Abstract
Bacillus cereus endospores germinate in response to particular nutrients. Spores are able to sense these nutrients in the environment by receptors encoded by the gerA family of operons. Analysis of the Bacillus cereus ATCC 14579 genome revealed seven gerA family homologues. Using a transposon Tn917-based insertional mutagenesis approach followed by an enrichment procedure to select for l-alanine-induced germination mutants, we isolated a mutant with a defect in the l-alanine germination pathway. The transposon disrupted the last gene of a tricistronic gerA family operon, designated gerR, with the order gerRA, gerRC, gerRB. A second mutant was created by insertion of pMUTIN4 in gerRC. Both mutants showed the same phenotype for nutrient-induced germination. Spores of the gerR mutant strains were blocked in their l-alanine-initiated germination pathway and showed a delayed inosine-induced germination response. Apparently, germination mediated by l-alanine and inosine cannot be compensated for completely by the other germinant receptors, and this points towards an essential role of the gerR-encoded receptor in the receptor complex. In food products, spores of the mutant strains showed a reduced germination response compared to spores of the parental strain. High-pressure-initiated germination was not affected by the gerR mutations, as experiments with 100 and 550 MPa showed no difference with spores of the parental strain.
Bacillus cereus is a ubiquitous gram-positive soil organism and is considered an opportunistic food pathogen. It is widely distributed in nature and frequently isolated as a contaminant of various food products. B. cereus cells growing in food products can produce a heat-stable toxin which causes vomiting. A second type of food poisoning is caused by enterotoxins that are produced during vegetative growth in the small intestine and are responsible for diarrheal syndromes (7, 12).
B. cereus is genetically very closely related to Bacillus anthracis and Bacillus thuringiensis, but these species show important phenotypic differences (10). B. anthracis is the cause of the often lethal disease anthrax, while B. thuringiensis is a useful source of insecticidal toxins and is widely used as a pesticide in agriculture.
Bacilli can form endospores when survival conditions for vegetative cells are limited, e.g., during nutrient depletion (26). Once formed, the spores remain stable for many years and exhibit no metabolic activity but are capable of sensing the environment to detect favorable germination and growing conditions. The first event in nutrient-induced spore germination is probably the activation of the germinant receptors, which are located in the inner membrane of the spore (8, 15, 19). This activation is followed by a cascade of reactions that include the release of dipicolinic acid and successive uptake of water from the core. Further rehydration of the core requires hydrolysis of the peptidoglycan cortex layer by cortex lytic enzymes, and after degradation of the cortex the core continues to swell through further water uptake. At this stage, enzymes in the core are reactivated, protective proteins are degraded, and ATP is synthesized, followed by the initiation of metabolic processes and vegetative growth (14, 16, 25).
In Bacillus and Clostridium species, germinant receptors are encoded by tricistronic operons. For B. cereus strain 569 (ATCC 10876), three gerA operon homologues have been identified so far (1, 2). These operons were found to be involved in the germination response initiated by l-alanine and inosine. The genome sequence of B. cereus type strain ATCC 14579 (10) reveals the presence of seven ger operon homologues, but none of these have been functionally characterized so far. The exact structure and function of the germinant receptors are unknown, but it is expected that proteins encoded by a ger operon act together in forming a germinant receptor (18). It has been suggested that more than one germinant receptor can act in concert during the germination responses of B. subtilis (13) and B. anthracis (9, 28).
High hydrostatic pressure is increasingly being used as a food preservation technique (11). Besides nutrient-mediated germination, high hydrostatic pressure can also initiate germination of bacterial spores (6, 11, 29). Spore suspensions that have been exposed to relatively low or moderate pressures (100 to 250 MPa) germinate more efficiently than spores exposed to high pressures (>550 MPa) (22, 29, 31). At pressures below 250 MPa the germinant receptors play a role in this germination pathway, and this process seems to involve the same pathways as nutrient-induced germination (17, 31). For relatively higher pressures (550 MPa) these receptors do not seem to be required, as germination of B. subtilis spores lacking all germinant receptors is similar to that of wild-type spores (17).
This communication describes the identification and characterization of one of the seven gerA operon homologues in the genome of B. cereus ATCC 14579. A transposon Tn917-based insertional mutagenesis approach was applied to identify this operon in B. cereus ATCC 14579, designated gerR. Using a directed gene inactivation approach based on plasmid pMUTIN4 (27), a second mutation was subsequently created in this operon. Furthermore, wild-type spores and spores of the mutant strains were germinated in model foods to mimic nutrient diverse environments. It was shown that the gerR-encoded receptor system plays a role in l-alanine- and inosine-induced germination and germination in model foods but not in high hydrostatic pressure-induced germination.
MATERIALS AND METHODS
B. cereus strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. B. cereus was routinely cultured in Luria broth (LB) medium (24) at 30°C with aeration at 225 rpm and with chloramphenicol at a concentration of 10 μg/ml and erythromycin in combination with lincomycin at a concentration of 1 and 25 μg/ml, respectively. Escherichia coli strains were cultured in Luria broth at 37°C supplemented with 100 μg of ampicillin per ml.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| B. cereus | ||
| ATCC 14579 | Wild-type strain for this study | BGSCa |
| ATCC 14579/pTV32Ts | Cmr Eryr | This study |
| LH104 | Tn917-TV32Ts::gerRB1 Eryr | This study |
| LH129 | pMUTIN4::gerRC1 Eryr | This study |
| Plasmids | ||
| pTV32Ts | Cmr Eryr | 32 |
| pMUTIN4 | Ampr Eryr | 27 |
| pMUTIN4/gerRC1 | Ampr Eryr | This study |
BGSC, Bacillus Genetic Stock Center.
Preparation of the spores.
B. cereus was grown overnight in 5 ml of LB at 30°C with aeration and subsequently resuspended in 100 ml of chemically defined sporulation medium (4). Spore development was followed by phase contrast microscopy. In general, after 48 h of shaking at 225 rpm and 30°C, the medium contained >99% free spores. Spores were harvested and washed 10 times by centrifugation and resuspension in ice-cold 10 mM phosphate buffer, pH 7.4, containing 0.1% Tween 20 until a pure spore suspension was obtained. The spore suspension was stored at 4°C in this buffer and washed once a week to prevent spontaneous germination.
Transposon mutagenesis and screening for germination mutants.
A fresh colony of B. cereus ATTC 14579 containing plasmid pTV32Ts was inoculated in 5 ml of LB, containing chloramphenicol, erythromycin, and lincomycin, and grown at 30°C. After overnight growth, this culture was diluted 25-fold in LB containing all antibiotics and grown at 30°C until an optical density at 600 nm of 0.5 was reached. Subsequently, the culture was diluted 20-fold in LB (with antibiotics except chloramphenicol) and the cultivation temperature was raised to 42°C to induce insertional mutagenesis, and incubation was performed overnight. Then, the culture was diluted 20-fold in LB containing erythromycin and lincomycin and incubated at 42°C until late log phase (typically 3 to 5 h).
Mutant cells were harvested and resuspended in sporulation medium. After 48 h. the spores were harvested, washed, and heat activated in sterile water by incubation at 70°C for 15 min. The spores were then resuspended in germination buffer (2) containing 100 mM l-alanine and germinated for 60 min. Germinated spores were killed by heat treatment at 70°C for 15 min. The surviving spores were washed and incubated in LB for 1 to 2 h to induce germination. The spores were used to inoculate sporulation medium, and the enrichment procedure was repeated. After two enrichment cycles for l-alanine, the remaining mutants were evaluated by a tetrazolium colony transfer test for their germination behavior (2). Clones with a blocked l-alanine germination pathway were selected for further study. The mutants obtained were checked by Southern hybridization for single insertion of transposon Tn917.
DNA isolation and inverse PCR.
DNA from B. cereus was routinely isolated by the method of Pospiech (20). The position of the transposon was determined by performing inverse PCR on both sites with the restriction enzyme AluI. Fragments were cloned, sequenced, and compared with the B. cereus ATCC 14579 genome to determine the position of the Tn917 insertion.
Gene inactivation with pMUTIN4.
To generate a mutation in the gerR operon, a 1,367-bp fragment spanning the first and second genes of gerR was amplified from genomic DNA isolated from the type strain ATCC 14579 by using the forward primer 5′-GCCAAGCTTTGGATGATTGGATCGTTCTTTCG-3′ (the HindIII site is italic) and reverse primer 5′-CGGGATCCTTATCGCTGCTTCGTAACGTCC-3′ (the BamHI site is italic). The PCR product was digested with HindIII and BamHI, ligated in pMUTIN4, and transformed into Escherichia coli JM-109 cells. Five micrograms of the isolated plasmid (plasmid mini kit; Qiagen Westburg, Leusden, The Netherlands) was used to transform B. cereus ATCC 14579 by electroporation with the following parameters: 25 μF, 400 Ω, and 1.2 kV in a Gene Pulser electroporation apparatus (Bio-Rad). After 5 h of recovery at 30°C with shaking at 200 rpm in Luria broth, the transformants were selected on LB plates containing erythromycin and lincomycin.
The position of integration was verified by PCR with pMUTIN4 primers and primers that were designed on flanking positions of the gerR operon and was found on the expected position of codon 233 of the second gene of the gerR operon. Transformants were analyzed by Southern hybridization to ensure that a single copy of the plasmid had integrated into the chromosome. The isopropylthiogalactopyranoside (IPTG)-inducible Pspac promoter of pMUTIN4 allows control of expression of the downstream gene gerRB, avoiding polar effects (27). To study the effect of the GerRB protein on germination, mutant strain LH129 and the wild-type strain were also sporulated in the presence of 1 and 0.01 mM IPTG.
Germination assays.
Spores were activated by incubation at 70°C for 15 min in distilled water. Subsequently, spores were washed with distilled water and resuspended in germination buffer (10 mM Tris-HCl pH 7.4, 10 mM NaCl) to an optical density at 600 nm of ≈1.0 (≈4.0 × 108 spores/ml) and incubated for 15 min at 30°C in a 96-well microplate. After adding the germinants, the germination process was followed by monitoring the optical density at 600 nm, which reflects the number of germination events in the whole spore population caused by changes in the refractility of the spore from phase bright to phase black.
The optical density at 600 nm of the samples was measured every 2 min in a Tecan Safire plate reader for 60 or 120 min. Before each measurement, the plate was shaken for 30 s to prevent settling of the spores. The pH of the germination buffer during the experiments varied between 7.3 and 7.4 as a result of the addition of germinants in different concentrations to the germination buffer. Spores were routinely checked for germination behavior by phase contrast microscopy. To mimic food products, spores were germinated in meat broth (Maggi; Nestlé S.A., Vevey, Switserland) and cooked rice water (Lassie B.V., Wormer, The Netherlands). These products were prepared according to the manufacturers' instructions.
High hydrostatic pressure treatment.
We transferred 800 μl of a spore suspension with an optical density at 600 nm of 1.0 in 50 mM phosphate buffer, pH 7.4, to a sterile plastic stomacher bag (Seward, London, United Kingdom) and heat-sealed while avoiding air bubbles in the bag. Pouches with spore suspensions were pressurized in a high-pressure unit (Resato, Roden, The Netherlands) containing glycol as the compressing fluid and cooled to 20°C. Spore suspensions were exposed to 100 MPa and 550 MPa pressure for 30 min. Adiabatic heating caused a temperature rise of 4°C at 100 MPa and 14°C at 550 MPa but settled quickly at 20°C. Therefore, adiabatic heating can be neglected as a cause for killing of (germinated) spores.
After the pressure treatment, the suspension containing both germinated and nongerminated spores was diluted and plated on LB plates to determine the number of survivors. To distinguish between germinated spores and nongerminated spores after pressure treatment, half the volume of the spore suspension was incubated for 15 min at 70°C to kill germinated spores, while the other half was not heated before plating.
RESULTS
Identification of the gerR operon.
After insertional mutagenesis, the mutant library was enriched twice for mutants with a defect in the l-alanine-initiated germination pathway, and 96 mutants were examined by a tetrazolium colony transfer procedure. A number of mutants showed a delayed germination response, and one mutant showed no germination after 2 h of incubation on germination plates containing 100 mM l-alanine. This mutant was analyzed by Southern hybridization to ensure single-copy integration of the Tn917 transposon. The integration site was determined by inverse PCR from both sides. Sequence analysis of the transposon-flanking regions showed integration of the transposon in the third gene of an operon of a gerA homologue, subsequently designated gerR.
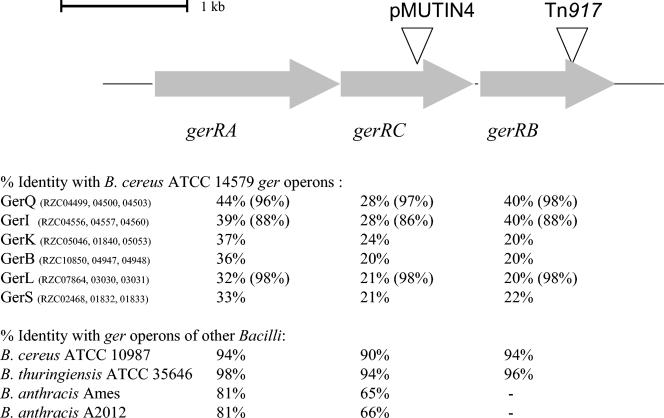
Like all ger operons in bacilli, gerR is tricistronic. However, the second gene shows homology to genes encoding GerAC proteins, while the third gene shows homology to GerAB proteins of other ger operons resulting in the gene order gerRA, gerRC, gerRB. The transposon had inserted in gerRB (Fig. 1).
FIG. 1.
Organization of the gerR operon and homology of the gerR operon with ger operons of B. cereus ATCC 14579 and other bacilli. Triangles show the positions of Tn917 insertion for mutant strain LH104 and of pMUTIN4 insertion for mutant strain LH129. The code numbers of the open reading frames of the gerR operon in the B. cereus ATCC 14579 genome database (10) are RZC04487 (A), RZC04488 (C), and RZC04490 (B). The code numbers of the open reading frames of the other ger operons and their percentages of amino acid identity are indicated. In contrast to gerR, these ger operons have the gene order gerA gerB gerC. The gene products encoded by the gerQ, gerI, and gerL operons are described for B. cereus 569 (1, 2), and the percentages of homology of these operons with the gerQ, gerI, and gerL operons of B. cereus ATCC 14579 are shown between brackets. Close homologues of the gerR operon can be found in other bacilli. The percentages of amino acid identity with homologues of the gerR operon in B. cereus ATCC 10987 (21), B. thuringiensis subsp. israelensis ATCC 35646 (http://www.ergo-light.com), B. anthracis Ames (http://www.tigr.org), and B. anthracis A2012 (http://www.ergo-light.com), are indicated.
Direct inactivation of the GerR receptor.
pMUTIN4 containing the cloned 1,367-bp PCR fragment internal to the gerR operon was introduced into B. cereus by electroporation. After transformation, erythromycin- and lincomycin-positive clones were obtained. Five clones were selected for further analysis, and single integration of pMUTIN4 was confirmed by Southern hybridization. PCR with primers designed on flanking regions and the pMUTIN4 sequence confirmed integration by homologous recombination in gerRC at the expected position for all five clones (Fig. 1).
gerR operon.
The gerR locus is located at position 769939 on the B. cereus ATCC 14579 genome and consists of three open reading frames. gerRA encodes a 505-amino-acid protein, gerRC encodes a 361-amino-acid protein, and gerRB encodes a 369-amino-acid protein. The gerR operon is flanked by an upstream gene encoding a 212-amino-acid protein and a downstream gene encoding a 170-amino-acid protein, both of unknown function. Upstream of each gerR gene, a probable ribosome-binding site could be identified, consisting of a GGTGA consensus sequence. ger operons have been shown to be expressed in the forespore compartment of the sporangium, and transcription is regulated by a σG-dependent promoter (3, 5). Upstream of the gerR locus, a typical σG consensus sequence could be detected, AGTATAN17AAAACTA.
Homology with the open reading frames of the six remaining ger operons found in the genome of the type strain is shown in Fig. 1. Close homologues of the gerR operon could be found in the recently sequenced genome of B. cereus ATCC 10987 (21) and in the close relatives B. anthracis and B. thuringiensis (Fig. 1). The homologue in B. thuringiensis subsp. israelensis ATCC 35646 is nearly identical to the gerR operon in B. cereus ATCC 14579, and this operon shows even higher similarity than the homologue in B. cereus ATCC 10987. Furthermore, homologues of gerRA and gerRC could be found in B. anthracis Ames and B. anthracis A2012. The gerRB homologues contained stop codons in both strains, and it is not clear whether this open reading frame encodes a functional protein. The order of the genes in these operons is similar to that of gerR.
The nutrient specificity of three ger receptor systems in B. cereus encoded by gerI, gerL, and gerQ have been described previously in strain B. cereus 569 (1, 2). The protein sequences of GerQ and GerL are nearly identical to their counterparts in B. cereus ATCC 14579. The homology of GerI is lower but still substantial (Fig. 1). In B. cereus 569, a defect in the gerI locus affects both l-alanine and inosine germination (2), a defect in the gerQ locus blocks the inosine germination pathway, and a defective gerL locus shows a slow l-alanine germination response (1). Whereas the homologies of the proteins of these receptors between B. cereus 569 and ATCC 14579 are high, the nutrient specificity in ATCC 14579 was not experimentally determined, and it is possible that this strain does not show the same nutrient specificities for these three operons.
l-Alanine- and inosine-induced germination.
First, the germination characteristics of B. cereus ATCC 14579 spores produced in chemically defined medium were determined. The dependence of the germination rate on l-alanine and inosine was measured by monitoring the reduction in optical density at 600 nm.
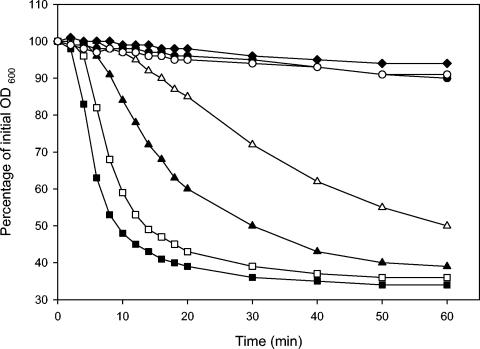
The germination response of wild-type spores to l-alanine is rapid, and the rate of germination is dependent on the l-alanine concentration (Fig. 2). Spores of the wild type are able to germinate at l-alanine concentrations as low as 0.1 mM, albeit at a reduced rate. Spores of both gerR mutant strains showed no significant germination in 100 mM l-alanine after 60 min of incubation (Fig. 2). Phase contrast microscopy after 60 min showed that the spores remained refractile, indicating that germination was not initiated in spores of the mutant strains. Both gerR mutant strains exhibited the same germination characteristics for l-alanine-induced germination.
FIG. 2.
Effect of l-alanine on germination of spores of the wild-type strain and of mutant strains LH104 (gerRB) and LH129 (gerRC). Germination was monitored as the fall in OD600 in response to l-alanine over the course of 60 min at 30°C and pH 7.4. ▪, wild-type strain, 100 mM l-alanine; □, wild-type strain, 10 mM l-alanine; ▴, wild-type strain, 1 mM l-alanine; ▵, wild-type strain, 0.1 mM l-alanine; ♦, wild-type strain, no germinant; •, LH104 (gerRB), 100 mM l-alanine; ○, LH129 (gerRC), 100 mM l-alanine. The data are means of values from duplicate experiments which were carried out with two independent spore batches.
Next, we examined inosine-induced germination; 10 and 1 mM inosine induced rapid germination of wild-type spores, and the germination rate is maximal and comparable to the germination rate in 100 mM l-alanine (Fig. 3). Germination in 0.1 mM inosine gives only a minor germination response (data not shown). Clear differences could be observed between the response of gerR mutant spores and wild-type spores (Fig. 3). Spores of the wild-type strain respond immediately to inosine, but spores of the mutant strains are delayed in their response and reach their maximum germination rate about 20 min after adding inosine. Furthermore, spores of the mutant strains show a reduced maximum germination rate compared with spores of the wild-type strain. Surprisingly, the maximum germination rate of the mutant spores is higher in 1 mM inosine than in 10 mM. This indicates an optimum inosine concentration for germination of gerR mutant spores; 0.1 mM inosine does not induce germination in the mutant spores (data not shown). Germination of spores of mutant strain LH129 prepared with IPTG to rescue expression of gerRB downstream did not differ from that of spores prepared without IPTG (data not shown).
FIG. 3.
Effect of inosine on germination of spores of the wild-type strain and of mutant strains LH104 (gerRB) and LH129 (gerRC) at inosine concentrations of 10 mM (A) and 1 mM (B). Germination was monitored as the reduction in OD600 in response to inosine over the course of 120 min at 30°C and pH 7.4. ▪, wild-type strain; •, LH104 (gerRB); ○, LH129 (gerRC).
Germination of bacterial spores in food products.
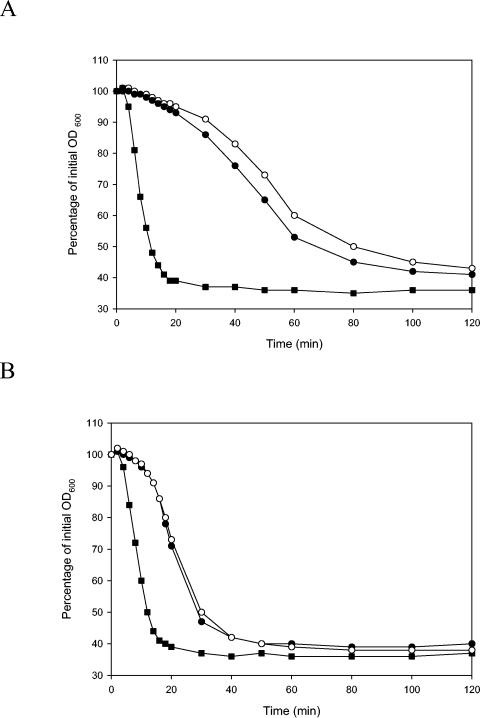
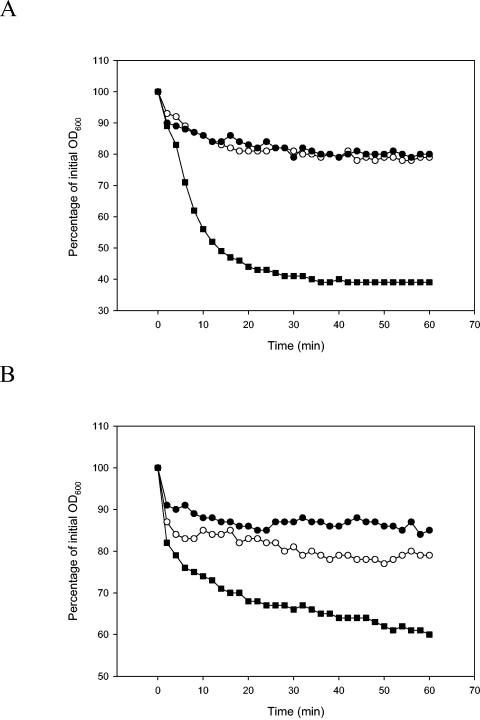
To mimic the natural situation of spore germination, spores of wild-type and mutant strains were inoculated in food products, and their germination in meat broth and cooked rice water was assessed. In both foods, spores of the parental strain responded quickly and germinated well (Fig. 4), whereas spores of both mutant strains showed a significant reduction in germination. This was confirmed visually by phase contrast microscopy. More than 90% of the spores from the gerR mutants were refractile, indicating that germination had not taken place in these spores. In contrast, more than 99% of the wild-type spores had changed to phase dark, indicating that they had germinated in the food products.
FIG. 4.
Effect of meat broth (A) and rice water (B) on germination of wild-type spores and spores of mutant strains LH104 (gerRB) and LH129 (gerRC). Germination was monitored as the fall in OD600 in response to meat broth over the course of 60 min at 30°C and pH 7.4. ▪, wild-type strain; •, LH104 (gerRB); ○, LH129 (gerRC).
High hydrostatic pressure-induced germination of spores from the parental strain and gerR mutants.
Because spores with a defect in the gerR locus show a clearly affected germination response in the nutrient-induced pathway (l-alanine, inosine, and food products), we assessed whether these spores were also affected in their germination response upon exposure to hydrostatic pressure of 100 and 550 MPa.
High pressure treatments of wild-type spores at 100 MPa for 30 min germinated >99.8% of the spores, of which 40% were killed during the treatment; 0.16% of the wild-type spores survived the heat treatment after pressurization, indicating that these spores did not germinate as a result of the pressure treatment. Spores of both mutant strains showed similar numbers; 0.13 and 0.36% of the spores of mutant strains LH104 and LH129, respectively, did not germinate because of this treatment. A high-pressure treatment at 550 MPa of the wild-type spore suspension germinated 37% and killed 10% of the spores; 63% of the spores survived the heat treatment after pressurization, i.e., these spores did not germinate as a result of this pressure. Again, no significant differences were observed between these mutants and the parental strain, as 71% of LH104 spores and 49% of LH129 spores did not germinate because of this treatment (data not shown).
Previous data from B. subtilis spore suspensions subjected to 550 MPa indicated a higher germination percentage as a result of this treatment (29). However, in these experiments, a higher initial temperature was used. Furthermore, adiabatic heating caused by pressurizing the sample will influence the germination and killing rate strongly (23, 29, 30). The equipment used for our high-pressure experiments is precooled, minimizing adiabatic heating. The minor temperature rise is not expected to have an important influence on spore germination and killing, so that we examined solely the high-pressure effect.
Spores of the wild-type and mutant strains pressurized at 100 and 550 MPa germinated with the same efficiency. Consequently, the germinant receptor encoded by the gerR operon does not play a role in the high-hydrostatic-pressure-induced germination pathway.
DISCUSSION
In this study we investigated the role of a novel germination receptor, termed gerR, in B. cereus ATCC 14579 type strain. This receptor was shown to be involved in l-alanine- and inosine-induced germination and also plays a role in germination of spores in food products.
The germination response upon addition of inosine differs from the l-alanine response: here the germinant concentration influences the germination rate within a very narrow range. Germination experiments with inosine as the germinant for spores of B. cereus 569 demonstrated a broader concentration range that influences the germination rate (2), and this might be characteristic for the type strain. Spores of both strains with a defective gerR operon germinated faster in 1 mM inosine than in 10 mM inosine. Another characteristic of both mutants is the delay in germination response after the addition of inosine. Normally, initiation of germination occurs within minutes upon addition of the germinant, but here a maximal response was not observed until 20 min after addition.
It cannot be excluded that mutant spores do not respond directly to inosine but that enzymes in the spore coat or cortex possibly convert inosine to another germinant molecule, which then causes germination of the spore, possibly in combination with inosine. The observed delayed response in germination could then be explained by the time needed to convert to the new germinant. Another explanation for the delay could be related to a reduction in the affinity of the receptor for inosine. This raises the question of why inosine-induced germination is not complemented by one of the other Ger receptors in B. cereus, in particular by the gene product of gerQ or gerI. Assuming that the germinant receptors in B. cereus ATCC 14579 and B. cereus 569 have the same nutrient specificity, B. cereus now contains three l-alanine receptors (GerL, GerI, and GerR) and three inosine receptors (GerI, GerQ, and GerR). By disrupting just one of these receptors, the l-alanine or inosine germination pathway is strongly inhibited or even blocked.
Receptors that play a role in the same germination pathway are not able to complement the function of a defective receptor. Various studies, including this one, suggest that the receptors can be part of a complex, probably in concert with the gene products of other ger operons (9, 13). Disturbance of this complex by disrupting one or more of its proteins will reduce or inhibit the ability to respond properly to nutrients. Due to the mere absence of the gerR encoded receptor system, the mutants are not able to germinate normally in the model foods tested, supporting the idea that the receptor complex might be disturbed by disruption of the gerR locus. The same may hold true for the gerI operon, as disruption of this receptor also affects both l-alanine- and inosine-induced germination (2). The elimination of certain germinant receptors causes a general inhibition in the nutrient-induced pathway, indicating that a single receptor might be an essential component in the nutrient-induced germination pathway.
Comparison of the germination characteristics with other germinant receptors in B. cereus 569 showed that all germinant receptors identified so far in B. cereus strains are involved in l-alanine- or inosine-induced germination. It appears that B. cereus requires a broad range of receptors that can all apparently be activated by l-alanine and/or inosine. However, it is not known if the germination characteristics of different strains can be compared. Furthermore, it is not known if all ger operons found in a bacterial genome encode a functional receptor or if these operons are differentially expressed under various sporulation conditions. These items remain a topic for further research.
Acknowledgments
Integrated Genomics (Chicago, Ill.) is acknowledged for use of the B. cereus genome sequence database at an early stage of this research. We thank Patrick de Leeuw for assistance during the high hydrostatic pressure experiments.
REFERENCES
- 1.Barlass, P. J., C. W. Houston, M. O. Clements, and A. Moir. 2002. Germination of Bacillus cereus spores in response to l-alanine and to inosine: the roles of gerL and gerQ operons. Microbiology 148:2089-2095. [DOI] [PubMed] [Google Scholar]
- 2.Clements, M. O., and A. Moir. 1998. Role of the gerI operon of Bacillus cereus 569 in the response of spores to germinants. J. Bacteriol. 180:6729-6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corfe, B. M., A. Moir, D. Popham, and P. Setlow. 1994. Analysis of the expression and regulation of the gerB spore germination operon of Bacillus subtilis 168. Microbiology 140:3079-3083. [DOI] [PubMed] [Google Scholar]
- 4.De Vries, Y. P., L. M. Hornstra, W. M. De Vos, and T. Abee. 2004. Growth and sporulation of Bacillus cereus ATCC 14579 under defined conditions: temporal expression of genes for key sigma factors. Appl Environ Microbiol. 70:2514-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feavers, I. M., J. Foulkes, B. Setlow, D. Sun, W. Nicholson, P. Setlow, and A. Moir. 1990. The regulation of transcription of the gerA spore germination operon of Bacillus subtilis. Mol Microbiol. 4:275-282. [DOI] [PubMed] [Google Scholar]
- 6.Gould, G. W., and A. J. Sale. 1970. Initiation of germination of bacterial spores by hydrostatic pressure. J. Gen Microbiol. 60:335-346. [DOI] [PubMed] [Google Scholar]
- 7.Granum, P. E. 2001. Bacillus cereus, p. 327-336. In M. P. Doyle, L. R. Beuchat and T. J. Montville (ed.), Food microbiology: fundamentals and frontiers, 2nd ed. American Society for Microbiology, Washington, D.C.
- 8.Hudson, K. D., B. M. Corfe, E. H. Kemp, I. M. Feavers, P. J. Coote, and A. Moir. 2001. Localization of GerAA and GerAC germination proteins in the Bacillus subtilis spore. J. Bacteriol. 183:4317-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ireland, J. A., and P. C. Hanna. 2002. Amino acid- and purine ribonucleoside-induced germination of Bacillus Anthracis DSterne endospores: gerS mediates responses to aromatic ring structures. J. Bacteriol. 184:1296-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivanova, N., A. Sorokin, I. Anderson, N. Galleron, B. Candelon, V. Kapatral, A. Bhattacharyya, G. Reznik, N. Mikhailova, A. Lapidus, L. Chu, M. Mazur, E. Goltsman, N. Larsen, M. D'Souza, T. Walunas, Y. Grechkin, G. Pusch, R. Haselkorn, M. Fonstein, S. D. Ehrlich, R. Overbeek, and N. Kyrpides. 2003. Genome sequence of Bacillus cereus and comparative analysis with Bacillus anthracis. Nature 423:87-91. [DOI] [PubMed] [Google Scholar]
- 11.Knorr, D. 1999. Novel approaches in food-processing technology: new technologies for preserving foods and modifying function. Curr. Opin. Biotechnol. 10:485-491. [DOI] [PubMed] [Google Scholar]
- 12.Lund, T., M. Buyser de, and P. E. Granum. 2000. A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol. Microbiol. 38:254-261. [DOI] [PubMed] [Google Scholar]
- 13.McCann, K. P., C. Robinson, R. L. Sammons, D. A. Smith, and B. M. Corfe. 1996. Alanine germination receptors of Bacillus subtilis. Lett. Appl. Microbiol. 23:290-294. [DOI] [PubMed] [Google Scholar]
- 14.Moir, A. 2003. Bacterial spore germination and protein mobility. Trends Microbiol. 11:452-454. [DOI] [PubMed] [Google Scholar]
- 15.Moir, A., E. H. Kemp, C. Robinson, and B. M. Corfe. 1994. The genetic analysis of bacterial spore germination. J. Appl. Bacteriol. 77:9S-16S. [PubMed] [Google Scholar]
- 16.Moir, A., and D. A. Smith. 1990. The genetics of bacterial spore germination. Annu. Rev. Microbiol. 44:531-553. [DOI] [PubMed] [Google Scholar]
- 17.Paidhungat, M., B. Setlow, W. B. Daniels, D. Hoover, E. Papafragkou, and P. Setlow. 2002. Mechanisms of induction of germination of Bacillus subtilis spores by high pressure. Appl. Environ. Microbiol. 68:3172-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paidhungat, M., and P. Setlow. 1999. Isolation and characterization of mutations in Bacillus subtilis that allow spore germination in the novel germinant d-alanine. J. Bacteriol. 181:3341-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paidhungat, M., and P. Setlow. 2001. Localization of a germinant receptor protein (GerBA) to the inner membrane of Bacillus subtilis spores. J. Bacteriol. 183:3982-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pospiech, A., and B. Neumann. 1995. A versatile quick-prep of genomic DNA from gram-positive bacteria. Trends Genet. 11:217-218. [DOI] [PubMed] [Google Scholar]
- 21.Rasko, D. A., J. Ravel, O. A. Okstad, E. Helgason, R. Z. Cer, L. Jiang, K. A. Shores, D. E. Fouts, N. J. Tourasse, S. V. Angiuoli, J. Kolonay, W. C. Nelson, A. B. Kolsto, C. M. Fraser, and T. D. Read. 2004. The genome sequence of Bacillus cereus ATCC 10987 reveals metabolic adaptations and a large plasmid related to Bacillus anthracis pXO1. Nucleic Acids Res. 32:977-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raso, J., G. Barbosa-Canovas, and B. G. Swanson. 1998. Sporulation temperature affects initiation of germination and inactivation by high hydrostatic pressure of Bacillus cereus. J. Appl. Microbiol. 85:17-24. [DOI] [PubMed] [Google Scholar]
- 23.Raso, J., M. M. Gongora-Nieto, G. V. Barbosa-Canovas, and B. G. Swanson. 1998. Influence of several environmental factors on the initiation of germination and inactivation of Bacillus cereus by high hydrostatic pressure. Int. J. Food Microbiol. 44:125-132. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550-556. [DOI] [PubMed] [Google Scholar]
- 26.Stragier, P., and L. Losick. 1996. Molecular genetics of sporulation in Bacillus subtilis. Annu. Rev. Genet. 30:297-341. [DOI] [PubMed] [Google Scholar]
- 27.Vagner, V., E. Dervyn, and S. D. Ehrlich. 1998. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology 144:3097-3104. [DOI] [PubMed] [Google Scholar]
- 28.Weiner, M. A., T. D. Read, and P. C. Hanna. 2003. Identification and characterization of the gerH operon of Bacillus anthracis endospores: a differential role for purine nucleosides in germination. J. Bacteriol. 185:1462-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wuytack, E. Y., S. Boven, and C. W. Michiels. 1998. Comparative study of pressure-induced germination of Bacillus subtilis spores at low and high pressures. Appl. Environ. Microbiol. 64:3220-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wuytack, E. Y., and C. W. Michiels. 2001. A study on the effects of high pressure and heat on Bacillus subtilis spores at low pH. Int. J. Food Microbiol. 64:333-341. [DOI] [PubMed] [Google Scholar]
- 31.Wuytack, E. Y., J. Soons, F. Poschet, and C. W. Michiels. 2000. Comparative study of pressure- and nutrient-induced germination of Bacillus subtilis spores. Appl. Environ. Microbiol. 66:257-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Youngman, P. J. 1987. Plasmid vectors for recovering and exploiting Tn917 transpositions in Bacillus and other Gram-positives, p. 79-103. In K. Hardy (ed.), Plasmids: a practical approach. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.