Abstract
Spinal cord injury is currently incurable. Treatment is limited to minimizing secondary complications and maximizing residual function by rehabilitation. Neurologic recovery is prevented by the poor intrinsic regenerative capacity of neurons in the adult central nervous system and by the presence of growth inhibitors in the adult brain and spinal cord. Here we identify three approaches to rewire the spinal cord after injury: axonal regeneration (direct endogenous reconnection), axonal sprouting (indirect endogenous reconnection) and neural stem cell transplantation (indirect exogenous reconnection). Regeneration and sprouting of axonal fibers can be both enhanced through the neutralization of myelin- and extracellular matrix-associated inhibitors described in the first part of this review. Alternatively, in the second part we focus on the formation of a novel circuit through the grafting of neural stem cells in the lesion site. Transplanted neural stem cells differentiate in vivo into neurons and glial cells which form an intermediate station between the rostral and caudal segment of the recipient spinal cord. In particular, here we describe how neural stem cells-derived neurons are endowed with the ability to extend long-distance axons to regain the transmission of motor and sensory information.
Introduction
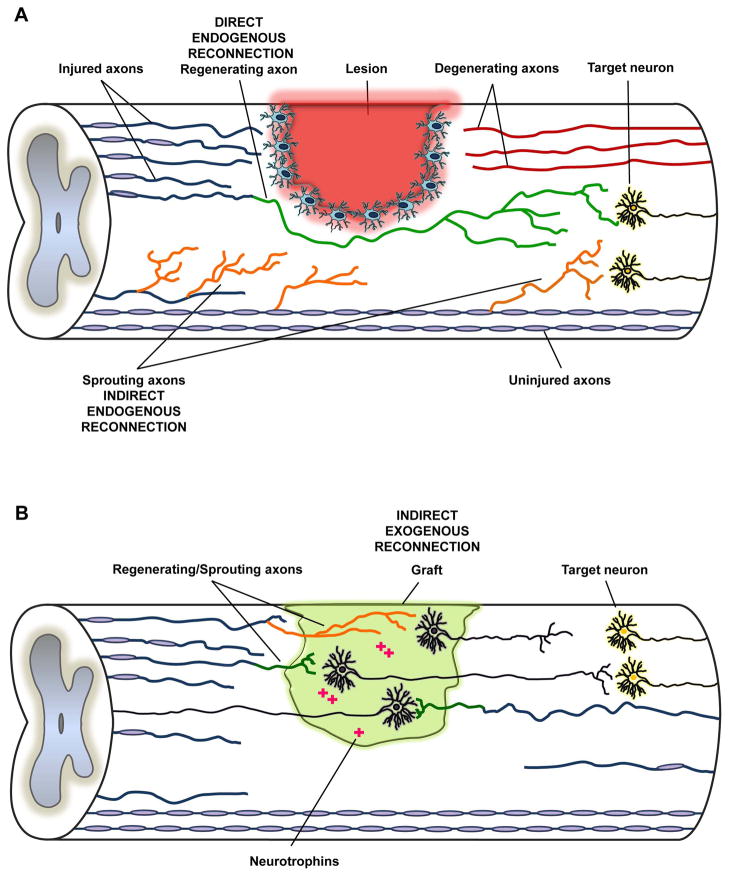
Traumatic spinal cord injury (SCI) is a debilitating condition characterized by the sudden loss of sensory, motor and autonomic functions distal to the level of the trauma. Despite major advances in the medical and surgical care of SCI patients, no effective treatment exists to relieve the neurological deficits [1]. In fact, current interventions include surgery to stabilize the lesioned area, prevention of secondary complications and rehabilitation. However, the neurological dysfunction is permanent and SCI patients often experience a lifelong disability. The lack of functional improvement has been traditionally attributed to the failure of long-distance regeneration of severed axons in the spinal cord. Once the tissue has been damaged, the axons show a poor regeneration capacity through the injured area, thus irreversibly compromising the transmission of motor and sensory information. Therefore, SCI is fundamentally a problem of interrupted communication between the brain and the distal spinal cord, and motor recovery ultimately depends on re-establishing a connection between cortical projection neurons and spinal motor neurons. Conceptually, we consider three distinct means to achieve reconnection: direct endogenous, indirect endogenous and indirect exogenous. Direct reconnection therapeutics are based on encouraging regeneration of damaged fibers in order to establish synaptic contacts with their original target neurons and restore the pre-existing circuit (Fig. 1A). Indirect endogenous reconnection refers to the establishment of new connections by sprouting axons either rostral or distal to the level of the trauma (Fig. 1A). Finally, indirect exogenous reconnection relies on the implantation of new cells at the lesion site and the establishment of novel circuits (Fig. 1B).
Figure 1. Schematic representation of direct and indirect interventions on the injured spinal cord.
(A) The neutralization of myelin and matrix associated inhibitors supports both regeneration (in green) and sprouting (in orange) of axonal fibers. Regenerating fibers make connections with the original target neurons (direct endogenous reconnection), whereas sprouting collaterals form new synaptic contacts (indirect endogenous reconnection). (B) Grafted neural stem cells, on the other hand, offer an alternative strategy (indirect exogenous reconnection) through cellualr differentiation at the injury site into neurons, astrocytes and oligodendrocytes. In particular, newly differentiated neurons are intrinsically primed for long-distance axonal growth which helps the indirect transmission of motor and sensory information. Descending supraspinal axons regenerate into and make synaptic connections with grafted neurons in the lesion site. Grafted neurons extend their axons into the caudal spinal cord and form new synaptic connections with host neurons. Similarly, grafted neurons can make a functional circuit for the ascending sensory system. Transplanted neural stem cells also help regeneration of severed axonal fibers by releasing neurotrophins in the damaged area.
Axonal regeneration may be achieved either by potentiating the intrinsic regenerative capacity of the severed neurons or by modifying the environment surrounding the injury. At present successful preclinical methods to boost the intrinsic growth capacity of axons rely on the genetic manipulation of cortical neurons, for instance through the overexpression of Kruppel-like factor 7 (Klf7) [2] or through the deletion of the mTOR regulator PTEN [3]. However, the functional consequences of unregulated stimulation of the neuronal growth program need to be carefully evaluated as it may lead to substantial unintended complications, including epilepsy, cancer and neuronal hypertrophy [4,5,6]. On the other hand, regeneration can be promoted by counteracting inhibitors present in the extracellular environment. The central nervous system (CNS) presents several molecules associated with myelin and extracellular matrix that impair regeneration of damaged axonal fibers. The neutralization of these inhibitory components, discussed in the first part of this review, represents one validated strategy to make the injured area more permissive for both axonal regeneration and sprouting [7, 8].
The implantation of new exogenous neurons can also support recovery of the motor function through a relay system. Regenerating descending fibers can form synapses with grafted cells, which in their turn connect with the target neuron distal to the injury site. Similarly, connection can be restored for ascending sensory pathways (Fig. 1B). The main advantage of this approach is that lesioned axons do not need to grow long distances. In fact, the onus for axon growth is placed on transplanted neurons which are specifically selected for their ability to extend very long axons in vivo [9]. The establishment of a new circuit is at the basis of neural stem cells (NSCs) transplantation in SCI and will be object of the second part of this review.
Myelin associated inhibitors (MAI)
Since early observations made by Ernesto Lugaro [10], the presence of inhibitory elements in the CNS has been extensively described in the literature. In particular, it has been pointed out that CNS myelin and oligodendrocytes expose molecules able to collapse of axonal growth cones and that these elements are absent in the peripheral nervous system (PNS), where axonal regrowth occurs more easily [11,12]. As an additional proof, when a peripheral nerve lacking neuronal cells is transplanted in a transected spinal cord, CNS axons are actually able to regenerate into the grafted tissue, avoiding the presence of CNS-specific components that otherwise hinder regeneration [13,14]. At present two major classes of CNS inhibitors have been identified: myelin associated inhibitors (MAI), and inhibitors associated with the extracellular matrix, typified by chondroitin sulfate proteoglycans (CSPGs). MAI include several molecules such as Myelin Associated Glycoprotein (MAG), Oligodendrocyte Myelin glycoprotein (OMgp) and Nogo A.
Nogo A and its receptor complex
Nogo A is a membrane-associated protein that belongs to the reticulon family [15]. Originally identified as a neurite growth inhibitory “activity” enriched in the spinal cord white matter fraction [16,17] the sequence of Nogo A gene was identified by three independent laboratories about 16 years ago [15,18,19]. Nogo A is one of three isoforms of Nogo including also Nogo B, generated by alternative splicing from the same gene, and Nogo C which is the product of an alternate promoter [15,18]. Nogo A is the principal isoform of Nogo in the CNS and it is not expressed in the PNS [20,21]. Its role in the limitation of axonal growth in the CNS has been proved by the ectopic expression of Nogo A in PNS Schwann cells where it restrains routine peripheral nerve regeneration after sciatic nerve injury [22]. Nogo A is expressed in many cell types, though it can be mostly found in the oligodendrocytes and neurons of brain regions with a high degree of network plasticity including the hippocampus and the cortex [21,23]. Although Nogo A has been well characterized as a myelin associated inhibitor, its physiological function in oligodendrocytes has not been fully elucidated yet. Data collected so far from genetically depleted mice show that the absence of Nogo A delays oligodendrocyte differentiation [24], myelin sheath formation and axonal caliber growth within the first postnatal month. Moreover, the combined deletion of Nogo A and MAG leads to delayed myelination [25]. Nogo presents two active domains: a 66 amino acid loop (Nogo-66) common to all the three isoforms of Nogo and a unique amino terminal region (amino Nogo) specific to Nogo A [19,26]. Amino Nogo and Nogo-66 have shown to inhibit neurite outgrowth in vitro through biochemically distinct mechanisms [15,18,19,26,27]. Amino Nogo disrupts integrin function [28], whereas Nogo-66 inhibits growth through its neuronal receptors, Nogo receptor 1 (NgR1) and Paired Immunoglobulin-like Receptor B (PirB) [7,27,29].
NgR1 is a glycosylphosphatidylinositol (GPI) anchored protein expressed in CNS neurons and specifically localized on axons terminals [20,27]. NgR1 gene deletion studies with comprehensive genetic labeling document corticospinal axon regeneration plus sprouting of multiple pathways after injury [30,31,32,33,95]. As a receptor without a transmembrane domain, activation of NgR1 must be transduced by co-receptors. Two have been described: the low-affinity neurotrophin receptor p75, which also interacts with the leucine-rich repeat (LRR)-protein LINGO-1 to mediate Nogo A signal in some neuronal populations [34], and the tumor necrosis factor-alpha (TNF-alpha) orphan receptor, TROY, which alternatively substitutes p75 in the NgR1-p75-LINGO-1 complex [8,35]. The interaction of Nogo-66 with NgR1 ultimately leads to the activation of the small GTPase RhoA and its effector ROCK, thus mediating growth cone collapse and growth arrest of neurites [36]. It has been recently found that RhoA is a downstream effector common to amino Nogo signaling pathway. Amino Nogo binds to the sphingosine-1-phosphate receptor 2 (S1PR2), thus activating the G-protein G13 which ultimately triggers RhoGEF LARG and RhoA. Knock down or pharmacological blockade of S1PR2 suppresses the effect of Nogo A on neurite outgrowth and hippocampal long-term potentiation (LTP) [37]. However, the potential role of S1PR2 as a pharmacological target for SCI is yet to be clarified.
As previously mentioned, PirB (LILRB2 in humans) is another major MAI receptor that binds to Nogo-66, MAG and OMgp [29]. PirB is expressed at low or undetectable levels in many parts of the adult CNS although its expression may be more prominent during development or after ischemia [38]. While it appears to have a major role in cortical plasticity [39] at present there is no evidence of a significant contribution of PirB receptor to axonal regeneration after SCI [40].
Suppression of the Nogo A/NgR1 receptor signaling
The Nogo A-NgR1 signal transduction system can be antagonized by different molecules which proved to be effective enhancing neural repair, through both axonal regeneration and sprouting after SCI. The Schwab group created an IgM monoclonal antibody, called IN-1, recognizing Nogo A. In adult injured rats injection of antibody IN-1 directly in the spinal cord promoted regeneration of axons in the corticospinal tract (CST), which ultimately led to an improvement in the sensory-motor function [41,42,43,44]. Subsequently, two additional IgG antibodies (m7B12 and m11C7) were raised against the Nogo A specific domain. When infused intrathecally, they penetrated into the adult CNS tissue within seven days reaching a broad distribution [45]. Anti-Nogo A antibodies were retained in the CNS tissue, they were internalized and co-localized intracellularly with lysosomal markers and endogenous Nogo A. This observation suggested that m7B12 and m11C7 could mediate the internalization and the overall downregulation of endogenous Nogo A levels [45]. When infused into the subdural space of SCI rats, both antibodies elicited regeneration and sprouting of CST fibers and enhanced functional recovery in a variety of motor tasks [46]. Similarly, anti-Nogo A antibodies proved to be effective in adult primates. Macaque monkeys subjected to unilateral spinal cord transection including the CST were treated with anti-Nogo A antibodies over a period of four weeks and showed recovery of hand function in comparison with control antibody-treated monkeys [47,48].
Alternatively, multiple strategies have been applied to antagonize the receptor NgR1, including a competitive antagonist for the Nogo-66 binding site (NEP 1-40) and a decoy receptor, NgR(310)ecto-Fc, designed to deprive endogenous NgR1 from ligand activation [7,49,50]. Both these molecules decrease myelin induced growth cone collapse, inhibition of neurite outgrowth in vitro and enhance CST regeneration and functional recovery in animal models of SCI in vivo [49,50,51]. Since NgR1 is also a functional receptor for MAG and OMgp [52,53,54] NgR1(310)ecto-Fc provides the only therapeutic method to target efficiently all three MAI ligands, thus resulting in an enhanced efficacy in comparison with Nogo A antagonists. In fact, contrary to anti-Nogo A therapy, NgR1(310)ecto-Fc has been shown to be effective also in acute and chronic SCI contusion models, which are generally considered to be more relevant for clinical translation [31,55, 56].
An indirect way to antagonize the Nogo receptor complex would be to target other components, namely p75 or LINGO-1. Immunohistochemical data showed that p75 is expressed in only a very small subset of ascending sensory axons in the dorsal column of either normal or SCI animals [57]. Accordingly, genetic deletion of p75 or local administration of a p75-Fc fusion protein did not improve regeneration of either descending CST fibers or ascending sensory neurons after SCI [57]. In contrast, LINGO-1 Fc treatment following rat SCI promoted a degree of axonal sprouting and increased oligodendrocytes and neuronal survival [58]. Another site of intervention to target the Nogo-NgR1 transduction pathway is the downstream effector ROCK. The ROCK inhibitor Y-27632 reverses myelin inhibition of axon outgrowth in culture and promotes axon regeneration and locomotor recovery in vivo [59,60]. A potential benefit of Y-27632 is that it also limits the inhibitory potential of CSPGs, which are among the main components of the glial scar [61]. However, the ubiquitous expression of ROCK, in contrast to the neuronal selectivity of NgR1, makes this target less specific for a designed therapeutic approach as its manipulation might alter numerous other physiological events.
Interestingly, these acute interventions using peptides, antibodies or fusion proteins yield more robust benefit in terms of regeneration than does mouse gene knockout for Nogo A, NgR1 or even double knockout for Nogo A and MAG and triple knockout targeting all MAIs. This observation suggests that in genetically depleted mice there might be the compensatory up-regulation of other inhibitory factors such as ephrinA3, EphA4, Sema 4D and 3F and plexin B2 [8].
MAG
MAG was the first myelin associated CNS growth inhibitory protein to be identified and its activity was discovered by two independent laboratories more than 20 years ago [62,63]. MAG is a transmembrane glycoprotein and a member of the sialic acid binding immunoglobulin superfamily. It is mostly expressed in the myelinating glia, Schwann cells in the periphery and oligodendrocytes in the CNS [64]. In particular, MAG has been found to be highly abundant in the periaxonal membrane of myelin sheath [65] suggesting its role in regulating axon-myelin interaction and myelin development. Accordingly, MAG knockout mice display numerous structural abnormalities, including aberrant myelin outfolds and uncompacted myelin wraps [25]. In vitro studies suggest that the inhibitory efficacy of MAG is limited compared to other MAIs. In fact, total CNS myelin derived from MAG knockout mice inhibits neurite outgrowth to a similar degree as total CNS myelin from wild type mice [66]. In agreement with these observations, genetic depletion of MAG did not improve axon regeneration in the CST of the lesioned spinal cord [67]. However, recent evidence combining both genetic deletion and intrathecal delivery of sialidase to interfere with MAG binding to its receptors GD1a and GT1b (brain gangliosides) enhanced serotonergic axon sprouting [68], thus suggesting a possible divergent role of MAG in different axonal systems.
OMgp
OMgp is a LRR protein linked to the cell membrane by a GPI anchor. OMgp is expressed by olygodendrocytes, neurons and astrocytes in the CNS [69]. Compared to MAG and Nogo A, significantly less is known about the physiological role of OMgp. However, there may be some degree of overlap as OMgp, similar to Nogo A and MAG, associates with NgR1 and PirB. OMgp is localized in the periaxonal processes of oligodendrocytes in the CNS with a more prominent concentration near the nodes of Ranvier where it reportedly blocks axon collateral sprouting from non-myelinated segments [70]. Consistent with this localization, OMgp null mice display defects of the nodal and perinodal architecture and hypomyelination of the spinal cord [71]. Although the subcellular localization of OMgp has been debated, it is clear that OMgp inhibits neurite outgrowth in vitro [54]. On the other hand, genetic deletion of OMgp does not promote CST axon regeneration, but only an enhancement in the sprouting of serotonergic fibers [72,73]. As a possible explanation, it is plausible that the presence of other more potent inhibitors mask the effect of OMgp deletion. Accordingly, Cafferty and colleagues show that when MAG and OMgp are deleted, neurite outgrowth is unaffected both in culture and in vivo. On the other hand, when MAG and OMgp are deleted in a Nogo A-null background there is a synergistic effect in decreasing growth inhibition in vitro and in promoting both CST regeneration and axonal sprouting after SCI [7, 74]. This finding suggests that while Nogo A has a prominent role in axonal growth inhibition, MAG and OMgp provide a secondary contribution. However, Lee and colleagues observed a more circumscribed effect of MAIs limited to axonal sprouting and not regeneration after injury with no evidence for greater axonal growth in triple knockout animals as compared to Nogo null mice [73]. Differences in the specific alleles or the genetic backgrounds or the lesion models might provide an explanation for the variable results; future work will be required to resolve the additivity of MAI in axonal growth after injury.
CSPGs
CSPGs are another prominent group of adult CNS axon growth inhibitors. They are extracellular matrix proteoglycans that consist of a protein core with covalently attached glycosaminoglycan (GAG) side chains [75]. CSPGs are secreted by astrocytes, neurons and oligodendrocytes [76], and they are strongly enriched at the glial scar after CNS injury where they inhibit regenerative growth and restrict plasticity [77]. The main method by which CSPG inhibition has been overcome to promote axon regeneration is through the application of the bacterial enzyme chondroitinase ABC (ChABC). ChABC catalyzes the removal the glycosyl side chains of CSPGs thus reducing the inhibitory nature of proteoglycans. Since the first observation by Bradbury and colleagues [77] who reported functional improvements in rats with injury to the dorsal columns upon treatment with ChABC, subsequent studies have confirmed the effect of this enzyme in more clinically relevant models of SCI [78]. Moreover, ChABC has also been combined experimentally with nerve or cellular transplantations and proved to potentiate the effect of the grafts in terms of axonal growth and motor function [79,80,81]. Treatment with ChABC in combination with a peripheral nerve graft has also proved to be effective to help the recovery of bladder function, one of the highest priorities for many SCI patients [82].
Despite substantial evidence that ChABC is beneficial to repair, many obstacles remain for clinical translation. First of all, concerns exist over the safety and immunogenicity of delivering a bacterial protein to human beings. Even though preclinical investigations did not report adverse effects, such as increase in pain sensitivity or changes in nerve conduction, long-term toxic effects need to be evaluated before ChABC can be used in human patients. Another critical issue with the use of ChABC as therapeutic agent is the instability of the enzyme. The biological activity of the enzyme decreases quickly when in solution and it is sensitive to temperature [83]. For this reason ChABC is generally given in multiple doses, through direct infusion into the brain ventricles or using soaked gelfoam onto the lesioned area. Some of these obstacles can be circumvented by thermostabilization of the enzyme [84] or with gene therapy to achieve long-term delivery. In one study, ChABC gene therapy actually promoted tissue repair and improved hindlimb function and spinal conduction in rats with spinal cord contusion [85]. However, because conventional pharmacological strategies can have more imminent clinical translation compared to gene therapy, other options have been explored. In this regard, the identification of receptors for CSPGs (PTPsigma, NgR1 and NgR3) has been very useful [86,87]. In particular, Lang and colleagues designed a PTPsigma-targeting peptide that showed to promote recovery of hindlimb and bladder function in rats with contusion SCI [88]. An alternative intervention is the inhibition of CSPGs synthesis using a DNA enzyme which proved to make the spinal cord less inhibitory to axon regeneration [89].
The mechanism behind the beneficial effect produced by the inactivation of CSPGs is still unclear. In fact, to date there is little anatomical evidence to suggest that the administration of ChABC has allowed for axonal regrowth over long distances. While substantial axonal growth can be observed into and even through SCI lesion, there is scant evidence that axons regrow to their original targets. Therefore, it is possible that measured improvements in motor function result from multiple events other than regeneration, such as formation of alternate neuronal circuits, improved survival of motor neurons, sprouting of spared axons as well as remyelination. Even though several mechanisms have been postulated, further analyses would be necessary to understand the exact molecular events taking place upon ChABC treatment.
Indirect effects of blocking the inhibitory environment
As noted above, while these inhibitors were originally described in the context of regeneration and direct endogenous reconnection, their blockade yields equal or greater benefit by promoting recovery of function after SCI through indirect endogenous reconnection. This fits with the emerging evidence from studies of greater experience-dependent plasticity when the myelin inhibitor or CSPG pathways are disrupted [7,90,91,92]. Consistent with a key role for these molecules in limiting sprouting and plasticity in uninjured CNS, the efficacy of extinction training in Post-Traumatic Stress Disorder models of conditioned fear is enhanced in their absence [93,94]. Moreover, recent work by Siegel and colleagues showed the impact of NgR1 ablation in the compensatory sprouting and consequent functional recovery in animals with a lesioned CST [95]. The discussion above highlights the role of endogenous molecular pathways titrating regeneration per se to also regulate indirect reconnection. While there is a cellular distinction between these mechanisms, molecular pathways appear to be shared. These observations lead to a focus on indirect reconnection as a means for recovery from SCI, and thence to the introduction of new cells by transplantation.
Indirect exogenous reconnection: neuronal grafts in the injured spinal cord
Cell therapy represents a promising strategy for SCI because it provides a combination of factors cooperating for axonal regrowth. In fact, grafted cells not only replace lost neurons and glia but also secrete growth factors, attenuate the glial scar and restrain the production of inhibitory proteoglycans [96,97]. Since the first attempts of transplantation reported in middle 80’s [98,99] many cellular grafting strategies have been studied in animal models of SCI. In this regard several types of cells have been used including Schwann cells [100,101], olfactory ensheating cells [102,103], bone marrow mesenchymal stromal cells [104], neuronal restricted progenitors [105,106,107] glial restricted progenitors [96] and NSCs [108]. In the present review we will specifically re-examine the application of NSCs in SCI focusing on the mechanisms underlying their beneficial effects upon transplantation in the lesioned tissue.
Neural stem cells in SCI: the establishment of a new neuronal circuit
Pioneering studies to analyze the outcome of transplantations for SCI were performed using whole segments of rat fetal spinal cord [109]. With the following development of culturing techniques it was later possible to identify the single cell populations composing the fetal tissue. In this way neuronal and glial restricted progenitors (NRP/GRP) were isolated from the rat fetal spinal cord and both proved to be effective in promoting regeneration and restoring the connectivity of injured fibers [106,107].
Distinct from NRP and GRP which are committed to a specific lineage, NSCs are multipotent populations with the ability to differentiate into neurons, oligodendrocytes and astrocytes. They generate the neurons and glia of the developing brain and also account for the limited regenerative capacity of the adult CNS [110,111]. Specifically, in the adult CNS they are located in specialized niches in the subventricular zone [112,113,114], in the dentate gyrus of the hippocampus [115] and in the periventricular zone of the spinal cord [116]. The main feature of NSCs which makes them highly eligible for SCI cell therapy in comparison with progenitors and other reported cells sources is their self-renewal capacity. In fact, NSCs are highly expandable in vitro and can be cultured as floating aggregates (called neurospheres) or as monolayers on adherent substrates. NSCs can be kept in proliferation through the exposure to mitogens such as fibroblast growth factor-2 (FGF-2) and epidermal growth factor (EGF) [110]. Neurosphere culture has been traditionally considered a classical method for NSCs expansion since it was firstly introduced in early 90’s [114]. However, recent evidences have shown that the preparation of floating aggregates is often accompanied by a loss of self-renewal and neurogenic capacity over time [117,118]. On the other hand, monolayer cultures can be expanded for long periods of time without any alteration of the original differentiation potential which is a highly desirable feature for transplantation studies [119,120,121,122]. Additionally, the preparation of NSCs as monolayers makes them suitable as platform for drug screening or for in vitro disease modeling [123].
It is generally accepted that NSCs can produce a functional benefit for SCI through the establishment of a new neuronal circuit. The rationale behind the formation of a new circuit at the lesion core is the achievement of a complete differentiation of NSCs into mature neurons and glial cells. Differentiated cells in the host spinal cord can establish a new relay through the formation of an intermediate station between the supraspinal axons and the denervated target below the lesion (Fig. 1B). Other transplantations including non-neuronal cell sources (olfactory ensheathing cells, Schwann cells, mesenchymal stromal cells) or peripheral nerves have been successfully performed into injured spinal cords allowing some regeneration of severed fibers [124,125]. However, non-neuronal cells can only provide a structural and trophic support to the injury site and damaged axons are still required to regenerate for long tracts. On the other hand, NSC-derived neurons can elongate axons and establish functional synapses with the intact rostral and caudal segments of the injured spinal cord, thus overcoming the inhibitory environment at the lesion epicenter [9,108]. For this purpose, the use of developmentally young cells which are intrinsically endowed with the capacity for long distance axon elongation has proven to be successful.
Transplant-derived axons must overcome the same inhibitory molecules that prevent endogenous axons growth, but it appears that graft-derived neurons have the intrinsic state to overcome those barriers [126]. A study conducted with brain derived NSCs grafted into the rat cervical CST revealed that the cells are able to extend long, straight and uniform axons into the host spinal cord white matter for distances as long as 8 mm [127]. Therefore, adult myelin is not necessarily an obstacle for the elongation of young developing axons. Recent evidence has demonstrated that axons express at least two receptors that respond to CSPGs [128,129] and that the distribution of CSPGs plays a role in the entrapment of growing axons in the glial scar [130]. However, in vitro experiments proved that neuronal precursors express low levels of CSPGs receptors, thus providing one possible mechanism to the extensive axonal elongation from NSCs observed in vivo [97]. To further support this hypothesis we recently found that the axonal elongation from the graft is not dependent on the degree of scarring at the lesion. Hemisection or transection models commonly used for transplantation studies are often minimally invasive, with little disruption of the blood brain barrier and a modest glial scar. However, NSCs can extend fibers for long tracts even in a contusion animal model where the glial scar usually covers a wider area (Dell’Anno et al., unpublished observations).
In vivo differentiation of NSCs has been observed after grafting in SCI models. NSCs derived from rodent embryonic brain and spinal cord give rise to neurons and glia in the host tissue resulting in enhanced motor recovery [108,131,132]. In particular, the possibility to obtain multiple neuronal phenotypes in vivo may be relevant for the formation of a new functional circuit through the graft. In fact, the restoration of the motor function does not only depend on the establishment of new synapses but also on their type and specificity. For instance, if the graft-derived neurons provide an inhibitory connection where the original circuit had an excitatory phenotype the relay will likely fail to restore the function. To this purpose, sources of transplants can be selected for their ability to generate particular neuronal subtypes in vivo [133,134] or different protocols may be used to promote specific neuronal populations [135]. At present, it is not possible to reconstruct the circuit with the same degree of cellular specificity it had before the lesion occurred. In fact, even if grafted cells can actually reconnect supraspinal axons with their original target, they can also establish some new connections, which were not present in the initial circuit [107]. In this regard, activity-dependent plasticity or training might play a role in the formation of the correct synapses. The achievement of a complete neuronal maturation is not only pivotal for the reconstruction of an interrupted circuit, but also for the safety of the transplant for future clinical applications. Yan and colleagues found that the expression of neuronal progenitors markers such as nestin and doublecortin in the graft declines over time [136]. Similarly, Tuszynski’s group showed that doublecortin and Ki67 (marker for proliferation) were rarely detected in the graft three months after implantation, suggesting that NSCs progressively exit the cell cycle in order to achieve a full maturation into neuronal and glial cells [137].
The differentiation of NSCs into astrocytes in vivo has also been observed [106], however their role in SCI is still a matter of debate. In fact, astrocytic differentiation has been associated with the development of allodynia and thermal hyperalgesia, two highly undesirable effects [138,139,140]. Additionally, astrocytes may contribute to the glial scar, which has been traditionally considered one of the main hindrances to axonal regeneration [141]. However, the inhibiting nature of the reactive astrocytes in the scar has been recently cast into doubt [144] and future studies will be required to understand their actual contribute to the graft integration. Other studies strengthened the idea that astrocytes grafted in the lesion core may produce different outcomes depending on the exposure to some specific growth factors. When supported by high levels of nerve growth factor (NGF), reactive astrocytes could provide a growth substrate for the elongation of adult axons [142]. Similarly, glial progenitors treated with either bone morphogenetic factor-4 (BMP-4) or ciliary neurotrophic factor (CNTF) produce opposite behavioral outcomes upon grafting in the lesioned spinal cord [140]. On this basis it is very likely that some refinements in the preparation of NSCs before the graft need to be considered in order to maximize the beneficial effects of the astrocytes and reduce the risk of adverse effects.
Alternative mechanisms behind NSCs application in SCI
The second mechanism through which NSCs could restore the connectivity after SCI is by improving the intrinsic regeneration capacity of severed axons. In this regard a very recent work by Kadoya and colleagues showed that the regional identity of the NSCs graft can produce a dramatic difference in terms of regeneration. In particular the authors have placed in the transected spinal cord grafts derived from the hindbrain, the telencephalon and the spinal cord of E14 rats. They found that CST fibers of the host regenerate for longer distances through the graft when it has been derived from the spinal cord rather than from more rostral anatomical areas. Spinal cord-derived grafts also allow CST fibers to cross the caudal interface between the graft and the host, thus supporting the idea that the implantation of homologous neural tissue should be elective to enhance the regrowth of severed axons [143]. The mechanisms involved in this tissue-specific interactions still needs to be fully understood, however it is plausible that some extracellular matrix molecules or cell-adhesion molecules may be responsible for this enhanced regeneration through the graft.
The lack of supporting or stimulating agents at the lesion core has always been considered one of the elements responsible of the poor regeneration of damaged fibers. Using RNA-sequencing technique Anderson and colleagues recently confirmed that cells at the lesion epicenter actually express very low levels of neurotrophins and that the application of neurotrophic factor-3 (NT3) and brain derived neurotrophic factor (BDNF) contributes to the regeneration of the ascending sensory tract in a damaged spinal cord [144]. NSCs do not only secrete neurotrophic factors by themselves [145,146,147], but also respond to the application of exogenous factors. Growth factors can be embedded in a fibrin matrix containing NSCs to help the cells filling the lesion cavity and maximizing the axonal elongation from the graft [108]. Alternatively, lentiviral vectors coding for BDNF or NT-3 can provide chemotropic guidance to reach a chosen target [106,148].
Finally, it has also been proved that NSCs can contribute to neuronal repair through the remyelination of severed fibers. To this purpose a recent experiment has been conducted using NSCs derived from a myelin-deficient (shiverer) mouse for transplantation in a SCI model. NSCs from shiverer or wild type animals share the same properties with the exception of the myelination potential. After grafting, NSCs-derived oligodendrocytes from shiverer mice formed much thinner myelin sheets in comparison to wild type NSCs-derived oligodendrocytes. Moreover, the functional benefit in hosts receiving shiverer mouse-derived NSCs was significantly restrained compared animals receiving wild type NSCs [149].
Concluding remarks
As previously discussed, both direct and indirect approaches for spinal cord repair relay on axonal elongation. Direct approaches aim to enhance axonal regeneration from severed fibers by counteracting myelin and matrix associated inhibitory molecules. Conversely, indirect approaches can either utilize the same inhibitor blockade, or be based on the incorporation of new pathways from grafted cells to reconnect supraspinal axons with downstream denervated targets. However, axonal elongation is only the first of several steps required to achieve behavioral recovery and in both cases the establishment of the correct synaptic contacts is crucial to restore the lost connectivity. Preliminary studies using dorsal root ganglion or dopaminergic neurons provided the proof of evidence that neurotrophic factors and repulsive cues can be combined to enhance axonal navigation toward designated targets [150,151]. Similar analyses on SCI models will be required to optimize the combination of attractive and repulsive cues with existing approaches and maximize the specificity of the newly formed synapses. Moreover, in SCI there are many adverse factors limiting the recovery of the neurological deficit and it is likely that a combination of therapeutic strategies might be more effective than a single approach. In fact, cell transplantation and neutralization of inhibitory molecules are not mutually exclusive. The integration of new cells in the spinal cord circuit still requires the regeneration of severed fibers as well as the sprouting of unlesioned ones within the graft area. Therefore, the above mentioned strategies to counteract the CNS growth inhibitors could be used to make the host axons more prone to establish functional connections with the transplanted cells. In this regard, the simultaneous application of NgR1 antagonists and NSCs transplantation has been recently explored [152,153]. However, data collected so far provided conflicting results in terms of regeneration and motor improvement. Given the high variability of experimental conditions, especially concerning the derivation of NSCs and the animal lesion models, further investigations will be needed in order to understand how to combine the neutralization of inhibitory molecules with NSCs transplantation and achieve more consistent results for the repair of the injured spinal cord. Finally, further experiments with well-defined populations of human NSCs derived from embryonic stem cells and/or induced pluripotent stem cells will improve the transition to the clinic.
Acknowledgments
This work was supported by grants from the NIH-NINDS and the Falk Medical Research Trust to S.M.S.
Footnotes
Conflicts Of Interest
S.M.S. is a co-founder of Axerion Therapeutics seeking to develop NgR1-based treatments.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mothe AJ, Tator CH. Advances in stem cell therapy for spinal cord injury. J Clin Invest. 2012;122:3824–3834. doi: 10.1172/JCI64124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, Xu B, Connolly L, Steward O, Zheng B, He Z. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pun RY, Rolle IJ, Lasarge CL, Hosford BE, Rosen JM, Uhl JD, Schmeltzer SN, Faulkner C, Bronson SL, Murphy BL, Richards DA, Holland KD, Danzer SC. Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy. Neuron. 2012;75:1022–1034. doi: 10.1016/j.neuron.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akhavan D, Cloughesy TF, Mischel PS. mTOR signaling in glioblastoma: lessons learned from bench to bedside. Neuro Oncol. 2010;12:882–889. doi: 10.1093/neuonc/noq052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutilla EA, Buyukozturk MM, Steward O. Long-term consequences of conditional genetic deletion of PTEN in the sensorimotor cortex of neonatal mice. Exp Neurol. 2016;279:27–39. doi: 10.1016/j.expneurol.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akbik F, Cafferty WBJ, Strittmatter SM. Myelin associated inhibitors: A link between injury-induced and experience-dependent plasticity. Experimental neurology. 2012;235:43–52. doi: 10.1016/j.expneurol.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwab ME, Strittmatter SM. Nogo limits neural plasticity and recovery from injury. Current opinion in neurobiology. 2014;27:53–60. doi: 10.1016/j.conb.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonner JF, Steward O. Repair of spinal cord injury with neuronal relays: from fetal grafts to eural stem cells. Brain Res. 2015;1619:115–123. doi: 10.1016/j.brainres.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraidakis MJ. Lugaro’s forgotten legacy: the hypothesis of negative neurotropism. J Hist Neurosci. 2010;19:239–52. doi: 10.1080/09647040903148621. [DOI] [PubMed] [Google Scholar]
- 11.Savio T, Schwab ME. Rat CNS white matter, but not gray matter, is non permissive for neuronal cell adhesion and fiber outgrowth. J Neurosci. 1989;9:1126–1133. doi: 10.1523/JNEUROSCI.09-04-01126.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bandtlow C, Zachleder T, Schwab ME. Oligodendrocytes arrest neurite growth by contact inhibition. J Neurosci. 1990;10:3837–3848. doi: 10.1523/JNEUROSCI.10-12-03837.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- 14.Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurons regenerate into PNS grafts. Nature. 1980;284:264–265. doi: 10.1038/284264a0. [DOI] [PubMed] [Google Scholar]
- 15.GrandPré T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- 16.Caroni P, Schwab ME. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol. 1988;106:1281–1288. doi: 10.1083/jcb.106.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caroni P, Savio T, Schwab ME. Central nervous system regeneration: oligodendrocytes and myelin as non-permissive substrates for neurite growth. Prog Brain Res. 1988;78:363–370. doi: 10.1016/s0079-6123(08)60305-2. [DOI] [PubMed] [Google Scholar]
- 18.Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 19.Prinjha R, Moore SE, Vinson M, Blake S, Morrow R, Christie G, Michalovich D, Simmons DL, Walsh FS. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Chun SJ, Treloar H, Vartanian T, Greer CA, Strittmatter SM. Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon-myelin and synaptic contact. J Neurosci. 2002;22:5505–5515. doi: 10.1523/JNEUROSCI.22-13-05505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber AB, Weinmann O, Brösamle C, Oertle T, Schwab ME. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J Neurosci. 2002;22:3553–3567. doi: 10.1523/JNEUROSCI.22-09-03553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pot C, Simonen M, Weinmann O, Schnell L, Christ F, Stoeckle S, Berger P, Rülicke T, Suter U, Schwab ME. Nogo-A expressed in Schwann cells impairs axonal regeneration after peripheral nerve injury. J Cell Biol. 2002;159:29–35. doi: 10.1083/jcb.200206068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-sequencing transcriptome, splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang JY, Wang YX, Gu WL, Fu SL, Li Y, Huang LD, Zhao Z, Hang Q, Zhu HQ, Lu PH. Expression and function of myelin-associated proteins and their common receptor NgR on oligodendrocyte progenitor cells. Brain Res. 2012;1437:1–15. doi: 10.1016/j.brainres.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Pernet V, Joly S, Christ F, Dimou L, Schwab ME. Nogo-A and myelin-associated glycoprotein differently regulate oligodendrocyte maturation and myelin formation. J Neurosci. 2008;28:7435–7444. doi: 10.1523/JNEUROSCI.0727-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oertle T, van der Haar ME, Bandtlow CE, Robeva A, Burfeind P, Buss A, Huber AB, Simonen M, Schnell L, Brösamle C, Kaupmann K, Vallon R, Schwab ME. Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J Neurosci. 2003;23:5393–5406. doi: 10.1523/JNEUROSCI.23-13-05393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- 28.Hu F, Strittmatter SM. The N-terminal domain of Nogo-A inhibits cell adhesion and axonal outgrowth by an integrin-specific mechanism. J Neurosci. 2008;28:1262–1269. doi: 10.1523/JNEUROSCI.1068-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- 30.Fink KL, Strittmatter SM, Cafferty WB. Comprehensive Corticospinal Labeling with mu-crystallin Transgene Reveals Axon Regeneration after Spinal Cord Trauma in ngr1−/− Mice. J Neurosci. 2015;35:15403–15418. doi: 10.1523/JNEUROSCI.3165-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Duffy P, McGee AW, Hasan O, Gould G, Tu N, Harel NY, Huang Y, Carson RE, Weinzimmer D, Ropchan J, Benowitz LI, Strittmatter WBSM. Recovery from chronic spinal cord contusion after Nogo receptor intervention. Ann Neurol. 2011;70:805–821. doi: 10.1002/ana.22527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cafferty WB, Strittmatter SM. The Nogo-Nogo receptor pathway limits a spectrum of adult CNS axonal growth. J Neurosci. 2006;26:12242–12250. doi: 10.1523/JNEUROSCI.3827-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JE, Liu BP, Park JH, Strittmatter SM. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44:439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 34.Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, Levesque M, Allaire N, Perrin S, Sands B, Crowell T, Cate RL, McCoy JM, Pepinsky RB. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- 35.Park JB, Yiu G, Kaneko S, Wang J, Chang J, He XL, Garcia KC, He Z. A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron. 2005;45:345–351. doi: 10.1016/j.neuron.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 36.Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kempf A, Tews B, Arzt ME, Weinmann O, Obermair FJ, Pernet V, Zagrebelsky M, Delekate A, Iobbi C, Zemmar A, Ristic Z, Gullo M, Spies P, Dodd D, Gygax D, Korte M, Schwab ME. The sphingolipid receptor S1PR2 is a receptor for Nogo-a repressing synaptic plasticity. PLoS Biol. 2014;12:e1001763. doi: 10.1371/journal.pbio.1001763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gou X, Zhang Q, Xu N, Deng B, Wang H, Xu L, Wang Q. Spatio-temporal expression of paired immunoglobulin-like receptor-B in the adult mouse brain after focal cerebral ischaemia. Brain Inj. 2013;27:1311–1315. doi: 10.3109/02699052.2013.812241. [DOI] [PubMed] [Google Scholar]
- 39.Syken J, GrandPre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313:1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura Y, Fujita Y, Ueno M, Takai T, Yamashita T. Paired immunoglobulin-like receptor B knockout does not enhance axonal regeneration or locomotor recovery after spinal cord injury. J Biol Chem. 2011;286:1876–1883. doi: 10.1074/jbc.M110.163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- 42.Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, Schwab ME. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378:498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- 43.Merkler D, Metz GA, Raineteau O, Dietz V, Schwab ME, Fouad K. Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A. J Neurosci. 2001;21:3665–3673. doi: 10.1523/JNEUROSCI.21-10-03665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brösamle C, Huber AB, Fiedler M, Skerra A, Schwab ME. Regeneration of lesioned corticospinal tract fibers in the adult rat induced by a recombinant, humanized IN-1 antibody fragment. J Neurosci. 2000;20:8061–8068. doi: 10.1523/JNEUROSCI.20-21-08061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinmann O, Schnell L, Ghosh A, Montani L, Wiessner C, Wannier T, Rouiller E, Mir A, Schwab ME. Intrathecally infused antibodies against Nogo-A penetrate the CNS and downregulate the endogenous neurite growth inhibitor Nogo-A. Mol Cell Neurosci. 2006;32:161–173. doi: 10.1016/j.mcn.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Liebscher T, Schnell L, Schnell D, Scholl J, Schneider R, Gullo M, Fouad K, Mir A, Rausch M, Kindler D, Hamers FP, Schwab ME. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol. 2005;58:706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
- 47.Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, Rouiller EM. Nogo-A-specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nat Med. 2006;12:790–792. doi: 10.1038/nm1436. [DOI] [PubMed] [Google Scholar]
- 48.Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, Rouiller EM. Anti-Nogo-A antibody treatment promotes recovery of manual dexterity after unilateral cervical lesion in adult primates--re-examination and extension of behavioral data. Eur J Neurosci. 2009;29:983–996. doi: 10.1111/j.1460-9568.2009.06642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.GrandPré T, Li S, Strittmatter SM. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- 50.Fournier AE, Gould GC, Liu BP, Strittmatter SM. Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin. J Neurosci. 2002;22:8876–8883. doi: 10.1523/JNEUROSCI.22-20-08876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li S, Liu BP, Budel S, Li M, Ji B, Walus L, Li W, Jirik A, Rabacchi S, Choi E, Worley D, Sah DW, Pepinsky B, Lee D, Relton J, Strittmatter SM. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J Neurosci. 2004;24:10511–10520. doi: 10.1523/JNEUROSCI.2828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu BP, Fournier A, GrandPré T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- 53.Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang K, Nikulina E, Kimura N, Cai H, Deng K, Gao Y, He Z, Filbin M. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- 54.Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- 55.Wang X, Baughman KW, Basso DM, Strittmatter SM. Delayed Nogo receptor therapy improves recovery from spinal cord contusion. Ann Neurol. 2006;60:540–549. doi: 10.1002/ana.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Yigitkanli K, Kim CY, Sekine-Komo T, Wirak D, Frieden E, Bhargava A, Maynard G, Cafferty WB, Strittmatter SM. Human NgR-Fc decoy protein via lumbar intrathecal bolus administration enhances recovery from rat spinal cord contusion. J Neurotrauma. 2014;31:1955–1966. doi: 10.1089/neu.2014.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song XY, Zhong JH, Wang X, Zhou XF. Suppression of p75NTR does not promote regeneration of injured spinal cord in mice. J Neurosci. 2004;24:542–546. doi: 10.1523/JNEUROSCI.4281-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ji B, Li M, Wu WT, Yick LW, Lee X, Shao Z, Wang J, So KF, McCoy JM, Pepinsky RB, Mi S, Relton JK. LINGO-1 antagonist promotes functional recovery and axonal sprouting after spinal cord injury. Mol Cell Neurosci. 2006;33:311–320. doi: 10.1016/j.mcn.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 59.Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J Neurosci. 2002;22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lehmann M, Fournier A, Selles-Navarro I, Dergham P, Sebok A, Leclerc N, Tigyi G, McKerracher L. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J Neurosci. 1999;19:7537–7547. doi: 10.1523/JNEUROSCI.19-17-07537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci. 2003;22:319–330. doi: 10.1016/s1044-7431(02)00035-0. [DOI] [PubMed] [Google Scholar]
- 62.Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 63.McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 64.Baldwin KT, Giger RJ. Insights into the physiological role of CNS regeneration inhibitors. Front Mol Neurosci. 2015;8:23. doi: 10.3389/fnmol.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trapp BD, Andrews SB, Cootauco C, Quarles R. The myelin-associated glycoprotein is enriched in multivesicular bodies and periaxonal membranes of actively myelinating oligodendrocytes. J Cell Biol. 1989;109:2417–2426. doi: 10.1083/jcb.109.5.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ng WP, Cartel N, Li C, Roder J, Lozano A. Myelin from MAG-deficient mice is a strong inhibitor of neurite outgrowth. Neuroreport. 1996;7:861–864. doi: 10.1097/00001756-199603220-00005. [DOI] [PubMed] [Google Scholar]
- 67.Bartsch U, Bandtlow CE, Schnell L, Bartsch S, Spillmann AA, Rubin BP, Hillenbrand R, Montag D, Schwab ME, Schachner M. Lack of evidence that myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron. 1995;15:1375–1381. doi: 10.1016/0896-6273(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 68.Mountney A, Zahner MR, Lorenzini I, Oudega M, Schramm LP, Schnaar RL. Sialidase enhances recovery from spinal cord contusion injury. Proc Natl Acad Sci U S A. 2010;107:11561–11566. doi: 10.1073/pnas.1006683107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vourc’h P, Dessay S, Mbarek O, Marouillat Védrine S, Müh JP, Andres C. The oligodendrocyte-myelin glycoprotein gene is highly expressed during the late stages of myelination in the rat central nervous system. Brain Res Dev Brain Res. 2003;144:159–168. doi: 10.1016/s0165-3806(03)00167-6. [DOI] [PubMed] [Google Scholar]
- 70.Huang JK, Phillips GR, Roth AD, Pedraza L, Shan W, Belkaid W, Mi S, Fex-Svenningsen A, Florens L, Yates JR, 3rd, Colman DR. Glial membranes at the node of Ranvier prevent neurite outgrowth. Science. 2005;310:1813–1817. doi: 10.1126/science.1118313. [DOI] [PubMed] [Google Scholar]
- 71.Lee X, Hu Y, Zhang Y, Yang Z, Shao Z, Qiu M, Pepinsky B, Miller RH, Mi S. Oligodendrocyte differentiation and myelination defects in OMgp null mice. Mol Cell Neurosci. 2011;46:752–761. doi: 10.1016/j.mcn.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 72.Ji B, Case LC, Liu K, Shao Z, Lee X, Yang Z, Wang J, Tian T, Shulga-Morskaya S, Scott M, He Z, Relton JK, Mi S. Assessment of functional recovery and axonal sprouting in oligodendrocyte-myelin glycoprotein (OMgp) null mice after spinal cord injury. Mol Cell Neurosci. 2008;39:258–267. doi: 10.1016/j.mcn.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, Kang B, Zheng B. Assessing spinal axon regeneration, sprouting in Nogo-MAG-, and OMgp-deficient mice. Neuron. 2010;66:663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cafferty WB, Duffy P, Huebner E, Strittmatter SM. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J Neurosci. 2010;30:6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Properzi F, Asher RA, Fawcett JW. Chondroitin sulphate proteoglycans in the central nervous system: changes and synthesis after injury. Biochem Soc Trans. 2003;31:335–336. doi: 10.1042/bst0310335. [DOI] [PubMed] [Google Scholar]
- 76.Ogawa T, Hagihara K, Suzuki M, Yamaguchi Y. Brevican in the developing hippocampal fimbria: differential expression in myelinating oligodendrocytes and adult astrocytes suggests a dual role for brevican in central nervous system fiber tract development. J Comp Neurol. 2001;432:285–295. doi: 10.1002/cne.1103. [DOI] [PubMed] [Google Scholar]
- 77.Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 78.Caggiano AO, Zimber MP, Ganguly A, Blight AR, Gruskin EA. Chondroitinase ABCI improves locomotion, bladder function following contusion injury of the rat spinal cord, Chondroitinase ABCI improves locomotion and bladder function following contusion injury of the rat spinal cord. J Neurotrauma. 2005;22:226–239. doi: 10.1089/neu.2005.22.226. [DOI] [PubMed] [Google Scholar]
- 79.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Schut D, Fehlings MG. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci. 2010;30:1657–1676. doi: 10.1523/JNEUROSCI.3111-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Houle D, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system “bridge”, matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee YS, Lin CY, Jiang HH, Depaul M, Lin VW, Silver J. Nerve regeneration restores supraspinal control of bladder function after complete spinal cord injury. J Neurosci. 2013;33:10591–10606. doi: 10.1523/JNEUROSCI.1116-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tester NJ, Plaas AH, Howland DR. Effect of body temperature on chondroitinase ABC’s ability to cleave chondroitin sulfate glycosaminoglycans. J Neurosci Res. 2007;85:1110–1118. doi: 10.1002/jnr.21199. [DOI] [PubMed] [Google Scholar]
- 84.Lee H, McKeon RJ, Bellamkonda RV. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2010;107:3340–3345. doi: 10.1073/pnas.0905437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bartus K, James ND, Didangelos A, Bosch JD, Verhaagen J, Yáñez-Muñoz RJ, Rogers JH, Schneider BL, Muir EM, Bradbury EJ. Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J Neurosci. 2014;34:4822–4836. doi: 10.1523/JNEUROSCI.4369-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, Wood A, Geoffroy CG, Zheng B, Liepmann CD, Katagiri Y, Benowitz LI, Geller HM, Giger RJ. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci. 2012;15:703–712. doi: 10.1038/nn.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lang BT, Cregg JM, DePaul MA, Tran AP, Xu K, Dyck SM, Madalena KM, Brown BP, Weng YL, Li S, Karimi-Abdolrezaee S, Busch SA, Shen Y, Silver J. Modulation of the proteoglycan receptor PTPσ promotes recovery after spinal cord injury. Nature. 2015;518:404–408. doi: 10.1038/nature13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grimpe B, Silver J. A novel DNA enzyme reduces glycosaminoglycan chains in the glial scar and allows microtransplanted dorsal root ganglia axons to regenerate beyond lesions in the spinal cord. J Neurosci. 2004;24:1393–1397. doi: 10.1523/JNEUROSCI.4986-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Akbik FV, Bhagat SM, Patel PR, Cafferty WB, Strittmatter SM. Anatomical plasticity of adult brain is titrated by Nogo Receptor 1. Neuron. 2013;77:859–866. doi: 10.1016/j.neuron.2012.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 93.Bhagat SM, Butler SS, Taylor JR, McEwen BS, Strittmatter SM. Erasure of fear memories is prevented by Nogo Receptor 1 in adulthood. Molecular psychiatry. 2015 doi: 10.1038/mp.2015.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gogolla N, Caroni P, Luthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- 95.Siegel CS, Fink KL, Strittmatter SM, Cafferty WB. Plasticity of intact rubral projections mediates spontaneous recovery of function after corticospinal tract injury. J Neurosci. 2015;35:1443–1457. doi: 10.1523/JNEUROSCI.3713-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hill CE, Proschel C, Noble M, Mayer-Proschel M, Gensel JC, Beattie MS, Bresnahan JC. Acute transplantation of glial-restricted precursor cells into spinal cord contusion injuries: survival, differentiation, and effects on lesion environment and axonal regeneration. Exp Neurol. 2004;190:289–310. doi: 10.1016/j.expneurol.2004.05.043. [DOI] [PubMed] [Google Scholar]
- 97.Ketschek AR, Haas C, Gallo G, Fischer I. The roles of neuronal and glial precursors in overcoming chondroitin sulfate proteoglycan inhibition. Exp Neurol. 2012;235:627–637. doi: 10.1016/j.expneurol.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bregman BS, Reier PJ. Neural tissue transplants rescue axotomized rubrospinal cells from retrograde death. J Comp Neurol. 1986;244:86–95. doi: 10.1002/cne.902440107. [DOI] [PubMed] [Google Scholar]
- 99.Reier PJ, Bregman BS, Wujek JR. Intraspinal transplantation of embryonic spinal cord tissue in neonatal and adult rats. J Comp Neurol. 1986;247:275–296. doi: 10.1002/cne.902470302. [DOI] [PubMed] [Google Scholar]
- 100.Xu XM, Guénard V, Kleitman N, Aebischer P, Bunge MB. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp Neurol. 1995;134:261–272. doi: 10.1006/exnr.1995.1056. [DOI] [PubMed] [Google Scholar]
- 101.Xu XM, Guénard V, Kleitman N, Bunge MB. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol. 1995;351:145–160. doi: 10.1002/cne.903510113. [DOI] [PubMed] [Google Scholar]
- 102.Cao L, Zhu YL, Su Z, Lv B, Huang Z, Mu L, He C. Olfactory ensheathing cells promote migration of Schwann cells by secreted nerve growth factor. Glia. 2007;55:897–904. doi: 10.1002/glia.20511. [DOI] [PubMed] [Google Scholar]
- 103.Ramón-Cueto A, Plant GW, Avila J, Bunge MB. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neurosci. 1998;134:3803–3815. doi: 10.1523/JNEUROSCI.18-10-03803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Himes BT, Neuhuber B, Coleman C, Kushner R, Swanger SA, Kopen GC, Wagner J, Shumsky JS, Fischer I. Recovery of function following grafting of human bone marrow-derived stromal cells into the injured spinal cord. Neurorehabil Neural Repair. 2006;20:278–296. doi: 10.1177/1545968306286976. [DOI] [PubMed] [Google Scholar]
- 105.Lepore AC, Neuhuber B, Connors TM, Han SS, Liu Y, Daniels MP, Rao MS, Fischer I. Long-term fate of neural precursor cells following transplantation into developing and adult CNS. Neuroscience. 2006;142:287–304. doi: 10.1016/j.neuroscience.2005.12.067. [DOI] [PubMed] [Google Scholar]
- 106.Bonner JF, Blesch A, Neuhuber B, Fischer I. Promoting directional axon growth from neural progenitors grafted into the injured spinal cord. J Neurosci Res. 2010;88:1182–1192. doi: 10.1002/jnr.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bonner JF, Connors TM, Silverman WF, Kowalski DP, Lemay MA, Fischer I. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J Neurosci. 2011;31:4675–4686. doi: 10.1523/JNEUROSCI.4130-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bregman BS, Reier PJ. Neural tissue transplants rescue axotomized rubrospinal cells from retrograde death. J Comp Neurol. 1986;244:86–95. doi: 10.1002/cne.902440107. [DOI] [PubMed] [Google Scholar]
- 110.Gage FH. Mammalian neural stem cells. Science. 2000;298:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 111.Conti L, Cattaneo E. Neural stem cell systems: physiological players or in vitro entities? Nat Rev Neurosci. 2010;11:176–187. doi: 10.1038/nrn2761. [DOI] [PubMed] [Google Scholar]
- 112.Chiasson BJ, Tropepe V, Morshead CM, van der Kooy D. Adult mammalian forebrain ependymal, subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J Neurosci. 1999;11:4462–4471. doi: 10.1523/JNEUROSCI.19-11-04462.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 114.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 115.Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- 116.Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16:7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ostenfeld T, Caldwell MA, Prowse KR, Linskens MH, Jauniaux E, Svendsen CN. Human neural precursor cells express low levels of telomerase in vitro and show diminishing cell proliferation with extensive axonal outgrowth following transplantation. Exp Neurol. 2000;164:215–226. doi: 10.1006/exnr.2000.7427. [DOI] [PubMed] [Google Scholar]
- 118.Reynolds BA, Rietze RL. Neural stem cells and neurospheres--re-evaluating the relationship. Nat Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- 119.Sun Y, Pollard S, Conti L, Toselli M, Biella G, Parkin G, Willatt L, Falk A, Cattaneo E, Smith A. A Long-term tripotent differentiation capacity of human neural stem (NS) cells in adherent culture. Mol Cell Neurosci. 2008;38:245–258. doi: 10.1016/j.mcn.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 120.Tailor J, Kittappa R, Leto K, Gates M, Borel M, Paulsen O, Spitzer S, Karadottir RT, Rossi F, Falk A, Smith A. Stem cells expanded from the human embryonic hindbrain stably retain regional specification and high neurogenic potency. J Neurosci. 2013;33:12407–12422. doi: 10.1523/JNEUROSCI.0130-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pollard SM, Conti L, Sun Y, Goffredo D, Smith A. Adherent neural stem (NS) cells from fetal and adult forebrain. Cereb Cortex. 2006:112–120. doi: 10.1093/cercor/bhj167. [DOI] [PubMed] [Google Scholar]
- 122.Conti L, Pollard SM, Gorba T, Reitano E, Toselli M, Biella G, Sun Y, Sanzone S, Ying QL, Cattaneo E, Smith A. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005;9 doi: 10.1371/journal.pbio.0030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Onorati M, Li Z, Liu F, Sousa AM, Nakagawa N, Li M, Dell’Anno MT, Gulden FO, Pochareddy S, Tebbenkamp AT, Han W, Pletikos M, Gao T, Zhu Y, Bichsel C, Varela L, Szigeti-Buck K, Lisgo S, Zhang Y, Testen A, Gao XB, Mlakar J, Popovic M, Flamand M, Strittmatter SM, Kaczmarek LK, Anton ES, Horvath TL, Lindenbach BD, Sestan N. Zika Virus Disrupts Phospho-TBK1 Localization and Mitosis in Human Neuroepithelial Stem Cells and Radial Glia. Cell Rep. 2016;16:2576–2592. doi: 10.1016/j.celrep.2016.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system “bridge”, matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tom VJ, Sandrow-Feinberg HR, Miller K, Santi L, Connors T, Lemay MA, Houlé JD. Combining peripheral nerve grafts and chondroitinase promotes functional axonal regeneration in the chronically injured spinal cord. J Neurosci. 2009;29:14881–14890. doi: 10.1523/JNEUROSCI.3641-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jin Y, Sura K, Fischer I. Differential effects of distinct central nervous system regions on cell migration and axonal extension of neural precursor transplants. J Neurosci Res. 2012;90:2065–2073. doi: 10.1002/jnr.23099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li Y, Raisman G. Long axon growth from embryonic neurons transplanted into myelinated tracts of the adult rat spinal cord. Brain Res. 1993;629:115–127. doi: 10.1016/0006-8993(93)90489-a. [DOI] [PubMed] [Google Scholar]
- 128.Fisher D, Xing B, Dill J, Li H, Hoang HH, Zhao Z, Yang XL, Bachoo R, Cannon S, Longo FM, Sheng M, Silver J, Li S. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J Neurosci. 2011;31:14051–14066. doi: 10.1523/JNEUROSCI.1737-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neurol. 2014;253:197–207. doi: 10.1016/j.expneurol.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ogawa Y, Sawamoto K, Miyata T, Miyao S, Watanabe M, Nakamura M, Bregman BS, Koike M, Uchiyama Y, Toyama Y, Okano H. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res. 2002;69:925–933. doi: 10.1002/jnr.10341. [DOI] [PubMed] [Google Scholar]
- 132.Abematsu M, Tsujimura K, Yamano M, Saito M, Kohno K, Kohyama J, Namihira M, Komiya K, Nakashima K. Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J Clin Invest. 2010;12:3255–3266. doi: 10.1172/JCI42957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bráz JM, Sharif-Naeini R, Vogt D, Kriegstein A, Alvarez-Buylla A, Rubenstein JL, Basbaum AI. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron. 2012;74:663–675. doi: 10.1016/j.neuron.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hou S, Tom VJ, Graham L, Lu P, Blesch A. Partial restoration of cardiovascular function by embryonic neural stem cell grafts after complete spinal cord transection. J Neurosci. 2013;33:17138–17149. doi: 10.1523/JNEUROSCI.2851-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kirkeby A, Nelander J, Parmar M. Generating regionalized neuronal cells from pluripotency, a step-by-step protocol. Front Cell Neurosci. 2013;6:64. doi: 10.3389/fncel.2012.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yan J, Xu L, Welsh AM, Hatfield G, Hazel T, Johe K, Koliatsos VE. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med. 2007;4:e39. doi: 10.1371/journal.pmed.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lu P, Woodruff G, Wang Y, Graham L, Hunt M, Wu D, Boehle E, Ahmad R, Poplawski G, Brock J, Goldstein LS, Tuszynski MH. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. 2014;83:789–796. doi: 10.1016/j.neuron.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hofstetter CP, Holmström NA, Lilja JA, Schweinhardt P, Hao J, Spenger C, Wiesenfeld-Hallin Z, Kurpad SN, Frisén J, Olson L. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci. 2005;8:346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- 139.Macias MY, Syring MB, Pizzi MA, Crowe MJ, Alexanian AR, Kurpad SN. Pain with no gain: allodynia following neural stem cell transplantation in spinal cord injury. Exp Neurol. 2006;201:335–348. doi: 10.1016/j.expneurol.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 140.Davies JE, Pröschel C, Zhang N, Noble M, Mayer-Pröschel M, Davies SJ. Transplanted astrocytes derived from BMP- or CNTF-treated glial-restricted precursors have opposite effects on recovery and allodynia after spinal cord injury. J Biol. 2008;7:24. doi: 10.1186/jbiol85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 142.Kawaja MD, Gage FH. Reactive astrocytes are substrates for the growth of adult CNS axons in the presence of elevated levels of nerve growth factor. Neuron. 1991;7:1019–1130. doi: 10.1016/0896-6273(91)90346-2. [DOI] [PubMed] [Google Scholar]
- 143.Kadoya K, Lu P, Nguyen K, Lee-Kubli C, Kumamaru H, Yao L, Knackert J, Poplawski G, Dulin JN, Strobl H, Takashima Y, Biane J, Conner J, Zhang SC, Tuszynski M. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat Med. 2016;22:479–487. doi: 10.1038/nm.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 146.Hawryluk GW, Mothe A, Wang J, Wang S, Tator C, Fehlings MG. An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells Dev. 2012;21:2222–2238. doi: 10.1089/scd.2011.0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yan J, Welsh AM, Bora SH, Snyder EY, Koliatsos VE. Differentiation and tropic/trophic effects of exogenous neural precursors in the adult spinal cord. J Comp Neurol. 2004;480:101–114. doi: 10.1002/cne.20344. [DOI] [PubMed] [Google Scholar]
- 148.Alto LT, Havton LA, Conner JM, Hollis ER, 2nd, Blesch A, Tuszynski MH. Chemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury. Nat Neurosci. 2009;12:1106–1113. doi: 10.1038/nn.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yasuda A, Tsuji O, Shibata S, Nori S, Takano M, Kobayashi Y, Takahashi Y, Fujiyoshi K, Hara CM, Miyawaki A, Okano HJ, Toyama Y, Nakamura M, Okano H. Significance of remyelination by neural stem/progenitor cells transplanted into the injured spinal cord. Stem Cells. 2011;29:1983–1994. doi: 10.1002/stem.767. [DOI] [PubMed] [Google Scholar]
- 150.Jin Y, Ziemba KS, Smith GM. Axon growth across a lesion site along a preformed guidance pathway in the brain. Exp Neurol. 2008;210:521–530. doi: 10.1016/j.expneurol.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ziemba KS, Chaudhry N, Rabchevsky AG, Jin Y, Smith GM. Targeting axon growth from neuronal transplants along preformed guidance pathways in the adult CNS. J Neurosci 28. 2008;28:340–348. doi: 10.1523/JNEUROSCI.3819-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xu CJ, Xu L, Huang LD, Li Y, Yu PP, Hang Q, Xu XM, Lu PH. Combined NgR vaccination and neural stem cell transplantation promote functional recovery after spinal cord injury in adult rats. Neuropathol Appl Neurobiol. 2011;37:135–155. doi: 10.1111/j.1365-2990.2010.01117.x. [DOI] [PubMed] [Google Scholar]
- 153.Guo X, Zahir T, Mothe A, Shoichet MS, Morshead CM, Katayama Y, Tator CH. The effect of growth factors and soluble Nogo-66 receptor protein on transplanted neural stem/progenitor survival and axonal regeneration after complete transection of rat spinal cord. Cell Transplant. 2012;21:1177–1197. doi: 10.3727/096368911X612503. [DOI] [PubMed] [Google Scholar]