Abstract
Objective
The purpose of this study was to investigate whether the craniofacial patterns of Korean children with snoring and adenotonsillar hypertrophy (ATH) could be categorized into characteristic clusters according to age.
Methods
We enrolled 236 children with snoring and ATH (age range, 5–12 years) in this study. They were subdivided into four age groups: 5–6, 7–8, 9–10, and 11–12 years. Based on cephalometric analysis, the sagittal and vertical skeletal patterns of each individual were divided into Class I, II, and III, as well as the normodivergent, hypodivergent, and hyperdivergent patterns, respectively. Cluster analysis was performed using cephalometric principal components in addition to the age factor.
Results
Three heterogeneous clusters of craniofacial patterns were obtained in relation to age: cluster 1 (41.9%) included patients aged 5–8 years with a skeletal Class I or mild Class II and hyperdivergent pattern; cluster 2 (45.3%) included patients aged 9–12 years with a Class II and hyperdivergent pattern; and cluster 3 (12.8%) included patients aged 7–8 years with a Class III and hyperdivergent pattern.
Conclusions
This study found that the craniofacial patterns of Korean children with snoring and ATH could be categorized into three characteristic clusters according to age groups. Although no significantly dominant sagittal skeletal discrepancy was observed, hyperdivergent vertical discrepancy was consistently evident in all clusters.
Keywords: Adenotonsillar hypertrophy, Cluster analysis, Craniofacial pattern, Snoring
INTRODUCTION
Snoring is the primary symptom of pediatric sleep-disordered breathing (SDB).1 Persistent snoring in children is considered a warning sign because it may progress into more severe obstructive sleep apnea (OSA),2 leading to irreparable problems such as somatic growth retardation, behavioral and cognitive disturbance, low performance in school, and craniofacial deformation.3,4 Current reviews have reported that the major cause of snoring in children is partial or complete upper airway obstruction caused by adenotonsillar hypertrophy (ATH).3,5 Adenoids and tonsils can be physiologically hypertrophied by the age of 11–12 years as the first immune barrier of host resistance against pathogens. However, children with ATH-related respiratory obstruction exhibit mouth breathing, which causes irritation and infection of the lymphatic tissues, thereby adversely leading to pathologic ATH.6
Mouth breathing caused by ATH is known to promote craniofacial deformation by neuromuscular and soft-tissue rearrangement in relation to abnormal head and tongue postures, resulting in the clockwise rotation of the maxillary-mandibular complex, retruded mandible, increased lower and total facial height, and downward displacement of the hyoid bone.7,8,9,10 Some studies have demonstrated no correlation between ATH and craniofacial abnormality, supporting the notion that genetic and epigenetic inheritance are predominantly responsible for craniofacial growth.11,12 However, functional disorder and postural imbalance of the stomatognathic system might alter craniofacial development, particularly in specific age groups. Therefore, early evaluation and intervention have been advocated for resolving pediatric snoring and mouth breathing.
Adenotonsillectomy (AT) is considered the first-line treatment for pediatric SDB3,13; however, there is still a lack of consensus regarding the optimal timing of the AT surgery. Early surgical resolution has been supported by some ear-nose-throat (ENT) surgeons in view of obtaining high surgical success with low recurrence rate and of preventing growth and developmental problems.13,14 In contrast, delayed surgery has been advocated considering the high postsurgical morbidity and low acceptance of nonemergency surgical procedures by parents.15,16 A recent systematic review reported no benefit of early AT in patients under 5 years of age.16 Huang et al.13 suggested that the upper age limit for AT should be less than 8 years old, based on their finding that age was a critical risk factor for recurrence after AT. From the perspective of craniofacial deformation, early AT can be also supported in terms of prevention or interruption of persistent orofacial neuromuscular problems and subsequent irreversible craniofacial deformation.17 Kim et al.18 suggested that the cutoff age for dentofacial abnormality should be 5.5 years in Korean children with ATH and 6.5 years in those without ATH, thereby insisting early surgical intervention. However, the parameters measured in their study were confined to dental aspects, which limited its use as an age reference for craniofacial alteration. Therefore, there is a need to determine the cutoff age at which craniofacial deformation develops significantly in snoring populations with ATH.
Many studies have reported the cephalometric craniofacial characteristics of children with SDB, but most of these studies have focused on Caucasian populations representing adenoid faces.19,20,21 Moreover, they compared the mean values of each group as a parallel integration of one-dimensional individual measurements. No study has categorized the craniofacial patterns of Asian children with snoring by using a multidimensional analysis, such as a cluster analysis, which can incorporate all features of a dataset while maintaining the autonomy of each individual measurement.22 Moreover, no report has considered that craniofacial alteration should be evaluated by the normative values of each age instead of the mean normative values of a broad age group, because the normative values of skeletal parameters keep changing yearly in actively growing children. Therefore, the purpose of this study was to investigate whether the craniofacial patterns of Korean children with snoring and ATH could be categorized into characteristic clusters according to age.
MATERIALS AND METHODS
We evaluated 280 patients aged between 5 and 12 years, who were referred from the Department of Otorhinolaryngology in Kyung Hee University Medical Center to the Department of Orthodontics in Kyung Hee University Dental Hospital, by using standardized history taking, physical examination, and subjective neurocognitive and psychosocial questionnaires administered by an otorhinolaryngologist. The patients were diagnosed as having chronic snoring with mouth breathing and ATH. This study included patients with tonsillar hypertrophy of grade 3 and above based on the grading scales suggested by Brodsky et al.23: grade 1, confined into the tonsillar pillars; grade 2, extended just outside the pillars; grade 3, extended outside the pillars but did not meet each other; and grade 4, large tonsils that met at the midline. Adenoid tissue was categorized into four grades: grade 0, 0–25%; grade 1, 25–50%; grade 2, 50–75%; and grade 3, 75–100% of hypertrophy.13 The exclusion criteria were as follows: previous ENT surgery, previous orthodontic treatment, other medical and genetic disorders, congenital craniofacial anomaly, severe psychological or behavioral problems, and severe obesity. The study protocol was approved by the Institutional Review Board of Kyung Hee University Dental Hospital, Seoul, Korea (KHD IRB 1506-1).
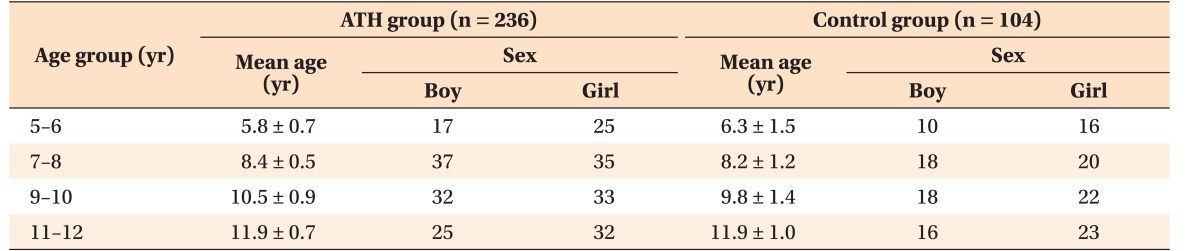
In total, 236 patients (114 boys and 122 girls) who had lateral cephalometric radiograms acquired in the natural head and lip postures 1 day before receiving AT surgery were finally selected (Figure 1). They were divided into four age groups: 5–6 years (n = 42), 7–8 years (n = 72), 9–10 years (n = 65), and 11–12 years (n = 57) (Table 1). All cephalograms were traced and measured twice by a single blinded orthodontic specialist. Twenty cephalometric parameters were chosen to analyze each compartment of the cranial base, maxilla, mandible, hyoid, and pharyngeal airway (Table 2) on the basis of the findings of previous studies on patients with SDB.24,25,26 The skeletal pattern of each individual was characterized as follows: the sagittal pattern was classified on the basis of the ANB angle as Class I (0° < ANB < 4°), Class II (ANB ≥ 4°), or Class III (ANB ≤ 0°); the vertical pattern was classified on the basis of the FMA angle as normodivergent (25° ≤ FMA ≤ 28°), hypodivergent (FMA < 25°), or hyperdivergent (FMA > 28°).
Figure 1. Flowchart illustrating the study design.
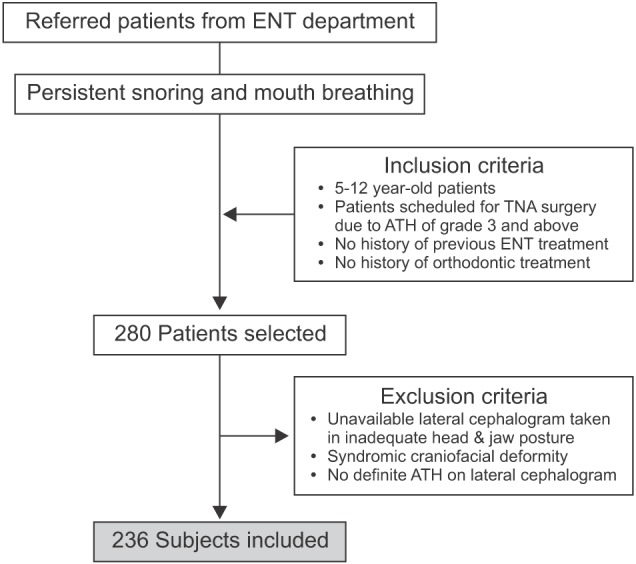
ENT, Ear-nose-throat; TNA, tonsillectomy and adenoidectomy; ATH, adenotonsillar hypertrophy.
Table 1. Demographic data of the patients enrolled in this study.
Data are reported as mean ± standard deviation or number only.
ATH, Adenotonsillar hypertrophy.
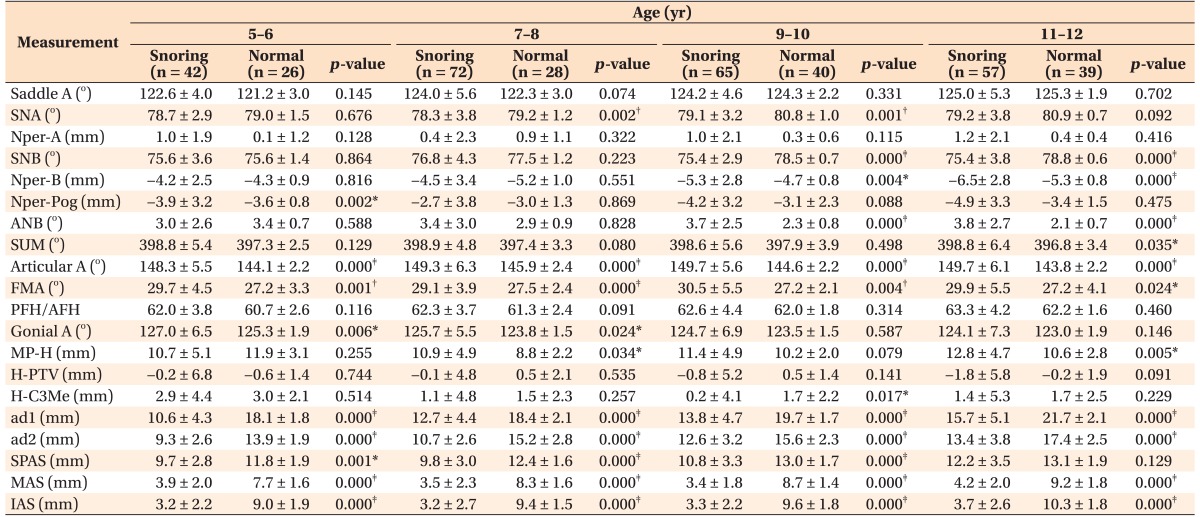
Table 2. Cephalometric analysis of patients with snoring in four age groups as compared to the age-matched normative values obtained from the normal samples.
Data are reported as mean ± standard deviation.
Saddle A, Angle formed between the lines Sella-Nasion and Sella-Articular; SNA, anteroposterior position of the maxilla in relation to the anterior cranial base (angle formed between the lines Sella-Nasion and Nasion-A point); Nper-A, distance between the A point and a line perpendicular to the Frankfort plane from the Nasion; SNB, anteroposterior position of the mandible in relation to the anterior cranial base (angle formed between the lines Sella-Nasion and Nasion-B point); Nper-B, distance between the B point and a line perpendicular to the Frankfort plane from the Nasion; Nper-Pog, distance between the Pogonion and a line perpendicular to the Frankfort plane from the Nasion; ANB, mathematical difference between the SNA and SNB; SUM, sum of the angles Saddle-Articular-Gonial; Articular A, angle formed between the lines Sella-Articular and Articular-Gonion; FMA, angle between the Frankfort plane and mandibular plane; PFH/AFH, ratio of posterior facial height relative to anterior facial height (S-Go/N-Me); Gonial A, angle formed between the lines Articular-Gonion and mandibular plane; MP-H, perpendicular distance of the hyoid point from the mandibular plane; H-PTV, distance of the hyoid point to the vertical pterygoid; H-C3Me, perpendicular distance of the hyoid point from the C3-Menton line; ad1, distance from the PNS to the nearest adenoid tissue measured along the line PNS-Ba; ad2, distance from the PNS to the nearest adenoid tissue measured along the line through the PNS perpendicular to the Sella-Basion; SPAS, superior posterior airway space (anteroposterior width of the pharynx measured between the posterior pharyngeal wall and the dorsum of the soft palate); MAS, middle airway space (anteroposterior width of the pharynx measured between the posterior pharyngeal wall and the palate tip); IAS, inferior airway space (anteroposterior width of the airway space along the Gonion-B point line).
Independent t-test was preliminarily performed to determine significantly different cephalometric parameters between the patients with snoring and the normal samples, for further principal component analysis; *p < 0.05, †p < 0.01, ‡p < 0.001.
In order to obtain normative values of each cephalometric parameter in the different age groups, a control sample of the lateral cephalograms of 104 normal Korean children were included and matched by age to the four age groups in this study. The normal children showed a balanced facial profile within the normal range of skeletal relationship, and normal occlusion. They had no history of snoring, persistent mouth breathing, deleterious oral habits, or ATH over grade 3 on the cephalograms.
Statistical analysis
The normality of the study sample and normal sample was tested using the Shapiro-Wilk test (p > 0.05). Independent t-test was primarily performed to assess and compare each cephalometric variable of the children with snoring to that of the age-matched normal sample. Cephalometric principal components were selected for subsequent principal component analysis in consideration of the result of the independent t-test. Based on a significant association between age and skeletal parameters by Pearson's chi-square test (p < 0.001), K-means cluster analysis was performed using the age factor in addition to cephalometric principal components to classify the study samples into characteristic skeletal patterns. All statistical analyses were performed using IBM SPSS Statistics for Windows (version 21.0.0.0; IBM Corp., Armonk, NY, USA) and p < 0.05 was considered significant.
RESULTS
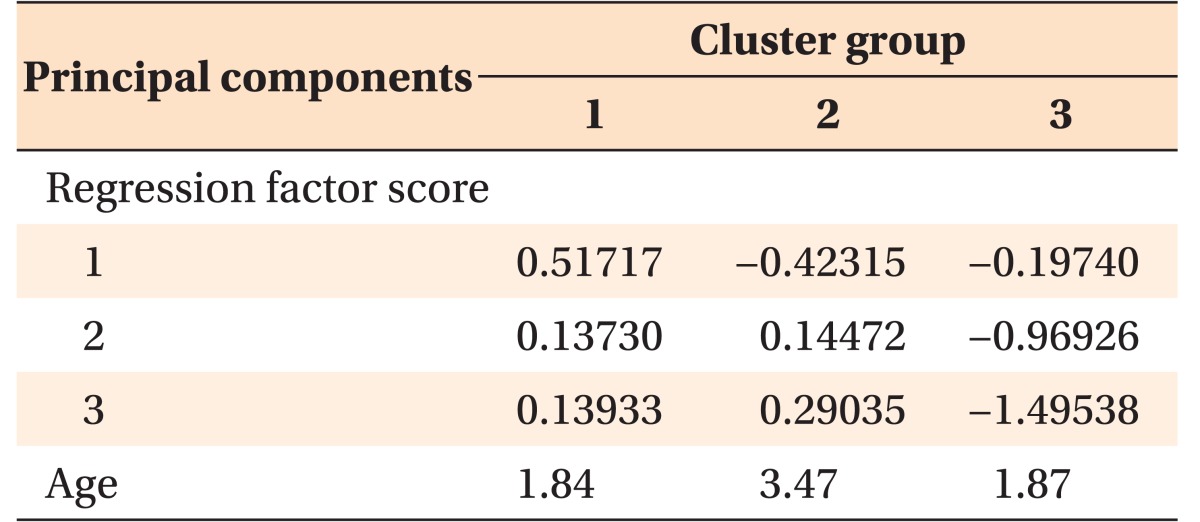
The intra-examiner reliability of the cephalometric values was examined by using the intraclass correlation coefficient (r = 0.815), which indicated high reproducibility of measurements. Based on the result of the independent t-test, the cephalometric variables that represented significant intergroup differences in all age groups were first selected for subsequent cluster analysis; these included the articular angle and FMA (Table 2). Then, the variables showing intergroup differences (p < 0.001) in at least two age groups were chosen, namely, the SNB and ANB angles. Although the SNA angle showed intergroup differences (p < 0.01) in two age groups (7–8 and 9–10 years), it was ruled out from the final cephalometric component. This was because the distance from the Na-perpendicular line to A point, which supports the SNA angle to evaluate the anteroposterior position of the maxilla, represented no significant intergroup differences (Table 2). In addition, the perpendicular distance of the hyoid point from the mandibular plane (MP-H) and saddle angle25,26 were included as cephalometric parameters of hyoid bone position and cranial base flexion, respectively. Finally, six cephalometric variables were used for principal component analysis, and three principal components were extracted, expressed as three linear combinations of two variables with minimum loss of information (Table 3).
Table 3. K-means cluster analysis using cephalometric principal components with the age factor.
Three cephalometric principal components had been extracted from the principal component analysis, expressed as linear combinations of the FMA-articular angle as component 1, saddle angle-SNB as component 2, and MPH-ANB as component 3.
MPH, Perpendicular distance of the hyoid point from the mandibular plane.
Extraction method, principal component analysis.
Rotation method, Varimax with Kaiser normalization.
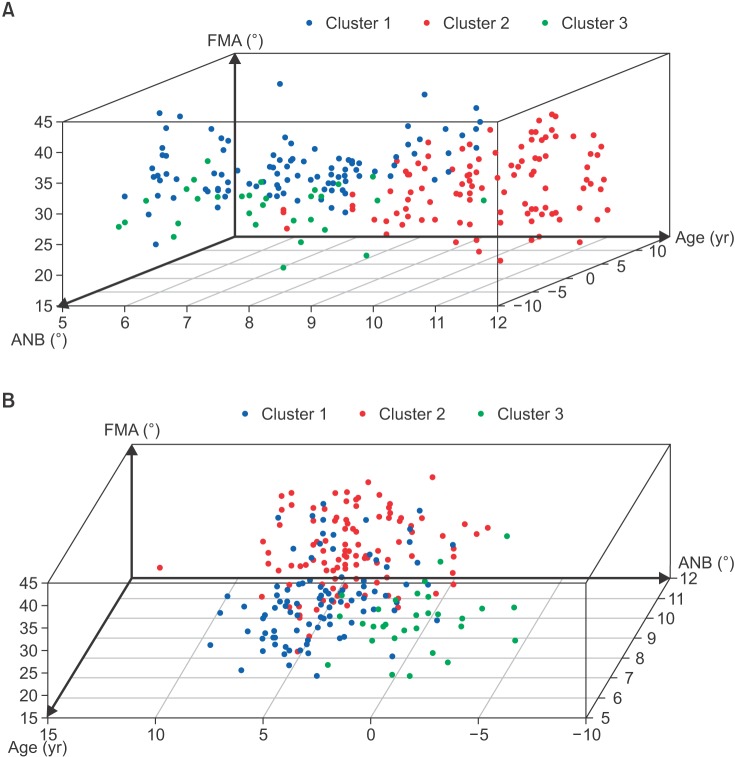
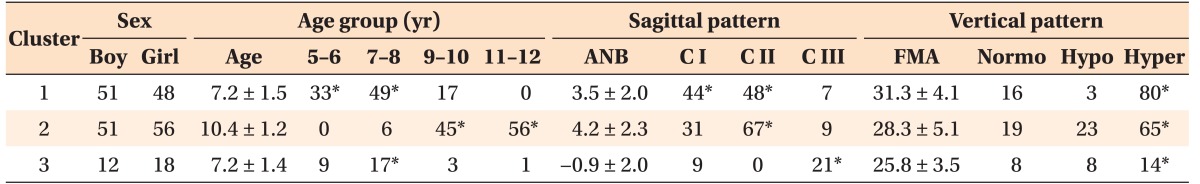
As a result of the cluster analysis, the children with snoring and ATH were categorized into three heterogeneous clusters (Figure 2 and Table 3). Cluster 1 (n = 99; 41.9%) included children aged 5–8 years (mean age, 7.2 ± 1.5 years; 82.8%) with a skeletal Class I or mild Class II (mean ANB, 3.5 ± 2.0; 92.9%) and hyperdivergent pattern (mean FMA, 31.3 ± 4.1; 80.8%). Cluster 2 (n = 107; 45.3%) included children aged 9–12 years (mean age, 10.4 ± 1.2 years; 94.4%) with a skeletal Class II (mean ANB, 4.2 ± 2.3; 62.6%) and hyperdivergent pattern (mean FMA, 28.3 ± 5.1; 61.4%). Cluster 3 (n = 30; 12.8%) included children aged 7–8 years (mean age, 7.2 ± 1.4 years; 56.7%) with a skeletal Class III (mean ANB, −0.9 ± 2.0; 70.0%) and hyperdivergent pattern (mean FMA, 25.8 ± 3.5; 46.6%). No significant sex difference was found among the clusters (Table 4).
Figure 2. Simplified three-dimensional scatter plots describing the result of a cluster analysis. A, A scatter plot constructed using the factors ANB (X-axis), age (Y-axis), and FMA (Z-axis). B, A scatter plot constructed using the factors age (X-axis), ANB (Y-axis), and FMA (Z-axis). Three clusters can be identified in the three dimensions, and clusters 1, 2, and 3 are indicated by blue, red, and green dots, respectively.
Table 4. Age-specific craniofacial patterns of the children with snoring and ATH obtained by cluster analysis.
Data are reported as mean ± standard deviation or the frequency of expression.
Cluster 1 included patients aged 5–8 years with a skeletal Class I (C I) or Class II (C II) and hyperdivergent pattern; cluster 2 included patients aged 9–12 years with a skeletal Class II and hyperdivergent pattern; and cluster 3 included patients aged 7–8 years with a skeletal Class III (C III) and hyperdivergent pattern.
Normo, Normodivergent; Hypo, hypodivergent; Hyper, hyperdivergent.
*Significant frequency representing the characteristic pattern in each cluster, analyzed by Pearson's chi-square test (p < 0.001).
DISCUSSION
The present study found that the craniofacial pattern of Korean children with snoring and ATH could be categorized into three characteristic clusters according to different age groups. Clusters 1 and 2 seemed to represent the same craniofacial pattern of Class II with hyperdivergency; however, these two clusters had different clinical interpretations since they were discriminated by different age groups having different cephalometric norms. Cluster 1 included younger children aged 5–8 years with progressed vertical discrepancy and without definite sagittal skeletal discrepancy when compared with the age-matched normative value of the ANB angle (Table 4). Cluster 2 included elder children aged 9–12 years with progressed sagittal discrepancy as well as vertical discrepancy, i.e., a skeletal Class II and hyperdivergent pattern. We assumed that ATH-related respiratory problems might have had an earlier influence on vertical growth rotation than on sagittal growth.
Interestingly, cluster 3 that included children aged 7–8 years with a skeletal Class III and hyperdivergent pattern which was obtained from our Korean snoring population, showed different findings from previous reports on Caucasians.5,6,7 The Class III pattern in cluster 3 was associated with true mandibular prognathism, even with a clockwise-rotated mandible, showing neither a significantly short cranial base nor maxillary retrusion (Table 4). Mandibular overgrowth or undergrowth might be more dominantly determined by ethnicity-related genetic traits than by ATH-related postural imbalance or by epigenetic hormonal insufficiency,12,26 even though it cannot be clearly demonstrated. Therefore, our clustering result should be interpreted as a combined consequence of genetic phenotypic expression and functional perpetuation by respiratory obstruction in different developmental ages of Korean children.
The cut-off age of craniofacial alteration in relation to ATH and mouth breathing has been investigated for providing clinical evidence on the latest age when AT surgery ought to be recommended to the patient. More importantly, the reversibility of craniofacial alterations after surgical removal of respiratory obstruction is highly relevant. Hultcrantz et al.27 observed favorable facial growth in children who underwent AT before the age of 6 years, even though significant craniofacial deformity had developed in the 5- to 6-year-old age group. Peltomäki28 followed up the 5-year growth of children after they underwent AT at 5 years of age and suggested that mandibular ramus growth was enhanced, and redirected in a counterclockwise rotation, by normalization of growth hormone secretion after the alteration of the mode of breathing following AT. Nevertheless, he acknowledged that orthodontic or orthopedic treatment was inevitable in many of these patients because the growth acceleration was not always sufficient to resolve the already-formed discrepancy.29 Valera et al.17 found significant postural and functional alterations in the 3- to 6-year-old group when skeletal and occlusal alterations were not prevalent except for the dolichofacial pattern with a steep mandibular plane. This corresponded to the findings of the present study showing that the hyperdivergent vertical pattern was distinct in all the tested age groups, even before the facial growth spurt. Taken together, ATH-related craniofacial alteration may develop before 5–6 years of age, particularly in the vertical aspect of growth rotation, and already-progressed alteration may be partly normalized after AT if performed before 6 years of age. Considering that progressed clockwise rotational growth tends to show poor response to later myofunctional therapy or orthopedic treatment,30 earlier resolution of ATH at 5–6 years of age could be considered.
Cluster analysis was superior to intergroup mean comparison for the purpose of our study,22,31 which aimed to classify the patterns within a population instead of comparing the patterns between populations. However, it is debatable whether the mean value of each cephalometric measurement could be used to describe the real skeletal pattern in the snoring population, because the population included various patients with extreme numbers of positive (+) and negative (−) values neutralizing the mean value. The normal sample was required not to serve as a control group to the snoring group by using the mean value, but instead to yield the normative values for all the measurements for each age group and to characterize the skeletal patterns of each individual and each cluster.
The results of our study should be interpreted with caution because of some limitations. (1) A cross-sectional design was used to understand the distribution of characteristic craniofacial patterns according to age in Korean children with snoring and ATH. (2) This was a two-dimensional cephalometric study which could not explain the transverse aspect of craniofacial discrepancy, and had inherent drawbacks because of the reliance on anatomic, static, and single-faceted information on parapharyngeal tissues. (3) No polysomnographic records were suggested because the target population was in a primary snoring state of SDB, not in OSA. Thus, the present findings should be distinguished from those of children with OSA when the clinical significance is evaluated.
CONCLUSION
The craniofacial patterns of Korean children with snoring and ATH were categorized into three heterogeneous clusters: cluster 1 included patients aged 5–8 years with a normal range of sagittal relationship and a hyperdivergent vertical pattern; cluster 2 included patients aged 9–12 years with a skeletal Class II and hyperdivergent pattern; and cluster 3 included patients aged 7–8 years with a skeletal Class III and hyperdivergent pattern. Hyperdivergent vertical discrepancy was consistently evident in all the tested groups, even though no significantly dominant sagittal skeletal pattern was observed in Korean children with ATH-related snoring.
Footnotes
The authors report no commercial, proprietary, or financial interest in the products or companies described in this article.
References
- 1.Li HY, Lee LA. Sleep-disordered breathing in children. Chang Gung Med J. 2009;32:247–257. [PubMed] [Google Scholar]
- 2.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–252. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinha D, Guilleminault C. Sleep disordered breathing in children. Indian J Med Res. 2010;131:311–320. [PubMed] [Google Scholar]
- 4.Katyal V, Pamula Y, Martin AJ, Daynes CN, Kennedy JD, Sampson WJ. Craniofacial and upper airway morphology in pediatric sleep-disordered breathing: Systematic review and meta-analysis. Am J Orthod Dentofacial Orthop. 2013;143:20–30.e3. doi: 10.1016/j.ajodo.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714–e755. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 6.Di Francesco RC, Passerotii G, Paulucci B, Miniti A. Mouth breathing in children: different repercussions according to the diagnosis. Rev Bras Otorrinolaringol. 2004;70:665–670. [Google Scholar]
- 7.Chung Leng Muñoz I, Beltri Orta P. Comparison of cephalometric patterns in mouth breathing and nose breathing children. Int J Pediatr Otorhinolaryngol. 2014;78:1167–1172. doi: 10.1016/j.ijporl.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 8.Feres MF, Hermann JS, Cappellette M, Jr, Pignatari SS. Lateral X-ray view of the skull for the diagnosis of adenoid hypertrophy: a systematic review. Int J Pediatr Otorhinolaryngol. 2011;75:1–11. doi: 10.1016/j.ijporl.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Baroni M, Ballanti F, Franchi L, Cozza P. Craniofacial features of subjects with adenoid, tonsillar, or adenotonsillar hypertrophy. Prog Orthod. 2011;12:38–44. doi: 10.1016/j.pio.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Huang YS, Guilleminault C. Pediatric obstructive sleep apnea and the critical role of oral-facial growth: evidences. Front Neurol. 2013;3:184. doi: 10.3389/fneur.2012.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Löfstrand-Tideström B, Hultcrantz E. Development of craniofacial and dental arch morphology in relation to sleep disordered breathing from 4 to 12 years. Effects of adenotonsillar surgery. Int J Pediatr Otorhinolaryngol. 2010;74:137–143. doi: 10.1016/j.ijporl.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 12.Chi L, Comyn FL, Keenan BT, Cater J, Maislin G, Pack AI, et al. Heritability of craniofacial structures in normal subjects and patients with sleep apnea. Sleep. 2014;37:1689–1698. doi: 10.5665/sleep.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang YS, Guilleminault C, Lee LA, Lin CH, Hwang FM. Treatment outcomes of adenotonsillectomy for children with obstructive sleep apnea: a prospective longitudinal study. Sleep. 2014;37:71–76. doi: 10.5665/sleep.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arun T, Isik F, Sayinsu K. Vertical growth changes after adenoidectomy. Angle Orthod. 2003;73:146–150. doi: 10.1043/0003-3219(2003)73<146:VGCAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell RB. Adenotonsillectomy for obstructive sleep apnea in children: outcome evaluated by pre- and postoperative polysomnography. Laryngoscope. 2007;117:1844–1854. doi: 10.1097/MLG.0b013e318123ee56. [DOI] [PubMed] [Google Scholar]
- 16.Venekamp RP, Hearne BJ, Chandrasekharan D, Blackshaw H, Lim J, Schilder AG. Tonsillectomy or adenotonsillectomy versus non-surgical management for obstructive sleep-disordered breathing in children. Cochrane Database Syst Rev. 2015;(10):CD011165. doi: 10.1002/14651858.CD011165.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valera FC, Travitzki LV, Mattar SE, Matsumoto MA, Elias AM, Anselmo-Lima WT. Muscular, functional and orthodontic changes in pre school children with enlarged adenoids and tonsils. Int J Pediatr Otorhinolaryngol. 2003;67:761–770. doi: 10.1016/s0165-5876(03)00095-8. [DOI] [PubMed] [Google Scholar]
- 18.Kim DK, Rhee CS, Yun PY, Kim JW. Adenotonsillar hypertrophy as a risk factor of dentofacial abnormal ity in Korean chi ldren. Eur Arch Otorhinolaryngol. 2015;272:3311–3316. doi: 10.1007/s00405-014-3407-6. [DOI] [PubMed] [Google Scholar]
- 19.Franco LP, Souki BQ, Cheib PL, Abrão M, Pereira TB, Becker HM, et al. Are distinct etiologies of upper airway obstruction in mouth-breathing children associated with different cephalometric patterns? Int J Pediatr Otorhinolaryngol. 2015;79:223–228. doi: 10.1016/j.ijporl.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Zhong Z, Tang Z, Gao X, Zeng XL. A comparison study of upper airway among different skeletal craniofacial patterns in nonsnoring Chinese children. Angle Orthod. 2010;80:267–274. doi: 10.2319/030809-130.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alessandri-Bonetti G, Ippolito DR, Bartolucci ML, D'Antò V, Incerti-Parenti S. Cephalometric predictors of treatment outcome with mandibular advancement devices in adult patients with obstructive sleep apnea: a systematic review. Korean J Orthod. 2015;45:308–321. doi: 10.4041/kjod.2015.45.6.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SJ, Lee S, Lim J, Ahn SJ, Kim TW. Cluster analysis of tooth size in subjects with normal occlusion. Am J Orthod Dentofacial Orthop. 2007;132:796–800. doi: 10.1016/j.ajodo.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 23.Brodsky L, Moore L, Stanievich JF. A comparison of tonsillar size and oropharyngeal dimensions in children with obstructive adenotonsillar hypertrophy. Int J Pediatr Otorhinolaryngol. 1987;13:149–156. doi: 10.1016/0165-5876(87)90091-7. [DOI] [PubMed] [Google Scholar]
- 24.Major MP, Flores-Mir C, Major PW. Assessment of lateral cephalometric diagnosis of adenoid hypertrophy and posterior upper airway obstruction: a systematic review. Am J Orthod Dentofacial Orthop. 2006;130:700–708. doi: 10.1016/j.ajodo.2005.05.050. [DOI] [PubMed] [Google Scholar]
- 25.Cistulli PA. Craniofacial abnormalities in obstructive sleep apnoea: implications for treatment. Respirology. 1996;1:167–174. doi: 10.1111/j.1440-1843.1996.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 26.Flores-Mir C, Korayem M, Heo G, Witmans M, Major MP, Major PW. Craniofacial morphological characteristics in children with obstructive sleep apnea syndrome: a systematic review and meta-analysis. J Am Dent Assoc. 2013;144:269–277. doi: 10.14219/jada.archive.2013.0113. [DOI] [PubMed] [Google Scholar]
- 27.Hultcrantz E, Larson M, Hellquist R, Ahlquist-Rastad J, Svanholm H, Jakobsson OP. The influence of tonsillar obstruction and tonsillectomy on facial growth and dental arch morphology. Int J Pediatr Otorhinolaryngol. 1991;22:125–134. doi: 10.1016/0165-5876(91)90032-7. [DOI] [PubMed] [Google Scholar]
- 28.Peltomäki T. The effect of mode of breathing on craniofacial growth--revisited. Eur J Orthod. 2007;29:426–429. doi: 10.1093/ejo/cjm055. [DOI] [PubMed] [Google Scholar]
- 29.Nelson S, Cakirer B, Lai YY. Longitudinal changes in craniofacial factors among snoring and nonsnoring Bolton-Brush study participants. Am J Orthod Dentofacial Orthop. 2003;123:338–344. doi: 10.1067/mod.2003.85. [DOI] [PubMed] [Google Scholar]
- 30.Sousa JB, Anselmo-Lima WT, Valera FC, Gallego AJ, Matsumoto MA. Cephalometric assessment of the mandibular growth pattern in mouth-breathing children. Int J Pediatr Otorhinolaryngol. 2005;69:311–317. doi: 10.1016/j.ijporl.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Park KH, Bayome M, Park JH, Lee JW, Baek SH, Kook YA. New classification of lingual arch form in normal occlusion using three dimensional virtual models. Korean J Orthod. 2015;45:74–81. doi: 10.4041/kjod.2015.45.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]