Abstract
Spinal cord injury (SCI) is a multifaceted phenomenon associated with alterations in both motor function and sensory function. A majority of patients with SCI report sensory disturbances, including not only loss of sensation, but in many cases enhanced abnormal sensation, dysesthesia and pain. Development of therapeutics to treat these abnormal sensory changes require common measurement tools that can enable cross-species translation from animal models to human patients. We review the current literature on translational nociception/pain measurement in SCI and discuss areas for further development. Although a number of tools exist for measuring both segmental and affective sensory changes, we conclude that there is a pressing need for better, integrative measurement of nociception/pain outcomes across species to enhance precise therapeutic innovation for sensory dysfunction in SCI.
Keywords: Common data elements, spinal cord injury, pain, sensory assessment
1. Introduction
Spinal cord injury (SCI) is characterized by disruption of ascending and descending spinal cord fiber tracts, leading to highly heterogeneous sensorimotor and autonomic impairments. SCI is followed by a complex cascade of multiple biological processes including secondary injury and delayed degeneration as well as compensatory neuroplasticity [1]. Evidence from animal models suggests that extensive plasticity along the neuroaxis occurs spontaneously and can be enhanced by specific therapeutic interventions [2–5]. However, spontaneous and experimentally-induced plasticity after a lesion to the spinal cord leads not only to beneficial recovery of motor and sensory function, but can also lead to maladaptive sensory changes like neuropathic pain (NP) [6–9].
Proper sensory function regarding body state and environment (cutaneous and proprioceptive) is crucial for recovery of motor function [10–14]. Sensory dysfunction is of major clinical relevance, as the majority of SCI patients report some pain [15] and NP occurs in over 50% of patients with a SCI [16–18]. Understanding how outcome measures of sensory plasticity change during recovery is essential for cross-species translation, especially when translating novel treatments emerging from preclinical research into the clinical setting [19]. Despite the importance of monitoring sensory changes after SCI, the assessment of sensory function is strongly under-represented in the SCI literature compared to assessment of the motor system.
Here we review the various measures that can shed light on sensory changes occurring after SCI at the different levels along the neuroaxis. We focus on tools for monitoring sensory (mal)-function in preclinical models of SCI and compare them with tools available for patients with SCI (Table 1). The overarching goal of our review is to assess the current state of the literature and gaps in cross-species measurement of pain after SCI, to promote translational alignment of outcomes and expedite clinical trials for precision pain medicine in SCI.
Table 1.
Sensory Outcome Measures Across Species.
| Rodent | Monkey | Human |
|---|---|---|
| Von Frey filament testing | Von Frey filament testing Mechanical allodynia measurements |
Von Frey filament testing Light touch sensation Pinprick sensation |
| Autotomy/picking Over-grooming Vocalization |
Autotomy/picking Over-grooming Vocalization |
Pain questionnaires
|
| Conditioned place preference |
||
| Facial expressions | ||
| Electrical motor threshold | Electrical operant escape response |
Electrical perception threshold |
| Electrical vocalization threshold |
Electrical vocalization threshold | Electrical pain threshold |
| Cold allodynia/hyperalgesia measurement |
Cold allodynia/hyperalgesia measurement |
Cold detection threshold Cold pain threshold |
| Heat motor threshold | Heat detection thresholds | |
| Heat vocalization threshold | Heat pain thresholds |
2.1 Behavioral Assessments
Behavioral assessments of the sensory system after SCI can measure evoked (stimulus-dependent) or spontaneous (stimulus-independent) sensory function. Behavioral assessments of evoked sensory function can be classified according to three main criteria: 1) stimulus modality, 2) site of stimulation relative to the level of lesion and 3) type of response that is measured. Modalities that are used include static and dynamic mechanical stimulation, thermal stimulation (cold and heat stimulation), chemical and electrical stimulation. The stimulation can be applied below-level, at-level or above the level of lesion. The responses can be measured at different levels along the neuroaxis: segmental reflex responses, brainstem reflex responses or cortical processing. Below we discuss each of these forms of behavioral assessment.
Evoked and Stimulus-Dependent Assessment Tools
Most sensory assessments in animal models focus on evoked sensory measures where a stimulus is delivered and a response is measured. These measurements can be segmentally-organized spinal reflexes or supraspinal and affective in nature.
Reflex-mediated sensory measures
Most commonly (mal)-adaptive sensory changes in preclinical models are measured by stimulus-evoked reflex responses. The external stimuli can either be nociceptive or non-nociceptive and of various modalities. Stimuli are most often applied to the plantar forepaw, plantar hindpaw or tail, as the corresponding segmental reflex responses are better detectable in the extremities than responses on the midline (e.g., thoracic cutaneous trunci muscles). The behaviors measured are spinal (i.e., withdrawal or tail flick) and/or brainstem reflex responses (i.e., licking, biting, struggling, guarding, jumping). Reflexes have often been discussed as inappropriate measures of pain and suffering in animal models. Segmental reflex responses such as withdrawal rely on a motor response and thus not only sensory but also motor function is assessed. Further, many of the reflex responses are also observed in decerebrated animals and therefore do not involve cortical processing [19]. In preclinical models of SCI spinal reflex responses can additionally be confounded by the presence of spasticity [20] and some motor function is necessary to assess the withdrawal response. Nevertheless, reflexes can give information about the segmental state of excitability within the spinal cord. For example, the Hoffman reflex (H-flex), an electrophysiological test of the muscle stretch reflex, is a representative procedure that has been used to evaluate the status of excitability of the spinal cord in a number of studies across species [21]. Although little is known about pathophysiological mechanisms of central NP after SCI [22], some measurements of H-reflex have the potential to reflect the state of NP/excitability in preclinical models.
Tactile allodynia/static mechanical hypersensitivity is primarily assessed with static application of von Frey hair (VFH) monofilaments using the up-down method [23]. The lowest gram force used to induce a spinal response in at least 50% of the applications is reported as the tactile threshold. In addition the incidence of supraspinal responses are recorded and the responses are presented as percentages of total response. Detloff et al. developed a method to assess below level (i.e. hindpaw) sensory thresholds acutely after rat SCI before motor functions, such as weight support and trunk stability, recovered [24]. The rat is wrapped in a towel during testing allowing spinal but not supraspinal reflex responses to be measured. Importantly, the acute dorsal reflex thresholds predicted chronic pain thresholds. Other studies have tested hind and forepaw responses as well as locations on the torso, in both rat and nonhuman primate (NHP) models of SCI [25,26]. In rats, in addition to suprasegmental responses (vocalization/guarding), segmental responses such as skin contraction and body flinch have been recorded after VFH tactile stimulation of the torso in low thoracic SCI.[25] This model of SCI induced both long-lasting suprasegmental responses to stimulation on the torso and decreased segmental hindpaw withdrawal thresholds to touch and cold [25]. In NHP, Salegio et al. used an electronic VFH filament to assess changes in sensory dysfunction above, at and below the level of lesion (i.e., shoulder, hand, thorax, knee and foot) by monitoring both segmental (flinch/withdrawal) and suprasegmental (facial activity/orientation) responses [26].
Affective/motivational measures of stimulus-dependent pain
In more recent years novel paradigms that assess affective and/ or motivational behavior induced by evoked or spontaneous maladaptive sensations have been adapted from the pain literature and applied in preclinical models of SCI [27–32]. Below we review these affective pain measures in SCI.
The place escape/ avoidance paradigm (PEAP) assesses the affective/ motivational behavior to avoid pain [27]. In rodents, this paradigm is applied in a dual chamber (dark and light room). An experimenter applies mechanical stimuli in the dark room to a location below or at the level of lesion potentially affected by maladaptive sensory changes [20,33,34]. In the light room the animal are either not stimulated or receive stimulation at a control site above the lesion [20,33,34]. The motivation to escape the mechanical aversive stimulus is opposed to rodents’ aversion to bright light, biasing the test against false positives. The time that the animal spends in the light room and the number of crossings between chambers is measured. The underlying assumption is that if mechanical stimuli induce an aversive response the animal will spend more time in the light chamber to avoid/ escape the stimulation overcoming rodents’ natural aversion to bright light. One limitation of this paradigm is that it is a learned task and can only be applied once [20]. Previous studies in both contusion and transection models of thoracic SCI were able to measure at-level hypersensitivity at the thorax and the forepaws [20,33,34]. The one study that assessed below-level hypersensitivity with the PEAP did not see a change in response due to below-level stimulation. The authors also observed no change in brain-stem mediated reflex thresholds [20]. Further work is needed to reconcile these findings with other work demonstrating below-level changes in tactile nociception in certain animal models of SCI [11,12].
In a similar dual-chamber PEAP paradigm the mechanical stimulation is replaced by thermal stimulation to assess cold and/ or heat sensitivity [28,35]. In the dark chamber hot, cold or neutral thermal stimuli are applied through a temperature regulated floor plate. In the bright chamber the floor plate is set to a neutral temperature. In this paradigm animals are trained to establish stimulus preference between avoidance of thermal stimulation and bright light [29]. In a model of low thoracic spinal cord compression Vierck et al. were able to measure cold and hot hyperalgesia in SCI [35].
In the PEAP paired with heat stimulation (temperature regulated floor plate) the thermal stimuli are simultaneously applied to fore and hindpaws. Depending on the level of SCI this model might not be able to distinguish between at and below level maladaptive sensory changes. In comparison in the mechanical stimulation PEAP, the stimuli can be applied to various locations by an experimenter. Whereas, an advantage of the thermal testing paradigm is that it can be accomplished in the absence of the experimenter, as the thermal stimulation can be automated.
Vocalization is another potentially useful and highly conserved measurement tool for assessing affective pain across species ranging from rodents to humans [36]. Ultrasonic vocalization (USV), which is emitted by rodents in response to noxious stimuli outside of the human audible range, can serve as a potential non-reflex behavioral measure to distinguish between positive and negative emotional-affective states of pain. USV has been mapped by stimulus type, associating specific frequency bands with particular stimulus modalities ranging from painful (~20–40 kHz) to pleasurable (~50 kHz) [37,38].
Stimulus-dependent sensory assessments in human SCI
Similar to preclinical models, stimuli of different modalities are used to assess evoked sensory function after human SCI. As part of the core SCI Common Data Elements, by the National Institute of Neurological Disorders and Stroke (NINDS), the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) have become a standard clinical tool to assess sensory function [39–41]. This exam uses light touch and pinprick stimuli applied to cervical, thoracic, lumbar, and sacral dermatomes. The quality of the sensation is scored on a 3-point ordinal scale (normal, impaired, absent). One limitation of this assessment is that dermatomes that have either decreased sensation or are hypersensitive fall into to the same category of impaired sensation. Only a few studies looked at the relation between the ISNCSCI sensory measures and the development of NP [42,43]. Hari et al. found that patients that suffer from NP have enhanced sensory recovery measured by pinprick sensation in comparison to SCI patients that do not develop NP [42]. Another study found that lower at-level pinprick scores were more predictive than light touch scores for NP development [43].
A well-established psychophysical method is quantitative sensory testing (QST) [44–46]. QST measures detection and pain thresholds of different stimulus modalities to assess sensory loss and sensory gain (e.g., allodynia, hyperalgesia) and depends on the patient’s subjective report of the perceived sensation. Included in the common data elements for SCI are both perceptual and pain threshold to cold and heat stimuli and perceptual threshold to mechanical vibration [41].
While in humans QST can assess sensory function along the entire neuroaxis using a vocalization/ verbal output, most published animal models have focused on fore and hindpaw, as segmental responses such as skin twitch (cutaneous trunci) reflexes are harder to detect. This distinction in measurement reflects a point for improvement for the goal of understanding the nature of pain sensitivity across the neuraxis after SCI.
Spontaneous (Stimulus-Independent) and Chronic Assessment Tools
Evoked pain responses such as reflexes, PEAP and vocalization measures are powerful tools for charting the basic neurobiology of sensory change after SCI for early stage therapeutic development. However, from the perspective of bench-to-bedside translation the role of evoked measures is less clear, as spontaneous and chronic pain is more common in SCI patients than evoked or stimulus-dependent pain [47–49]. Although measuring spontaneous and chronic pain, such as central NP can be challenging in preclinical models, a few papers have demonstrated approaches for doing so in the SCI preclinical model. Below we will discuss these emerging approaches and review their utility as tools for targeting affective pain as a translational therapeutic approach.
Conditioned place preference
Conditioned place preference (CPP) is a paradigm that was recently adapted from the pain literature to assess sensory dysfunction after SCI [30,50]. This paradigm is designed to measure spontaneous and chronic pain rather than evoked pain. The CPP unmasks the affective component of chronic pain (unpleasantness), by taking advantage of the fact that rapid pain relief is rewarding [51]. Based on this concept, non-narcotic analgesic treatments should only be rewarding in the presence of chronic pain [30] and therefore only animals in chronic pain should demonstrate pavlovian CPP for stimuli paired with pain-relieving treatment. During CPP conditioning, distinct black or white chambers are paired with manipulation that have proven to alleviate pain (e.g., lidocaine in the rostroventral medulla) or saline. On test days the animals are tested in a drug-free state. They are placed in the CPP box with access to both black and white chambers and time spent in the different chambers is recorded to assess chamber preference. In SCI animals it has been shown that analgesic treatments are rewarding to animals with spinal cord lesions but not to sham operated animals [50]. This place preference provides strong evidence that SCI is associated with chronic pain and provides a platform for testing therapies that mitigate or prevent this chronic pain.
Facial expressions
The coding of facial expressions is another way to assess the affective component of spontaneous pain. This paradigm was first developed in mice [31] and later modified for the use in rats [32]. In the mouse grimace scale five facial features are coded on a 3-point scale (not present, moderate and severe): orbital tightening, nose bulge, cheek bulge, ear position and whisker change [31]. In the rat the nose and cheek specific features are collapsed into one category, whereby pain results in increased flatting of this facial area [32]. Studies in mice highlighted that the grimace scale might not be sensitive to assess pain assays of longer duration, such as chronic NP [31]. In a recent SCI study the mouse grimace scale detected early-phase spontaneous pain up to 3 weeks after injury. In comparison, in the same study mechanical and thermal sensitivity continued to be increased at 2-month post lesion [52]. It is unclear if distinct (mal)-adaptive sensory changes with distinct temporal courses are measured with these different measurement tools or if animals learn to control their facial response over time.
In addition to assessment tools that measure ongoing spontaneous pain, spontaneous behavioral events such as sleep behavior, picking/ overgrooming and autotomy can be monitored. However, these spontaneous behaviors can reflect multiple underlying causes not solely related to (mal)-adaptive sensory changes [19]. For example picking/ overgrooming could also relate to numbness of specific body areas at or below the level of lesion independent of pain. Lee-Kubli et al. measured spontaneous lifting of forepaws unrelated to locomotion, grooming or change in position after a thoracic SCI in rats [33]. They found that animals with a complete spinal transection or compression have increased foot lifting in comparison to sham-operated animals. The increased spontaneous foot lifts persisted for several weeks and were alleviated by the applications of gabapentin, a drug used to treat NP.
Stimulus-independent sensory assessments in human SCI
A common problem in preclinical assessment of spontaneous pain is that the location that relates to the pain experience is not clearly defined and therefore below and at-level NP are indistinguishable. In human SCI, the patient’s personal description of spontaneous and also evoked-pain gives valuable detailed information including intensity, location and duration of the perceived pain sensation. Visual analog scales, numeric rating scales and pain questionnaires are used to capture the subjective pain perception. There are numerous different measures that have been recommended to assess NP in humans [53]. The pain working group from the NINDS SCI Common Data Elements [41] recommends the International SCI Pain Basic Dataset (ISCIPBDS) [54,55] which includes the International SCI Pain Classification (ISCIP) [56,57]. The goal of the ISCIP classification is to discern between pain types (i.e., neuropathic, nociceptive, other or unknown pain). Within the NP classification at-level, below-level and other NP types that are unrelated to SCI are coded [55]. In addition to the pain types, pain presence, interference, location, intensity, onset and treatment are assessed [54,55]. Developing animal correlates for gathering this type of information remains a challenge requiring further work.
Disconnect Between Different Pain Assessments Along the Neuroaxis
In preclinical models, one important question is whether interneuronal circuits connecting nociceptive neurons to motor neurons segmentally are similarly affected by anti-nociceptive interventions as behavioral outcome measures that involve brainstem or cortical processing. Some studies have shown discrepancies between spinally-mediated reflex responses and supraspinal (i.e., brainstem or cerebral-dependent) responses [20,52]. This disconnect between the sensory measures at different processing levels along the neuroaxis suggests that these tests reflect different components of pain processing (affective versus sensory component) [34,58]. In the context of SCI, segmental responses might also reflect ongoing spasticity rather than alterations in the nociceptive system [20]. This disconnect of measures along the neuroaxis implies that different behavioral assessments can give distinct insights into (mal)-adaptive sensory changes after SCI, and studies applying multiple behavioral assessments may reflect most accurately the complex underlying syndrome [59]. In studies with the aim of targeting NP in preclinical models of SCI, assessment assays that include supra-segmental processing (ideally cortical processing) are needed.
2.2 Biomarkers
Although behavioral assessment remains the most common method for measuring sensory dysfunction after SCI, recent research has been focused on identifying biomarkers that may provide objective translational measures of sensory changes. At the outset, it should be stated that there is little currently known about the specific biomarkers for SCI pain. Yet, emerging biomarkers for SCI severity exist, and some of these molecular pathways overlap with known pain mechanisms. Thus, the search for SCI NP biomarkers may be partially informed by screening molecular mechanisms of injury pathophysiology and cross-referencing them with molecular pain signatures. Recent work has revealed candidate cerebrospinal fluid (CSF) or serum biomarkers for objectively stratifying injury severity, including the structural proteins S100Beta, tau and pNF-H, and the cytokines IL-6 and IL-8 [60–64]. Although biomarkers of injury severity may aid in overall disease prognosis, their potential for predicting the development of neuropathic pain is limited by the unreliable correlation between injury severity and NP. Reports of below-level NP prevalence following SCI range from 34 to 67% [17,48], yet the precise constellation of factors that determines whether a patient will develop NP is still unknown, and to date, little clinical research on SCI pain biomarkers has been undertaken [60,65]. In the following section we review a number of promising preclinical studies aimed at early biomarker discovery that may shed light on the biological and morphological indicators of NP development after SCI.
Immune Factors
The immune response following SCI is believed to alter sensory plasticity through the release of inflammatory cytokines from activated microglia, macrophages and astrocytes. A large body of preclinical work has identified these factors in mediating allodynia following peripheral injury, and recent preclinical work has highlighted similar effects following SCI [66–69]. Hains and Waxman demonstrated that microglia play a pivotal role in the maintenance of hyper-responsiveness in lumbar dorsal horn neurons after SCI [70]. Detloff and colleagues showed that the severity of SCI-induced allodynia below the level of injury was highly correlated with activation of microglia, and the expression of inflammatory cytokines TNFa and Il-1B in the lumbar dorsal horn, up to 21 days after SCI [71].
Based on these and other preclinical studies indicating a role for cytokines as potentially potent pain biomarkers, these factors have been measured in CSF and serum of SCI patients [62]. Kwon and colleagues found that among inflammatory cytokines measured 24 hours after SCI, IL-6 and IL-8 were elevated in an injury severity-dependent fashion, but only one inflammatory factor (the TNFa receptor TNFR1) was correlated with NP symptoms. Cytokine expression following SCI is highly spatially and temporally dynamic, and future work on the time course of cytokine expression will likely be needed to elucidate a serum or CSF-derived pain biomarker in the clinic.
Imaging Biomarkers
Another promising avenue for pain biomarkers lies in the rapidly evolving field of neuroimaging. In a study of 23 SCI patients, Finnerup and colleagues used T2-weighted MRI to assess the relationship between gross morphological changes in white matter integrity in SCI patients with NP (61%) and without NP (39%), and found a higher instance of spinothalamic tract lesions (in conjunction with hyperexcitability) in patients with NP [72]. Others have used functional magnetic resonance imaging (fMRI), which allows for the detection of neural activation (albeit indirectly) in morphologically distinct areas of the brain and spinal cord. As this technique can sensitively capture the robust SCI-induced reorganization of the somatosensory cortex in rats, it may be an essential tool in measuring maladaptive sensory plasticity in a translational fashion across species [73,74]. Wrigley and colleagues demonstrated that light brush of the little finger in human SCI patients produced neural activation in different areas of the contralateral post-central gyrus, depending on whether the patient was exhibiting NP symptoms, indicating cortical reorganization in the pain patients. Further, the extent of reorganization was found to be significantly correlated with pain intensity [75]. In contrast, Jutzeler et al found greater reorganization in SCI individuals without NP in comparison to with NP [76].
More recent work has employed MR spectroscopy to detect changes on specific metabolites that may be correlated with increased pain. Stanwell and colleagues showed that the concentration of myoinositol (Ins), a presumptive marker of glial activation in the anterior cingulate cortex, was a potent indicator of the presence of NP following experimental SCI [77]. This finding was supported in a study of human SCI patients, which showed that when combined with the marker N-acetyl aspartate and glutamate-glutamine, levels of Ins could be used to differentiate between patients with and without NP [78,79].
Biomarkers of structural plasticity
As NP following SCI is characterized as a form of nociceptive (mal)-adaptive plasticity, the search for a biological predictor of this behavioral change has also led to the investigation of structural changes on nociceptive neurons as a biomarker. Waxman and colleagues have demonstrated that the development of nociceptive hypersensitivity following SCI is highly correlated with changes in the quantity and quality of dendritic spines on wide dynamic range neurons in the spinal dorsal horns of spinal cord injured rats [80]. They found that rats exhibited increased dendritic spine density as well as abnormal morphology (“mushroom spines”) that was closely associated with SCI-induced decrease in withdrawal thresholds from tactile stimuli and increased single unit spike firing, indicative of neuropathic allodynia and hypersensitivity [80]. They have recently shown that the structural protein RAC1 may be a key mediator in producing this spine dysgenesis, demonstrating a therapeutic effect of RAC1 inhibition to reduce allodynia and restore dendritic spines to a normal state [81]. This type of tissue-based measurement is enticing as a mechanistic pain measure, however it remains unclear how such detailed morphology could be measured in a non-invasive manner, which would be required for translational assessments.
A recent study using voxel-wise analysis of anatomical magnet resonance imaging data looked at cross-sectional cervical cord area and volumetric brain changes after human SCI patients with and without below-level NP [82]. In individuals with paraplegia a reduction in the cross-sectional cervical cord area was associated with below-level NP, whereby cortical changes were bidirectional [82].
Although there are many emerging biomarker candidates for pain and nociceptive changes in SCI, to date there has been little work demonstrating their sensitivity and specificity in human SCI. Further, SCI pain biomarker studies have largely focused on identifying acute pathophysiological changes. Given the complexity and heterogeneity of time-dependent secondary injury processes after SCI, it is not known whether these early candidate biomarkers would be effective if measured in chronic SCI. Fortunately, preclinical genomic and proteomic and image-based screening techniques continue to evolve. Future developments in these areas have the potential to expedite cross-species translation and accelerate therapeutic discovery.
3. Discussion
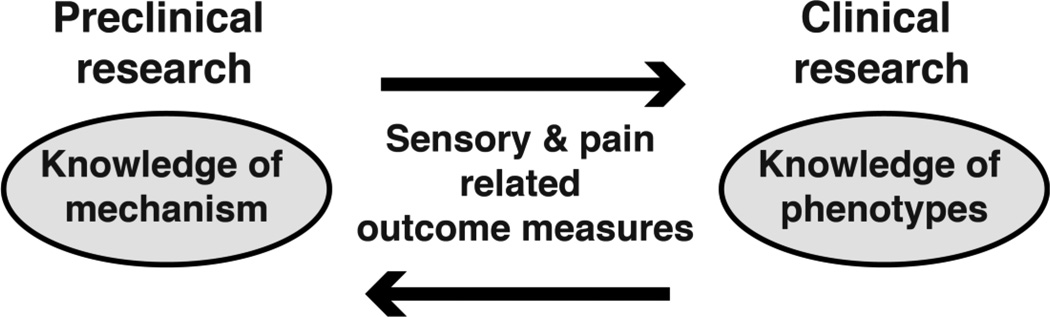
In the context of sensory changes/pain in SCI both forward-translation (preclinical to clinical) and back-translation (clinical to preclinical) is important to link clinical phenotypes with preclinical mechanisms (Figure 1). Clinical NP phenotypes in SCI have been well described and can include spontaneous pain and/ or stimulus-evoked pain such as allodynia (i.e., pain in response to a non-nociceptive stimulus) and hyperalgesia (i.e., increased pain sensitivity) [83–85]. Typical human pain descriptors include: hot-burning, tingling, pricking, pins and needles, sharp, shooting, squeezing, painful-cold and electric-shock-like [56]. This broad and heterogeneous spectrum of phenotypes is proposed to reflect the diversity of the underlying mechanisms which are currently not fully understood [47,86,87]. The relationship between preclinically-revealed mechanisms and clinical symptoms might also not be bivariate (e.g. one mechanism may give rise to more than one symptom) [88,89]. Recent research highlights the importance of appreciating distinct sensory profiles resulting from multiple variables to draw conclusions about the underlying mechanism/ combination of mechanisms [19,22,49,89–92]. As modulation and processing of sensory stimuli occurs on different levels along the neuroaxis the combined evaluation of multiple readouts can help link preclinical mechanism to clinical phenotypes and accelerate translation.
Figure 1.
To relate knowledge of mechanism gained in preclinical research to clinical phenotypes (e.g., burning pain) efforts to link sensory and pain related outcome measures across species are crucial.
Efforts to promote translation also must acknowledge model limitations. Currently, most preclinical research assessing maladaptive sensory changes after SCI mainly uses rodent animal models. However, SCI researchers recommend that NHP are placed within the preclinical discovery pathway as there are several factors that limit direct translation from rodents to humans [93– 95]. One crucial difference is the neuroanatomical arrangement of the tracts within the spinal cord [95,96]. Given the phylogenetic difference between rodents and humans, some pain-related targets function differently between species [97,98]. Studies assessing maladaptive sensory changes after SCI in NHP suggest sensory changes resembling certain human features of central pain including: supraspinal hyper-responsiveness to at-level stimulation, evidence of below-level dysesthesia (depilitation, overgrooming), symptoms that wax and wane over time, and unresponsiveness to opioids[26,99]. However, future studies need to further investigate how the observed sensory changes reflect neuropathic pain phenotypes observed in humans after SCI [100].
4. Conclusion
Pain in SCI is a pervasive and complex phenomenon. Yet development of outcome measures for SCI pain that translate across species is less well developed than motor endpoints. We review the existing literature on outcome measures for sensory changes after SCI, with a specific goal of aligning measures across species. Further development of translational sensory measures after SCI is area for ongoing work that has potential to improve development of therapies to improve sensory outcomes.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grants NS069537, NS088475, NS067092 (Adam R. Ferguson), Wings for Life Spinal Cord Research Foundation Grants WFLUS008/12, WFLUS006/14 (Adam R. Ferguson) and WFLUS013/13 (Kazuhito Morioka), Craig H. Neilsen Foundation 224308 (Adam R. Ferguson) and 313739 (Jenny Haefeli), Japan Society for the Promotion of Science (JSPS) KAKENHI Grants 21800092 and 23700659 (Kazuhito Morioka), Mitsui Sumitomo Insurance Welfare Foundation Research Grant (Kazuhito Morioka).
Abbreviations
- CSF
cerebrospinal fluid
- NHP
nonhuman primate
- NP
neuropathic pain
- QST
quantitative sensory testing
- SCI
spinal cord injury
- USV
Ultrasonic vocalization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat. Rev. Neurosci. 2001;2:263–273. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- 2.Bradbury EJ, McMahon SB. Spinal cord repair strategies: why do they work? Nat Rev Neurosci. 2006;7:644–653. doi: 10.1038/nrn1964. [DOI] [PubMed] [Google Scholar]
- 3.Thuret S, Moon LDF, Gage FH. Therapeutic interventions after spinal cord injury. Nat. Rev. Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 4.Blesch A, Tuszynski MH. Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 2009;32:41–47. doi: 10.1016/j.tins.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Pernet V, Schwab ME. The role of Nogo-A in axonal plasticity, regrowth and repair. Cell Tissue Res. 2012;349:97–104. doi: 10.1007/s00441-012-1432-6. [DOI] [PubMed] [Google Scholar]
- 6.Hofstetter CP, a V Holmström N, a Lilja J, Schweinhardt P, Hao J, Spenger C, Wiesenfeld-Hallin Z, Kurpad SN, Frisén J, Olson L. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat. Neurosci. 2005;8:346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- 7.Deumens R, Joosten Ea J, Waxman SG, Hains BC. Locomotor dysfunction and pain: the scylla and charybdis of fiber sprouting after spinal cord injury. Mol. Neurobiol. 2008;37:52–63. doi: 10.1007/s12035-008-8016-1. [DOI] [PubMed] [Google Scholar]
- 8.Fouad K, Krajacic A, Tetzlaff W. Spinal cord injury and plasticity: opportunities and challenges. Brain Res. Bull. 2011;84:337–342. doi: 10.1016/j.brainresbull.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Brown A, Weaver LC. The dark side of neuroplasticity. Exp. Neurol. 2012;235:133–141. doi: 10.1016/j.expneurol.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frigon A, Rossignol S. Functional plasticity following spinal cord lesions. Prog. Brain Res. 2006;157:231–260. doi: 10.1016/s0079-6123(06)57016-5. [DOI] [PubMed] [Google Scholar]
- 11.Grau JW, Huie JR, Lee KH, Hoy KC, Huang Y-J, Turtle JD, Strain MM, Baumbauer KM, Miranda RM, Hook Ma, Ferguson AR, Garraway SM. Metaplasticity and behavior: how training and inflammation affect plastic potential within the spinal cord and recovery after injury. Front. Neural Circuits. 2014;8 doi: 10.3389/fncir.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson AR, Crown ED, Grau JW. Nociceptive plasticity inhibits adaptive learning in the spinal cord. Neuroscience. 2006;141:421–431. doi: 10.1016/j.neuroscience.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 13.Dorofeev IY, Avelev VD, Shcherbakova NA, Gerasimenko YP. The role of cutaneous afferents in controlling locomotion evoked by epidural stimulation of the spinal cord in decerebrate cats. Neurosci. Behav. Physiol. 2008;38:695–701. doi: 10.1007/s11055-008-9034-1. [DOI] [PubMed] [Google Scholar]
- 14.Bouyer LJG, Rossignol S. Contribution of Cutaneous Inputs From the Hindpaw to the Control of Locomotion. II. Spinal Cats. J. Neurophysiol. 2003;90:3640–3653. doi: 10.1152/jn.00497.2003. [DOI] [PubMed] [Google Scholar]
- 15.van Gorp S, Kessels aG, a Joosten E, van Kleef M, Patijn J. Pain prevalence and its determinants after spinal cord injury: A systematic review. Eur. J. Pain. 2014;19:1–10. doi: 10.1002/ejp.522. [DOI] [PubMed] [Google Scholar]
- 16.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 17.Siddall PJ, Mcclelland JM, Rutkowski SB, Cousins MJ. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–257. doi: 10.1016/S0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- 18.Burke D, Fullen BM, Stokes D, Lennon O. Neuropathic pain prevalence following spinal cord injury: A systematic review and meta-analysis. Eur. J. Pain. 2016 doi: 10.1002/ejp.905. [DOI] [PubMed] [Google Scholar]
- 19.Vierck CJ, Hansson PT, Yezierski RP. Clinical and pre-clinical pain assessment: are we measuring the same thing? Pain. 2008;135:7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Baastrup C, Maersk-Moller CC, Nyengaard JR, Jensen TS, Finnerup NB. Spinal-, brainstem- and cerebrally mediated responses at- and below-level of a spinal cord contusion in rats: Evaluation of pain-like behavior. Pain. 2010;151:670–679. doi: 10.1016/j.pain.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 21.Knikou M. The H-reflex as a probe: Pathways and pitfalls. J. Neurosci. Methods. 2008;171:1–12. doi: 10.1016/j.jneumeth.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Costigan M, Scholz J, Woolf CJ. A Maladaptive Response of the Nervous System to Damage. Annu Rev Neurosci. 2010;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 24.Detloff MR, Clark LM, Hutchinson KJ, Kloos AD, Fisher LC, Basso DM. Validity of acute and chronic tactile sensory testing after spinal cord injury in rats. Exp. Neurol. 2010;225:366–376. doi: 10.1016/j.expneurol.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindsey AE, LoVerso RL, Tovar CA, Hill CE, Beattie MS, Bresnahan JC. An Analysis of Changes in Sensory Thresholds to Mild Tactile and Cold Stimuli after Experimental Spinal Cord Injury in the Rat. Neurorehabil. Neural Repair. 2000 doi: 10.1177/154596830001400405. [DOI] [PubMed] [Google Scholar]
- 26.Salegio EA, Bresnahan JC, Sparrey CJ, Camisa W, Fischer J, Leasure J, Buckley J, Nout-Lomas YS, Rosenzweig E, Moseanko R, Strand S, Hawbecker S, Lemoy M-J, Haefeli J, Ma X, Nielson JL, Edgerton VR, Ferguson AR, Tuszynski MH, Beattie MS. A unilateral cervical spinal cord contusion injury model in non-human primates (macaca mulatta) J. Neurotrauma. 2015;7900:1–80. doi: 10.1089/neu.2015.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaBuda CJ, Fuchs PN. A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp. Neurol. 2000;163:490–494. doi: 10.1006/exnr.2000.7395. [DOI] [PubMed] [Google Scholar]
- 28.Vierck CJ, Kline R, Wiley RG. Comparison of operant escape and innate reflex responses to nociceptive skin temperatures produced by heat and cold stimulation of rats. Behav. Neurosci. 2004;118:627–635. doi: 10.1037/0735-7044.118.3.627. [DOI] [PubMed] [Google Scholar]
- 29.Mauderli AP, Acosta-Rua A, Vierck CJ. An operant assay of thermal pain in conscious, unrestrained rats. J. Neurosci. Methods. 2000;97:19–29. doi: 10.1016/s0165-0270(00)00160-6. [DOI] [PubMed] [Google Scholar]
- 30.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat. Neurosci. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7:447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 32.Sotocinal SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, Mapplebeck JC, Wei P, Zhan S, Zhang S, McDougall JJ, King OD, Mogil JS. The Rat Grimace Scale: A partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain. 2011;7:55. doi: 10.1186/1744-8069-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee-Kubli CA, Ingves M, Henry KW, Shiao R, Collyer E, Tuszynski MH, Campana WM. Analysis of the behavioral, cellular and molecular characteristics of pain in severe rodent spinal cord injury. Elsevier B.V. 2016 doi: 10.1016/j.expneurol.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Baastrup C, Jensen TS, Finnerup NB. Pregabalin attenuates place escape/avoidance behavior in a rat model of spinal cord injury. Brain Res. 2011;1370:129–135. doi: 10.1016/j.brainres.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Vierck CJ, Baastrup C, Maersk-Moller C, Roth M, Cannon RL, Finnerup NB, Yezierski RP. A preclinical model of hyperalgesia following spinal stenosis/compression. Eur. J. Pain. 2015;19 doi: 10.1002/ejp.640. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 36.Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat. Rev. Neurosci. 2008;9:314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- 37.Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol. Bull. 2002;128:961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- 38.Oliveira AR, Barros HMT. Ultrasonic rat vocalizations during the formalin test: A measure of the affective dimension of pain? Anesth. Analg. 2006;102:832–839. doi: 10.1213/01.ane.0000196530.72813.d9. [DOI] [PubMed] [Google Scholar]
- 39.Grinnon ST, Miller K, Marler JR, Lu Y, Stout A, Odenkirchen J, Kunitz S. National Institute of Neurological Disorders and Stroke Common Data Element Project - approach and methods. Clin. Trials. 2012;9:322–329. doi: 10.1177/1740774512438980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirshblum SC, Waring W, Biering-Sorensen F, Burns SP, Johansen M, Schmidt-Read M, Donovan W, Graves D, Jha A, Jones L, Mulcahey MJ, Krassioukov A. International Standards for Neurological Classification of Spinal Cord Injury (Revised 2011) J. Spinal Cord Med. 2011;34:547–554. doi: 10.1179/204577211X13207446293695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biering-Sørensen F, Alai S, Anderson K, Charlifue S, Chen Y, DeVivo M, Flanders AE, Jones L, Kleitman N, Lans A, Noonan VK, Odenkirchen J, Steeves J, Tansey K, Widerström-Noga E, Jakeman LB. Common data elements for spinal cord injury clinical research: a National Institute for Neurological Disorders and Stroke project. Spinal Cord. 2015;53:265–277. doi: 10.1038/sc.2014.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hari AR, Wydenkeller S, Dokladal P, Halder P. Enhanced recovery of human spinothalamic function is associated with central neuropathic pain after SCI. Exp. Neurol. 2009;216:428–430. doi: 10.1016/j.expneurol.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 43.Levitan Y, Zeilig G, Bondi M, Ringler E, Defrin R. Predicting the Risk for Central Pain Using the Sensory Components of the International Standards for Neurological Classification of Spinal Cord Injury. J. Neurotrauma. 2015;9:150805132220000. doi: 10.1089/neu.2015.3947. [DOI] [PubMed] [Google Scholar]
- 44.Backonja MM, Attal N, Baron R, Bouhassira D, Drangholt M, Dyck PJ, Edwards RR, Freeman R, Gracely R, Haanpaa MH, Hansson P, Hatem SM, Krumova EK, Jensen TS, Maier C, Mick G, Rice AS, Rolke R, Treede RD, Serra J, Toelle T, Tugnoli V, Walk D, Walalce MS, Ware M, Yarnitsky D, Ziegler D. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain. 2013;154:1807–1819. doi: 10.1016/j.pain.2013.05.047. [DOI] [PubMed] [Google Scholar]
- 45.Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, Treede RD. Quantitative sensory testing: A comprehensive protocol for clinical trials. Eur. J. Pain. 2006;10:77–88. doi: 10.1016/j.ejpain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Finnerup NB. Predictors of Spinal Cord Injury Neuropathic Pain: The Role of QST. Top. Spinal Cord Inj. Rehabil. 2007;13:35–42. [Google Scholar]
- 47.Finnerup NB. Pain in patients with spinal cord injury. Pain. 2013;154(Suppl):S71–S76. doi: 10.1016/j.pain.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 48.Finnerup NB, Johannesen IL, Sindrup SH, Bach FW, Jensen TS. Pain and dysesthesia in patients with spinal cord injury: A postal survey. Spinal Cord. 2001;39:256–262. doi: 10.1038/sj.sc.3101161. [DOI] [PubMed] [Google Scholar]
- 49.Finnerup NB, Norrbrink C, Trok K, Piehl F, Johannesen IL, Sørensen JC, Jensen TS, Werhagen L. Phenotypes and predictors of pain following traumatic spinal cord injury: a prospective study. J. Pain. 2014;15:40–48. doi: 10.1016/j.jpain.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Davoody L, Quiton RL, Lucas JM, Ji Y, Keller A, Masri R. Conditioned place preference reveals tonic pain in an animal model of central pain. J. Pain. 2011;12:868–874. doi: 10.1016/j.jpain.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Navratilova E, Porreca F. Reward and motivation in pain and pain relief. Nat. Neurosci. 2014;17:1304–1312. doi: 10.1038/nn.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu J, Zhao Z, Zhu X, Renn CL, Dorsey SG, Faden AI. Cell cycle inhibition limits development and maintenance of neuropathic pain following spinal cord injury. Pain. 2016;157:488–503. doi: 10.1097/j.pain.0000000000000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bryce TN, Budh CN, Cardenas DD, Dijkers M, Felix ER, Finnerup NB, Kennedy P, Lundeberg T, Richards JS, Rintala DH, Siddall P, Widerstrom-Noga E. Pain after spinal cord injury: an evidence-based review for clinical practice and research. Report of the National Institute on Disability and Rehabilitation Research Spinal Cord Injury Measures meeting. J. Spinal Cord Med. 2007;30:421–440. doi: 10.1080/10790268.2007.11753405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Widerström-Noga E, Biering-Sørensen F, Bryce T, Cardenas DD, Finnerup NB, Jensen MP, Richards JS, Siddall PJ. The international spinal cord injury pain basic data set. Spinal Cord. 2008;46:818–823. doi: 10.1038/sc.2008.64. [DOI] [PubMed] [Google Scholar]
- 55.Widerstrom-Noga E, Biering-Sorensen F, Bryce TN, Cardenas DD, Finnerup NB, Jensen MP, Richards JS, Siddall PJ, Widerström-Noga E, Biering-Sørensen F, Bryce TN, Cardenas DD, Finnerup NB, Jensen MP, Richards JS, Siddall PJ. The International Spinal Cord Injury Pain Basic Data Set (version 2.0) Spinal Cord. 2014;52:282–286. doi: 10.1038/sc.2014.4. [DOI] [PubMed] [Google Scholar]
- 56.Bryce TN, Biering-Sørensen F, Finnerup NB, Cardenas DD, Defrin R, Lundeberg T, Norrbrink C, Richards JS, Siddall P, Stripling T, Treede R-D, Waxman SG, Widerström-Noga E, Yezierski RP, Dijkers M. International spinal cord injury pain classification: part I. Background and description. Spinal Cord. 2012;50:413–417. doi: 10.1038/sc.2011.156. [DOI] [PubMed] [Google Scholar]
- 57.Bryce TN, Biering-Sørensen F, Finnerup NB, Cardenas DD, Defrin R, Ivan E, Lundeberg T, Norrbrink C, Richards JS, Siddall P, Stripling T, Treede R-D, Waxman SG, Widerström-Noga E, Yezierski RP, Dijkers M. International Spinal Cord Injury Pain (ISCIP) Classification: Part 2. Initial validation using vignettes. Spinal Cord. 2012;50:404–412. doi: 10.1038/sc.2012.2. [DOI] [PubMed] [Google Scholar]
- 58.King T, Porreca F. Preclinical Assessment of Pain: Improving Models in Discovery Research. Curr. Top. Behav Neurosci. 2014:101–120. doi: 10.1007/7854_2014_330. [DOI] [PubMed] [Google Scholar]
- 59.Yezierski RP, Vierck CJ. Reflex and pain behaviors are not equivalent: Lessons from spinal cord injury. Pain. 2010;151:569–570. doi: 10.1016/j.pain.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 60.Pouw MH, Hosman AJF, van Middendorp JJ, Verbeek MM, Vos PE, van de Meent H. Biomarkers in spinal cord injury. Spinal Cord. 2009;47:519–525. doi: 10.1038/sc.2008.176. [DOI] [PubMed] [Google Scholar]
- 61.Kwon BK, Streijger F, Fallah N, Noonan VK, Bélanger LM, Paquette SJ, Ailon T, Boyd MC, Street J, Fisher CG, Dvorak MF. Cerebrospinal Fluid Biomarkers to Stratify Injury Severity and Predict Outcome in Human Traumatic Spinal Cord Injury. J. Neurotrauma. 2016:1–44. doi: 10.1089/neu.2016.4435. [DOI] [PubMed] [Google Scholar]
- 62.Kwon BK, Stammers AMT, Belanger LM, Bernardo A, Chan D, Bishop CM, Slobogean GP, Zhang H, Umedaly H, Giffin M, Street J, Boyd MC, Paquette SJ, Fisher CG, Dvorak MF. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J. Neurotrauma. 2010;27:669–682. doi: 10.1089/neu.2009.1080. [DOI] [PubMed] [Google Scholar]
- 63.Ueno T, Ohori Y, Ito J, Hoshikawa S, Yamamoto S, Nakamura K, Tanaka S, Akai M, Tobimatsu Y, Ogata T. Hyperphosphorylated neurofilament NF-H as a biomarker of the efficacy of minocycline therapy for spinal cord injury. Spinal Cord. 2011;49:333–336. doi: 10.1038/sc.2010.116. [DOI] [PubMed] [Google Scholar]
- 64.Hayakawa K, Okazaki R, Ishii K, Ueno T, Izawa N, Tanaka Y, Toyooka S, Matsuoka N, Morioka K, Ohori Y, Nakamura K, Akai M, Tobimatsu Y, Hamabe Y, Ogata T. Phosphorylated neurofilament subunit NF-H as a biomarker for evaluating the severity of spinal cord injury patients, a pilot study. Spinal Cord. 2012;50:493–496. doi: 10.1038/sc.2011.184. [DOI] [PubMed] [Google Scholar]
- 65.Guéz M, Hildingsson C, Rosengren L, Karlsson K, Toolanen G. Nervous tissue damage markers in cerebrospinal fluid after cervical spine injuries and whiplash trauma. J. Neurotrauma. 2003;20:853–858. doi: 10.1089/089771503322385782. [DOI] [PubMed] [Google Scholar]
- 66.Tsuda M, Yukari K, Shigemoto-Mogami Schuichi, Mizokoshi A, Kohsaka S, Salter MW, Kazuhide I. P2×4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:1–6. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 67.Clark AK, Gentry C, Bradbury EJ, McMahon SB, Malcangio M. Role of spinal microglia in rat models of peripheral nerve injury and inflammation. Eur. J. Pain. 2007;11:223–230. doi: 10.1016/j.ejpain.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 68.Peng X, Zhou Z, Glorioso JC, Fink DJ, Mata M. Tumor necrosis factor-alpha contributes to below-level neuropathic pain after spinal cord injury. Ann. Neurol. 2006;59:843–851. doi: 10.1002/ana.20855. [DOI] [PubMed] [Google Scholar]
- 69.Crown ED, Gwak YS, Ye Z, Johnson KM, Hulsebosch CE. Activation of p38 MAP kinase is involved in central neuropathic pain following spinal cord injury. Exp. Neurol. 2008;213:257–267. doi: 10.1016/j.expneurol.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci. 2006;26:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Detloff MR, Fisher LC, McGaughy V, Longbrake EE, Popovich PG, Basso DM. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp. Neurol. 2008;212:337–347. doi: 10.1016/j.expneurol.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Finnerup NB. Sensory function in spinal cord injury patients with and without central pain. Brain. 2003;126:57–70. doi: 10.1093/brain/awg007. [DOI] [PubMed] [Google Scholar]
- 73.Kaas JH, Qi HX, Burish MJ, Gharbawie OA, Onifer SM, Massey JM. Cortical and subcortical plasticity in the brains of humans, primates, and rats after damage to sensory afferents in the dorsal columns of the spinal cord. Exp. Neurol. 2008;209:407–416. doi: 10.1016/j.expneurol.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghosh A, Sydekum E, Haiss F, Peduzzi S, Zörner B, Schneider R, Baltes C, Rudin M, Weber B, Schwab ME. Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats. J. Neurosci. 2009;29:12210–12219. doi: 10.1523/JNEUROSCI.1828-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wrigley PJ, Press SR, Gustin SM, Macefield VG, Gandevia SC, Cousins MJ, Middleton JW, Henderson LA, Siddall PJ. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain. 2009;141:52–59. doi: 10.1016/j.pain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 76.Jutzeler CR, Freund P, Huber E, Curt A, Kramer JLK. Neuropathic pain and functional reorganization in the primary sensorimotor cortex after spinal cord injury. J. Pain. 2015;16:1256–1267. doi: 10.1016/j.jpain.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 77.Stanwell P, Siddall P, Keshava N, Cocuzzo D, Ramadan S, Lin A, Herbert D, Craig A, Tran Y, Middleton J, Gautam S, Cousins M, Mountford C. Neuro magnetic resonance spectroscopy using wavelet decomposition and statistical testing identifies biochemical changes in people with spinal cord injury and pain. Neuroimage. 2010;53:544–552. doi: 10.1016/j.neuroimage.2010.06.051. [DOI] [PubMed] [Google Scholar]
- 78.Widerstrom-Noga E, Pattany PM, Cruz-Almeida Y, Felix ER, Perez S, Cardenas DD, Martinez-Arizala A. Metabolite concentrations in the anterior cingulate cortex predict high neuropathic pain impact after spinal cord injury. Pain. 2013;154:204–212. doi: 10.1016/j.pain.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Widerström-Nogaa E, Cruz-Almeidaa Y, Felixa ER, Pattany PM. Somatosensory phenotype is associated with thalamic metabolites and pain intensity after spinal cord injury. Pain. 2015;33:395–401. doi: 10.1016/j.pain.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tan AM, Waxman SG. Spinal cord injury, dendritic spine remodeling, and spinal memory mechanisms. Exp. Neurol. 2012;235:142–151. doi: 10.1016/j.expneurol.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 81.Zhao P, Hill M, Liu S, Chen L, Bangalore L, Waxman SG, Tan AM. Dendritic Spine Remodeling Following Early and Late Rac1-Inhibition after Spinal Cord Injury: Evidence for a Pain Biomarker. J. Neurophysiol. 2016 doi: 10.1152/jn.01057.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jutzeler CR, Huber E, Callaghan MF, Luechinger R, Curt A, Kramer JLK, Freund P. Association of pain and CNS structural changes after spinal cord injury. Sci. Rep. 2016;6:18534. doi: 10.1038/srep18534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Loeser JD, Treede R-D. The Kyoto protocol of IASP Basic Pain Terminology. Pain. 2008;137:473–477. doi: 10.1016/j.pain.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 84.Finnerup NB, Sørensen L, Biering-Sørensen F, Johannesen IL, Jensen TS. Segmental hypersensitivity and spinothalamic function in spinal cord injury pain. Exp. Neurol. 2007;207:139–149. doi: 10.1016/j.expneurol.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 85.Christensen MD, Everhart AW, Pickelman JT, Hulsebosch CE. Mechanical and thermal allodynia in chronic central pain following spinal cord injury. Pain. 1996;68:97–107. doi: 10.1016/S0304-3959(96)03224-1. [DOI] [PubMed] [Google Scholar]
- 86.Campbell JN, Meyer RA, Considerations A. Mechanisms of Neuropathic Pain Review. Pain. 2006:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Widerstrom-Noga E, Felix ER, Adcock JP, Escalona M, Tibbett J. Multidimensional neuropathic pain phenotypes after spinal cord injury. J. Neurotrauma. 2015;11 doi: 10.1089/neu.2015.4040. neu.2015.4040. [DOI] [PubMed] [Google Scholar]
- 88.Finnerup NB, Jensen TS. Mechanisms of disease: mechanism-based classification of neuropathic pain-a critical analysis. Nat. Clin. Pract. Neurol. 2006;2:107–115. doi: 10.1038/ncpneuro0118. [DOI] [PubMed] [Google Scholar]
- 89.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- 90.Nakae A, Nakai K, Yano K, Hosokawa K, Shibata M, Mashimo T. The animal model of spinal cord injury as an experimental pain model. J. Biomed. Biotechnol. 2011;2011:939023. doi: 10.1155/2011/939023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baron R, Förster M, Binder A. Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: a first step to a stratified treatment approach. Lancet Neurol. 2012;11:999–1005. doi: 10.1016/S1474-4422(12)70189-8. [DOI] [PubMed] [Google Scholar]
- 92.Freeman R, Baron R, Bouhassira D, Cabrera J, Emir B. Sensory profiles of patients with neuropathic pain based on the neuropathic pain symptoms and signs. Pain. 2014;155:367–376. doi: 10.1016/j.pain.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 93.Kwon BK, Hillyer J, Tetzlaff W. Translational Research in Spinal Cord Injury: A Survey of Opinion from the SCI Community. J. Neurotrauma. 2010;33:21–33. doi: 10.1089/neu.2009.1048. [DOI] [PubMed] [Google Scholar]
- 94.Kwon BK, Streijger F, Hill CE, Anderson AJ, Bacon M, Beattie MS, Blesch A, Bradbury EJ, Brown A, Bresnahan JC, Case CC, Colburn RW, David S, Fawcett JW, Ferguson AR, Fischer I, Floyd CL, Gensel JC, Houle JD, Jakeman LB, Jeffery ND, Jones LAT, Kleitman N, Kocsis J, Lu P, Magnuson DSK, Marsala M, Moore SW, Mothe AJ, Oudega M, Plant GW, Rabchevsky AS, Schwab JM, Silver J, Steward O, Xu XM, Guest JD, Tetzlaff W. Large animal and primate models of spinal cord injury for the testing of novel therapies. Exp. Neurol. 2015;269:154–168. doi: 10.1016/j.expneurol.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 95.Courtine G, Bunge MB, Fawcett JW, Grossman RG, Kaas JH, Lemon R, Maier I, Martin J, Nudo RJ, Ramon-Cueto A, Rouiller EM, Schnell L, Wannier T, Schwab ME, Edgerton VR. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat. Med. 2007;13:561–566. doi: 10.1038/nm1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Darian-smith C. Synaptic Plasticity, Neurogenesis, and Functional Recovery after Spinal Cord Injury. Neurosci. 2009;15:149–165. doi: 10.1177/1073858408331372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Serrano A, Mo G, Grant R, Pare M, Donnell DO, Yu XH, Tomaszewski MJ, Perkins MN, Se P. Differential Expression and Pharmacology of Native P2X Receptors in Rat and Primate Sensory Neurons Differential Expression and Pharmacology of Native P2X Receptors in Rat and Primate Sensory Neurons. J. Neurophysiol. 2016 [Google Scholar]
- 98.De La Roche J, Eberhardt MJ, Klinger AB, Stanslowsky N, Wegner F, Koppert W, Reeh PW, Lampert A, Fischer MJM, Leffler A. The molecular basis for species-specific activation of human TRPA1 protein by protons involves poorly conserved residues within transmembrane domains 5 and 6. J. Biol. Chem. 2013;288:20280–20292. doi: 10.1074/jbc.M113.479337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Levitt M, Levitt JH. The deafferentation syndrome in monkeys: Dysesthesias of spinal origin. Pain. 1981;10:129–147. doi: 10.1016/0304-3959(81)90190-1. [DOI] [PubMed] [Google Scholar]
- 100.Mercier C, Roosink M, Bouffard J, Bouyer LJ. Promoting Gait Recovery and Limiting Neuropathic Pain After Spinal Cord Injury: Two Sides of the Same Coin?, Neurorehabil. Neural Repair. 2016 doi: 10.1177/1545968316680491. [DOI] [PMC free article] [PubMed] [Google Scholar]