the stronger association in initial use suggests a facilitating effect.
Keywords: Parkinson’s disease, statins, cholesterol, large data
Introduction
Approximately 43 US million adults receive or are eligible for statin therapy; this may increase based on new cardiovascular prevention guidelines and new generic statins. Several preclinical studies have suggested statins may protect against Parkinson’s disease (PD),1–5 yet epidemiological findings on this relationship have been inconsistent: some reported lower PD risk among statin users,6–8 but others no difference9–13 or even higher risk.14
The interpretation of these data, however, is not straightforward. First, a recent meta-analysis suggested a significant publication bias toward reporting protective effects of statins in PD.15 Second, even in those studies reporting protective effects, the interpretation of the results is confounded by the use of statins for hyperlipidemia.16 This is relevant because several case-control10–12 and prospective studies13,17 suggest that higher serum/plasma total- and/or LDL-cholesterol levels are related to lower occurrence of PD. Lastly, statins differ in their ability to cross the blood-brain-barrier, and the number of PD subjects was too small in prior studies to allow stratification.
Despite this uncertainty,14,18 there has been a prevailing sentiment to use statins as neuroprotective agents,19–21 indeed simvastatin (a lipophilic statin) is in current clinical trial.19 It is, therefore, timely to evaluate carefully the relationship of statins and PD risk.
Methods
Data Source
The MarketScan® Commercial Claims and Encounters database (Truven Health Analytics) consists of individual reimbursed healthcare claim information from more than 130 payers describing the healthcare use and expenditures for >50 million employees and family members per year. Ages range from birth to 64 y, when most individuals switch from private insurance to Medicare.22 Because PD is age-related and mainly affects older adults, we limited our search to all individuals in the database who had one year or more of continuous enrollment during January 1, 2008 to December 31, 2012 and were 40 or older at any time during their enrollment period.
PD case identification and confirmation
Possible prevalent PD cases were identified using the following criteria: 1) primary or secondary diagnosis of PD (ICD-9 332); 2) use of any anti-PD medication containing carbidopa/levodopa (Sinemet, Parcopa, Stalevo, Duodopa), pramipexole (Mirapex), ropinirole (Requip), pergolide (Permax), rotigotine (Neupro), entacapone (Comtan), selegiline (Eldepryl, Zelapar), rasagiline (Azilect), carbidopa (Lydosyn), or amantadine (Symmetrel); and 3) deep brain stimulation (DBS) surgery (CPT-4 codes 61850, 61860, 61863, 61864, 61867, 61868, 61870, 61875, 61880, 61885, 61886, 61888, 64573, 64580, 95970, 95971, 95972, 95973, 95974, 95975, 95978, 95979). To increase accuracy of PD classification, we excluded any patient who had PD indications from only one of the three aforementioned criteria.
To ascertain incident PD patients, we narrowed our search to those prevalence cases who had a minimum of 2.5 y of continuous enrollment prior to the index date defined as the earliest PD indication by diagnosis or prescription of antiparkinson medication. This ensured a reasonable observation window. Controls were matched individually to cases by age, gender, and a “follow-up window” that was defined as the time from a patient’s initial insurance coverage date during our study period to the index date of PD indication. The control in the matched pair must have had a follow-up window that was at least as long as the PD case. The matched control then was assigned the same index date as the matched patient case.
Ascertainment of hyperlipidemia and comorbidities
Although cholesterol may play a role in the hypothesized statin-PD association, cholesterol lab data were not included in the MarketScan database. Thus, we used the diagnosis of hyperlipidemia (272.0–272.4) as a surrogate marker of history of high cholesterol level. Statins can be prescribed for conditions other than hyperlipidemia, so we identified the following disorders by diagnosis codes (ICD-9 codes): hypertension (401); coronary artery disease (CAD; 410); and diabetes (250).
Ascertainment of statins and other cholesterol-lowing drug usage
Statins and other cholesterol-lowering drugs (i.e., unspecified anti-hyperlipidemics, bile sequestrants, or fibrates) usage were defined as greater than two separate prescriptions. Statins were subgrouped into hydrophilic (pravastatin, rosuvastatin) and lipophilic (atorvastatin, fluvastatin, lovastatin, pitavastatin, simvastatin). Fenofibrate, ezetimibe, and niacin were considered as non-statin cholesterol lowering drugs. For the matched case-control analysis, we determined whether a patient had been prescribed statins and/or non-statin cholesterol-lowering drugs. If so, we then calculated the statin exposure window as the period between the earliest date of a filed-claim for a statin prescription and the index date. We categorized statin users into three groups according to the length of their statin exposure (i.e., <1 y, 1–2.5 y, or ≥2.5 y) prior to first PD indication.
Data analysis
Cross-sectional analysis
We performed descriptive analyses on the entire study cohort, summarizing the cohort demographics (age, gender), comorbidities, and cholesterol-lowering drug use (statins and non-statins). We then ran a multivariable logistic regression to assess the significance of the association between cholesterol-lowering drug use (vs. non-user as a reference) and prevalent PD, adjusting for age, gender, and comorbid diseases such as hyperlipidemia, diabetes, hypertension, and CAD. Cholesterol-lowering drugs were divided into statin only, non-statin cholesterol lowering drugs only, and the use of both statin and non-statin cholesterol-lowering drugs.
Matched incident case-control analysis
We ascertained the usage of cholesterol-lowering drugs prior to index dates for PD cases and controls, and statins also were analyzed based on their lipophilic or hydrophilic properties. We also categorized the earliest time of drug exposure epochs as <1 y, 1–2.5 y, or ≥2.5 y before the index date. We ran a conditional logistic regression to determine the significance of the associations between PD incidence and the earliest prior statin exposure (i.e., <1 y, 1–2.5 y, or ≥2.5 y) in conjunction with or without use of non-statin cholesterol-lowering drugs. The analyses were adjusted for all of the aforementioned covariates except for age and gender that had been used for matching cases and controls.
All statistical analyses were performed using SAS version 9.3 software (SAS Institute, Cary, NC). Statistical tests were two-sided, with α≤0.05 being statistically significant.
Results
Population characteristics
Of the 30,343,035 subjects eligible for the analysis, a total of 21,599 individuals met our prevalence case definition (Supplemental Table 1). As expected, PD patients were older and more likely to be males. PD prevalence was associated positively with age, male gender, hypertension, CAD, usage of cholesterol lowering drugs (statins or non-statins), and negatively with hyperlipidemia.
Case-control analysis of prior statin exposure and PD
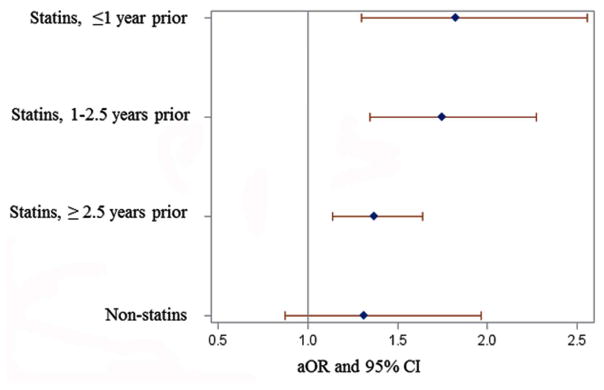
Prior statin usage was associated significantly with higher PD risk (Table 1, Figure 1). The association was strongest for lipophilic- (OR [95% CI]: 1.58 [1.33–1.89]), and weaker for hydrophilic-statins (1.19 [0.88–1.61]) and non-statin cholesterol lowering drugs (1.34 [0.90–2.02]). The combined use of both statins and non-statin cholesterol-lowering drugs (marking aggressive cholesterol management) had the highest PD incidence (1.95 [1.56–2.43]). Moreover, the association was stronger for the initial period of statin use with OR: 1.82 [1.30–2.56] <1 y prior to index date, 1.75 [1.35–2.27] for 1–2.5 y, and 1.37 [1.14–1.64] for ≥2.5 y (ptrend<0.0001, Figure 1).
Table 1.
The association between prior use of cholesterol-lowering drugs and Parkinson’s disease.
| Type of cholesterol lowering drugs | Controls N (%) | PD cases N (%) | Multivariable logistic regression model | |||
|---|---|---|---|---|---|---|
| aOR* | 95% CI | P-value | ||||
| Lipophilic only | 375 (16.2%) | 507 (21.8%) | 1.58 | 1.33 | 1.89 | <0.0001 |
| Hydrophilic only | 100 (4.3%) | 105(4.5%) | 1.19 | 0.88 | 1.61 | 0.25 |
| Both statins | 75 (3.2%) | 104 (4.5%) | 1.61 | 1.17 | 2.21 | 0.004 |
| Non-statin only | 48 (2.1%) | 57 (2.5%) | 1.34 | 0.90 | 2.02 | 0.15 |
| Both statin+non-statin | 185 (8.0%) | 315 (13.6%) | 1.95 | 1.56 | 2.43 | <0.0001 |
| None | 1,539 (66.3%) | 1,234(53.1%) | Reference | |||
| Comorbidity | ||||||
| Hyperlipidemia | 1,254 (54.1%) | 1,445 (62.2%) | 1.07 | 0.93 | 1.23 | 0.37 |
| Hypertension | 1,046 (45.1%) | 1,241 (53.5%) | 1.21 | 1.07 | 1.38 | 0.004 |
| Diabetes | 423 (18.2%) | 558 (24.0%) | 1.10 | 0.94 | 1.28 | 0.24 |
| Coronary artery disease | 37 (1.6%) | 59 (2.5%) | 1.22 | 0.79 | 1.88 | 0.37 |
Adjusted for history of hypertension, hyperlipidemia, diabetes, and coronary arterial disease. CI: Confident Intervals
Figure 1.
Forest plot of the association between Parkinson’s disease and exposure to statins prior to the earliest disease indication in a case-control analysis. Reference group: non-users of cholesterol lowering drugs. Abbreviations: aOR = Adjusted Odds Ratio; CI = confidence interval
Discussion
Statin use was associated with higher, not lower, PD risk, and the association was more pronounced for lipophilic statins, an observation inconsistent with the current hypothesis that these statins are neuroprotective.19–21 In addition, the statin-PD association was most robust for the initial phase of statin treatment (<2.5 y), suggesting that statins may facilitate the onset of preclinical PD. Because new guidelines23 may lead to broader statin use, additional studies should assess the potentially adverse effect of statins (particularly lipophilic ones) on certain populations vulnerable to PD.
Although not unanimous,24 the weight of the literature favors an association of high lipid cholesterol levels with lower risk of PD via both case control 9,25–28 and prospective14,29–31 studies. Our cross-sectional analysis of the Marketscan Database (supplemental Table 1) also found that the diagnosis of hyperlipidemia (a marker of high cholesterol) was associated with lower PD prevalence, consistent with the large majority of literature.
Most literature used data collected in the post-statin era where hyperlipidemia would prime statin usage. Some reports have suggested lower PD risk among statin users,6–8,17 but none factored in this confound (i.e., hyperlipidemia). Indeed, a recent meta-analysis reported that “protective effects” of statins disappear if hyperlipidemia is considered.16 Moreover, prospective data from the Atherosclerosis in Community cohort (ARIC) showed that after accounting for total- and LDL-cholesterol levels, statin usage was associated with higher future risk of PD.14 The current analysis also found that statin use was associated with higher PD risk after adjusting for all potential confounders, including hyperlipidemia.
We explored the timing of initial statin exposure relative to the earliest indication of PD onset. PD risk was highest during the initial period after statin use (<2.5 y) compared to exposure further from PD diagnosis (≥2.5 y). Various environmental factors may trigger an otherwise dormant disease or medical condition, particularly in high-risk populations, and our data suggest that the statin-PD link may represent one such scenario. The large sample size in the current study also permitted estimation of associations with different statin types (lipophilic vs. hydrophilic). In contrast to prior studies17,18 with much smaller sample sizes, we found that lipophilic statins were associated with highest PD risk. Conversely, a significant association was not found for non-statin cholesterol lowering agents, but the less frequent use of these drugs left this aspect of the study underpowered.
A recent study using the Taiwanese NHI database reported that discontinuing statins (especially lipophilics) was more likely to lead to diagnosis of PD,32 and this was interpreted as compelling evidence for neuroprotection.17,21 The current study, however, leads to an alternate interpretation -- statins may have “unmasked” symptoms in those with pre-PD,17 explaining why those subjects discontinued statins. In the future, it is critical to obtain both diagnosis and lipid status for those discontinuing statins.
The study has several limitations. First, the claims data did not include Medicare, Medicaid, or the uninsured. Because it was based on private insurance, the population did not include individuals 65 y or older (the transition age to Medicare), hence, we are unable to generalize our findings to older populations. We ascertained PD cases from the claims records alone, without having clinical details or case confirmation, and misdiagnosis may have occurred due to record entry, coding, etc. To minimize diagnostic uncertainty, we required that PD cases had at least two separate criteria supporting the diagnosis. Additionally, the claims data lacked lab values, preventing quantitative assessment of lipid cholesterol levels, something of importance for future studies.
Supplementary Material
Acknowledgments
Funding Sources: Tobacco CURE funds grant from the Pennsylvania Department of Health; NIH (NS060722 and NS082151 to XH); the Hershey Medical Center GCRC (National Institute of Health M01RR10732); the GCRC Construction Grant (C06RR016499); the Intramural Research Program of the National Institute of Environmental Health Sciences and Z01 ES101986; The Center for Applied Studies in Health Economics (CASHE).
Footnotes
Financial Disclosures: The authors have nothing to disclose as it relates to this research.
Documentation of Author Roles
Guodong Liu: Conception, organization, execution of the project; statistical design and execution; writing of the first draft, and review and critique of the manuscript;
Nicholas W. Sterling: Organization of the project, review of statistical analysis, and review and critique of the manuscript.
Lan Kong: Review of statistical design, review and critique of the manuscript.
Mechelle M. Lewis: Organization of the project, review of the statistical design, extensive review and critique of the manuscript.
Richard B. Mailman: Review of statistical design, extensive review and critique of the manuscript.
Honglei Chen: Organization of the project, review and critique of the statistical design and manuscript.
Douglas Leslie: Organization and execution of the project, review and critique of the statistical design and manuscript.
Xuemei Huang: Conception, organization, and execution of the project; review of the statistical design; writing of the first draft, and review and critique of the manuscript.
Financial Disclosures for last three years:
| Xuemei Huang: | Has received consultant fees from the National Institute of Environmental Health Sciences unrelated to this study. |
| Richard B. Mailman: | Patents related to D1 dopamine agonists as therapeutic agents, including in Parkinson’s disease (assigned to University foundations). Expert witness or consultant to several law firms unrelated to this research. Consultant to Pfizer and Jazz Pharma on projects unrelated to this study. |
| Nicholas W. Sterling: | Nothing to disclose |
| Guangwei Du: | Nothing to disclose |
| Mechelle M. Lewis: | Nothing to disclose |
| Lan Kong: | Nothing to disclose |
| Honglei Chen: | Nothing to disclose |
References
- 1.Bar-On P, Crews L, Koob AO, et al. Statins reduce neuronal alpha-synuclein aggregation in in vitro models of Parkinson’s disease. J Neurochem. 2008;105(5):1656–1667. doi: 10.1111/j.1471-4159.2008.05254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar A, Sharma N, Gupta A, Kalonia H, Mishra J. Neuroprotective potential of atorvastatin and simvastatin (HMG-CoA reductase inhibitors) against 6-hydroxydopamine (6-OHDA) induced Parkinson-like symptoms. Brain Res. 2012;1471:13–22. doi: 10.1016/j.brainres.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh A, Roy A, Matras J, Brahmachari S, Gendelman HE, Pahan K. Simvastatin inhibits the activation of p21ras and prevents the loss of dopaminergic neurons in a mouse model of Parkinson’s disease. J Neurosci. 2009;29(43):13543–13556. doi: 10.1523/JNEUROSCI.4144-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koob AO, Ubhi K, Paulsson JF, et al. Lovastatin ameliorates alpha-synuclein accumulation and oxidation in transgenic mouse models of alpha-synucleinopathies. Exp Neurol. 2010;221(2):267–274. doi: 10.1016/j.expneurol.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan J, Xu Y, Zhu C, et al. Simvastatin prevents dopaminergic neurodegeneration in experimental parkinsonian models: the association with anti-inflammatory responses. PLoS One. 2011;6(6):e20945. doi: 10.1371/journal.pone.0020945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao X, Simon KC, Schwarzschild MA, Ascherio A. Prospective study of statin use and risk of Parkinson disease. Arch Neurol. 2012;69(3):380–384. doi: 10.1001/archneurol.2011.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wahner AD, Bronstein JM, Bordelon YM, Ritz B. Statin use and the risk of Parkinson disease. Neurology. 2008;70(16 Pt 2):1418–1422. doi: 10.1212/01.wnl.0000286942.14552.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolozin B, Wang SW, Li NC, Lee A, Lee TA, Kazis LE. Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med. 2007;5(1):20. doi: 10.1186/1741-7015-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang X, Chen H, Miller WC, et al. Lower low-density lipoprotein cholesterol levels are associated with Parkinson’s disease. Mov Disord. 2007;22(3):377–381. doi: 10.1002/mds.21290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker C, Jick SS, Meier CR. Use of statins and the risk of Parkinson’s disease: a retrospective case-control study in the UK. Drug Saf. 2008;31(5):399–407. doi: 10.2165/00002018-200831050-00004. [DOI] [PubMed] [Google Scholar]
- 11.Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197. doi: 10.1136/bmj.c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ritz B, Manthripragada AD, Qian L, et al. Statin use and Parkinson’s disease in Denmark. Mov Disord. 2010;25(9):1210–1216. doi: 10.1002/mds.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samii A, Carleton BC, Etminan M. Statin use and the risk of Parkinson disease: a nested case control study. J Clin Neurosci. 2008;15(11):1272–1273. doi: 10.1016/j.jocn.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Huang X, Alonso A, Guo X, et al. Statins, plasma cholesterol, and risk of Parkinson’s disease: a prospective study. Mov Disord. 2015;30(4):552–559. doi: 10.1002/mds.26152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Undela K, Gudala K, Malla S, Bansal D. Statin use and risk of Parkinson’s disease: a meta-analysis of observational studies. J Neurol. 2013;260(1):158–165. doi: 10.1007/s00415-012-6606-3. [DOI] [PubMed] [Google Scholar]
- 16.Bykov K, Yoshida K, Weisskopf MG, Gagne JJ. Confounding of the association between statins and Parkinson disease: systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. 2016 doi: 10.1002/pds.4079. [DOI] [PubMed] [Google Scholar]
- 17.Lee YC, Lin CH, Wu RM, et al. Discontinuation of statin therapy associates with Parkinson disease: a population-based study. Neurology. 2013;81(5):410–416. doi: 10.1212/WNL.0b013e31829d873c. [DOI] [PubMed] [Google Scholar]
- 18.Singh NK, Banerjee BD, Bala K, Mitrabasu, Dung Dung AA, Chhillar N. APOE and LRPAP1 gene polymorphism and risk of Parkinson’s disease. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2014;35(7):1075–1081. doi: 10.1007/s10072-014-1651-6. [DOI] [PubMed] [Google Scholar]
- 19.Venosa A. Medical Daily. New York: IBT Media Inc; 2016. Cholesterol Medication Simvastatin Tested As Potential Treatment For Parkinson’s Disease In New Clinical Trial. [Google Scholar]
- 20.Plymouth Uo. Could a cholesterol-lowering drug be a potential treatment for Parkinson’s? Cholesterol-lowering drug Simvastatin trialled as a potential neuroprotective treatment for Parkinson’s. ScienceDaily 2016. 2016 Jan 11; [Google Scholar]
- 21.Tan EK, Tan LC. Holding on to statins in Parkinson disease. Neurology. 2013;81(5):406–407. doi: 10.1212/WNL.0b013e31829d87bb. [DOI] [PubMed] [Google Scholar]
- 22.Anonymous. Putting Research Data Into Your Hands with the MarketScan Databases. Truven Health Analytics; 2016. http://truvenhealth.com/markets/life-sciences/products/data-tools/marketscan-databases. [Google Scholar]
- 23.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;63(25 Pt B):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Hu G, Antikainen R, Jousilahti P, Kivipelto M, Tuomilehto J. Total cholesterol and the risk of Parkinson disease. Neurology. 2008;70(21):1972–1979. doi: 10.1212/01.wnl.0000312511.62699.a8. [DOI] [PubMed] [Google Scholar]
- 25.Huang X, Miller WC, et al. Cardiovascularly “desirable” cholesterol levels associated with Parkinson’s disease. Ann Neurol. 2005;58(Suppl 9):S24. [Google Scholar]
- 26.Scigliano G, Musicco M, Soliveri P, Piccolo I, Ronchetti G, Girotti F. Reduced risk factors for vascular disorders in Parkinson disease patients: a case-control study. Stroke. 2006;37(5):1184–1188. doi: 10.1161/01.STR.0000217384.03237.9c. [DOI] [PubMed] [Google Scholar]
- 27.Miyake Y, Tanaka K, Fukushima W, et al. Case-control study of risk of Parkinson’s disease in relation to hypertension, hypercholesterolemia, and diabetes in Japan. J Neurol Sci. 2010;293(1–2):82–86. doi: 10.1016/j.jns.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Mollenhauer B, Trautmann E, Sixel-Doring F, et al. Nonmotor and diagnostic findings in subjects with de novo Parkinson disease of the DeNoPa cohort. Neurology. 2013;81(14):1226–1234. doi: 10.1212/WNL.0b013e3182a6cbd5. [DOI] [PubMed] [Google Scholar]
- 29.de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum cholesterol levels and the risk of Parkinson’s disease. Am J Epidemiol. 2006;164(10):998–1002. doi: 10.1093/aje/kwj283. [DOI] [PubMed] [Google Scholar]
- 30.Simon KC, Chen H, Schwarzschild M, Ascherio A. Hypertension, hypercholesterolemia, diabetes, and risk of Parkinson disease. Neurology. 2007;69(17):1688–1695. doi: 10.1212/01.wnl.0000271883.45010.8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang X, Abbott RD, Petrovitch H, Mailman RB, Ross GW. Low LDL cholesterol and increased risk of Parkinson’s disease: prospective results from Honolulu-Asia Aging Study. Mov Disord. 2008;23(7):1013–1018. doi: 10.1002/mds.22013. [DOI] [PubMed] [Google Scholar]
- 32.Lin KD, Yang CY, Lee MY, Ho SC, Liu CK, Shin SJ. Statin therapy prevents the onset of Parkinson disease in patients with diabetes. Ann Neurol. 2016;80(4):532–540. doi: 10.1002/ana.24751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.