Abstract
Background
Moxibustion therapy has been used historically for thousands of years and there are many clinical trials supporting its efficacy and effectiveness for various conditions. Moxa smoke has been a major reason for avoiding moxibustion due to its smell and potential risks to the human body.
Methods
10 units of commercial indirect moxa (CIM) from six manufacturers (A–F) were burnt in a 2.5×2.5×2.5 m chamber without ventilation, and concentrations of carbon oxides (CO and CO2), nitrogen oxides (NOx), and volatile organic compounds (VOCs) from the indoor air samples were measured.
Results
For brands A, B, C, D, E, and F, respectively, relative to baseline values, we observed an increase in CO (from 0.002 to 0.007, 0.006, 0.005, 0.006, 0.005, and 0.006 parts per billion (ppb)), NOx (from 0.009 to 0.051, 0.025, 0.015, 0.050, 0.019, and 0.020 ppb), and total VOCs (TVOC; from 48.06 to 288.83, 227.93, 140.82, 223.22, 260.15, and 161.35 μg/m3), while the concentration of CO2 was not elevated. Each CIM brand demonstrated different VOC emission characteristics, which could be divided into three groups. On average, we estimated that 20 units of CIM or 2.41 g moxa floss would need to be combusted in order to exceed the safe levels set by Korean environmental law. This limit is likely to be greater in the case of a larger room or use of ventilation.
Conclusions
Despite increased CO/NOx/VOC concentrations, overall levels remained within safe limits. These findings may help clinicians to maintain safe moxibustion treatment conditions to help keep both patients and clinicians safe from the pollutants generated by moxa combustion.
Keywords: MOXIBUSTION
Introduction
Two types of smoke are generated when moxa is burned. The first type of smoke, called moxa smoke, has a white colour and rises straight upwards. The second type of smoke, called moxi-tar1 or moxa tar,2 has a light yellow colour, moves downward, and pools on the surface of the skin. According to Korean medical doctors, moxi-tar is believed to have important therapeutic effects3–5 while moxa smoke contains many toxic substances.6 More data are available regarding the composition and efficacy of moxi-tar than moxa smoke. Moxi-tar contains a range of components, including tricosariol, hentriacontane, arachinalkohol, thujone, n-nonacosane, n-hentriacontane, heptatriacontane (C37H76), tannin, and catechols.7 In addition, moxi-tar is believed to have various health benefits, including antioxidative,3 neuroprotective,4 and colitis-relieving5 effects.
However, the available data for moxa smoke are not comprehensive. Wheeler et al8 previously investigated the toxic substances in moxa smoke generated from burning moxa rolls, and Mo et al9 calculated the risk of carcinoma from moxa smoke generated by various moxa rolls. Similarly, Sakagami et al10 and Matsumoto et al11 studied the cancer cell-specific cytotoxicity and anti-inflammatory effects of moxa smoke, respectively. Huang et al12 measured the concentration of PM2.5 (particulate matter with an aerodynamic diameter <2.5 μm) in moxa smoke and examined the relationship between PM2.5 and oxidative stress. However, few studies other than these have focused specifically on moxa smoke and we still have insufficient evidence regarding the basic characteristics of moxibustion,13 the amount of commercial indirect moxa (CIM; the most commonly used form of moxa in South Korea) that is used in the treatment room, and how much ventilation is necessary.
To consider further the safety of moxa smoke, it is important to determine the types and concentrations of potentially harmful substances generated; however, there are no nationally or internationally standardised methods of testing. The aim of the present study was to analyse the toxic substances in moxa smoke based on standard guidelines for indoor air quality (IAQ) and an established process test method.14 In addition, we aimed to estimate the amount of moxa that would need to be burnt in order to exceed safe levels of potentially harmful compounds, to help guide the limitation of CIM combustion in a given room.
Methods
Moxa combustion chamber
A chamber test was conducted, whereby indirect moxa (IM) combustion gas was deposited in a wallpapered space of 2.5×2.5×2.5 m. The size of the chamber was calculated as 17.08 L for 10 CIM from the calculated oxygen consumption (oxygen volume/moxa weight) while combusting dried leaves (40 kg/kg),15 the weight of CIM (0.12 g), oxygen concentration in the air (21%), and density of oxygen gas (1.35 g/L). No interior construction had been performed in the chamber for 3 years, which was free from reactive chemical substances (flammable, acid or base, etc). Furthermore, ventilation and air conditioning were not used during IM combustion. The average temperature and humidity inside the chamber were 26.7±1.9°C and 39.0±5.7%, respectively. To minimise the effects of volatile organic compounds (VOCs), the chamber contained no furniture other than two beds, which were used to determine the deposition height of moxa and VOCs in the clinical environment.
CIM combustion
CIM was performed by combusting wormwood that was rolled in thin paper and bound to a thick paper stake. Six different brands of IM were used for combustion gas analysis and were labelled A to F. The IM samples were stored in desiccators containing silica gel for more than 48 hours. The humidity of the desiccator was maintained at 11±1%. The weights of samples A, B, C, D, E, and F were 0.148±0.035 g, 0.082±0.016 g, 0.128±0.015 g, 0.120±0.023 g, 0.115±0.014 g, and 0.135±0.015 g, respectively.16 To prevent fire, 10 IM units were combusted during each test on a glass dish above the bed. A gas lighter was used for IM ignition and applied for <30 s. After igniting the IM, the chamber door was closed and human access was prohibited for 30 min. The combustion durations of samples A, B, C, D, E, and F were 313.57±38.23 s, 294.14±33.05 s, 291.56±50.06 s, 368.27±39.63 s, 187.97±41.24 s, and 276.72±45.52 s, respectively, and the mean combustion time of all samples was 288.7 s (4 min 48.7 s). IAQ in the chamber at baseline was determined and used as control data (blank) before any CIM was combusted.
CO, CO2, and NOx concentration analyses
Carbon monoxide (CO), carbon dioxide (CO2), and nitrogen oxide (NOx) densities were determined based on the standard guidelines for IAQ and the process test method of the Korean Ministry of Environment.14 Real-time CO and CO2 densities were measured in the field with a non-dispersive infrared sensor (300E, Teledyne Advanced Pollution Instrumentation Inc, USA). The nitric oxide (NO), nitrogen dioxide (NO2), and NOx concentrations were measured using a chemiluminescence method from the electroluminescence that was generated from the reaction of NO with ozone (O3). The measurement system (400E, Teledyne Advanced Pollution Instrumentation Inc, USA) monitored the NO concentration for 30 min after IM ignition.
Capture and analysis of VOCs
VOCs were absorbed at a flow rate of 50–100 mL/min, using an adhesion tube and sampling pump (GilAir3 kit, Gilian, USA), for 30 min after ignition. The adhesion tube and sampling pump were placed on the bed. After changing the VOCs that were absorbed on the adhesion tube to gas phase with a thermal desorber (STD-1000, DANI, Italy), the VOC density was measured with gas chromatography mass spectrometry (GC-MS; GC/MS-2010, Shimadzu, Japan). The GC-MS settings for the VOC concentration measurements are presented in table 1. The total concentration of VOCs (TVOC) was calculated from the dimension of all materials between the VOC range and toluene curve of each sample following the standard equation method.17 CO, CO2, NOx, and VOC concentrations were compared with IAQ standards and guidelines from the Korean Ministry of Environment.14 These guidelines contain standard CO, CO2, NO2, radon (Rn), VOC, formaldehyde (HCHO), PM10 (particulate matter with an aerodynamic diameter <10 μm), O3, asbestos, and total bacteria colony limits. Only CO, CO2, NO2, and VOC concentrations were observed because it was hypothesised that these components would change during combustion. The currently acceptable upper limits for CO, CO2, NO2, and TVOC are 0.05 parts per billion (ppb), 1000 parts per million (ppm), 0.05 ppb, and 400 μg/m3, respectively.
Table 1.
GC-MS settings for measurement of VOC concentrations in moxa smoke
| Tuning | |||
|---|---|---|---|
| GC column | SPB-1 (0.25 mm, 60 m, 1.0 μm) | ||
| Detector (kV) | 0.85 | ||
| Low vacuum | 8.6e+000 Pa | ||
| High vacuum | 1.4e−004 Pa | ||
| m/z | Intensity (area) | Ratio (%) | |
| Perfluoro-tributylamine (PFTBA) | 69 | 613 409 | 100.00 |
| 219 | 318 096 | 51.86 | |
| 502 | 40 274 | 6.57 | |
GC-MS, gas chromatography mass spectrometry; VOC, volatile organic compound.
Estimation of maximum number of units
Finally, given that >10 IM units would likely be used during actual moxibustion treatments in clinical settings, we estimated the number of acceptable IMs for a single moxibustion treatment using each product (A to F) based on the assumption that burning >10 IM units is similar to burning 10 IM units. Given that alternative forms of moxibustion (eg, moxa rolls) are also used at clinics, the CIM unit was converted into a maximum combustible weight of moxa floss to aid extrapolation of the data.
Results
Carbon and nitrogen oxide concentrations
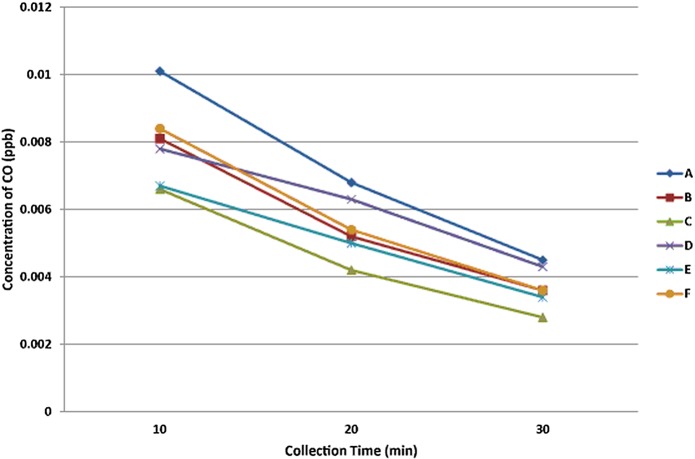
The CO and CO2 concentrations were below the limits of 0.05 ppb and 1000 ppm, respectively. Although CO2 concentrations did not increase, CO concentrations increased relative to the baseline concentrations (table 2, Blank) after CIM combustion. NO2 was the only NOx with a published acceptable limit, and concentrations were within this limit. For all of the tested IMs, the NO2 concentrations were far below the acceptable limits. However, these concentrations significantly increased relative to baseline after combustion. When changes in CO concentrations over time were observed (figure 1), it was noted that they decreased by approximately 50% between 10 and 30 min after the beginning of IM combustion. Based on this finding, the half-life of CO during moxa combustion was estimated to be approximately 20 min.
Table 2.
Carbon and nitrogen oxide concentrations after combustion of six brands of indirect moxa
| CO2 (ppm) | CO (ppb) | NO (ppb) | NO2 (ppb) | NOx (ppb) | |
|---|---|---|---|---|---|
| Blank | 748 | 0.002 | 0.009 | 0.008 | 0.012 |
| A | 688 | 0.007 | 0.051 | 0.026 | 0.077 |
| B | 600 | 0.006 | 0.025 | 0.025 | 0.058 |
| C | 647 | 0.005 | 0.015 | 0.021 | 0.036 |
| D | 636 | 0.006 | 0.050 | 0.031 | 0.081 |
| E | 671 | 0.005 | 0.019 | 0.025 | 0041 |
| F | 653 | 0.006 | 0.020 | 0.031 | 0.051 |
| Limit | 1000 | 0.05 | 0.05 |
Blank refers to indoor air condition before combustion. Limit refers to upper safety threshold according to Korean environmental law. A to F represent six different brands of indirect moxa.
CO2, carbon dioxide; CO, carbon monoxide; NO, nitric oxide; NO2, nitrogen dioxide; NOx, nitrogen oxide; ppb, parts per billion; ppm, parts per million.
Figure 1.
Serial measurements of carbon dioxide (CO) concentration in a sealed chamber over a 30-min period during combustion of six different brands (A–F) of indirect moxa. There were no significant differences in CO concentration between the products. ppb, parts per billion.
VOC concentrations of IM smoke
Concentrations of all measured VOCs increased relative to baseline concentrations after IM combustion. The extent of this increase varied for each VOC and IM brand (table 3). The TVOC concentration was based on the toluene concentration and remained within the acceptable limits for a health facility (400 μg/m3) for all tested products. For products A and E, concentrations of toluene, ethylbenzene, and O-xylene were higher than the other brands. In contrast, products B and D had higher benzene, chlorobenzene, and 1,2-dichlorobenzene concentrations than the other brands. However, no obvious differences were observed for any of the VOCs in products C and F.
Table 3.
Volatile organic compound (VOC) concentrations after combustion of six brands of indirect moxa
| VOCs (μg/m3) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Benzene | Toluene | Chloro-benzene | Ethyl-benzene | m.p-Xylene | Styrene | o-Xylene | 1,3-Dichloro-benzene | 1,4-Dichloro-benzene | 1,2-Dichloro-benzene | TVOCs | |
| Blank | 3.20 | 1.47 | 0.05 | 0.36 | 0.78 | 0.11 | 0.24 | 2.31 | 0.28 | 0.04 | 48.06 |
| A | 33.97 | 31.21 | 0.31 | 16.70 | 39.36 | 3.22 | 10.14 | 3.57 | 0.37 | 0.11 | 288.83 |
| B | 45.17 | 24.61 | 1.83 | 5.18 | 10.50 | 4.32 | 3.10 | 2.80 | 0.00 | 0.37 | 227.93 |
| C | 23.50 | 14.07 | 0.15 | 3.43 | 6.95 | 2.33 | 1.99 | 3.80 | 0.28 | 0.13 | 140.82 |
| D | 41.92 | 25.28 | 2.18 | 5.86 | 12.70 | 4.13 | 3.62 | 3.34 | 0.82 | 0.38 | 223.22 |
| E | 29.10 | 30.23 | 0.25 | 18.73 | 44.65 | 4.27 | 11.73 | 3.45 | 0.74 | 0.00 | 260.15 |
| F | 33.22 | 18.23 | 0.12 | 3.14 | 6.52 | 3.37 | 1.95 | 3.16 | 0.00 | 0.00 | 161.35 |
| Limit | 400 | ||||||||||
Blank refers to indoor air condition before combustion. Limit refers to upper safety threshold according to Korean environmental law. A to F represent six different brands of indirect moxa.
TVOCs, total volatile organic compounds (estimated from toluene).
Estimation of maximum number of units
Based on our assumption that burning >10 IM units is similar to burning 10 IM units, we extrapolated the above data and estimated that 14.6, 19.6, 32.3, 18.3, 16.6, and 18.3 units would be required to exceed the limits of at least one component (based on the standard limits and IAQ process test) for products A, B, C, D, E, and F, respectively (table 4). On average, it was estimated that burning more than approximately 20 IM units of any brand could result in chemical levels exceeding the acceptable limits. After conversion of these data into a maximum combustible weight of moxa floss, the respective values were 2.16, 1.61, 4.13, 2.20, 1.90, and 2.47 g for brands A, B, C, D, E, and F. On average, it was estimated that burning more than approximately 2.4 g of moxa floss could result in chemical levels exceeding the acceptable limits.
Table 4.
Estimated maximum permissible amounts of indirect moxa per treatment and limits per item
| CO2 | CO | NO2 | TVOCs | Limit | Mean weight | Limit (g) | |
|---|---|---|---|---|---|---|---|
| A | – | 96 | 23.3 | 14.6 | 14.6 | 0.148 | 2.16 |
| B | – | 120 | 24.7 | 19.6 | 19.6 | 0.082 | 1.61 |
| C | – | 160 | 32.3 | 37.9 | 32.3 | 0.128 | 4.13 |
| D | – | 120 | 18.3 | 20.1 | 18.3 | 0.12 | 2.20 |
| E | – | 160 | 24.7 | 16.6 | 16.6 | 0.115 | 1.91 |
| F | – | 120 | 18.3 | 31.1 | 18.3 | 0.135 | 2.47 |
| Average | 19.95 | 2.41 |
A to F represent six different brands of indirect moxa.
CO2, carbon dioxide; CO, carbon monoxide; NO2, nitrogen dioxide; TVOCs, total volatile organic compounds (estimated from toluene concentrations).
Discussion
Although it has been more than a decade since concerns were first raised by oriental medicine professionals regarding the safety of human exposure to moxa smoke, relatively little information exists regarding these substances, their concentrations in IM smoke, and their impact on the human body. Currently we do not have any standard test method to detect these harmful substances in moxa smoke; however, recent research has focused on their identification and quantification. Wheeler et al8 investigated chemical compounds and their concentrations in moxa smoke. They compared the effects of short- and long-term exposure against the IAQ and exposure guidelines of the UK Department of Health. As a result of the experiments, which followed ISO (International Organization for Standardization) standard test methods for cigarette smoke, they came to the conclusion that moxa smoke is harmless.
However, Mo et al9 investigated the characteristics of indoor air pollutants produced from both smoking and smokeless moxa roll combustion and reached a different conclusion. They found that the chemical characteristics of smoking moxa were similar to those of cigarettes, while the chemical characteristics of smokeless moxa roll were similar to those of an incense stick. Hsu et al18 noted benzene and formaldehyde to be major carcinogenic substances, which is an important consideration for calculations using moxa smoke data.
It is important to consider the safety and health risks of moxa smoke, especially given that there is evidence of efficacy of moxibustion therapy for various diseases13 Most prior safety studies have analysed moxa smoke generated by moxa rolls, which are much less popular than CIM in Korea. To date, it has been assumed that there are no differences in additives (such as glue, herbs, etc) between the moxa roll and CIM. Wheeler et al8 tested moxa rolls based on the ISO smoking regimen standards for cigars to minimise the influence of other chemicals. However, cigar smoke, for which testing must be performed separately from mainstream and sidestream smoke, uses inhalation of air to combust the test product. This process is different from that used for moxa roll combustion in clinics. These authors failed to collect the smoke generated from combustion due to air inhalation in this test. Furthermore, moxa rolls are rarely used in Korea. Instead, Korean oriental clinicians frequently use IM, which contains glue for woodworking. Therefore, the study by Wheeler et al is unlikely to provide sufficient evidence regarding the safety of moxa smoke released by IM for clinical use.
Consequently, we determined the types and concentrations of the chemical compounds produced during the combustion of CIM products that are generally used at Korean clinics. In addition, we evaluated the toxicity of moxa smoke based on oxide concentrations to establish a safe dosage. A chamber test was performed to test moxa smoke in a controlled environment. We deliberately chose a room with wallpaper to reflect absorption of chemicals in a real clinical setting. The ventilation system was stopped and ventilation openings were sealed tightly to control airflow, especially the airflow between the inside and outside of the room, to measure the concentrations of chemical compounds emitted during CIM combustion. Measurement of the baseline air quality in a room without ventilation was deliberate in order to help calculate a conservative maximum limit of usage.
When carbon oxide concentrations in the moxa smoke were examined, both CO and CO2 were within the limits defined by the standards and guidelines for IAQ based on the process test method. CO2 concentrations decreased relative to baseline after combustion. The lower baseline CO2 concentrations were hypothesised to have resulted from the respiration of individuals who entered the chamber to set up the test equipment. These results suggest that the CO2 concentrations generated by moxa combustion are harmless for humans because they are lower than the concentrations produced by human respiration.
By contrast, CO concentrations significantly increased relative to baseline following moxa combustion, but remained within the acceptable limit. This result suggests that a substantial amount of incomplete combustion occurred during moxa combustion. Based on the concentration change of CO over time, its half-life was likely to be <20 min. Therefore, adequate ventilation is recommended when performing IM treatments over intervals of 30 min to minimise the potentially harmful effects of an increasing CO concentration. Thus, we would recommend that moxibustion be performed in spaces with sufficient ventilation.
In addition, the NO2 concentration increased after IM combustion; however, it remained below the standard limit based on the IAQ guideline process test method. Although the harmful effects of NO2 are minimal, excessive IM in an enclosed area could potentially produce harmful effects including airway irritation, fatigue, pulmonary oedema, and skin corrosion.19
VOCs are a group of organic compounds with boiling points between 50°C and 250°C. Thousands of different VOCs are present in air, water and earth.20 VOCs can be generated from human activity and within the natural environment.21 Therefore, although VOCs facilitate environmental destruction (such as ozone depletion20) and can negatively affect the human body (by potentially causing cancer and lung disease),22 simple exposure to VOCs is not considered dangerous. Rather, the type and amount of VOC exposure is more likely to be important.23 The potentially harmful effects of the individual VOCs are as follows: benzene may harm blood, the central nervous system (CNS), skin, bone marrow, eyes, and the respiratory system; chlorobenzene may cause respiratory system, eye, skin, CNS, and liver diseases; ethyl benzene may cause eye, upper respiratory system, skin, and CNS disorders; styrene may harm the CNS, respiratory system, eyes, and skin; toluene may negatively impact the CNS, liver, kidneys, and skin; and xylene may cause CNS, eye, gastrointestinal tract, blood, liver, kidney, and skin problems.24
When TVOC concentrations were calculated from the toluene concentrations in our study,14 these increased three- to six-fold after IM combustion of all the tested products relative to baseline. However, overall TVOC concentrations remained within the acceptable limit in each case. Regarding products A and E, the toluene, ethylbenzene, and O-xylene concentrations were greater than for the other products. Products B and D had greater benzene, chlorobenzene, and 1,2-dichlorobenzene concentrations than the other products. No concentration differences were observed for products C and F. The observed differences between the products could have resulted from various factors, including their origin, the burning mechanism, the use of mugwort as a raw material, potential contamination with agricultural chemicals or heavy metals, and the use of chemicals (glue, additives, etc) during the CIM manufacturing process. Although the chemicals in the closed door that were used during the CIM manufacturing process were hypothesised to be the largest cause of these differences, these results will be verified in the future by analysing the CIM extract.
Overall, our results suggest that moxa smoke from IM combustion is not a health risk because all analysed oxides were within the acceptable standard limits and guidelines for IAQ according to the process test method. Although >10 IM units would be used during actual moxibustion treatments in clinical settings, we estimated the number of acceptable IMs for a single moxibustion treatment (assuming that burning >10 IM units is similar to burning 10 IM units) to be between 14.6 and 32.3 units, depending on the brand. This result indicated that burning approximately 20 IM units of any brand may result in chemical levels that exceed the acceptable limits. Because combustion conditions may change as the amount of burning material changes, this simple calculation may not reflect actual conditions and result in errors. Nonetheless, our estimation may help ensure safer moxibustion treatments because it gives an indication of the limitations required on the number of IM units per treatment and considers ventilation needs based on the amount of moxa used.
A ventilation system is likely to be a very important part of clinic design for practitioners planning to provide moxibustion therapy for their patients. Given the relative lack of evidence or current guidelines for moxibustion, we would suggest that treatment takes place in an adequately sized room. Our data may or may not be extrapolatable to different clinical environments. For example, it is possible that integration of a ventilation system may allow clinicians to safely use more moxa products in a given session.
Conclusions
Following previous studies, further research into and discussion around the safety of moxa smoke is needed, despite the evidence for efficacy of moxibustion therapy. This study has provided novel data that may help clinicians and patients to use moxibustion safely. We estimated an upper limit of CIM usage in clinics based on concentrations of VOCs, carbon oxides and NOs in moxa smoke, which may contribute to ensuring a safer environment in clinics using moxibustion.
Footnotes
Contributors: OSK wrote the main text. SJC and K-HC prepared tables 2 and 4. SHY, S-YK, J-hK and S-HA prepared the manuscript. YR corrected the data analysis and manuscript text. All authors approved the final version accepted for publication.
Funding: This study was funded by Korea Institute of Oriental Medicine (KIOM) (grant no. K16070) and the Korea Evaluation Institute of Industrial Technology (grant no. D15090).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ahn SH, Koo ST, Do JW, et al. The effects of moxi tar on inos synthase in raw 264.7 cell. Korean J Acupunct 2000;17:33–46. [Google Scholar]
- 2.Tohya K, Urabe S, Igarashi J, et al. Appearance of peculiar vessels with immunohistological features of high endothelial venules in the dermis of moxibustion-stimulated rat skin. Am J Chin Med 2000;28:425–33. 10.1142/S0192415X00000507 [DOI] [PubMed] [Google Scholar]
- 3.Seo SR, Yang SB, Kim JH, et al. Antioxidant effects of the moxi with ginger tar produced by moxibustion with ginger combustion. Korean J Acupunct 2011;28:29–40. [Google Scholar]
- 4.Ahn YN, An SH, Yang KZ, et al. The effects of moxi tar on nitric oxide in c6-glioma cell. Korean J Acupunct 2000;17:25–44. [Google Scholar]
- 5.Song MY, Kwon OS, Jang JH, et al. Effects of moxi-tar herbal acupuncture of LI11 on inflammatory bowel disease induced by tnbs in mice. Korean J Acupunct 2007;24:131–49. [Google Scholar]
- 6.Kim EJ, Kim YJ, Hwang JH, et al. Study of density of smoke and harmful gases of adhesive indirect moxibustion. J Korean Oriental Med 2012;33:42–51. [Google Scholar]
- 7.Cheon YS, Kim CH, Kang SK, et al. Reviews of research on the standardization of aeyung. J Korean Acupunct Moxibust Soc 1997;14:55–69. [Google Scholar]
- 8.Wheeler J, Coppock B, Chen C. Does the burning of moxa (Artemisia vulgaris) in traditional Chinese medicine constitute a health hazard? Acupunct Med 2009;27:16–20. 10.1136/aim.2009.000422 [DOI] [PubMed] [Google Scholar]
- 9.Mo F, Chi C, Guo M, et al. Characteristics of selected indoor air pollutants from moxibustion. J Hazard Mater 2014;270:53–60. 10.1016/j.jhazmat.2014.01.042 [DOI] [PubMed] [Google Scholar]
- 10.Sakagami H, Matsumoto H, Satoh K, et al. Cytotoxicity and radical modulating activity of moxa smoke. In Vivo 2005;19:391–7. [PubMed] [Google Scholar]
- 11.Matsumoto H, Shimada J, Nagasaka H, et al. Inhibition by moxa smoke of no production and inos expression in mouse macrophage-like cells raw 264.7. In Vivo 2005;19:471–4. [PubMed] [Google Scholar]
- 12.Huang J, Lim MY, Zhao B, et al. PM2.5 and ash residue from combustion of moxa floss. Acupunct Med 2016;34:101–6. 10.1136/acupmed-2015-010914 [DOI] [PubMed] [Google Scholar]
- 13.Lim MY, Huang J, Zhao B. Standardisation of moxibustion: challenge and future development. Acupunt Med 2015;33:142–7. 10.1136/acupmed-2014-010688 [DOI] [PubMed] [Google Scholar]
- 14.Korean Ministry of Environment. Indoor air quality control in public use facilities. Korean Goverment, 2010. [Google Scholar]
- 15.Park YJ, Lee SY, Sin YJ, et al. The combustion characteristics of tree branches, barks, living leaves and dead leaves in Pinus densiflora and Quercus dentata. J Korean Inst Fire Sci Eng 2008;22:84–9. [Google Scholar]
- 16.Kwon OS, Lee SH, Cho SJ, et al. Research about spec and uniformity of commercial indirect moxibustion. Korean J Acupunct 2011;28:53–62. [Google Scholar]
- 17.International Standard Organization. ISO 16000-6, Indoor air—Part 6: Determination of volatile organic compounds in indoor and test chamber air by active sampling on Tenax TA sorbent, thermal desorption and gas chromatography using MS or MS-FID. ISO, 2011. [Google Scholar]
- 18.Hsu YC, Chao HR, Shih SI. Human exposure to airborne aldehydes in Chinese medicine clinics during moxibustion therapy and its impact on risks to health. J Environ Sci Health A Tox Hazard Subst Environ Eng 2015;50:260–71. 10.1080/10934529.2015.981112 [DOI] [PubMed] [Google Scholar]
- 19.Gardner DE, Miller FJ, Blommer EJ, et al. Influence of exposure mode on the toxicity of NO2. Environ Health Perspect 1979;30:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atkinson R. Atmospheric chemistry of VOCs and NOx. Atmospheric Environ 2000;34:2063–101. 10.1016/S1352-2310(99)00460-4 [DOI] [Google Scholar]
- 21.Goldstein AH, Galbally IE. Known and unknown organic constituents in the Earth's atmosphere. Environ Sci Technol 2007;41:1514–21. 10.1021/es072476p [DOI] [PubMed] [Google Scholar]
- 22.Pirkle JL, Sampson EJ, Needham LL, et al. Using biological monitoring to assess human exposure to priority toxicants. Environ Health Perspect 1995;103(Suppl 3):45 10.1289/ehp.95103s345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashley DL, Bonin MA, Cardinali FL, et al. Measurement of volatile organic compounds in human blood. Environ Health Perspect 1996;104:871 10.1289/ehp.96104s5871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamrin MA. Toxicology-A primer on toxicology principles and applications. CRC Press, 1988. [Google Scholar]