Abstract
Monoglyerides (MGs) are short-lived, intermediary lipids deriving from the degradation of phospho- and neutral lipids, and monoglyceride lipase (MGL), also designated as monoacylglycerol lipase (MAGL), is the major enzyme catalyzing the hydrolysis of MGs into glycerol and fatty acids. This distinct function enables MGL to regulate a number of physiological and pathophysiological processes since both MGs and fatty acids can act as signaling lipids or precursors thereof. The most prominent MG species acting as signaling lipid is 2-arachidonoyl glycerol (2-AG) which is the most abundant endogenous agonist of cannabinoid receptors in the body. Importantly, recent observations demonstrate that 2-AG represents a quantitatively important source for arachidonic acid, the precursor of prostaglandins and other inflammatory mediators. Accordingly, MGL-mediated 2-AG degradation affects lipid signaling by cannabinoid receptor-dependent and independent mechanisms. Recent genetic and pharmacological studies gave important insights into MGL’s role in (patho-)physiological processes, and the enzyme is now considered as a promising drug target for a number of disorders including cancer, neurodegenerative and inflammatory diseases. This review summarizes the basics of MG (2-AG) metabolism and provides an overview on the therapeutic potential of MGL.
Keywords: monoglyceride lipase, MGL, MAGL, 2-arachidonoyl glycerol, arachidonic acid, cannabinoid receptor, eicosanoids
1. Introduction
1.1. Milestones in monoglyceride lipase research
Early work from the 1960s suggested the presence of a distinct enzymatic activity that hydrolyzes monoglycerides (MGs) to glycerol and fatty acids in the intestine (Senior and Isselbacher, 1963) and in adipose tissue (Vaughan et al., 1964) of rats. This activity was later ascribed to monoglyceride lipase [MGL, also known as monoacylglycerol lipase (MAGL)], a 33 kDa serine hydrolase that cleaves MGs at neutral pH (Kupiecki, 1966). Subsequently, MGL was purified, cloned, and enzymatically characterized (Tornqvist and Belfrage, 1976; Karlsson et al., 1997). The enzyme harbors an alpha/beta hydrolase fold and a catalytic triade with an active serine located within a GXSXG consensus sequence commonly found in lipases (Karlsson et al., 1997). MGL returned into the spotlight of research when Dinh et al. demonstrated that it degrades 2-arachidonoyl glycerol (2-AG), the most abundant endocannabinoid (EC) in the body (Dinh et al., 2002). This finding led to the development of powerful tools used today in MGL research, including selective MGL inhibitors (Table 1) and genectic mouse models of MGL deficiency or overexpression (Table 2). Thereby, MGL was identified as the major enzyme providing arachidonic acid (AA) for eicosanoid synthesis in certain tissues (Nomura et al., 2011b). Furthermore, MGL’s crystal stucture was solved unveiling how the enzyme interacts with membranes, substrates, and inhibitors (Bertrand et al., 2010; Labar et al., 2010a; Schalk-Hihi et al., 2011). Genetic as well as pharmacological studies gave important insights into MGL’s role in (patho-) physiological processes, and the enzyme is now considered as a promising therapeutic target for the treatment of a number of disorders including cancer, neurodegenerative and inflammatory diseases.
Table 1. MGL Inhibitors.
| INHIBITOR | ORGANISM | IC50 | REFERENCE |
|---|---|---|---|
| URB602 | Rat/mouse | 28 µM | (Hohmann et al., 2005) |
| NAM | Rat/mouse | 0.14 µM | (Saario et al., 2005) |
| JZL184 | Mouse /(rat, human) | 0.008 µM | (Long et al., 2009c) |
| OMDM169 | Rat/mouse | 0.34 µM/7.3µM | (Bisogno et al., 2009) |
| JZL195 (MGL and FAAH) | Mouse | 0.013 and 0.019 µM | (Long et al., 2009b) |
| KML29 | Rat (mouse, human) | 0.015 µM | (Chang et al., 2012) |
| MJN110 | Mouse | 0.0095 µM | (Chang et al., 2013) |
| sar127303 | Mouse/human | 0.0038 and 0.029 µM | (Griebel et al., 2015) |
Table 2. Genetic mouse models of MGL deficiency or overexpression.
| ORIGIN | KNOCKOUT/TRANSGENE | BACKGROUND | OBSERVATION | REFERENCE |
|---|---|---|---|---|
| MGL-ko | global | 129SvEv x C57BL/6J | CB1 receptor desensitization | (Schlosburg et al., 2010) |
| MGL-ko | global | C57BL/6NTac | Decreased CB1 receptor density | (Chanda et al., 2010) |
| MGL-ko | global | C57BL/6J | Impaired lipolysis Increased insulin sensitivity on HFD CB1 receptor desensitization |
(Taschler et al., 2011) |
| MGL-ko | global | C57BL/6 | Tissue expression analyses of MGL | (Uchigashima et al., 2011) |
| MGL-ko | global | C57BL/6 | Delayed lipid absorption; resistance to HFD induced obesity | (Douglass et al., 2015) |
| MGL-ko/ApoE-ko | global | C57BL/6J | Increased plaque stability on Western type diet | (Vujic et al., 2016) |
| MGLflox/GluN2Cre | granula cells | C57BL/6 | (Tanimura et al., 2012) | |
|
MGLflox/Rosa26-Cre MGLflox/Eno2-Cre MGLflox/GFAP-Cre MGLflox/LysM-Cre |
global neurons astrocytes microglia |
Astrocytes and neurons collaborate to terminate endocannabinoid signaling; Astrocytes couple 2-AG hydrolysis to neuroinflammatory prostaglandin production |
(Viader et al., 2015) | |
| MGLflox/GFAP-Cre | astrocytes | 129Sv x C57BL6/J | Protected from LPS induced inflammation | (Grabner et al., 2016) |
| CaMKIIA-MGL Transgene | forebrain | C57BL/6 | Decreased 2-AG in forebrain, resistance to HFD induced obesity | (Jung et al., 2012) |
| IFABP-MGL Transgene | intestine | SJL, F4-F10 | Development of obesity on HFD | (Chon et al., 2012) |
1.2. Overview on monoglyceride metabolism
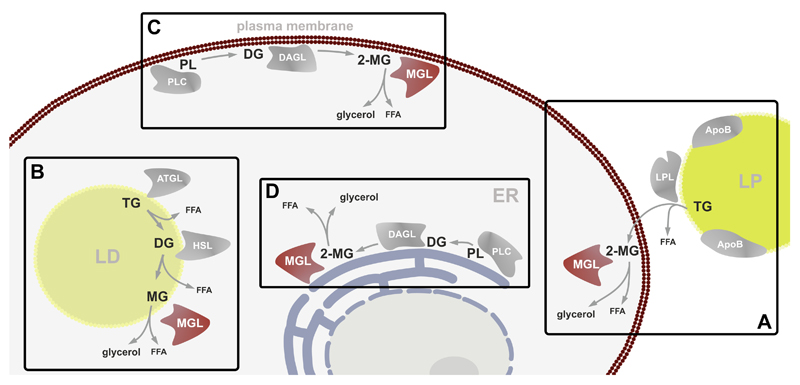
MGs are short-lived lipids deriving from intra- and extracellular sources. The extracellular quantitatively most important source of MGs are triglyceride- (TG)-rich lipoproteins since lipoprotein lipase (LPL) hydrolyzes TGs in sn-1- and sn-3-position, generating 2-MG species (Goldberg and Merkel, 2001). Similarly, digestion of dietary TG by pancreatic lipase results in the generation of 2-MG (Lowe, 1994). Extracellularly generated MGs are internalized by cells and may be degraded by MGL (Figure 1A) or re-esterified to TG by acyl-CoA:monoacylglycerol and acyl-CoA:diacylglycerol acyltransferase (MGAT and DGAT) reactions. The latter pathway is specifically important in the small intestine where MGAT enzymes synthesize about 80% of the TG incorporated into chylomicrons (reviewed in (Shi and Cheng, 2009)). MGs can also derive from intracellular TGs stored in cytosolic lipid droplets (Figure 1B). These TGs are hydrolyzed by the sequential action of two lipases. The first and rate-limiting step is catalyzed by adipose triglyceride lipase (ATGL) generating diglycerides (DGs) and fatty acids. These DGs are then hydrolyzed by hormone-sensitive lipase (HSL), releasing another fatty acid and thereby generating MG (Schweiger et al., 2006). Finally, MGL cleaves the last fatty acid off the MG and releases glycerol. Membrane bound glycerophospholipids are precursors for the generation of MGs (Figure 1C, D). Glycerophospholipids are hydrolyzed by phospholipase C (PLC) to generate DGs which are further metabolized by sn-1-specific DG lipases (DAGLα and DAGLβ) (Bisogno et al., 2003) to generate 2-MG (Stella et al., 1997; Bisogno et al., 1999b). This is the major pathway for the generation of the EC 2-AG. AA and other polyunsaturated fatty acids (PUFAs) are commonly found esterified in sn-2 position of glycerophospholipids and TGs. Accordingly, by degrading 2-MG from different sources, MGL can affect PUFA metabolism. It is currently unknown whether also 2-AG, deriving from TG of extra- or intracellular sources, can contribute to EC signaling. As summarized in Figure 1, the heterogeneity of MG sources correlates with the subcellular distribution of MGL. The enzyme has been shown to localize to plasma membranes, endoplasmic reticulum, and lipid droplets (Tornqvist and Belfrage, 1976; Sakurada and Noma, 1981; Dinh et al., 2002; Blankman et al., 2007). Upon over-expression of MGL in cells, it equally distributes between membrane and cytosolic fractions (Dinh et al., 2002), however, brain tissue fractionation revealed that more than 90 % of endogenous MGL activity is found in the membrane fraction (Blankman et al., 2007).
Figure 1. Sources of monoglycerides.
Monoglycerides (MGs) are ubiquitously occurring intermediate lipids derived from various cellular processes. (A) The major extracellular source are triglycerides (TG) from lipoproteins (LP) which are hydrolyzed by lipoprotein lipase (LPL) to generate sn-2 monoglycerides (2-MGs). (B) Also, intracellular TGs stored in lipid droplets (LD) are a source of MGs. Sequential hydrolysis of TGs via adipose triglyceride lipase (ATGL) generating free fatty acids (FFAs) and diglycerides (DGs), which are subsequently hydrolyzed by hormone sensitive lipase (HSL) to MGs and FFAs. (C, D) On cellular membranes, including plasma membrane and membranes of the endoplasmic reticulum (ER), 2-MGs are generated from phospholipids (PL) by hydrolysis through phospholipase C (PLC) to DGs and subsequent hydrolysis by diglyceride lipases (DAGL) to MGs.
1.3. Biochemical properties, tissue distribution, and regulation of MGL
MGL is capable of hydrolyzing MG species with different fatty acid chain length and saturation. The enzyme has no positional preference for sn-1(3) or 2-MGs, yet a slight preference for MG species containing unsaturated fatty acids (Ghafouri et al., 2004; Vandevoorde et al., 2005). Recent data suggest that MGL also degrades prostaglandin glycerol esters, which represent still poorly characterized inflammatory mediators (Savinainen et al., 2014). Finally, MGL has been implicated in non-oxidative ethanol metabolism by hydrolyzing fatty acid ethyl esters which are produced in the body in response to alcohol consumption (Heier et al., 2016).
Genetic as well as pharmacological inactivation of MGL in mice causes a strong increase of MGs in many tissues including brain, liver, adipose tissue, intestine, and others, demonstrating a major role of the enzyme in MG catabolism (Long et al., 2009c; Chanda et al., 2010; Schlosburg et al., 2010). Accordingly, MGL is expressed in many cell types of various tissues. In mice, highest expression is observed in brown and white adipose tissue (Karlsson et al., 1997) and the brain (Dinh et al., 2002). MGL is expressed throughout the major cell types of the brain, including neurons, astrocytes, oligodendrocytes, and to a lower extent in microglia (Dinh et al., 2002; Stella, 2004). Several studies demonstrated the existence of MGL proteins with different molecular weight by Western Blot analysis. In murine adipose tissue, liver, heart, lung, spleen, kidney, and adrenal glands, a single MGL band is observed at 33 kDa, whereas in skeletal muscle, MGL can be detected at 40 kDa. In testis, MGL is found at 30 kDa, and in the brain even two bands are observed at 33 and 35 kDa (Karlsson et al., 2001; Blankman et al., 2007). Although it is not fully understood how these variations occur, they very likely derive from differential use of start codons in the 5’ leader sequence of the Mgll gene as well as alternative splicing (Karlsson et al., 2001). The existence of different splice variants might also explain the variation in the subcellular localization of MGL. For human Mgll, splice variants have been described that lack exon 5 which encodes for the cap region which is implicated in substrate selectivity and membrane localization (Bertrand et al., 2010; Labar et al., 2010a; Scalvini et al., 2016).
Surprisingly, little is known about the regulation of MGL. At the transcriptional level, microarray analysis of murine liver revealed that MGL expression is regulated via the transcription factor peroxisome proliferator activated receptor α (PPARα) (Rakhshandehroo et al., 2007). PPARs are nuclear receptors that heterodimerize with retinoid X receptor upon ligand binding and thereby activate transcription of genes, most of which are involved in energy metabolism (Grygiel-Górniak, 2014). In wild-type mice, PPARα agonist treatment led to a 6-fold increase in Mgll gene expression, a response that was absent in PPARα-deficient mice (Rakhshandehroo et al., 2007).Furthermore, Chon et al. demonstrated that intestinal and hepatic MGL protein levels are induced in mice upon feeding a diet that contains a high amount of fat, indicating nutritional regulation of MGL expression (Chon et al., 2007).
At the posttranslational level, recent data suggest that MGL activity is regulated via sulfenylation of cysteine residues by H2O2. The involved cysteines are located in the cap region that controls entry of the substrate into the active site of the enzyme. H2O2 exposure leads to a rapid, reversible, non-competitive inhibition of MGL activity. This inhibition may be relevant under conditions associated with increased oxidative stress such as brain ischemia in which increased 2-AG levels may have protective effects (Dotsey et al., 2015).
1.4. Other MG hydrolases
Although MGL is clearly the major enzyme in MG catabolism in most tissues, other MG hydrolases may contribute to this process in cell types or cellular compartments that do not contain MGL. HSL was the first intracellular enzyme characterized with MG hydrolase activity (Fredrikson et al., 1986; Yeaman, 1990). This enzyme is highly expressed in adipose tissue and plays an important role in the mobilization of fatty acids from TG stores. Yet, HSL has substantial MG hydrolase activity and can therefore partially compensate MGL deficiency in lipolysis (Taschler et al., 2011). Fatty acid amide hydrolase (FAAH), responsible for the degradation of the EC arachidonoyl ethanolamine (AEA, also anandamide), was also shown to hydrolyze 2-AG in vitro (Goparaju et al., 1998). However, FAAH-deficient mice have unaltered 2-AG levels, questioning a physiological role of FAAH as a MG hydrolase in vivo (Cravatt et al., 2001). In 2007, α/β hydrolase domains containing protein 6 and 12 (ABHD6 and ABHD12) were identified as additional MG hydrolases in the brain, accounting for about 15% of total membrane activity (Blankman et al., 2007). Recent reports suggest that ABHD12 is involved in phospholipid rather than MG metabolism. ABHD12 acts as a potent lyso-phosphatidyl serine hydrolase, and human ABHD12 mutations or genetic deletion in mice are associated with the neurodegenerative disease PHARC (polyneuropathy, hearing loss, ataxia, retinitis pigmentosa, and cataract disease) (Fiskerstrand et al., 2010; Blankman et al., 2013). ABHD6 was proposed to acts as the major 2-AG hydrolase in cell types in which MGL is not expressed (Marrs et al., 2010; Alhouayek et al., 2013). Nevertheless, MG hydrolase activity of ABHD6 may also play a role in cells expressing MGL, via distinct cellular distribution of both enzymes. E.g., in neurons, ABHD6 is localized at postsynaptic membranes while MGL is predominantly found presynaptically. Accordingly, ABHD6 may counteract 2-AG formation at the post-synapse (Marrs et al., 2010). Moreover, recent evidence suggests that ABHD6 plays a role in the hydrolysis of MGs esterified in sn-1 position, especially 1-palmitoyl glycerol and 1-stearoyl glycerol. Zhao et al. suggested that these saturated MG species activate Munc13-1 that is required for the priming of insulin containing vesicles, thereby promoting exocytosis of insulin in pancreatic β-cells (Zhao et al., 2014, 2015). Conversely, inhibition of MGL decreases glucose-stimulated insulin secretion in INS-1 cells and rat islets (Berdan et al., 2016). In contrast to MGL, ABHD6 can also hydrolyze acidic lysophospholipids and the late endosomal/lysosomal lipid bis(monoacylglycero)phosphate (Thomas et al., 2013; Pribasnig et al., 2015). The enzyme co-localizes with acidic organelles and might be therefore involved in the remodeling of outer membranes of these organelles (Pribasnig et al., 2015). Recently, another member of the ABHD family, namely ABHD2, was identified as a 2-AG hydrolase in spermatozoa. This highly interesting study suggests that ABHD2 is directly activated by progesterone and responsible for the genome-independent action of the hormone via 2-AG signaling (Miller et al., 2016). This observation implicates that lipid-metabolizing enzymes can represent direct targets of hormones. Besides ABHD family members, triglyceride hydrolase 2, rat esterase 4 and 10, carboxyl esterase 1, and neuropathy target esterase have been shown to hydrolyze MG in vitro (Lehner and Vance, 1999; van Tienhoven et al., 2002; Xie et al., 2010). While all these hydrolases are active at neutral pH, lysosomal acid lipase was reported to hydrolyze MG in acidic organelles (Sheriff et al., 1995).
2. MGL in endocannabinoid signaling
After initial characterization, MGL returned into the spotlight of research when Dinh et al. demonstrated that MGL is the primary enzyme in the brain hydrolyzing 2-AG. This MG species belongs to a class of signaling lipids called ECs which are primarily derivatives of AA. In general, it is thought that Δ9-tetrahydrocannabinol (Δ9-THC), the major psychoactive component of Cannabis sativa (Gaoni and Mechoulam, 1964), mimics the action of ECs. Δ9-THC, synthetic cannabinoids, and ECs bind and activate cannabinoid (CB) receptors of which two have been characterized. The CB1 receptor is expressed mainly in central and peripheral neurons (Howlett et al., 1990), but also in various other cell types including adipocytes and hepatocytes (Pagotto et al., 2006). Its activation is involved in the regulation of various physiological processes including appetite, activity, emotions, pain processing, and energy homeostasis (de Kloet and Woods, 2009; Maccarrone et al., 2011; Silvestri and Di Marzo, 2013; Hasenoehrl et al., 2016). The CB2 receptor is predominantly expressed in cells of the hematopoietic system and important in the regulation of immune response (Munro et al., 1993; Galiègue et al., 1995). Besides 2-AG, AEA acts as an EC, however its tissue abundance is more than 100 times lower in the brain as compared to 2-AG (Bisogno et al., 1999a). Generally, ECs are produced “on demand” and are rapidly degraded by intracellular enzymes after receptor binding. ECs, together with the two receptors and the EC metabolizing enzymes, constitute the EC system. CB receptors mainly signal through Gi/o proteins, inhibit voltage gated Ca2+ channels and activate A-type and inwardly rectifying K+ currents (Howlett, 2005). Whether other receptors, like the orphan G-protein coupled receptor 55 (GPR55) and the transient receptor potential cation channel subfamily V member 1 (TRPV1) are EC-responsive receptors, and contribute to CB1/CB2 receptor independent effects of ECs requires further investigation (Di Marzo et al., 1998; Ryberg et al., 2007). Furthermore, PPARs respond to synthetic cannabinoids and ECs, and may mediate some effects (O’Sullivan, 2016).
Although both AEA and 2-AG derive from phospholipids, they have distinct pathways of synthesis and degradation. AEA is generated by a Ca2+-activated N-arachidonoyl-phosphatidylethanolamine selective phospholipase D (NAPE-PLD) (Okamoto et al., 2004), or alternatively by the phospholipase ABHD4 and glycerophospho-diesterase 1 (Simon and Cravatt, 2008). AEA and other N-acyl ethanolamines are hydrolyzed by FAAH (Cravatt et al., 2001; Deutsch et al., 2002). AEA synthesis and degradation pathways are reviewed in detail elsewhere (Cascio and Marini, 2015).
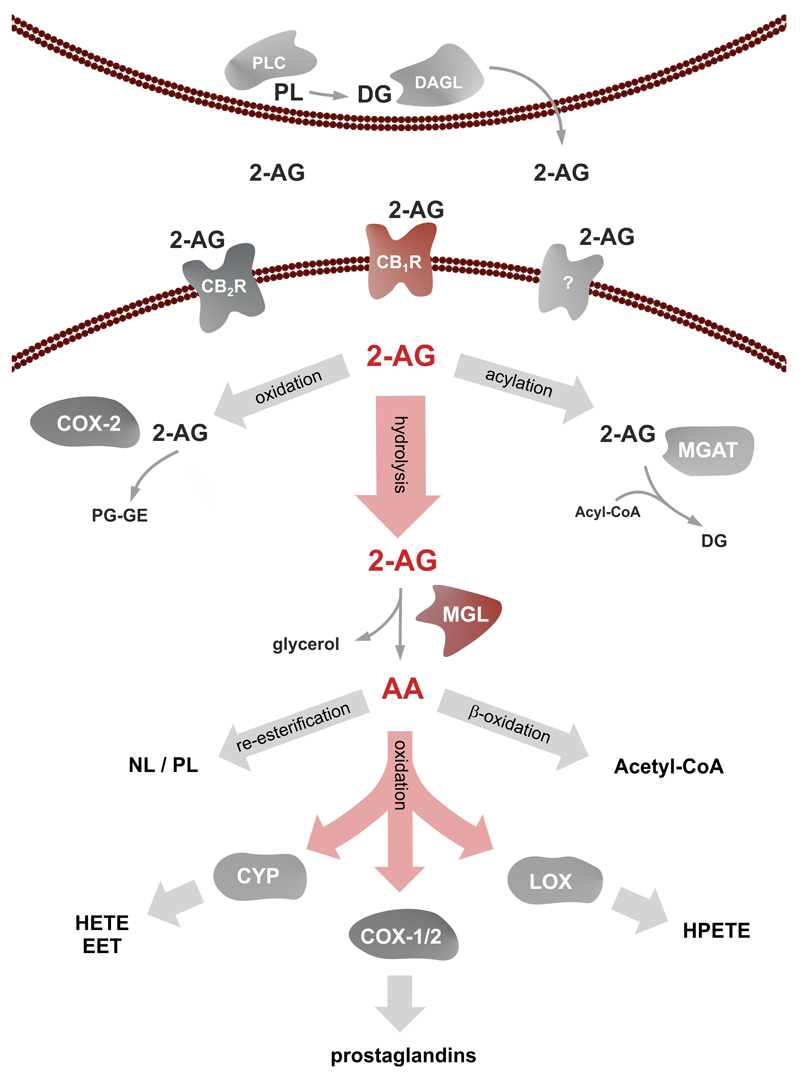
2-AG is synthesized by the consecutive action of a PLC generating 2-arachidonoyl DG which is further hydrolyzed by sn-1 specific DAGLs. Very recently, DDH domain containing 2 has been shown to possess DG hydrolase activity and its overexpression in CHO cells results in an increased generation of 2-AG (Araki et al., 2016). 2-AG may undergo different metabolic pathways resulting in inactivation or in the generation of other signaling molecules. The strong increase in 2-AG levels in MGL-knockout (ko) mice implicates that most of the EC is degraded by hydrolysis. However, 2-AG can be phosphorylated to lyso-phosphatidic acid by MG kinase(s) (Simpson et al., 1991) and metabolized by cyclooxygenase-2 (COX-2) resulting in the formation of prostaglandin glycerol esters (Gopez et al., 2005; Telleria-Diaz et al., 2010). Alternatively, 2-AG may be acylated by acyltransferases to generate DG (Yen et al., 2002, 2003, Cao et al., 2003, 2007). A schematic overview of 2-AG synthesis and degradation is illustrated in Figure 2.
Figure 2. Monoglyceride lipase in 2-arachidonoyl glycerol signaling and arachidonic acid metabolism.
2-AG is generated by hydrolysis of membrane phospholipids (PL) to diglycerides (DGs) by phospholipase C (PLC) and subsequent hydrolysis by diglyceride lipase (DAGL). 2-AG is secreted and binds to CB1 receptors (CB1R), CB2 receptors (CB2R), or other yet uncharacterized receptors. 2-AG is then metabolized by oxidation through cyclooxygenase 2 (COX-2) generating prostaglandin-glyceryl esters (PG-GE) or acylation through monoacylglycerol acyltransferase (MGAT) to DG. The majority of 2-AG is hydrolyzed by monoglyceride lipase (MGL) to glycerol and arachidonic acid (AA). AA is either re-esterified into cellular neutral lipid (NL) or PLs or conducted to β-oxidation to generate acetyl-CoA. AA can also be subjected to oxidation by COX-1, COX-2, lipoxygenases (LOX), or cytochrome p450 (CYP) generating important signaling lipids like prostaglandins, hydroxyeicosatetraenoic acid/epoxy-eicosatrienoic acid (HETE, EET), and hydroperoxyeicosatetraenoic acid (HPETE).
2.1. MGL blockade and CB receptor desensitization
Within recent years, different strategies have been established to investigate the physiological role of MGL in vivo. Several MGL inhibitors have been synthetized that block MGL activity, as reviewed extensively elsewhere (Labar et al., 2010b; Kohnz and Nomura, 2014) (Table 1), and different genetically modified mouse models have been generated (Table 2). One of the first selective inhibitors developed was JZL184 which inhibits MGL by irreversible active site carbamylation (Long et al., 2009c). JZL184 inhibits around 90% of 2-AG hydrolase activity in the brain and leads to strong accumulation of 2-AG and other MGs 30 min after administration (Long et al., 2009c). However, MGs did not increase in all tissues which could either indicate that MGL activity is compensated by other MG hydrolases in certain tissues or that JZL184 shows a tissue-specific distribution pattern and MGL is not fully inhibited in all tissues (Long et al., 2009a).
The in vivo effects of MGL inhibitors have been investigated using the “tetrad test for cannabinoid activity” which consists of four individual tests: analgesia, sedation, catalepsy, and hypothermia (Wiley and Martin, 2003). Acute blockade of MGL induces analgesia, hypomotility, and hypothermia, while catalepsy was not observed (Long et al., 2009c). The effects of JZL184 are dependent on CB1 receptor activation since they were absent in CB1 receptor-deficient (CB1-ko) mice. None of the MGL inhibitors developed so far produced catalepsy which might require the inhibition of both MGL and FAAH as shown with the dual inhibitor JZL195 (Long et al., 2009b). However, these results clearly demonstrate that CB1 receptor agonists and MGL inhibitors have similar effects in vivo.
As mentioned above, MGL-ko mice exhibit dramatically increased 2-AG levels in the brain and in peripheral tissues. Notably, increased 2-AG levels do not evoke cannabimimetic effects in these mice as observed in response to acute inhibitor treatment. The reason for this discrepancy is that chronically elevated 2-AG in MGL-ko mice causes desensitization of CB1 receptors in the brain similar to what may occur in response to chronic, heavy cannabis abuse (González et al., 2005; Borgelt et al., 2013). Accordingly, MGL-deficiency is associated with decreased CB1 receptor density, reduced CB1 receptor ligand binding (Chanda et al., 2010; Schlosburg et al., 2010), and resistance to the hypometabolic effects of CB receptor agonists (Taschler et al., 2011). A recent study suggests that chronically increased 2-AG induced basal CB1 receptor/Gi/o signaling in the brain but dampened agonist-evoked responses (Navia-Paldanius et al., 2015). Impaired CB1 receptor signaling due to desensitization in the brain is accompanied by increased β-arrestin 2 binding and a significant reduction of ERK1/2 phosphorylation in different brain regions, such as the amygdala, medial prefrontal cortex, and hippocampus (CA1 and CA3), but not in the cerebellum (Imperatore et al., 2015). Interestingly, β-arrestin2 mediated CB1 receptor desensitization was observed in brain areas controlling emotional and stress related states, pain sensation, memory and learning, whereas brain areas associated with motor function were not affected by desensitization. Receptor internalization/desensitization is a process generally observed for G-protein coupled receptors, and the CB1 receptor was shown to internalize only minutes after receptor activation in cell culture (Hsieh et al., 1999). Recently, also desensitization of peripherally expressed CB1 receptors in the enteric nervous system has been demonstrated by chronic pharmacological inhibition or genetic deletion of MGL. While MGL-ko mice show normal gut motility under physiological conditions, they are insensitive to CB receptor agonist- induced intestinal hypomotility (Taschler et al., 2015). Whether CB1 receptor activity/expression is impaired in other peripheral tissues and non-neuronal cells remains elusive.
In contrast to the CB1 receptor, data on CB2 receptor desensitization/internalization are scarce. Previous reports suggest that CB2 receptors are not desensitized in MGL-ko mice. Chanda et al. showed by CB receptor agonist binding studies that CB2 receptor density is unaltered in the spleen, however, a functional characterization of the receptor activity was not performed (Chanda et al., 2010). In a more recent study, Vujic et al. suggest that CB2 receptors are not desensitized in MGL-ko macrophages isolated from mice on an ApoE-deficient background. These conclusions are based on Ca2+-flux experiments in response to 2-AG stimulation (Vujic et al., 2016). Yet, cell culture experiments show that CB2 receptors undergo internalization after activation (Grimsey et al., 2011). Since 2-AG is a physiologically relevant agonist of CB2 receptors, it is reasonable to assume that also these receptors desensitize in MGL-ko mice. Thus, careful evaluation of inhibitor concentrations may be the key to avoid CB receptor desensitization upon MGL inhibition. Indeed, while the injection of JZL184 at concentrations of 8mg/kg mouse and higher over a period of 5 days causes CB1 receptor desensitization (Schlosburg et al., 2010), similar to observations in MGL-ko mice, the injection of 4mg/kg mouse did not promote CB1 receptor desensitization (Kinsey et al., 2013). Burston et al. investigated the effects of acute and chronic MGL inhibition in a rat model of osteoarthritis. Repeated administration of MJN110 at high doses (5mg/kg) resulted in anti-nociceptive tolerance whereas lower concentrations (1mg/kg) preserved antinociception (Burston et al., 2016).
Despite CB1 receptor desensitization caused by chronic MGL inhibition, blockade of the enzyme has still protective effects in a variety of pathophysiological states such as inflammation and neurodegeneration, mostly via CB1 receptor independent mechanisms as discussed in the following section (Nomura et al., 2011b; Viader et al., 2015; Grabner et al., 2016).
3. MGL in inflammation and pain
Cannabinoids have a long history as analgesics (Touw, 1981; Mechoulam and Parker, 2013) and research of the last decades suggests close connections between the EC system and inflammation (Turcotte et al., 2015). MGL is a highly interesting target for the treatment of inflammation since it conversely regulates 2-AG and AA levels in several tissues (Dinh et al., 2002; Long et al., 2009c). Accordingly, MGL deficiency exerts a dual anti-inflammatory function. First, MGL ablation leads to augmented 2-AG levels, leading to increased CB receptor activation. Activation of CB1 or CB2 receptors by endogenous or exogenous agonists has been shown to have beneficial effects on various diseases harboring an inflammatory component (Klein, 2005; Turcotte et al., 2015). Second, MGL limits AA availability causing CB receptor independent anti-inflammatory effects in the brain and some peripheral tissues (Figure 2). Notably, cytosolic PLA2 (cPLA2) has been considered as mainly responsible for mobilizing AA for prostaglandin synthesis in the brain (Buczynski et al., 2009). Yet, cPLA2-deficient mice do not show a reduction in brain free AA levels indicating that other enzymes mediate this process (Rosenberger et al., 2003). Upon characterization of mice treated with the MGL inhibitor JZL184, a significant, dose dependent reduction in brain AA was observed (Long et al., 2009c). This effect was also confirmed in MGL-deficient mice (Chanda et al., 2010; Schlosburg et al., 2010). Further, it was demonstrated that global genetic deletion or pharmacological inhibition of MGL protects mice from LPS-induced neuroinflammation independent of CB receptor activation, due to reduced AA availability for prostaglandin synthesis (Nomura et al., 2011b). Diminished prostaglandin levels attenuate cytokine production and microglia activation (Nomura et al., 2011b). Since then, numerous studies demonstrated beneficial effects of MGL ablation on various disease models harboring an inflammatory component. These effects are mediated by CB receptor dependent and/or independent effects. MGL inhibition improves pathologies of Parkinson’s (Nomura et al., 2011b) and Alzheimer’s disease (Chen et al., 2012; Piro et al., 2012), multiple sclerosis (Pryce et al., 2013; Hernández-Torres et al., 2014), and Down syndrome (Lysenko et al., 2014). Interestingly, MGL in neurons and astrocytes equally contributes to brain AA availability upon inflammatory stimuli, while MGL in microglia plays a minor role (Viader et al., 2015; Grabner et al., 2016). In peripheral tissues, MGL inhibition leads to attenuated liver (Cao et al., 2013), lung (Costola-de-Souza et al., 2013), and muscle injury (Jiang et al., 2015). MGL deficiency also extenuates colitis and related systemic inflammation (Alhouayek et al., 2011).
Pharmacological inhibition of MGL thus represents a highly interesting strategy to treat inflammation. To date, the majority of anti-inflammatory drugs used are COX inhibitors, prescribed in many of the aforementioned diseases. As an example, pharmacological inhibition or genetic deletion of COX-1 has beneficial effects on neuroinflammation and neurodegenerative disorders (Choi et al., 2008; Vlad et al., 2008; Choi and Bosetti, 2009). A severe drawback of COX inhibitors however is that they cause gastrointestinal (GI) injury (Singh, 1998; Ng and Chan, 2010). Side effects of selective COX-2 inhibitors (coxibs) are less severe in the GI tract, however, wide usage of these drugs is restricted owing to cardiovascular complications (Cannon and Cannon, 2012). Inhibition of MGL could thus represent an anti-inflammatory strategy, which avoids adverse effects of COX inhibition. The beneficial effects of MGL ablation are thereby comparable to those of COX inhibitors, however, MGL inhibition by JZL184 does not lead to GI injury, but protects from COX-1 inhibitor induced GI damage (Kinsey et al., 2011a). Recently, a Phase 1b study has been initiated to evaluate the effects of a single dose of the orally active human MGL inhibitor ABX-1431 (ABIDE Therapeutics) for the treatment of functional dyspepsia.
Cannabinoids and (naturally occurring) COX inhibitors have a long common history, likely being the first analgesics used by humans. To date, it is well established that CB receptor agonists exhibit analgesic effects (Manzanares et al., 2006; Fine and Rosenfeld, 2013). Concordantly, pharmacological MGL inhibition leads to anti-nociceptive effects in various models of pain, including inflammatory, neuropathic, thermal, and chemically induced pain in a 2-AG/CB1 receptor dependent manner (Kinsey et al., 2009; Long et al., 2009c; Guindon et al., 2011; Woodhams et al., 2012; Ghosh et al., 2013; Griebel et al., 2015). The orally active MGL inhibitor SAR127303 has been reported to produce anti-nociceptive properties and even repeated administration caused no tolerance since the effects were maintained in the formalin test. However, the authors also demonstrate that inhibition of MGL impaired memory performance (Griebel et al., 2015). Interestingly, administration of the highly selective MGL inhibitor KML29 produces analgesia without cannabimimetic side effects, its chronic administration, however, leads to CB1 receptor desensitization, as similarly observed for other MGL inhibitors (Ignatowska-Jankowska et al., 2014). Also, genetic mouse models of MGL deficiency, do not show analgesic properties due to constantly increased 2-AG levels and subsequent CB1 receptor desensitization (Chanda et al., 2010; Schlosburg et al., 2010), as mentioned previously. Nevertheless, by combining anti-inflammatory and anti-nociceptive properties, MGL inhibitors may represent a promising strategy to counteract inflammation and pain.
4. MGL in metabolic disorders
Metabolic syndrome is a major public health care concern and combines several pathological conditions, including abdominal obesity, elevated blood pressure, insulin resistance and high blood glucose, high serum TG and low high-density lipoprotein levels. It is closely associated with the risk to develop type 2 diabetes and cardiovascular disease. A large number of studies implicates that metabolic disorders are associated with the EC system. In general, activation of CB1 receptors is associated with increased lipid deposition, increased lipogenesis, hyperphagy, and hypomotility as reviewed in (Quarta et al., 2011). In line, acute treatment of mice with isopropyl dodecylfluorophosphonate (IDFP), an inhibitor of MGL and FAAH, caused hepatic steatosis and impaired glucose tolerance by CB1 receptor dependent and independent mechanisms (Ruby et al., 2011). Moreover, a single administration of JZL184 (40mg/kg) significantly increased food intake (Woodhams et al., 2012). In contrast, CB1-ko mice are resistant to diet induced obesity, hepatic steatosis and insulin resistance. These effects are mostly caused by reduced caloric intake and increased energy expenditure (Cota et al., 2003; Quarta et al., 2010). Similarly, CB1 receptor blockade with rimonabant (Acomplia®), a selective inverse CB1 receptor agonist (Rinaldi-Carmona et al., 1994), ameliorates hepatic steatosis and dyslipidemia in obese mice and rats (Gary-Bobo et al., 2007; Osei-Hyiaman et al., 2008; Tam et al., 2010). Important evidence was provided by studies in obese and diabetic patients treated with rimonabant. The drug promoted weight loss and improved cardiovascular risk factors (Van Gaal et al., 2005; Scheen et al., 2006). Yet, rimonabant was withdrawn from the market due to serious side-effects such as depression and even suicidal tendencies (Christensen et al., 2007). Based on these observations, it can be assumed that inactivation of MGL increases EC signaling, promoting obesity and co-morbidities. Yet, MGL-ko mice are lean and show no alterations in plasma lipid parameters in the fed state while fasted animals exhibit reduced plasma glycerol and TG levels. Moreover, MGL-deficiency protects from high fat diet (HFD) induced insulin resistance despite similar weight gain (Taschler et al., 2011). A more recent study suggests that MGL-ko mice exhibit decreased body weight and fat mass upon HFD feeding (Douglass et al., 2015). It is important to mention that the fat rich diets used in both studies vary in fat content (55% vs 25% fat w/w), possibly explaining the discrepancy in diet induced obesity. Also, Douglass et al. demonstrated even higher differences in body fat mass when using a diet with lower fat content (4,3% w/w) (Douglass et al., 2015). Together, these observations suggest that the metabolic effects of MGL inactivation cannot be explained by hyperactivation of the EC system. As mentioned above, MGL is involved in TG hydrolysis in adipocytes, and reduced lipolysis is known to counteract the development of insulin resistance (Boden, 2011). Furthermore, MGL inactivation has anti-inflammatory effects and insulin resistance is associated with inflammation in insulin-sensitive tissues (Samuel and Shulman, 2012). Finally, MGL inactivation can affect lipid signaling independent of CB receptors. 2-oleoyl glycerol (2-OG) has been identified as a ligand of the G-protein coupled receptor GPR119. Activation of this receptor stimulates the intestinal release of incretin hormones, like glucagon-like peptide-1 (GLP-1), having well-known beneficial effects in the development and progression of diabetic complications (Hansen et al., 2011). Important effects of GLP-1 are augmentation of insulin secretion and inhibition of glucagon release. Yet, MGL has also been directly implicated in the regulation of insulin release in pancreatic β-cells. A recently published study suggests that MGL inactivation inhibits glucose-stimulated and depolarization-induced insulin secretion (Berdan et al., 2016). Thus, in addition to changes in EC signaling, MGL inactivation may have complex effects on metabolic disorders by affecting lipolysis, inflammation, incretin and insulin signaling.
Evidence for an important role of MGL in energy homeostasis comes also from animal models overexpressing the enzyme. Transgenic mice that overexpress MGL in the intestine develop obesity and liver steatosis already after three weeks of HFD feeding, caused by decreased energy expenditure and hyperphagia. The authors speculated that 2-AG might act as a satiety signal (Chon et al., 2012). In contrast, other studies demonstrate that 2-AG in the intestine is increased upon fat intake and acts as hunger signal which is abandoned upon local CB1 receptor antagonism or surgical transection of the vagus nerve (DiPatrizio et al., 2011). Interestingly, this effect seems to be dependent on distinct fatty acids (DiPatrizio et al., 2013). Another study demonstrated that MGL, expressed in the central nervous system (CNS), is able to influence peripheral energy balance (Jung et al., 2012). Transgenic mice overexpressing MGL specifically in the forebrain are resistant to HFD-induced obesity mostly via elevated brown adipose tissue thermogenesis. Interestingly, these mice maintained leanness and insulin sensitivity despite increased food intake and decreased locomotor activity (Jung et al., 2012). Thus, MGL overexpression has different effects on energy homeostasis depending on the site of expression.
EC signaling has also been implicated in the pathogenesis of atherosclerosis. Initially, it was observed that low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice (Steffens et al., 2005). The beneficial effects of Δ9-THC were completely blocked by a selective CB2 receptor antagonist, implicating that CB2 receptors located on immune cells play a central role in this process. Subsequent studies showed that rimonabant has anti-atherosclerotic effects in low density lipoprotein (LDL) receptor–deficient mice (Dol-Gleizes et al., 2008). These effects are due to an enhancement of reverse cholesterol transport (Sugamura et al., 2010). Yet, clinical trials with rimonabant did not show any significant benefit of this agent in preventing progression of atherosclerosis (Nissen et al., 2008). A recent study investigated atherosclerosis-susceptibility of MGL/ApoE double knockout mice. Mice exhibited a body weight gain and plasma lipoprotein profile similar to their wild-type littermates. However, MGL-ko/ApoE-ko mice displayed larger lesion size and changes in plaque composition indicating increased plaque stability (Vujic et al., 2016). Treatment with a CB2 receptor inverse agonist prevented these changes suggesting that MGL inactivation provokes increased CB2 receptor signaling.
Together, all these studies emphasize an important role for MGL in the regulation of energy homeostasis. Acute blockade of MGL induces hyperphagy, while long-term blockade of MGL attenuates diet induced obesity and insulin resistance. Therefore, the potential of MGL inhibitors as therapeutic strategy to treat obesity have to be evaluated carefully. The effects of MGL inhibition cannot be limited to changes in EC signaling. Further studies are required to outline MGL’s role in the regulation of incretin/insulin secretion and inflammation in insulin-sensitive tissues. Nevertheless, inhibition of MGL might represent an interesting therapeutic option for the treatment of metabolic disease.
5. MGL in stress, emotion, and addiction
EC effects on neuronal transmission have a deep impact on stress response, mood, and emotion (Hill and Gorzalka, 2009; Lutz, 2009). Activation of CB1 receptors leads to anxiolysis and improves mood and depression like behavior in humans and animal models (Berrendero and Maldonado, 2002; Denson and Earleywine, 2006; Patel and Hillard, 2006). Conversely, administration of the CB1 receptor inverse agonist rimonabant leads to increased anxiety and depression in humans (Samat et al., 2008). The impact of (endo-)cannabinoid signaling on acute and chronic stress (Riebe and Wotjak, 2011; Hillard, 2014; Morena et al., 2015), emotion (McLaughlin and Gobbi, 2012), mood and depression (Gaetani et al., 2009; Micale et al., 2013) have been extensively reviewed recently, thus we only briefly summarize the role of MGL therein. MGL inhibition by JZL184 exhibits beneficial effects in various behavioral tests of anxiety via elevated 2-AG and activation of CB1 receptors (Busquets-Garcia et al., 2011; Kinsey et al., 2011b; Sumislawski et al., 2011; Aliczki et al., 2012, 2013; Lomazzo et al., 2015), but impairs short-term fear extinction in mice (Hartley et al., 2016). Reduction of 2-AG levels by genetic deletion of DAGLα leads to increased anxiety which is ameliorated upon restoration by pharmacological MGL inhibition (Shonesy et al., 2014). JZL184 treatment also improves chronic stress-induced depression-like behavior (Zhong et al., 2014) and attenuates plasma corticosterone levels in the early recovery from restraint stress (Roberts et al., 2014). Conversely, CB1 receptor desensitization in MGL-deficient mice leads to anxiety-like behavior (Imperatore et al., 2015). Impaired CB1 receptor signaling could thus be a major drawback of 2-AG elevation by chronic MGL inhibition, as discussed above.
Another pharmacologically highly relevant area of EC action is drug abuse and withdrawal. ECs play a major role in regulating addictive behavior, and their involvement in opioid, nicotine, and alcohol dependence has been extensively investigated (Parolaro et al., 2007; Scavone et al., 2013). Although it has long been known that Cannabis sativa ameliorates opioid withdrawal symptoms (Birch, 1889), MGL’s particular role therein has been investigated in detail just recently. Several studies demonstrated that the MGL inhibitor JZL184 attenuates Δ9-THC (Schlosburg et al., 2009), nicotine (Muldoon et al., 2015), and opioid (Ramesh et al., 2011, 2013) withdrawal in mice, but does not improve aversive behavioral aspects of withdrawal (Gamage et al., 2015). Taken together, pharmacological inhibition of MGL represents a promising strategy to ameliorate stress, depression, and drug withdrawal symptoms.
6. MGL in cancer
First evidence for a pathophysiological role of MGL in cancer emerged when Nomura et al. demonstrated that MGL was highly elevated in aggressive ovarian, breast and melanoma cancer cells. The enzyme was involved in cellular growth and survival, in vitro migration, and invasion of these cells (Nomura et al., 2010). The study suggests that MGL affects tumor growth through involvement of a pool of fatty acids that promotes pathogenicity, and that blockade of MGL reduces this pool leading to decreased cancer cell aggressiveness independently of EC signaling and CB receptor activation (Nomura et al., 2010). Interestingly, several MGL-derived bioactive lipid mediators affecting tumor invasiveness were identified in this study, including lyso-phosphatidic acid and prostaglandin E2. Both lipids are known to promote cancer aggressiveness (Tsujiuchi et al., 2014; O’Callaghan and Houston, 2015), and their supplementation reestablished malignancy of MGL deficient cancer cells (Nomura et al., 2010). In a subsequent study, the group further demonstrated that MGL activity was also high in aggressive prostate cancer cells and that anti-tumorigenic EC signaling was terminated by MGL via free fatty acid reduction and by CB1 receptor activation (Nomura et al., 2011a). High MGL expression was also detected in nasopharyngeal (Hu et al., 2014) and hepatocellular carcinoma (Zhang et al., 2016; Zhu et al., 2016). Knockdown of MGL inhibited migration of these cells further indicating a tumor-promoting role of MGL. This may also apply for neuroblastoma cells because inhibition of MGL and type II topoisomerase also decreased proliferation (Matuszak et al., 2012).
In contrast, the role of MGL in the oncogenesis of colorectal cancer is controversial. In line with the previous data, Ye et al. demonstrated high expression of MGL in colorectal cancerous tissues and a tumor-promoting effect of MGL in colorectal cancer cell lines (Ye et al., 2011). Conversely, Sun et al. described reduced MGL expression in colorectal tumor tissues (and also other human malignancies) and a colony formation suppressive effect in MGL overexpressing colon cancer cells (Sun et al., 2013). The authors of this study suggested that the underlying mechanism by which MGL may affect tumor growth could be through constitutive suppression of Akt phosphorylation because shRNA mediated knockdown of MGL expression enhanced phosphorylation of Akt in both HT29 colon cancer and MDA-MB-231 breast cancer cells (Sun et al., 2013). The discrepancy between these studies is unclear and requires further research on the role of MGL in colorectal cancer.
Taken together, studies in various cancer cell lines suggest that MGL may drive cancer malignancy. However, the picture may be different in colon cancer in which a tumor suppressive role for MGL was proposed. Certainly, more research is needed to define the role of MGL in human malignancies and whether MGL’s role involves CB receptor dependent or independent mechanisms.
7. Conclusions and Outlook
Starting from the first description of MGL activity more than 50 years ago, MGL research has shifted from fat breakdown to endocannabinoid signaling and further to AA metabolism. Apparently, MGL has a central function in feeding AA into COX and LOX pathways. The investigation of these pathways will be of paramount importance to understand the role of MGL in various diseases. Accordingly, the pharmacological inhibition of MGL evokes two different effects: First, levels of the EC 2-AG rise, inducing anti-inflammatory and analgesic effects via activation of CB1 and CB2 receptors and, second, the pool of AA is lowered counteracting the synthesis of prostaglandins. Based on these mechanisms, MGL inhibitors represent an alternative to COX inhibitors in the treatment of inflammation and pain.
Yet, it has to be considered that the EC system has a major impact on neuronal signaling and consequently CB receptor activation or desensitization upon MGL inhibition may result in adverse psychological effects. Peripherally restricted MGL inhibitors may circumvent this issue. Nevertheless, MGL inhibitors may prove useful in pathologies where moderate adverse effects are acceptable. Studies in various disease models, such as intestinal inflammation, hepatic injury, endotoxemia, insulin resistance, depression and stress, opioid and nicotine withdrawal already suggest that inhibition of MGL may be a powerful anti-inflammatory pharmacological strategy. In addition, MGL inhibitors may have high potential as anticancer drugs, however, studies on the role of MGL in cancer are conflicting and the role of MGL in the metabolism of cancers cells is incompletely understood. Furthermore, it should be kept in mind that MGL not only degrades 2-AG but also other 2-MGs esterified with PUFAs and thus may affect the mobilization of docosahexaenoic acid (DHA) or eicosapentaenoic acid (EPA). These PUFAs are precursors of potent inflammation-resolving mediators such as resolvins and protectins. From preclinical studies we have to assume that therapy with MGL inhibitors may cause CB1 receptor desensitization in long term use which hampers therapeutic success. This obstacle might be overcome by assessing the lowest effective dose avoiding CB1 receptor desensitization. In conclusion, the therapeutic potential of MGL inhibition is vast and promising, but warrants further research. Since highly selective inhibitors targeting human MGL have been developed, studies in the near future will disclose whether MGL inhibitors can become an alternative to COX inhibitors in the treatment of inflammation, pain, and other disorders.
Funding
This study was supported by Austrian Science Fund (FWF) grants P21166 and KLI 521-B31 and the doctoral program Molecular Enzymology Grant W 09.
Abbreviations
- ABHD
α/β hydrolase domain containing protein
- Δ9-THC
Δ9-tetrahydrocannabinol
- 2-AG
2-arachidonoyl glycerol
- 2-OG
2-oleoyl glycerol
- DGAT
acyl-CoA:diacylglycerol acyltransferase
- MGAT
acyl-CoA:monoacylglycerol acyltransferase
- ATGL
adipose triglyceride lipase
- AA
arachidonic acid
- AEA
arachidonoyl ethanoamine
- CB
cannabinoid
- CNS
central nervous system
- COX
cyclooxygenase
- cPLA2
cytosolic phospholipase A2
- DAGL
diglyceride lipase
- DG
diglyceride
- EC
endocannabinoid
- FAAH
fatty acid amide hydrolase
- GI
gastrointestinal
- GLP-1
glucagon-like peptide-1
- GPR55
G-protein coupled receptor 55
- HFD
high-fat diet
- HSL
hormone-sensitive lipase
- LPL
lipoprotein lipase
- LDL
low density lipoprotein
- MAGL
monoacylglycerol lipase
- MGL
monoglyceride lipase
- MG
monoglycerides
- NAPE-PLD
N-arachidonoyl-phosphatidylethanolamine selective phospholipase D
- PPAR
peroxisome proliferator activated receptor
- PHARC
polyneuropathy, hearing loss, ataxia, retinitis pigmentosa, and cataract disease
- PLC
phospholipase C
- PUFA
polyunsaturated fatty acid
- TRPV1
transient receptor potential cation channel subfamily V member 1
- TG
triglyceride
8
Conflict of interest statement
The authors declare that there are no conflicts of interest.
9
- Alhouayek M, Lambert DM, Delzenne NM, Cani PD, Muccioli GG. Increasing endogenous 2-arachidonoylglycerol levels counteracts colitis and related systemic inflammation. FASEB J. 2011;25:2711–21. doi: 10.1096/fj.10-176602. [DOI] [PubMed] [Google Scholar]
- Alhouayek M, Masquelier J, Cani PD, Lambert DM, Muccioli GG. Implication of the anti-inflammatory bioactive lipid prostaglandin D2-glycerol ester in the control of macrophage activation and inflammation by ABHD6. Proc Natl Acad Sci U S A. 2013;110:17558–63. doi: 10.1073/pnas.1314017110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliczki M, Balogh Z, Tulogdi A, Haller J. The temporal dynamics of the effects of monoacylglycerol lipase blockade on locomotion, anxiety, and body temperature. Behav Pharmacol. 2012;23:348–357. doi: 10.1097/FBP.0b013e3283564dfa. [DOI] [PubMed] [Google Scholar]
- Aliczki M, Zelena D, Mikics E, Varga ZK, Pinter O, Bakos NV, Varga J, Haller J. Monoacylglycerol lipase inhibition-induced changes in plasma corticosterone levels, anxiety and locomotor activity in male CD1 mice. Horm Behav. 2013;63:752–758. doi: 10.1016/j.yhbeh.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Araki M, Ohshima N, Aso C, Konishi A, Obinata H, Tatei K, Izumi T. Enzymatic characterization of recombinant rat DDHD2: a soluble diacylglycerol lipase. J Biochem. 2016;160:269–279. doi: 10.1093/jb/mvw034. [DOI] [PubMed] [Google Scholar]
- Berdan CA, Erion KA, Burritt NE, Corkey BE, Deeney JT. Inhibition of Monoacylglycerol Lipase Activity Decreases Glucose-Stimulated Insulin Secretion in INS-1 (832/13) Cells and Rat Islets. PLoS One. 2016;11:e0149008. doi: 10.1371/journal.pone.0149008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, Maldonado R. Involvement of the opioid system in the anxiolytic-like effects induced by Δ9-tetrahydrocannabinol. Psychopharmacology (Berl) 2002;163:111–117. doi: 10.1007/s00213-002-1144-9. [DOI] [PubMed] [Google Scholar]
- Bertrand T, Augé F, Houtmann J, Rak A, Vallée F, Mikol V, Berne PF, Michot N, Cheuret D, Hoornaert C, Mathieu M. Structural basis for human monoglyceride lipase inhibition. J Mol Biol. 2010;396:663–73. doi: 10.1016/j.jmb.2009.11.060. [DOI] [PubMed] [Google Scholar]
- Birch E. The use of Indian hemp in the treatment of chronic chloral and chronic opium poisoning. Lancet. 1889;133:625. [Google Scholar]
- Bisogno T, Berrendero F, Ambrosino G, Cebeira M, Ramos JA, Fernandez-Ruiz JJ, Di Marzo V. Brain regional distribution of endocannabinoids:implications for their biosynthesis and biological function. Biochem Biophys Res Commun. 1999a;256:377–80. doi: 10.1006/bbrc.1999.0254. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Melck D, De Petrocellis L, Di Marzo V. Phosphatidic acid as the biosynthetic precursor of the endocannabinoid 2-arachidonoylglycerol in intact mouse neuroblastoma cells stimulated with ionomycin. J Neurochem. 1999b;72:2113–2119. doi: 10.1046/j.1471-4159.1999.0722113.x. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Ortar G, Petrosino S, Morera E, Palazzo E, Nalli M, Maione S, Di Marzo V. Development of a potent inhibitor of 2-arachidonoylglycerol hydrolysis with antinociceptive activity in vivo. Biochim Biophys Acta - Mol Cell Biol Lipids. 2009;1791:53–60. doi: 10.1016/j.bbalip.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Blankman JL, Long JZ, Trauger Sa, Siuzdak G, Cravatt BF. ABHD12 controls brain lysophosphatidylserine pathways that are deregulated in a murine model of the neurodegenerative disease PHARC. Proc Natl Acad Sci U S A. 2013;110:1500–5. doi: 10.1073/pnas.1217121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. A Comprehensive Profile of Brain Enzymes that Hydrolyze the Endocannabinoid 2-Arachidonoylglycerol. Chem Phys Lipids. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139–43. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgelt LM, Franson KL, Nussbaum AM, Wang GS. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy. 2013;33:195–209. doi: 10.1002/phar.1187. [DOI] [PubMed] [Google Scholar]
- Buczynski MW, Dumlao DS, Dennis EA. Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology. J Lipid Res. 2009;50:1015–38. doi: 10.1194/jlr.R900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burston JJ, Mapp PI, Sarmad S, Barrett DA, Niphakis MJ, Cravatt BF, Walsh DA, Chapman V. Robust anti-nociceptive effects of monoacylglycerol lipase inhibition in a model of osteoarthritis pain. Br J Pharmacol. 2016;173:3134–3144. doi: 10.1111/bph.13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquets-Garcia A, Puighermanal E, Pastor A, de la Torre R, Maldonado R, Ozaita A. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol Psychiatry. 2011;70:479–86. doi: 10.1016/j.biopsych.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Cannon CP, Cannon PJ. COX-2 Inhibitors and Cardiovascular Risk. Science. 2012;336:1386–7. doi: 10.1126/science.1224398. [DOI] [PubMed] [Google Scholar]
- Cao J, Cheng L, Shi Y. Catalytic properties of MGAT3, a putative triacylgycerol synthase. J Lipid Res. 2007;48:583–591. doi: 10.1194/jlr.M600331-JLR200. [DOI] [PubMed] [Google Scholar]
- Cao J, Lockwood J, Burn P, Shi Y. Cloning and Functional Characterization of a Mouse Intestinal Acyl-CoA:Monoacylglycerol Acyltransferase, MGAT2. J Biol Chem. 2003;278:13860–13866. doi: 10.1074/jbc.M300139200. [DOI] [PubMed] [Google Scholar]
- Cao Z, Mulvihill MM, Mukhopadhyay P, Xu H, Erdélyi K, Hao E, Holovac E, Haskó G, Cravatt BF, Nomura DK, Pacher P. Monoacylglycerol lipase controls endocannabinoid and eicosanoid signaling and hepatic injury in mice. Gastroenterology. 2013;144:808–817.e15. doi: 10.1053/j.gastro.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio MG, Marini P. Biosynthesis and Fate of Endocannabinoids. Handb Exp Pharmacol. 2015;231:39–58. doi: 10.1007/978-3-319-20825-1_2. [DOI] [PubMed] [Google Scholar]
- Chanda PK, Gao Y, Mark L, Btesh J, Strassle BW, Lu P, Piesla MJ, Zhang M, Bingham B, Uveges A, Kowal D, et al. Monoacylglycerol Lipase Activity Is a Critical Modulator of the Tone and Integrity of the Endocannabinoid System. Mol Pharmacol. 2010;78:996–1003. doi: 10.1124/mol.110.068304. [DOI] [PubMed] [Google Scholar]
- Chang JW, Cognetta AB, Niphakis MJ, Cravatt BF, Cravatt BF. Proteome-wide reactivity profiling identifies diverse carbamate chemotypes tuned for serine hydrolase inhibition. ACS Chem Biol. 2013;8:1590–9. doi: 10.1021/cb400261h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JW, Niphakis MJ, Lum KM, Cognetta AB, Wang C, Matthews ML, Niessen S, Buczynski MW, Parsons LH, Cravatt BF. Highly Selective Inhibitors of Monoacylglycerol Lipase Bearing a Reactive Group that Is Bioisosteric with Endocannabinoid Substrates. Chem Biol. 2012:1–10. doi: 10.1016/j.chembiol.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Zhang J, Wu Y, Wang D, Feng G, Tang Y-P, Teng Z, Chen C. Monoacylglycerol lipase is a therapeutic target for Alzheimer’s disease. Cell Rep. 2012;2:1329–39. doi: 10.1016/j.celrep.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S-H, Bosetti F. Cyclooxygenase-1 null mice show reduced neuroinflammation in response to beta-amyloid. Aging (Albany NY) 2009;1:234–44. doi: 10.18632/aging.100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S-H, Langenbach R, Bosetti F. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. FASEB J. 2008;22:1491–501. doi: 10.1096/fj.07-9411com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chon S-H, Douglass JD, Zhou YX, Malik N, Dixon JL, Brinker A, Quadro L, Storch J. Over-Expression of Monoacylglycerol Lipase (MGL) in Small Intestine Alters Endocannabinoid Levels and Whole Body Energy Balance, Resulting in Obesity. PLoS One. 2012;7:e43962. doi: 10.1371/journal.pone.0043962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chon S-H, Zhou YX, Dixon JL, Storch J. Intestinal monoacylglycerol metabolism: developmental and nutritional regulation of monoacylglycerol lipase and monoacylglycerol acyltransferase. J Biol Chem. 2007;282:33346–57. doi: 10.1074/jbc.M706994200. [DOI] [PubMed] [Google Scholar]
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–13. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- Costola-de-Souza C, Ribeiro A, Ferraz-de-Paula V, Calefi AS, Aloia TPA, Gimenes-Júnior JA, de Almeida VI, Pinheiro ML, Palermo-Neto J. Monoacylglycerol Lipase (MAGL) Inhibition Attenuates Acute Lung Injury in Mice. PLoS One. 2013;8:e77706. doi: 10.1371/journal.pone.0077706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschöp M, Grübler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thöne-reineke C, Ortmann S, Tomassoni F, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman aH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denson TF, Earleywine M. Decreased depression in marijuana users. Addict Behav. 2006;31:738–742. doi: 10.1016/j.addbeh.2005.05.052. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Ueda N, Yamamoto S. The fatty acid amide hydrolase (FAAH) Prostaglandins Leukot Essent Fatty Acids. 2002;66:201–210. doi: 10.1054/plef.2001.0358. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–24. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPatrizio NV, Joslin A, Jung K-M, Piomelli D. Endocannabinoid signaling in the gut mediates preference for dietary unsaturated fats. FASEB J. 2013;27:2513–2520. doi: 10.1096/fj.13-227587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPatrizio NV, Astarita G, Schwartz G, Li X, Piomelli D. Endocannabinoid signal in the gut controls dietary fat intake. Proc Natl Acad Sci. 2011;108:12904–12908. doi: 10.1073/pnas.1104675108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dol-Gleizes F, Paumelle R, Visentin V, Marés A-M, Desitter P, Hennuyer N, Gilde A, Staels B, Schaeffer P, Bono F. Rimonabant, a Selective Cannabinoid CB1 Receptor Antagonist, Inhibits Atherosclerosis in LDL Receptor–Deficient Mice. Arterioscler Thromb Vasc Biol. 2008;29:12–8. doi: 10.1161/ATVBAHA.108.168757. [DOI] [PubMed] [Google Scholar]
- Dotsey EY, Jung K-M, Basit A, Wei D, Daglian J, Vacondio F, Armirotti A, Mor M, Piomelli D. Peroxide-Dependent MGL Sulfenylation Regulates 2-AG-Mediated Endocannabinoid Signaling in Brain Neurons. Chem Biol. 2015;22:619–628. doi: 10.1016/j.chembiol.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass JD, Zhou YX, Wu A, Zadrogra JA, Gajda AM, Lackey AI, Lang W, Chevalier KM, Sutton SW, Zhang S-P, Flores CM, et al. Global deletion of monoacylglycerol lipase in mice delays lipid absorption and alters energy homeostasis and diet-induced obesity. J Lipid Res. 2015;56:1153–71. doi: 10.1194/jlr.M058586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine PG, Rosenfeld MJ. The endocannabinoid system, cannabinoids, and pain. Rambam Maimonides Med J. 2013;4:e0022. doi: 10.5041/RMMJ.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskerstrand T, H’mida-Ben Brahim D, Johansson S, M’zahem A, Haukanes BI, Drouot N, Zimmermann J, Cole AJ, Vedeler C, Bredrup C, Assoum M, et al. Mutations in ABHD12 cause the neurodegenerative disease PHARC: An inborn error of endocannabinoid metabolism. Am J Hum Genet. 2010;87:410–417. doi: 10.1016/j.ajhg.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrikson G, Tornqvist H, Belfrage P. Hormone-sensitive lipase and monoacylglycerol lipase are both required for complete degradation of adipocyte triacylglycerol. Biochim Biophys Acta. 1986;876:288–293. doi: 10.1016/0005-2760(86)90286-9. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S, Group R-ES Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- Gaetani S, Dipasquale P, Romano A, Righetti L, Cassano T, Piomelli D, Cuomo V. The Endocannabinoid System as A Target for Novel Anxiolytic and Antidepressant Drugs. Int Rev Neurobiol. 2009;85:57–72. doi: 10.1016/S0074-7742(09)85005-8. [DOI] [PubMed] [Google Scholar]
- Galiègue S, Mary S, Marchand J, Dussossoy D, Carrière D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Gamage TF, Ignatowska-Jankowska BM, Muldoon PP, Cravatt BF, Damaj MI, Lichtman AH. Differential effects of endocannabinoid catabolic inhibitors on morphine withdrawal in mice. Drug Alcohol Depend. 2015;146:7–16. doi: 10.1016/j.drugalcdep.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J Am Chem Soc. 1964;86:1646–1647. [Google Scholar]
- Gary-Bobo M, Elachouri G, Gallas JF, Janiak P, Marini P, Ravinet-Trillou C, Chabbert M, Cruccioli N, Pfersdorff C, Roque C, Arnone M, et al. Rimonabant reduces obesity-associated hepatic steatosis and features of metabolic syndrome in obese Zucker fa/fa rats. Hepatology. 2007;46:122–129. doi: 10.1002/hep.21641. [DOI] [PubMed] [Google Scholar]
- Ghafouri N, Tiger G, Razdan RK, Mahadevan A, Pertwee RG, Martin BR, Fowler CJ. Inhibition of monoacylglycerol lipase and fatty acid amide hydrolase by analogues of 2-arachidonoylglycerol. Br J Pharmacol. 2004;143:774–84. doi: 10.1038/sj.bjp.0705948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Wise LE, Chen Y, Gujjar R, Mahadevan A, Cravatt BF, Lichtman AH. The monoacylglycerol lipase inhibitor JZL184 suppresses inflammatory pain in the mouse carrageenan model. Life Sci. 2013;92:498–505. doi: 10.1016/j.lfs.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg IJ, Merkel M. Lipoprotein lipase: physiology, biochemistry, and molecular biology. Front Biosci. 2001;6:D388–405. doi: 10.2741/goldberg. [DOI] [PubMed] [Google Scholar]
- González S, Cebeira M, Fernández-Ruiz J. Cannabinoid tolerance and dependence: A review of studies in laboratory animals. Pharmacol Biochem Behav. 2005;81:300–318. doi: 10.1016/j.pbb.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Goparaju SK, Ueda N, Yamaguchi H, Yamamoto S. Anandamide amidohydrolase reacting with 2-arachidonoylglycerol, another cannabinoid receptor ligand. FEBS Lett. 1998;422:69–73. doi: 10.1016/s0014-5793(97)01603-7. [DOI] [PubMed] [Google Scholar]
- Gopez JJ, Yue H, Vasudevan R, Malik AS, Fogelsanger LN, Lewis S, Panikashvili D, Shohami E, Jansen SA, Narayan RK, Strauss KI. Cyclooxygenase-2-specific inhibitor improves functional outcomes, provides neuroprotection, and reduces inflammation in a rat model of traumatic brain injury. Neurosurgery. 2005;56:590–603. doi: 10.1227/01.NEU.0000154060.14900.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner GF, Eichmann TO, Wagner B, Gao Y, Farzi A, Taschler U, Radner FPW, Schweiger M, Lass A, Holzer P, Zinser E, et al. Deletion of Monoglyceride Lipase in Astrocytes Attenuates Lipopolysaccharide-induced Neuroinflammation. J Biol Chem. 2016;291:913–23. doi: 10.1074/jbc.M115.683615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Pichat P, Beeské S, Leroy T, Redon N, Jacquet A, Françon D, Bert L, Even L, Lopez-Grancha M, Tolstykh T, et al. Selective blockade of the hydrolysis of the endocannabinoid 2-arachidonoylglycerol impairs learning and memory performance while producing antinociceptive activity in rodents. Sci Rep. 2015;5:7642. doi: 10.1038/srep07642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsey NL, Goodfellow CE, Dragunow M, Glass M. Cannabinoid receptor 2 undergoes Rab5-mediated internalization and recycles via a Rab11-dependent pathway. Biochim Biophys Acta - Mol Cell Res. 2011;1813:1554–1560. doi: 10.1016/j.bbamcr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Grygiel-Górniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications - a review. Nutr J. 2014;13:17. doi: 10.1186/1475-2891-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Guijarro A, Piomelli D, Hohmann AG. Peripheral antinociceptive effects of inhibitors of monoacylglycerol lipase in a rat model of inflammatory pain. Br J Pharmacol. 2011;163:1464–78. doi: 10.1111/j.1476-5381.2010.01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Rosenkilde MM, Knop FK, Wellner N, Diep Ta, Rehfeld JF, Andersen UB, Holst JJ, Hansen HS. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J Clin Endocrinol Metab. 2011;96:E1409–17. doi: 10.1210/jc.2011-0647. [DOI] [PubMed] [Google Scholar]
- Hartley ND, Gunduz-Cinar O, Halladay L, Bukalo O, Holmes A, Patel S. 2-arachidonoylglycerol signaling impairs short-term fear extinction. Transl Psychiatry. 2016;6:e749. doi: 10.1038/tp.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenoehrl C, Taschler U, Storr M, Schicho R. The gastrointestinal tract - a central organ of cannabinoid signaling in health and disease. Neurogastroenterol Motil. 2016;28:1765–80. doi: 10.1111/nmo.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heier C, Taschler U, Radulovic M, Aschauer P, Eichmann TO, Grond S, Wolinski H, Oberer M, Zechner R, Kohlwein SD, Zimmermann R. Monoacylglycerol Lipases Act as Evolutionarily Conserved Regulators of Non-oxidative Ethanol Metabolism. J Biol Chem. 2016;291:11865–75. doi: 10.1074/jbc.M115.705541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Torres G, Cipriano M, Hedén E, Björklund E, Canales A, Zian D, Feliú A, Mecha M, Guaza C, Fowler CJ, Ortega-Gutiérrez S, et al. A reversible and selective inhibitor of monoacylglycerol lipase ameliorates multiple sclerosis. Angew Chemie - Int Ed. 2014;53:13765–13770. doi: 10.1002/anie.201407807. [DOI] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. The endocannabinoid system and the treatment of mood and anxiety disorders. CNS Neurol Disord Drug Targets. 2009;8:451–8. doi: 10.2174/187152709789824624. [DOI] [PubMed] [Google Scholar]
- Hillard CJ. Stress regulates endocannabinoid-CB1 receptor signaling. Semin Immunol. 2014;26:380–388. doi: 10.1016/j.smim.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Michael Walker J, Holmes PV, Crystal JD, Duranti A, et al. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Howlett AC. Cannabinoid receptor signaling. Handb Exp Pharmacol. 2005:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Bidaut-Russell M, Devane WA, Melvin LS, Johnson MR, Herkenham M. The cannabinoid receptor: biochemical, anatomical and behavioral characterization. Trends Neurosci. 1990;13:420–423. doi: 10.1016/0166-2236(90)90124-s. [DOI] [PubMed] [Google Scholar]
- Hsieh C, Brown S, Derleth C, Mackie K. Internalization and recycling of the CB1 cannabinoid receptor. J Neurochem. 1999;73:493–501. doi: 10.1046/j.1471-4159.1999.0730493.x. [DOI] [PubMed] [Google Scholar]
- Hu W-R, Lian Y-F, Peng L-X, Lei J-J, Deng C-C, Xu M, Feng Q-S, Chen L-Z, Bei J-X, Zeng Y-X. Monoacylglycerol lipase promotes metastases in nasopharyngeal carcinoma. Int J Clin Exp Pathol. 2014;7:3704–13. [PMC free article] [PubMed] [Google Scholar]
- Ignatowska-Jankowska BM, Ghosh S, Crowe MS, Kinsey SG, Niphakis MJ, Abdullah R a, Tao Q, O’ Neal ST, Walentiny DM, Wiley JL, Cravatt BF, et al. In vivo characterization of the highly selective monoacylglycerol lipase inhibitor KML29: antinociceptive activity without cannabimimetic side effects. Br J Pharmacol. 2014;171:1392–407. doi: 10.1111/bph.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperatore R, Morello G, Luongo L, Taschler U, Romano R, De Gregorio D, Belardo C, Maione S, Di Marzo V, Cristino L. Genetic deletion of monoacylglycerol lipase leads to impaired cannabinoid receptor CB 1 R signaling and anxiety-like behavior. J Neurochem. 2015;135:799–813. doi: 10.1111/jnc.13267. [DOI] [PubMed] [Google Scholar]
- Jiang S-K, Zhang M, Tian Z-L, Wang M, Zhao R, Wang L-L, Li S-S, Liu M, Li J-Y, Zhang M-Z, Guan D-W, et al. The monoacylglycerol lipase inhibitor JZL184 decreases inflammatory response in skeletal muscle contusion in rats. Eur J Pharmacol. 2015;761:1–10. doi: 10.1016/j.ejphar.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Jung K-M, Clapper JR, Fu J, D’Agostino G, Guijarro A, Thongkham D, Avanesian A, Astarita G, DiPatrizio NV, Frontini A, Cinti S, et al. 2-Arachidonoylglycerol Signaling in Forebrain Regulates Systemic Energy Metabolism. Cell Metab. 2012;15:299–310. doi: 10.1016/j.cmet.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson M, Contreras JA, Hellman U, Tornqvist H, Holm C. cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. Evolutionary relationship to esterases, lysophospholipases, and haloperoxidases. J Biol Chem. 1997;272:27218–27223. doi: 10.1074/jbc.272.43.27218. [DOI] [PubMed] [Google Scholar]
- Karlsson M, Reue K, Xia YR, Lusis AJ, Langin D, Tornqvist H, Holm C. Exon-intron organization and chromosomal localization of the mouse monoglyceride lipase gene. Gene. 2001;272:11–18. doi: 10.1016/s0378-1119(01)00559-5. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Long JZ, Neal STO, Abdullah RA, Poklis JL, Boger DL, Cravatt BF, Lichtman AH. Blockade of Endocannabinoid-Degrading Enzymes Attenuates Neuropathic Pain. Pharmacology. 2009;330:902–910. doi: 10.1124/jpet.109.155465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Nomura DK, O’Neal ST, Long JZ, Mahadevan A, Cravatt BF, Grider JR, Lichtman AH. Inhibition of monoacylglycerol lipase attenuates nonsteroidal anti-inflammatory drug-induced gastric hemorrhages in mice. J Pharmacol Exp Ther. 2011a;338:795–802. doi: 10.1124/jpet.110.175778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, O’Neal ST, Long JZ, Cravatt BF, Lichtman AH. Inhibition of endocannabinoid catabolic enzymes elicits anxiolytic-like effects in the marble burying assay. Pharmacol Biochem Behav. 2011b;98:21–7. doi: 10.1016/j.pbb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Wise LE, Ramesh D, Abdullah R, Selley DE, Cravatt BF, Lichtman AH. Repeated low-dose administration of the monoacylglycerol lipase inhibitor JZL184 retains cannabinoid receptor type 1-mediated antinociceptive and gastroprotective effects. J Pharmacol Exp Ther. 2013;345:492–501. doi: 10.1124/jpet.112.201426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005;5:400–411. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- de Kloet AD, Woods SC. Endocannabinoids and Their Receptors as Targets for Obesity Therapy. Endocrinology. 2009;150:2531–2536. doi: 10.1210/en.2009-0046. [DOI] [PubMed] [Google Scholar]
- Kohnz RA, Nomura DK. Chemical approaches to therapeutically target the metabolism and signaling of the endocannabinoid 2-AG and eicosanoids. Chem Soc Rev. 2014;43:6859–6869. doi: 10.1039/c4cs00047a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupiecki FP. Partial purification of monoglyceride lipase from adipose tissue. J Lipid Res. 1966;7:230–235. [PubMed] [Google Scholar]
- Labar G, Bauvois C, Borel F, Ferrer J-L, Wouters J, Lambert DM. Crystal structure of the human monoacylglycerol lipase, a key actor in endocannabinoid signaling. Chembiochem. 2010a;11:218–27. doi: 10.1002/cbic.200900621. [DOI] [PubMed] [Google Scholar]
- Labar G, Wouters J, Lambert DM. A review on the monoacylglycerol lipase: at the interface between fat and endocannabinoid signalling. Curr Med Chem. 2010b;17:2588–2607. doi: 10.2174/092986710791859414. [DOI] [PubMed] [Google Scholar]
- Lehner R, Vance DE. Cloning and expression of a cDNA encoding a hepatic microsomal lipase that mobilizes stored triacylglycerol. Biochem J. 1999;343(Pt 1):1–10. [PMC free article] [PubMed] [Google Scholar]
- Lomazzo E, Bindila L, Remmers F, Lerner R, Schwitter C, Hoheisel U, Lutz B. Therapeutic potential of inhibitors of endocannabinoid degradation for the treatment of stress-related hyperalgesia in an animal model of chronic pain. Neuropsychopharmacology. 2015;40:488–501. doi: 10.1038/npp.2014.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J, Nomura D, Cravatt B. Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem Biol. 2009a;16:744–753. doi: 10.1016/j.chembiol.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X, Burston JJ, Sim-Selley LJ, Lichtman AH, Wiley JL, Cravatt BF. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci U S A. 2009b;106:20270–5. doi: 10.1073/pnas.0909411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Weiwei L, Lamont B, Burston J, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Loren H. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009c;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe ME. Pancreatic triglyceride lipase and colipase: insights into dietary fat digestion. Gastroenterology. 1994;107:1524–36. doi: 10.1016/0016-5085(94)90559-2. [DOI] [PubMed] [Google Scholar]
- Lutz B. Endocannabinoid signals in the control of emotion. Curr Opin Pharmacol. 2009;9:46–52. doi: 10.1016/j.coph.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Lysenko LV, Kim J, Henry C, Tyrtyshnaia A, Kohnz RA, Madamba F, Simon GM, Kleschevnikova NE, Nomura DK, Ezekowitz RAB, Kleschevnikov AM. Monoacylglycerol lipase inhibitor JZL184 improves behavior and neural properties in Ts65Dn mice, a model of down syndrome. PLoS One. 2014;9:1–25. doi: 10.1371/journal.pone.0114521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Bernardi G, Agrò AF, Centonze D. Cannabinoid receptor signalling in neurodegenerative diseases: a potential role for membrane fluidity disturbance. Br J Pharmacol. 2011;163:1379–90. doi: 10.1111/j.1476-5381.2011.01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares J, Julian M, Carrascosa A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr Neuropharmacol. 2006;4:239–57. doi: 10.2174/157015906778019527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs WR, Blankman JL, Horne Ea, Thomazeau A, Lin YH, Coy J, Bodor AL, Muccioli GG, Hu SS-J, Woodruff G, Fung S, et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951–7. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, Melck D, Ross R, Brockie H, Stevenson L, Pertwee R, De Petrocellis L. Interactions between synthetic vanilloids and the endogenous cannabinoid system. FEBS Lett. 1998;436:449–454. doi: 10.1016/s0014-5793(98)01175-2. [DOI] [PubMed] [Google Scholar]
- Matuszak N, Hamtiaux L, Baldeyroux B, Muccioli GG, Poupaert JH, Lansiaux A, Lambert DM. Dual inhibition of MAGL and type II topoisomerase by N-phenylmaleimides as a potential strategy to reduce neuroblastoma cell growth. Eur J Pharm Sci. 2012;45:263–271. doi: 10.1016/j.ejps.2011.11.011. [DOI] [PubMed] [Google Scholar]
- McLaughlin RJ, Gobbi G. Cannabinoids and emotionality: a neuroanatomical perspective. Neuroscience. 2012;204:134–144. doi: 10.1016/j.neuroscience.2011.07.052. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Parker La. The Endocannabinoid System and the Brain. Annu Rev Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- Micale V, Di Marzo V, Sulcova A, Wotjak CT, Drago F. Endocannabinoid system and mood disorders: Priming a target for new therapies. Pharmacol Ther. 2013;138:18–37. doi: 10.1016/j.pharmthera.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Miller MR, Mannowetz N, Iavarone AT, Safavi R, Gracheva EO, Smith JF, Hill RZ, Bautista DM, Kirichok Y, Lishko PV. Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone. Science. 2016;352:555–559. doi: 10.1126/science.aad6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M, Patel S, Bains JS, Hill MN. Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology. 2015;41:80–102. doi: 10.1038/npp.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon PP, Chen J, Harenza JL, Abdullah RA, Sim-Selley LJ, Cravatt BF, Miles MF, Chen X, Lichtman AH, Damaj MI. Inhibition of monoacylglycerol lipase reduces nicotine withdrawal. Br J Pharmacol. 2015;172:869–82. doi: 10.1111/bph.12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Navia-Paldanius D, Aaltonen N, Lehtonen M, Savinainen JR, Taschler U, Radner FPW, Zimmermann R, Laitinen JT. Increased tonic cannabinoid CB1R activity and brain region-specific desensitization of CB1R Gi/o signaling axis in mice with global genetic knockout of monoacylglycerol lipase. Eur J Pharm Sci. 2015;77:180–8. doi: 10.1016/j.ejps.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Ng SC, Chan FKL. NSAID-induced gastrointestinal and cardiovascular injury. Curr Opin Gastroenterol. 2010;26:611–7. doi: 10.1097/MOG.0b013e32833e91eb. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Nicholls SJ, Wolski K, Rodés-Cabau J, Cannon CP, Deanfield JE, Després J-P, Kastelein JJP, Steinhubl SR, Kapadia S, Yasin M, et al. Effect of Rimonabant on Progression of Atherosclerosis in Patients With Abdominal Obesity and Coronary Artery Disease. JAMA. 2008;299:1547. doi: 10.1001/jama.299.13.1547. [DOI] [PubMed] [Google Scholar]
- Nomura DK, Lombardi DP, Chang JW, Niessen S, Anna M, Long JZ, Hoover HH, Cravatt BF. Monoacylglycerol lipase exerts dual control over endocannabinoid and fatty acid pathways to support prostate cancer. Chem Biol. 2011a;18:846–856. doi: 10.1016/j.chembiol.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Long JZ, Niessen S, Hoover HS, Ng S-W, Cravatt BF. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MCG, Ward AM, Hahn YK, Lichtman AH, Conti B, Cravatt BF. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011b;334:809–13. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan G, Houston A. Prostaglandin E2 and the EP receptors in malignancy: possible therapeutic targets? Br J Pharmacol. 2015;172:5239–5250. doi: 10.1111/bph.13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan SE. An update on PPAR activation by cannabinoids. Br J Pharmacol. 2016;173:1899–1910. doi: 10.1111/bph.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–5305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, Jeong WI, Batkai S, Marsicano G, Lutz B, Buettner C, Kunos G. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest. 2008;118:3160–3169. doi: 10.1172/JCI34827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006;27:73–100. doi: 10.1210/er.2005-0009. [DOI] [PubMed] [Google Scholar]
- Parolaro D, Vigano D, Realini N, Rubino T. Role of endocannabinoids in regulating drug dependence. Neuropsychiatr Dis Treat. 2007;3:711–21. doi: 10.2147/ndt.s976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Pharmacological Evaluation of Cannabinoid Receptor Ligands in a Mouse Model of Anxiety: Further Evidence for an Anxiolytic Role for Endogenous Cannabinoid Signaling. J Pharmacol Exp Ther. 2006;318:304–11. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- Piro JR, Benjamin DI, Duerr JM, Pi Y, Gonzales C, Wood KM, Schwartz JW, Nomura DK, Samad TA. A Dysregulated Endocannabinoid-Eicosanoid Network Supports Pathogenesis in a Mouse Model of Alzheimer’s Disease. Cell Rep. 2012;1:617–623. doi: 10.1016/j.celrep.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribasnig MA, Mrak I, Grabner GF, Taschler U, Knittelfelder O, Scherz B, Eichmann TO, Heier C, Grumet L, Kowaliuk J, Romauch M, et al. α/β Hydrolase Domain-Containing 6 (ABHD6) Degrades the Late Endosomal/Lysosomal Lipid Bis(monoacylglycero)phosphate. J Biol Chem. 2015;290:29869–81. doi: 10.1074/jbc.M115.669168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce G, Cabranes A, Fernández-Ruiz J, Bisogno T, Di Marzo V, Long JZ, Cravatt BF, Giovannoni G, Baker D. Control of experimental spasticity by targeting the degradation of endocannabinoids using selective fatty acid amide hydrolase inhibitors. Mult Scler. 2013;19:1896–904. doi: 10.1177/1352458513485982. [DOI] [PubMed] [Google Scholar]
- Quarta C, Bellocchio L, Mancini G, Mazza R, Cervino C, Braulke LJ, Fekete C, Latorre R, Nanni C, Bucci M, Clemens LE, et al. CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab. 2010;11:273–85. doi: 10.1016/j.cmet.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Quarta C, Mazza R, Obici S, Pasquali R, Pagotto U. Energy balance regulation by endocannabinoids at central and peripheral levels. Trends Mol Med. 2011;17:518–526. doi: 10.1016/j.molmed.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Rakhshandehroo M, Sanderson LM, Matilainen M, Stienstra R, Carlberg C, de Groot PJ, Müller M, Kersten S. Comprehensive analysis of PPARalpha-dependent regulation of hepatic lipid metabolism by expression profiling. PPAR Res. 2007;2007:26839. doi: 10.1155/2007/26839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh D, Gamage TF, Vanuytsel T, Owens RA, Abdullah RA, Niphakis MJ, Shea-Donohue T, Cravatt BF, Lichtman AH. Dual Inhibition of Endocannabinoid Catabolic Enzymes Produces Enhanced Antiwithdrawal Effects in Morphine-Dependent Mice. Neuropsychopharmacology. 2013;38:1039–1049. doi: 10.1038/npp.2012.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh D, Ross GR, Schlosburg JE, Owens RA, Abdullah RA, Kinsey SG, Long JZ, Nomura DK, Sim-Selley LJ, Cravatt BF, Akbarali HI, et al. Blockade of endocannabinoid hydrolytic enzymes attenuates precipitated opioid withdrawal symptoms in mice. J Pharmacol Exp Ther. 2011;339:173–85. doi: 10.1124/jpet.111.181370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebe CJ, Wotjak CT. Endocannabinoids and stress. Stress. 2011;14:384–397. doi: 10.3109/10253890.2011.586753. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Néliat G, Caput D. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–4. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Roberts C, Stuhr K, Hutz M, Raff H, Hillard C. Endocannabinoid Signaling in Hypothalamic-Pituitary-Adrenocortical Axis Recovery Following Stress: Effects of Indirect Agonists and Comparison of Male and Female Mice. Pharmacol Biochem Behav. 2014;117:17–24. doi: 10.1016/j.pbb.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger Ta, Villacreses NE, Contreras Ma, Bonventre JV, Rapoport SI. Brain lipid metabolism in the cPLA2 knockout mouse. J Lipid Res. 2003;44:109–117. doi: 10.1194/jlr.m200298-jlr200. [DOI] [PubMed] [Google Scholar]
- Ruby Ma, Nomura DK, Hudak CSS, Barber A, Casida JE, Krauss RM. Acute overactive endocannabinoid signaling induces glucose intolerance, hepatic steatosis, and novel cannabinoid receptor 1 responsive genes. PLoS One. 2011;6:e26415. doi: 10.1371/journal.pone.0026415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson N-O, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saario SM, Salo OMH, Nevalainen T, Poso A, Laitinen JT, Järvinen T, Niemi R. Characterization of the sulfhydryl-sensitive site in the enzyme responsible for hydrolysis of 2-arachidonoyl-glycerol in rat cerebellar membranes. Chem Biol. 2005;12:649–56. doi: 10.1016/j.chembiol.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Sakurada T, Noma A. Subcellular localization and some properties of monoacylglycerol lipase in rat adipocytes. J Biochem. 1981;90:1413–9. doi: 10.1093/oxfordjournals.jbchem.a133607. [DOI] [PubMed] [Google Scholar]
- Samat A, Tomlinson B, Taheri S, Thomas GN. Rimonabant for the treatment of obesity. Recent Pat Cardiovasc Drug Discov. 2008;3:187–93. doi: 10.2174/157489008786264014. [DOI] [PubMed] [Google Scholar]
- Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–71. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savinainen JR, Kansanen E, Pantsar T, Navia-Paldanius D, Parkkari T, Lehtonen M, Laitinen T, Nevalainen T, Poso A, Levonen A-L, Laitinen JT. Robust Hydrolysis of Prostaglandin Glycerol Esters by Human Monoacylglycerol Lipase (MAGL) Mol Pharmacol. 2014;86:522–35. doi: 10.1124/mol.114.094284. [DOI] [PubMed] [Google Scholar]
- Scalvini L, Piomelli D, Mor M. Monoglyceride lipase: Structure and inhibitors. Chem Phys Lipids. 2016;197:13–24. doi: 10.1016/j.chemphyslip.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavone JL, Sterling RC, Van Bockstaele EJ. Cannabinoid and opioid interactions: implications for opiate dependence and withdrawal. Neuroscience. 2013;248:637–54. doi: 10.1016/j.neuroscience.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalk-Hihi C, Schubert C, Alexander R, Bayoumy S, Clemente JC, Deckman I, DesJarlais RL, Dzordzorme KC, Flores CM, Grasberger B, Kranz JK, et al. Crystal structure of a soluble form of human monoglyceride lipase in complex with an inhibitor at 1.35 Å resolution. Protein Sci. 2011;20:670–83. doi: 10.1002/pro.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF, Group R-DS Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet. 2006;368:1660–1672. doi: 10.1016/S0140-6736(06)69571-8. [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, Nguyen PT, Ramesh D, Booker L, James J, Thomas EA, et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Carlson BLA, Ramesh D, Abdullah RA, Long JZ, Cravatt BF, Lichtman AH. Inhibitors of endocannabinoid-metabolizing enzymes reduce precipitated withdrawal responses in THC-dependent mice. AAPS J. 2009;11:342–52. doi: 10.1208/s12248-009-9110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem. 2006;281:40236–41. doi: 10.1074/jbc.M608048200. [DOI] [PubMed] [Google Scholar]
- Senior JR, Isselbacher KJ. Demonstration of an intestinal monoglyceride lipase: An enzyme with a possible role in the intracellular completion of fat digestion. J Clin Invest. 1963;42:187–195. doi: 10.1172/JCI104705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheriff S, Du H, Grabowski GA. Characterization of lysosomal acid lipase by site-directed mutagenesis and heterologous expression. J Biol Chem. 1995;270:27766–27772. doi: 10.1074/jbc.270.46.27766. [DOI] [PubMed] [Google Scholar]
- Shi Y, Cheng D. Beyond triglyceride synthesis: the dynamic functional roles of MGAT and DGAT enzymes in energy metabolism. Am J Physiol Endocrinol Metab. 2009;297:E10–E18. doi: 10.1152/ajpendo.90949.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonesy BC, Bluett RJ, Ramikie TS, Báldi R, Hermanson DJ, Kingsley PJ, Marnett LJ, Winder DG, Colbran RJ, Patel S. Genetic disruption of 2-arachidonoylglycerol synthesis reveals a key role for endocannabinoid signaling in anxiety modulation. Cell Rep. 2014;9:1644–53. doi: 10.1016/j.celrep.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri C, Di Marzo V. The Endocannabinoid System in Energy Homeostasis and the Etiopathology of Metabolic Disorders. Cell Metab. 2013;17:475–490. doi: 10.1016/j.cmet.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF. Anandamide biosynthesis catalyzed by the phosphodiesterase GDE1 and detection of glycerophospho-N-acyl ethanolamine precursors in mouse brain. J Biol Chem. 2008;283:9341–9349. doi: 10.1074/jbc.M707807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CM, Itabe H, Reynolds CN, King WC, Glomset JA. Swiss 3T3 cells preferentially incorporate sn-2-arachidonoyl monoacylglycerol into sn-1-stearoyl-2-arachidonoyl phosphatidylinositol. J Biol Chem. 1991;266:15902–15909. [PubMed] [Google Scholar]
- Singh G. Recent considerations in nonsteroidal anti-inflammatory drug gastropathy. Am J Med. 1998;105:31S–38S. doi: 10.1016/s0002-9343(98)00072-2. [DOI] [PubMed] [Google Scholar]
- Steffens S, Veillard NR, Arnaud C, Pelli G, Burger F, Staub C, Zimmer A, Frossard J-L, Mach F. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature. 2005;434:782–786. doi: 10.1038/nature03389. [DOI] [PubMed] [Google Scholar]
- Stella N. Cannabinoid signaling in glial cells. Glia. 2004;48:267–77. doi: 10.1002/glia.20084. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Sugamura K, Sugiyama S, Fujiwara Y, Matsubara J, Akiyama E, Maeda H, Ohba K, Matsuzawa Y, Konishi M, Nozaki T, Horibata Y, et al. Cannabinoid 1 receptor blockade reduces atherosclerosis with enhances reverse cholesterol transport. J Atheroscler Thromb. 2010;17:141–7. doi: 10.5551/jat.2865. [DOI] [PubMed] [Google Scholar]
- Sumislawski JJ, Ramikie TS, Patel S. Reversible Gating of Endocannabinoid Plasticity in the Amygdala by Chronic Stress: A Potential Role for Monoacylglycerol Lipase Inhibition in the Prevention of Stress-Induced Behavioral Adaptation. Neuropsychopharmacology. 2011;36:2750–2761. doi: 10.1038/npp.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Jiang L, Luo X, Jin W, He Q, An J, Lui K, Shi J, Rong R, Su W, Lucchesi C, et al. Potential tumor-suppressive role of monoglyceride lipase in human colorectal cancer. Oncogene. 2013;32:234–41. doi: 10.1038/onc.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J, Vemuri VK, Liu J, Bátkai S, Mukhopadhyay B, Godlewski G, Osei-hyiaman D, Ohnuma S, Ambudkar SV, Pickel J, Makriyannis A, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010;120:2953–66. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A, Uchigashima M, Yamazaki M, Uesaka N, Mikuni T, Abe M, Hashimoto K, Watanabe M, Sakimura K, Kano M. Synapse type-independent degradation of the endocannabinoid 2-arachidonoylglycerol after retrograde synaptic suppression. Proc Natl Acad Sci U S A. 2012;109:12195–200. doi: 10.1073/pnas.1204404109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschler U, Eichmann TO, Radner FPW, Grabner GF, Wolinski H, Storr M, Lass A, Schicho R, Zimmermann R. Monoglyceride lipase deficiency causes desensitization of intestinal cannabinoid receptor type 1 and increased colonic μ-opioid receptor sensitivity. Br J Pharmacol. 2015;172:4419–29. doi: 10.1111/bph.13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschler U, Radner FPW, Heier C, Schreiber R, Schweiger M, Schoiswohl G, Preiss-Landl K, Jaeger D, Reiter B, Koefeler HC, Wojciechowski J, et al. Monoglyceride lipase deficiency in mice impairs lipolysis and attenuates diet-induced insulin resistance. J Biol Chem. 2011;286:17467–77. doi: 10.1074/jbc.M110.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telleria-Diaz A, Schmidt M, Kreusch S, Neubert A-K, Schache F, Vazquez E, Vanegas H, Schaible H-G, Ebersberger A. Spinal antinociceptive effects of cyclooxygenase inhibition during inflammation: Involvement of prostaglandins and endocannabinoids. Pain. 2010;148:26–35. doi: 10.1016/j.pain.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Thomas G, Betters JL, Lord CC, Brown AL, Marshall S, Ferguson D, Sawyer J, Davis MA, Melchior JT, Blume LC, Howlett AC, et al. The Serine Hydrolase ABHD6 Is a Critical Regulator of the Metabolic Syndrome. Cell Rep. 2013;5:508–20. doi: 10.1016/j.celrep.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tienhoven M, Atkins J, Li Y, Glynn P. Human neuropathy target esterase catalyzes hydrolysis of membrane lipids. J Biol Chem. 2002;277:20942–20948. doi: 10.1074/jbc.M200330200. [DOI] [PubMed] [Google Scholar]
- Tornqvist H, Belfrage P. Purification hydrolyzing and Some Properties of a Monoacylglycerol- Enzyme of Rat Adipose Tissue. Electrophoresis. 1976;251:813–819. [PubMed] [Google Scholar]
- Touw M. The religious and medicinal uses of Cannabis in China, India and Tibet. J Psychoactive Drugs. 1981;13:23–34. doi: 10.1080/02791072.1981.10471447. [DOI] [PubMed] [Google Scholar]
- Tsujiuchi T, Araki M, Hirane M, Dong Y, Fukushima N. Lysophosphatidic acid receptors in cancer pathobiology. Histol Histopathol. 2014;29:313–21. doi: 10.14670/HH-29.313. [DOI] [PubMed] [Google Scholar]
- Turcotte C, Chouinard F, Lefebvre JS, Flamand N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J Leukoc Biol. 2015;97:1049–70. doi: 10.1189/jlb.3RU0115-021R. [DOI] [PubMed] [Google Scholar]
- Uchigashima M, Yamazaki M, Yamasaki M, Tanimura A, Sakimura K, Kano M, Watanabe M. Molecular and morphological configuration for 2-arachidonoylglycerol-mediated retrograde signaling at mossy cell-granule cell synapses in the dentate gyrus. J Neurosci. 2011;31:7700–14. doi: 10.1523/JNEUROSCI.5665-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandevoorde S, Saha B, Mahadevan A, Razdan RK, Pertwee RG, Martin BR, Fowler CJ. Influence of the degree of unsaturation of the acyl side chain upon the interaction of analogues of 1-arachidonoylglycerol with monoacylglycerol lipase and fatty acid amide hydrolase. Biochem Biophys Res Commun. 2005;337:104–9. doi: 10.1016/j.bbrc.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Vaughan M, Berger JE, Steinberg D. Hormone-Sensitive Lipase and Monoglyceride Lipase Activities in Adipose Tissue. J Biol Chem. 1964;239:401–409. [PubMed] [Google Scholar]
- Viader A, Blankman JL, Zhong P, Liu X, Schlosburg JE, Joslyn CM, Liu Q-S, Tomarchio AJ, Lichtman AH, Selley DE, Sim-Selley LJ, et al. Metabolic Interplay between Astrocytes and Neurons Regulates Endocannabinoid Action. Cell Rep. 2015;12:798–808. doi: 10.1016/j.celrep.2015.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlad SC, Miller DR, Kowall NW, Felson DT. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology. 2008;70:1672–7. doi: 10.1212/01.wnl.0000311269.57716.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujic N, Schlager S, Eichmann TO, Madreiter-Sokolowski CT, Goeritzer M, Rainer S, Schauer S, Rosenberger A, Woelfler A, Doddapattar P, Zimmermann R, et al. Monoglyceride lipase deficiency modulates endocannabinoid signaling and improves plaque stability in ApoE-knockout mice. Atherosclerosis. 2016;244:9–21. doi: 10.1016/j.atherosclerosis.2015.10.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Martin BR. Cannabinoid pharmacological properties common to other centrally acting drugs. Eur J Pharmacol. 2003;471:185–193. doi: 10.1016/s0014-2999(03)01856-9. [DOI] [PubMed] [Google Scholar]
- Woodhams S, Wong A, Barrett D, Bennett A, Chapman V, Alexander S. Spinal administration of the monoacylglycerol lipase inhibitor JZL184 produces robust inhibitory effects on nociceptive processing and the development of central sensitization in the rat. Br J Pharmacol. 2012;167:1609–1619. doi: 10.1111/j.1476-5381.2012.02179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S, Borazjani A, Hatfield MJ, Edwards CC, Potter PM, Ross MK. Inactivation of Lipid Glyceryl Ester Metabolism in Human THP1 Monocytes/Macrophages by Activated Organophosphorus Insecticides: Role of Carboxylesterases 1 and 2. Chem Res Toxicol. 2010;23:1890–1904. doi: 10.1021/tx1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Zhang B, Seviour EG, Tao K-X, Liu X-H, Ling Y, Chen J-Y, Wang G-B. Monoacylglycerol lipase (MAGL) knockdown inhibits tumor cells growth in colorectal cancer. Cancer Lett. 2011;307:6–17. doi: 10.1016/j.canlet.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Yeaman SJ. Hormone-sensitive lipase--a multipurpose enzyme in lipid metabolism. Biochim Biophys Acta. 1990;1052:128–132. doi: 10.1016/0167-4889(90)90067-n. [DOI] [PubMed] [Google Scholar]
- Yen C-LEL, Farese RV, Farese RV., Jr MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J Biol Chem. 2003;278:18532–7. doi: 10.1074/jbc.M301633200. [DOI] [PubMed] [Google Scholar]
- Yen C-LEL, Stone SJ, Cases S, Zhou P, Farese RV, Jr, Farese RV. Identification of a gene encoding MGAT1, a monoacylglycerol acyltransferase. Proc Natl Acad Sci U S A. 2002;99:8512–7. doi: 10.1073/pnas.132274899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Liu Z, Lian Z, Liao R, Chen Y, Qin Y, Wang J, Jiang Q, Wang X, Gong J. Monoacylglycerol Lipase: A Novel Potential Therapeutic Target and Prognostic Indicator for Hepatocellular Carcinoma. Sci Rep. 2016;6:35784. doi: 10.1038/srep35784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Mugabo Y, Iglesias J, Xie L, Delghingaro-Augusto V, Lussier R, Peyot M-L, Joly E, Taïb B, Davis Ma, Brown JM, et al. α/β-Hydrolase Domain-6-Accessible Monoacylglycerol Controls Glucose-Stimulated Insulin Secretion. Cell Metab. 2014;6:1–15. doi: 10.1016/j.cmet.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Zhao S, Poursharafi P, Mugabo Y, Levens EJ, Vivot K, Attane C, Iglesias J, Peyot M, Joly E, Madiraju SRM, Prentki Marc. A/B-Hydrolase Domain-6 and Saturated Long Chain Monoacylglycerol Regulate Insulin Secretion Promoted By Both Fuel and Non-Fuel Stimuli. Mol Metab. 2015;4:940–950. doi: 10.1016/j.molmet.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P, Wang W, Pan B, Liu X, Zhang Z, Long JZ, Zhang H, Cravatt BF, Liu Q. Monoacylglycerol lipase inhibition blocks chronic stress-induced depressive-like behaviors via activation of mTOR signaling. Neuropsychopharmacology. 2014;39:1763–76. doi: 10.1038/npp.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Zhao Y, Zhou J, Wang X, Pan Q, Zhang N, Wang L, Wang M, Zhan D, Liu Z, He X, et al. Monoacylglycerol lipase promotes progression of hepatocellular carcinoma via NF-κB-mediated epithelial-mesenchymal transition. J Hematol Oncol. 2016;9:127. doi: 10.1186/s13045-016-0361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]