Abstract
Objective
N-methyl-D-aspartate receptor (NMDAR) activation and downstream transduction pathways are crucial for pain signalling. Fibromyalgia (FM) is a common pain syndrome of unclear aetiology that is often drug-refractory but may benefit from treatment with electroacupuncture (EA). We examined the contributions of NMDAR signalling to FM pain and EA responses in a mouse model.
Methods
A model of FM was established by acid saline injection in 32 mice and subgroups (n=8 each) were treated with EA (2 Hz, 15 min daily for 4 days) or minimal acupuncture (MA). Expression of NMDAR subunits, calmodulin-dependent protein kinase II (CaMKII), cyclic AMP response element binding protein (pCREB) and their corresponding phospho-activated forms were measured by Western blotting and immunohistochemistry.
Results
Acid saline injection induced significant mechanical hyperalgesia (paw withdrawal threshold 2.18±0.27 g, p<0.05 vs controls), which was reversed by EA (4.23±0.33 g, p<0.05 vs FM group) but not by MA (2.37±0.14 g, p<0.05 vs EA group). Expression levels of phosphorylated N-methyl-D-aspartate receptor (pNR)1 and pNR2B were significantly increased in the dorsal root ganglion of FM model mice (132.21±14.4% and 116.69±3.22% of control values), whereas NR1 and NR2B levels were unchanged (97.31±3.79% and 97.07%±2.27%, respectively). Expression levels of pCaMKIIα and pCREB were also higher in the FM group, and these changes were reversed by EA but not by MA. Similar changes in expression were observed in spinal cord neurons.
Conclusions
Reduced NMDAR−CaMKIIα−pCREB signalling is implicated in the positive effects of EA in FM. NMDAR signalling components may represent promising therapeutic targets for FM treatment.
Keywords: ACUPUNCTURE, ANAESTHETICS
Introduction
Fibromyalgia (FM) is a complex syndrome characterised by chronic widespread mechanical pain. It is also relatively common, with an overall prevalence of approximately 2–8%.1 2 Moreover, this chronic pain is associated with fatigue, depression, memory problems, anxiety, sleep disturbances, and headaches. Despite its high prevalence, the underlying disease mechanisms are unclear. Recent studies have indicated that FM results from imbalances in neurotransmitters, such as serotonin, dopamine and norepinephrine, in the brain.3 4 Lower serotonin levels have been reported in the cerebrospinal fluid (CSF) of FM patients, indicating that central inhibition of pain is reduced.5 In addition, hypothalamic−pituitary−adrenal (HPA) axis hypofunction and chronic stress have also been implicated in FM.6
FM is often difficult to treat because of a generally poor understanding of basic disease mechanisms. Many therapeutic approaches have been adopted, such as drug therapy, exercise and dietary changes, but their efficacy remains unclear. Pregabalin is a presynaptic voltage-gated calcium channel blocker that is used to reduce FM-related pain and sleep disturbances.7 8 Duloxetine also decreases FM-related pain symptoms but may have potentially serious side effects.9 Milnacipran is a serotonin-norepinephrine reuptake inhibitor that is also used to treat FM but frequently induces nausea.10 Although research has improved FM pain treatment, our understanding of FM pathogenesis remains insufficient for the optimal development of effective yet safe therapies.11 12
Injections of acidic saline into the muscles of mice can reliably induce pain symptoms similar to those of FM patients with minimal histopathological changes. This FM mouse model exhibits sustained and widespread mechanical hyperalgesia without muscle damage, fatigue, sympathetic predominance or altered central sensitisation.13–15 Moreover, these FM animal models are sensitive to antidepressants and anticonvulsants but not to non-steroidal anti-inflammatory drugs.12 This FM-like syndrome may result from activation of acid sensing ion channels (ASICs) or vanilloid type 1 receptors (TRPV1s). ASIC3 and TRPV1 are voltage-insensitive cationic channels gated by extracellular protons.16 Decreased peripheral tissue pH has been observed in FM, and protons have been shown to activate the terminals of nociceptors. Recordings of peripheral dorsal root ganglion (DRG) neurons have shown that acid induces several inward currents differing in kinetics, ion selectivity and pH dependence. Though much is known about how these ion channels give rise to peripheral and central sensitisation leading to FM pain, little is known about the mechanisms involved in FM pain-related signalling.
Glutamate is the major excitatory neurotransmitter in the mammalian central nervous system. Glutamate released from presynaptic terminals binds to four types of receptors: AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptors; NDMA (N-methyl-D-aspartate) receptors (NMDARs); kainate (KA) receptors; and metabotropic receptors (mGluRs),17 18 of which the NMDAR is most closely linked to peripheral pain signalling and central sensitisation. NMDARs are heteromeric protein complexes that are comprised of four subunits, which together form a Ca2+-permeable ion channel. These receptors can be divided into subtypes based on their subunits, of which there are three main families: NR1, NR2, and NR3. There are eight different NR1 subunits, four NR2 subunits (NR2A-2D), and two NR3 subunits (NR3A and NR3B).19 Subunits NR1, NR2A, NR2B, and NR2D have been detected in the dorsal horn of the spinal cord (SC),20 21 and are considered to be involved in peripheral pain signalling. Increased presynaptic glutamate release or enhanced postsynaptic receptor activation can induce long-lasting pain signalling and this pain is often attributed to long-term potentiation (LTP) of glutamatergic synapses or central sensitisation. In contrast, induction of long-term depression (LTD) in the SC dorsal horn can reduce pain. Importantly, these long-term synaptic alterations (LTP and LTD) are often dependent on NMDAR signalling.
Acupuncture has been used in Asia for over 3000 years to treat pain, and the analgesic efficacy of acupuncture is recognised worldwide.22–26 Many studies have shown that the anti-nociceptive effect of acupuncture may be related to changes in expression of various ionotropic receptor channels or voltage-gated channels including NMDARs, acid-sensing (protein gated) ion channel (ASIC)-3, transient receptor potential cation channel subfamily V (TRPV)-1, TRPV4, and voltage-gated sodium channels.22–26 Hurt and Zylka showed that injection of an adenosine receptor type 1 (A1R) agonist at BL40 had a short-term anti-nociceptive effect. Interestingly, peripheral injection of prostatic acid phosphatase induced a longer lasting A1R-dependent analgesic effect than agonist injection.27 Yang et al28 reported that acupuncture therapy for FM is superior to pharmacological treatment and that acupuncture combined with drugs and exercise significantly increased pain thresholds. Additionally, Stival et al29 found that acupuncture promoted immediate pain reduction in FM patients.
Accordingly, we hypothesised that acupuncture would effectively treat FM-related mechanical hyperalgesia. We have previously demonstrated that electroacupuncture (EA) can reduce mechanical hyperalgesia in the FM mouse model through the TRPV1 pathway.26 The aim of the current study was to examine whether EA can mitigate mechanical hyperalgesia in this FM model by reducing NMDAR signalling via the phosphorylated (p)NR1/pNR2B-pCaMKIIα (calmodulin-dependent protein kinase IIα)-pCREB (cyclic AMP (cAMP) response element binding protein) pathway in both peripheral and central neurons.
Methods
Animals
Experiments were conducted on C57/B6 mice (age 8–12 weeks) purchased from BioLASCO Co, Ltd (Taipei, Taiwan). Mice were randomly subdivided into four groups (n=8 each): (1) Control (Con); (2) FM; (3) FM+EA; and (4) FM+MA (minimal acupuncture). Assuming an SD in pain level of 2.5 units, it was estimated that eight animals per group would be required to detect an effect size of 0.65 at 80% power with an α of 0.05. After arrival, mice were housed under a 12/12 hour light/dark cycle with water and food provided ad libitum. All procedures were approved by the Institute of Animal Care and Use Committee of China Medical University (permit no. 101-116-N) and conducted in accordance with the National Research Council's ‘Guide for the Care and Use of Laboratory Animals’ and the ethical guidelines of the International Association for the Study of Pain. All possible measures were taken to reduce the number of animals used and to minimise their suffering.
FM induction and behavioural assessment of mechanical hyperalgesia
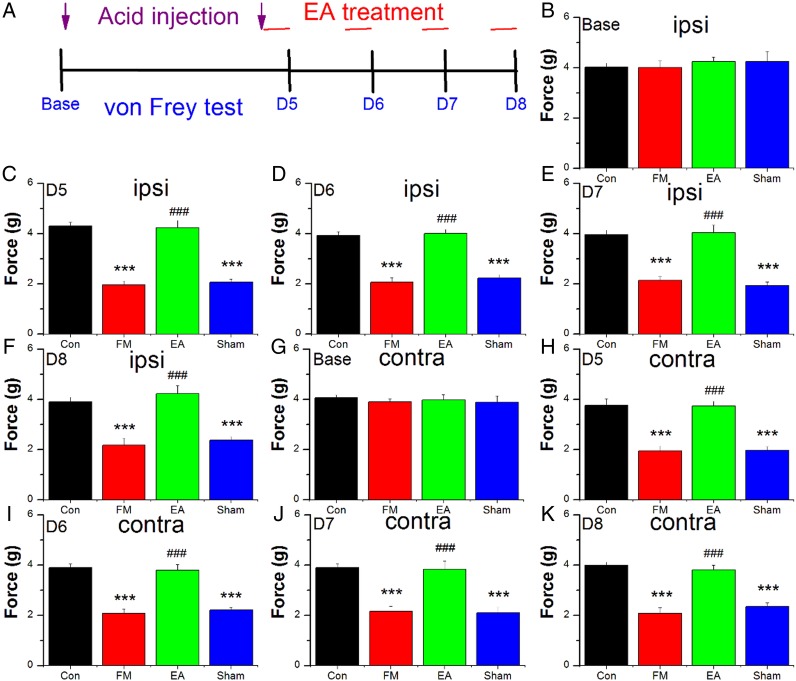
On day 1, all mice except controls received a 20 μL injection of acid saline (pH 4.0) into the right gastrocnemius muscle (GM) under isoflurane (1%) anaesthesia (figure 1A). A second acid saline injection was performed on day 5 to establish the FM mouse model. Mechanical sensitivity was tested on days 5–8. All mechanical pain measurements were performed at room temperature (approximately 25°C) and the stimuli were applied only when the animals were calm and not sleeping or grooming. Mechanical sensitivity was measured as the threshold force required (g) for three paw withdrawal responses to electrical stimulation of the hind paw using von Frey filaments (North Coast Medical, Gilroy, California, USA). All measurements were conducted at approximately the same time of day. For mice in the FM+EA and FM+MA groups, the test was conducted 30 min after EA or MA, respectively. Mice were first placed on a metal mesh and allowed to adapt to the new environment for at least 30 min. Mechanical hyperalgesia of the hind paw was measured at baseline and daily from days 5 to 8 after the first acid saline injection.
Figure 1.
Withdrawal thresholds of the ipsilateral (B–F) and contralateral (G–K) paw of 32 mice receiving control saline injection (Con group, n=8) or acid injection to model fibromyalgia (FM) followed by no treatment (FM group, n=8) or 4 days of electroacupuncture (FM+EA group, n=8) or minimal acupuncture (FM+MA group, n=8) on days 5–8 as detailed in (A). ***p<0.001 vs baseline. ###p<0.001 vs FM group.
Acupuncture treatment
EA was applied using stainless steel needles (0.5 inch, 32 G, Yu Kuang, Taiwan), which were inserted into the muscle layer at ST36 bilaterally to a depth of 2–3 mm. ST36 is one of the most commonly used acupuncture points in Traditional Chinese Medicine and its analgesic effects are well established.26 28 29 EA was administered at roughly the same time of day (10:00−12:00) immediately after the second injection of acid saline (figure 1A) from days 5 to 8. A stimulator (Trio 300, Ito, Japan) delivered 100 μs square pulses of 1 mA for 15 min at a frequency of 2 Hz. The MA group received acupuncture at the ST36 acupuncture point but without electrical stimulation and without elicitation of a de qi response.
Necropsy and tissue sampling
At the end of the experiment, animals were euthanased with 2% isoflurane and intracardially perfused with saline followed by 4% paraformaldehyde. L3−L5 DRG neurons were immediately dissected and either snap-frozen in liquid nitrogen and stored at −80°C (pending protein extraction and Western blotting) or post-fixed with 4% paraformaldehyde (pending immunohistochemistry). Post-fixed tissues were placed in 30% sucrose overnight for cryoprotection. The DRGs were then embedded in optimal cutting temperature compound and rapidly frozen at −20°C.
Immunohistochemistry
Frozen sections of L3−L5 DRG neurons (12 μm thickness) were cut on a cryostat. Samples were next incubated with blocking solution containing 3% bovine serum albumin (BSA), 0.1% Triton X-100, and 0.02% sodium azide in phosphate-buffered saline for 120 min at room temperature. After blocking, the DRGs were incubated overnight at 4°C with primary antibodies (Cell Signaling, Danvers, Massachusetts, USA) against the following: pNR1, pNR2B, pCaMKIIα, and pCREB (all 1:1000), which were prepared in blocking solution. The secondary antibody was fluorescence-conjugated goat anti-rabbit 488 (Molecular Probes, Carlsbad, California, USA). Slides were finally mounted and coverslipped before being examined under a fluorescent microscope (Olympus, BX-51, Japan) with a 40× high numerical aperture (NA=1.4) objective for imaging the distribution of proteins in DRG neurons. Images were analysed using the National Institutes of Health (NIH) Image J software (Bethesda, Maryland, USA).
Western blot analysis
Total protein was extracted from snap-frozen DRG neurons by homogenisation in lysis buffer containing 50 mM Tris-HCl pH 7.4, 250 mM NaCl, 1% NP-40, 5 mM EDTA, 50 mM NaF, 1 mM Na3VO4, 0.02% NaN3, and 1× protease inhibitor cocktail (AMRESCO, Cleveland, Ohio, USA). The extracted proteins (30 μg per sample as quantified by a bicinchoninic acid (BCA) protein assay) were subjected to 8% sodium dodecyl sulfate-Tris glycine gel electrophoresis and transferred onto a polyvinylidene fluoride membrane. The membrane was blocked with 5% non-fat milk in Tris-buffered saline (TBS)-T buffer (10 mM Tris pH 7.5, 100 mM NaCl, 0.1% Tween 20), incubated with anti-pNR1, NR1, pNR2B, NR2B, pCaMKIIα, calmodulin-dependent protein kinase IIδ (CaMKIIδ), CaMKIIγ, pCREB, and CREB (all 1:1000, cell signalling) in TBS-T with 1% BSA and incubated for 1 hour at room temperature. Peroxidase-conjugated anti-rabbit antibody (1:5000) was used as a secondary antibody. The bands were visualised using an enhanced chemiluminescent substrate kit (Pierce, Thermo Fisher Scientific, Waltham, Massachusetts, USA) and an LAS-3000 Fujifilm camera (Fuji Photo Film Co Ltd). Where applicable, the image intensities of specific bands were quantified with NIH Image J software.
Statistical analysis
Data are presented as the mean±SEM. Control, FM, FM+EA, and FM+MA groups were compared using one-way analysis of variance (ANOVA) followed by post hoc Tukey's test. A value of p<0.05 was considered statistically significant.
Results
The withdrawal thresholds of the ipsilateral and contralateral paw, a marker of mechanical hyperalgesia, are illustrated in figure 1B–K. In control mice, dual injection of saline (pH 7.0) did not induce mechanical hyperalgesia of the ipsilateral hind paw from days 5 to 8 after the first injection (p>0.05 vs baseline). By contrast, mechanical hyperalgesia was observed from days 5 to 8 in mice receiving dual injection of acid saline (p<0.05, FM vs control groups; figure 1C–F). Low frequency EA administered immediately following the second acid saline injection reduced mechanical hyperalgesia on days 5 to 8 (p<0.05, FM+EA vs FM groups), while such analgesic effects were not observed in FM+MA mice (p>0.05, FM+MA vs FM groups), suggesting a specific effect of electrical stimulation on induced hyperalgesia rather than a general reduction in mechanical pain threshold. Similar results were also observed in the contralateral hind paw, suggesting additional central effects of the FM paradigm and EA (figure 1G–K).
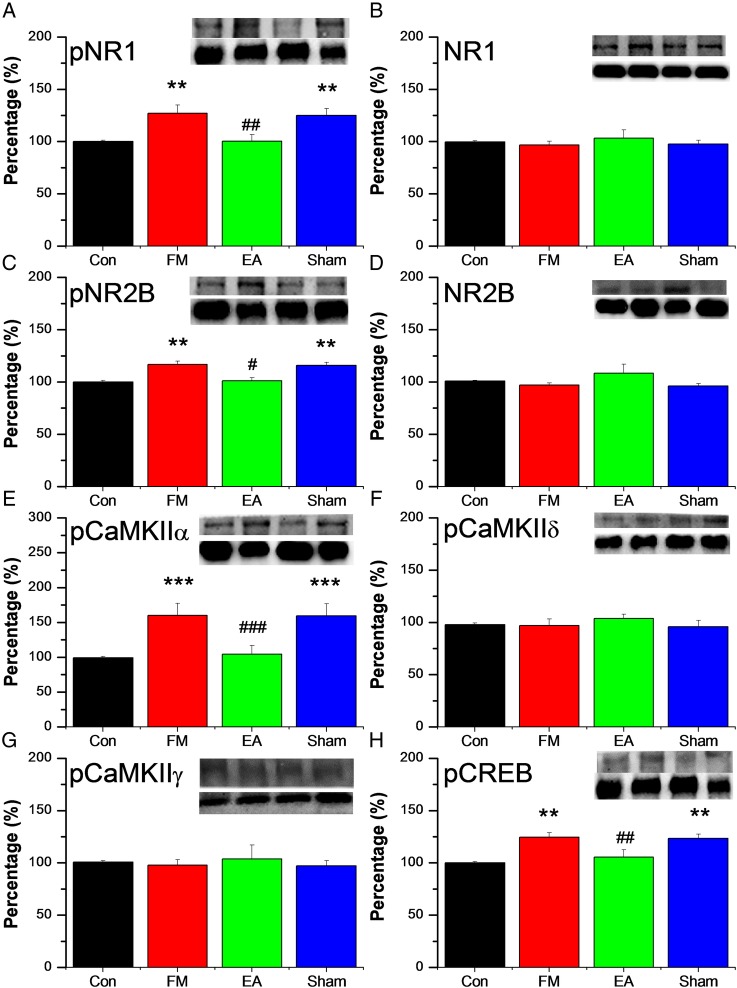
Figure 2A–D illustrates phosphorylated and unphosphorylated peripheral NMDA receptor protein expression, measured by Western blotting. Mean pNR1-immunoreactive band density was significantly higher in the FM group on day 8 (132.21±14.4% of control values; figure 2A), while mean band density in the FM+EA group was similar to controls (95.31±8.84%), suggesting reversal of the pNR1 upregulation induced by FM modelling. No such reversal of pNR1 upregulation was observed in the FM+MA group (125.19±6.52%). By contrast, there were no differences in NR1 immunoreactivity between groups (figure 2B). Similar to pNR1, expression of pNR2B was also increased after FM modelling (116.69±3.22% of control values; figure 2C) and reversed by EA (100.61±2.96%) but not by MA (115.69±3.22%). Expression of NR2B was similar in all groups (p>0.05, figure 2D).
Figure 2.
Protein expression of phosphorylated N-methyl-D-aspartate receptor (pNR)-1 (A), NR1 (B) pNR2B (C), NR2B (D), phosphorylated calmodulin-dependent protein kinase II (pCaMKII)α (E), pCaMKIIδ (F), pCaMKIIγ (G), and phosphorylated cyclic AMP response element binding protein pCREB (H), measured by Western blotting, in the dorsal root ganglion of 32 mice receiving control saline injection (Con group, n=8) or acid injection to model fibromyalgia (FM) followed by no treatment (FM group, n=8) or 4 days of electroacupuncture (FM+EA group, n=8) or minimal acupuncture (FM+MA group, n=8). All results are expressed relative to the Con group. ** p<0.01 *** p<0.001 vs Con group. # p<0.05 ## p<0.01 ### p<0.001 vs FM group.
Figure 2E–G shows expression of several CaMKII subtypes in the DRG, which reflect potential downstream signalling mechanisms activated by enhanced NMDA phosphorylation. The expression of pCaMKIIα was higher in the DRG after FM modelling (160.1±17.6% of control values; figure 2E) and reversed by EA (104.67±11.91%) but not by MA (159.11±17.59%). In contrast to pCaMKIIα, DRG expression levels of CaMKIIδ and CaMKIIγ were similar in all treatment groups (figure 2F, G). Figure 2H shows expression of pCREB in the DRG, as a marker of nuclear transcription. Expression of pCREB was higher after FM modelling (123.04±3.98% of control values) and similarly reversed by EA (104.34±7.27%) but not by MA (123.23±4.36%).
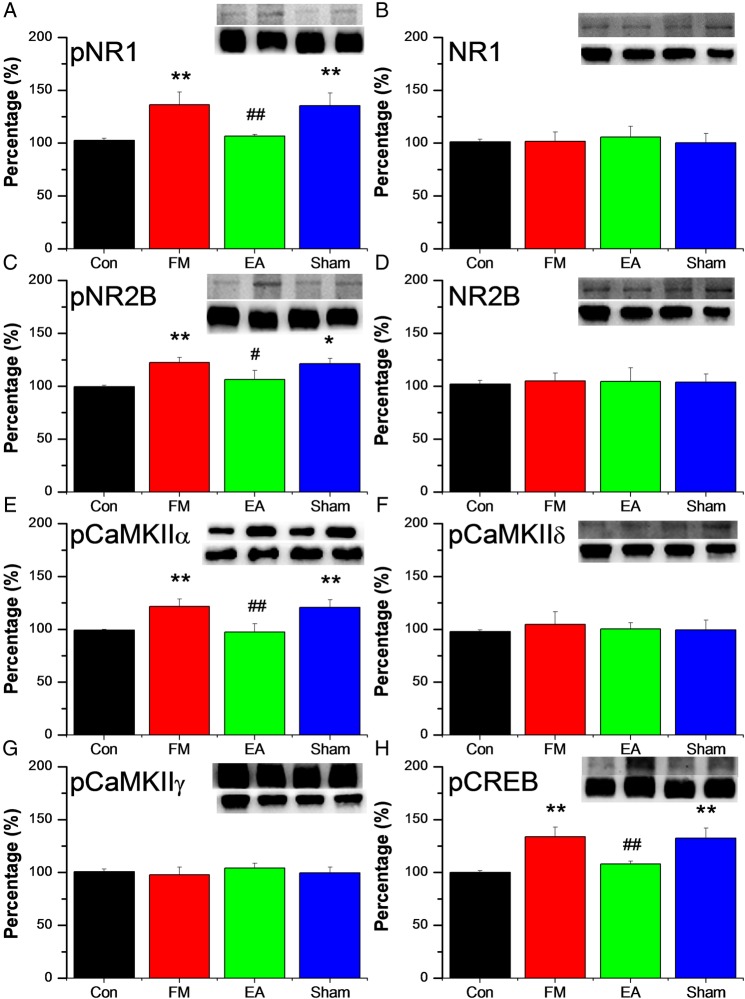
Figure 3A–D shows the results from SC neurons immunostained with NMDA subtype antibodies. Expression of pNR1 was higher in the SC of FM mice (134.03±10.17% of control values; figure 3A), and this over-activation was reduced by EA (113.41±5.26%) but not by MA (135.3±11.99%). However, total SC NR1 expression was similar in all groups (figure 3B). Similar to pNR1, pNR2B was increased in the SC of FM group mice (122.46±3.47% of control values; figure 3C) and this overexpression was reversed by EA (106.4±8.76%) but not by MA (121.34±4.72%). By contrast, total expression of NR2B was similar in all groups (figure 3D). Figure 3E–F illustrates levels of expression of pCaMKII subtypes and pCREB in the SC. Similar to the DRG, pCaMKIIα levels were increased in the SC from FM mice on day 8 (126.86±5.81% of control values, figure 3E) and also reversed by EA (92.91±7.56%) but not by MA (120.71±7.21%). Spinal levels of CaMKIIδ and CaMKIIγ remained unchanged in all groups of mice on day 8 (figure 3F, G). Expression of pCREB was increased in FM mice (133.55±9.53% of control values, figure 3H) and this effect was reduced by EA (107.78±2.97%) but not by MA (132.55±9.53%).
Figure 3.
Protein expression of phosphorylated N-methyl-D-aspartate receptor (pNR)-1 (A), NR1 (B), pNR2B (C), NR2B (D), phosphorylated calmodulin-dependent protein kinase II (pCaMKII)α (E), pCaMKIIδ (F), pCaMKIIγ (G), and phosphorylated cyclic AMP response element binding protein (pCREB) (H), measured by Western blotting, in the spinal cord of 32 mice receiving control saline injection (Con group, n=8) or acid injection to model fibromyalgia (FM) followed by no treatment (FM group, n=8) or 4 days of electroacupuncture (FM+EA group, n=8) or minimal acupuncture (FM+MA group, n=8). All results are expressed relative to the Con group. * p<0.05 ** p<0.01 vs Con group. # p<0.05 ## p<0.01 vs FM group.
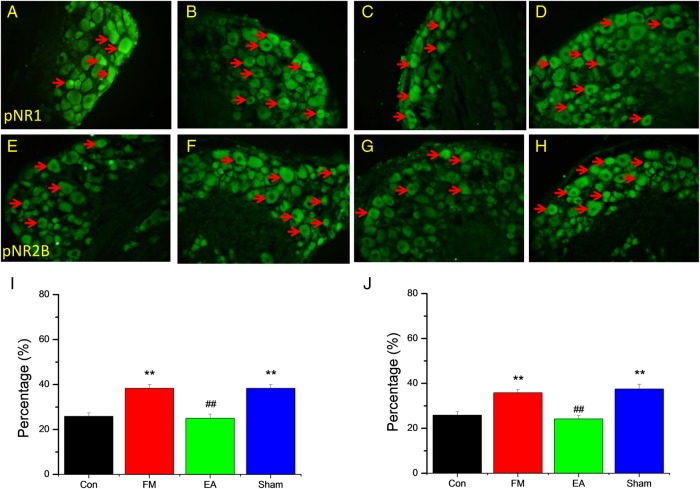
Figure 4 shows the expression of pNR1 and pNR2B at the single DRG neuron level as measured by immunofluorescence staining. Consistent with the Western blotting results, the number of pNR1 immunopositive neurons, expressed relative to control mice, was increased by FM modelling and this increase was reversed by EA but not by MA (figure 4A–D, I). Similarly, the number of pNR2B-immunopositive DRG neurons, expressed relative to the control group, was enhanced in FM mice and this increase was attenuated by EA but not by MA (figure 4E–H, J).
Figure 4.
Representative immunofluorescence images and quantification of neurons immunostained for phosphorylated N-methyl-D-aspartate receptor (pNR)-1 (A–D and I) and pNR2B (E–H and J) in the L3−L5 dorsal root ganglion neurons of 32 mice receiving control saline injection (Con group, n=8) or acid injection to model fibromyalgia (FM) followed by no treatment (FM group, n=8) or 4 days of electroacupuncture (FM+EA group, n=8) or minimal acupuncture (FM+MA group, n=8). Arrows indicate immunopositive neurons. **p<0.01 vs Con group. ##p<0.01 vs FM group.
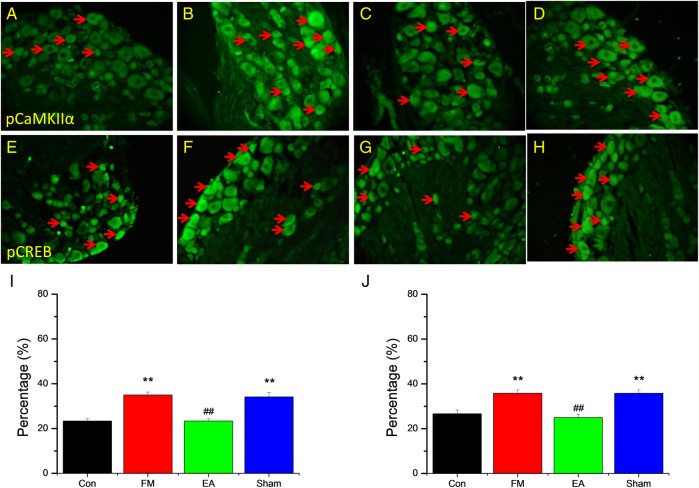
Figure 5 shows the results of DRG neurons labelled with pCaMKIIα and pCREB antibodies. pCaMKIIα-positive neurons were distributed throughout the DRG and the total number was higher in the FM group than in controls but near the control level in the EA group (figure 5A–C, I). Again, the number of immunopositive neurons remained elevated in the MA group (figure 5D, I). A similar response pattern was observed for pCREB labelling (figure 5E–H, J); the number of immunoreactive neurons was increased after FM modelling and the increase was reversed by EA but not MA.
Figure 5.
Representative immunofluorescence images and quantification of neurons immunostained for phosphorylated calmodulin-dependent protein kinase II (pCaMKII)-α (A–D and I) and phosphorylated cyclic AMP response element binding protein (pCREB) (E–H and J) in the dorsal root ganglion neurons of 32 mice receiving control saline injection (Con group, n=8) or acid injection to model fibromyalgia (FM) followed by no treatment (FM group, n=8) or 4 days of electroacupuncture (FM+EA group, n=8) or minimal acupuncture (FM+MA group, n=8). Arrows indicate immunopositive neurons. **p<0.01 vs Con group. ##p<0.01 vs FM group.
Discussion
In the present study, EA at bilateral ST36 effectively decreased mechanical hyperalgesia in mice induced by intramuscular dual acid saline injection, a well-characterised model of FM. We hypothesised that EA at ST36 could attenuate FM pain through modulation of NMDARs and downstream signalling molecules and our results support this. Paw withdrawal thresholds were reduced and both pNR1 and pNR2B protein expression levels were increased 8 days after dual acid saline injection, indicating development of mechanical hyperalgesia at both the peripheral and central levels. EA at ST36 for 4 consecutive days starting on day 5 successfully reversed pNR1 and pNR2B overexpression and increased withdrawal thresholds, suggesting that EA could effectively suppress mechanical pain in this mouse model of FM. The phospho-activated forms of both CaMKIIα and CREB, which are major transducers of NMDAR-mediated calcium influx, were also upregulated after acid saline injection, suggesting that hyperalgesia was maintained by both kinase pathways and changes in gene expression. Moreover, overexpression of both pCaMKIIα and pCREB was reversed by EA, paralleling the change in mechanical pain threshold. Our findings suggest that suppression of pNR1/pNR2B-pCaMKIIα-pCREB signalling is a major therapeutic mechanism of EA; however, it remains unclear if other NR subtypes and mechanisms are additionally involved.
Attenuation of the overexpression of NMDARs may be crucial in pain management. Enhanced expression of NR2B-containing NMDARs can potentiate chronic pain in cases of tissue or nerve injury and antagonism of NMDARs has been established as an effective approach for pain management.30 For example, Wu and Zhou reported that NR2B-containing NMDARs are potential targets for treatment of neuropathic pain.31 Recently, several articles have suggested that EA can reduce pain by suppressing NMDAR phosphorylation.32 This same anti-nociceptive effect was also achieved by injection of dizocilpine, an NMDAR antagonist. Furthermore, combined administration of EA and dizocilpine potentiated the anti-nociceptive effects of the individual treatments. The role of NMDARs in inflammatory pain is well established but unclear in FM. Our results showed that EA at ST36 can reliably reduce FM hyperalgesia and attenuate the pNR1 and pNR2B signalling pathways.
Xing et al33 found high baseline field potential amplitudes and a lower C-fibre LTP threshold in the SC dorsal horn of a rat neuropathic pain model. Intriguingly, 2 Hz EA delivered at ST36 and SP6 reliably reduced neuropathic pain by inducing central LTD in C-fibres and the analgesic effect was increased by administration of the NMDAR antagonist MK-801 or the opioid receptor antagonist naloxone. Additionally, central sensitisation of nociceptive transmission may be initiated in inflammatory, neuropathic and postoperative pain syndromes, and NMDAR activation may further increase Ca2+ influx and activation of second messenger pathways underlying painful sensations.34 Jung et al34 reported that intracellular Ca2+ is a crucial target of the analgesia produced by 2 Hz EA and can be modulated through spinal NMDARs. NMDARs can also be regulated by several protein kinases and phosphatases, and it has been suggested that protein phosphatases 1 and 2A play crucial roles in EA-mediated analgesia through regulation of NMDAR phosphorylation in the SC.32 The present study also showed that EA can alter pNR1 and pNR2B expression at both the peripheral (DRG) and central (SC) levels.
Multiple physiological mechanisms may contribute to FM pain. NMDARs, ASIC3, TRPV1, voltage-gated calcium channels, and substance P have all been implicated in this FM model.35–39 Dual acid saline injections have been shown to activate the cAMP pathway in the SC.40 Activation of extracellular signal-related kinase (ERK), a member of the mitogen-activated protein kinase (MAPK) family, has also been reported in the anterior paraventricular nucleus of the thalamus and hippocampus.39 41 Administration of neurotrophin-3 can reduce acid-induced chronic muscle pain,42 and the calcium channel antagonist pregabalin and the M-type voltage-gated potassium channel activator flupirtine have been shown to be effective for treatment of muscle pain.43 44 The development and maintenance of this type of chronic muscle hyperalgesia is also associated with changes in the amygdala.45 Here we demonstrated that pCaMKIIα and pCREB are involved in FM pain and can be further regulated by EA in both the peripheral and central nervous systems.
There is controversy surrounding the therapeutic efficacy and safety of opioids for treating FM pain syndromes. Mounting evidence suggests that opioids offer little help to FM patients suffering from widespread pain, although some FM patients do experience relief following opioid administration. In contrast, μ- or δ-opioid receptor agonists can reduce pain in an animal model of FM,46 as can glutamate receptor antagonists delivered to the SC.47 Even if they achieve an anti-nociceptive effect in FM, opioid use is still not ideal because of the significant potential for tolerance and addiction. Wang et al48 used immunohistochemistry to demonstrate that the number of DRG neurons expressing both isolectin B4 (IB4) and NR1 are dramatically increased in CFA (complete Freund's adjuvant)-mediated inflammatory pain. This increase was reversed by EA treatment, suggesting a specific role of NMDARs in IB4-positive nociceptive neurons. Furthermore, medium- to high- frequency EA (10 and 100 Hz) reliably reduced CFA-initiated inflammatory pain, and when EA was combined with a sub-effective dose of MK-801, the anti-nociceptive effect was prolonged.48 Similarly, Zhang et al reported that the NMDAR antagonist MK-801 enhanced the antinociceptive effect of both low-frequency (10 Hz) and high-frequency (100 Hz) EA in a rat model of neuropathic pain.49 The analgesic effect of EA can be reversed by a serotonin synthesis inhibitor delivered to the descending serotonergic spinal pathway, suggesting a central effect.50 A recent study indicated that acupuncture is superior to drugs for FM patients, while acupuncture combined with drugs further increased pain thresholds.28 Acupuncture also has therapeutic efficacy and can achieve immediate pain reduction in FM patients.29 Our results suggest that EA may be able to treat FM-associated pain by suppressing pNR1/pNR2B-pCaMKIIα-pCREB signalling in both the peripheral and central nervous systems. Pharmaceutical antagonism of this pathway is also a potential therapeutic strategy that warrants further investigation.
Conclusion
We demonstrated that EA at bilateral ST36 can significantly reduce mechanical hyperalgesia in a mouse FM model, likely by reversing the increase in pNR1/pNR2B-pCaMKIIα-pCREB signalling observed in the peripheral and central nervous systems. These results reveal EA-related analgesic mechanisms that could be relevant for clinical practice, particularly suppression of the NMDA pathway.
Footnotes
Contributors: C-LH and Y-WL wrote the manuscript. K-WL and YJ performed the experiments. All authors reviewed the manuscript, agreed to submission and approved the final version for publication.
Funding: This work was supported by CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan, MOST 104-2320-B-039-010, CMU104-S-45, and in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.English B. Neural and psychosocial mechanisms of pain sensitivity in fibromyalgia. Pain Manag Nurs 2014;15:530–8. 10.1016/j.pmn.2012.07.009 [DOI] [PubMed] [Google Scholar]
- 2.Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311:1547–55. 10.1001/jama.2014.3266 [DOI] [PubMed] [Google Scholar]
- 3.Riva R, Mork PJ, Westgaard RH, et al. Catecholamines and heart rate in female fibromyalgia patients. J Psychosom Res 2012;72:51–7. 10.1016/j.jpsychores.2011.09.010 [DOI] [PubMed] [Google Scholar]
- 4.Valim V, Natour J, Xiao Y, et al. Effects of physical exercise on serum levels of serotonin and its metabolite in fibromyalgia: a randomized pilot study. Rev Bras Reumatol 2013;53:538–41. 10.1016/j.rbr.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 5.Dadabhoy D, Crofford LJ, Spaeth M, et al. Biology and therapy of fibromyalgia. Evidence-based biomarkers for fibromyalgia syndrome. Arthritis Res Ther 2008;10:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker AJ, Wessely S, Cleare AJ. The neuroendocrinology of chronic fatigue syndrome and fibromyalgia. Psychol Med 2001;31:1331–45. 10.1017/S0033291701004664 [DOI] [PubMed] [Google Scholar]
- 7.Roth T, Arnold LM, Garcia-Borreguero D, et al. A review of the effects of pregabalin on sleep disturbance across multiple clinical conditions. Sleep Med Rev 2014;18:261–71. 10.1016/j.smrv.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 8.Smith MT, Moore BJ. Pregabalin for the treatment of fibromyalgia. Expert Opin Pharmacother 2012;13:1527–33. 10.1517/14656566.2012.687373 [DOI] [PubMed] [Google Scholar]
- 9.Hauser W, Walitt B, Fitzcharles MA, et al. Review of pharmacological therapies in fibromyalgia syndrome. Arthritis Res Ther 2014;16:201 10.1186/ar4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trugman JM, Palmer RH, Ma Y. Milnacipran effects on 24-hour ambulatory blood pressure and heart rate in fibromyalgia patients: a randomized, placebo-controlled, dose-escalation study. Curr Med Res Opin 2014;30:589–97. 10.1185/03007995.2013.861812 [DOI] [PubMed] [Google Scholar]
- 11.Vierck CJ., Jr Mechanisms underlying development of spatially distributed chronic pain (fibromyalgia). Pain 2006;124:242–63. 10.1016/j.pain.2006.06.001 [DOI] [PubMed] [Google Scholar]
- 12.DeSantana JM, Sluka KA. Central mechanisms in the maintenance of chronic widespread noninflammatory muscle pain. Curr Pain Headache Rep 2008;12:338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve 2001;24:37–46. [DOI] [PubMed] [Google Scholar]
- 14.Pratt D, Fuchs PN, Sluka KA. Assessment of avoidance behaviors in mouse models of muscle pain. Neuroscience 2013;248:54–60. 10.1016/j.neuroscience.2013.05.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeSantana JM, da Cruz KM, Sluka KA. Animal models of fibromyalgia. Arthritis Res Ther 2013;15:222 10.1186/ar4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steen KH, Reeh PW, Anton F, et al. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J Neurosci 1992;12:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnes JM, Henley JM. Molecular characteristics of excitatory amino acid receptors. Prog Neurobiol 1992;39:113–33. 10.1016/0301-0082(92)90007-2 [DOI] [PubMed] [Google Scholar]
- 18.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci 1994;17:31–108. 10.1146/annurev.ne.17.030194.000335 [DOI] [PubMed] [Google Scholar]
- 19.Zhou HY, Chen SR, Pan HL. Targeting N-methyl-D-aspartate receptors for treatment of neuropathic pain. Expert Rev Clin Pharmacol 2011;4:379–88. 10.1586/ecp.11.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yung KK. Localization of glutamate receptors in dorsal horn of rat spinal cord. Neuroreport 1998;9:1639–44. 10.1097/00001756-199805110-00069 [DOI] [PubMed] [Google Scholar]
- 21.Nagy GG, Watanabe M, Fukaya M, et al. Synaptic distribution of the NR1, NR2A and NR2B subunits of the N-methyl-d-aspartate receptor in the rat lumbar spinal cord revealed with an antigen-unmasking technique. Eur J Neurosci 2004;20:3301–12. 10.1111/j.1460-9568.2004.03798.x [DOI] [PubMed] [Google Scholar]
- 22.Lu K-W, Hsu C-K, Hsieh C-L, et al. Probing the effects and mechanisms of electroacupuncture at ipsilateral or contralateral ST36-ST37 acupoints on CFA-induced inflammatory pain. Sci Rep 2016;6:22123 10.1038/srep22123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choowanthanapakorn M, Lu KW, Yang J, et al. Targeting TRPV1 for body weight control using TRPV1−/− mice and electroacupuncture. Sci Rep 2016;5:17366 10.1038/srep17366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin YW, Hsieh CL. Auricular electroacupuncture reduced inflammation-related epilepsy accompanied by altered TRPA1, pPKCα, pPKCε, and pERk1/2 signaling pathways in kainic acid-treated rats. Mediators Inflamm 2014;2014:493480 10.1155/2014/493480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu SY, Chen WH, Hsieh CL, et al. Abundant expression and functional participation of TRPV1 at Zusanli acupoint (ST36) in mice: mechanosensitive TRPV1 as an “acupuncture-responding channel”. BMC Complement Altern Med 2014;14:96 10.1186/1472-6882-14-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin JG, Hsieh CL, Lin YW. Analgesic effect of electroacupuncture in a mouse fibromyalgia model: roles of TRPV1, TRPV4, and pERK. PLoS One 2015;10:e0128037 10.1371/journal.pone.0128037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hurt JK, Zylka MJ. PAPupuncture has localized and long-lasting antinociceptive effects in mouse models of acute and chronic pain. Mol Pain 2012;8:28 10.1186/1744-8069-8-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang B, Yi G, Hong W, et al. Efficacy of acupuncture on fibromyalgia syndrome: a meta-analysis. J Tradit Chin Med 2014;34:381 10.1016/S0254-6272(15)30037-6 [DOI] [PubMed] [Google Scholar]
- 29.Stival RS, Cavalheiro PR, Stasiak CE, et al. Acupuncture in fibromyalgia: a randomized, controlled study addressing the immediate pain response. Rev Bras Reumatol 2014;54:431 10.1016/j.rbr.2014.06.001 [DOI] [PubMed] [Google Scholar]
- 30.Zhuo M. Plasticity of NMDA receptor NR2B subunit in memory and chronic pain. Mol Brain 2009;2:4 10.1186/1756-6606-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu LJ, Zhuo M. Targeting the NMDA receptor subunit NR2B for the treatment of neuropathic pain. Neurotherapeutics 2009;6:693–702. 10.1016/j.nurt.2009.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryu JW, Lee JH, Choi YH, et al. Effects of protein phosphatase inhibitors on the phosphorylation of spinal cord N-methyl-D-aspartate receptors following electroacupuncture stimulation in rats. Brain Res Bull 2008;75:687–91. 10.1016/j.brainresbull.2007.11.005 [DOI] [PubMed] [Google Scholar]
- 33.Xing GG, Liu FY, Qu XX, et al. Long-term synaptic plasticity in the spinal dorsal horn and its modulation by electroacupuncture in rats with neuropathic pain. Exp Neurol 2007;208:323–32. 10.1016/j.expneurol.2007.09.004 [DOI] [PubMed] [Google Scholar]
- 34.Jung TG, Lee JH, Lee IS, et al. Involvement of intracellular calcium on the phosphorylation of spinal N-methyl-D-aspartate receptor following electroacupuncture stimulation in rats. Acta Histochem 2010;112:127–32. 10.1016/j.acthis.2008.09.009 [DOI] [PubMed] [Google Scholar]
- 35.Sluka KA, Price MP, Breese NM, et al. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain 2003;106:229–39. 10.1016/S0304-3959(03)00269-0 [DOI] [PubMed] [Google Scholar]
- 36.Chen WN, Lee CH, Lin SH, et al. Roles of ASIC3, TRPV1, and NaV1.8 in the transition from acute to chronic pain in a mouse model of fibromyalgia. Mol Pain 2014;10:40 10.1186/1744-8069-10-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen WN, Chen CC. Acid mediates a prolonged antinociception via substance P signaling in acid-induced chronic widespread pain. Mol Pain 2014;10:30 10.1186/1744-8069-10-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin CC, Chen WN, Chen CJ, et al. An antinociceptive role for substance P in acid-induced chronic muscle pain. Proc Natl Acad Sci USA 2012;109:E76–83. 10.1073/pnas.1108903108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen WK, Liu IY, Chang YT, et al. Ca(v)3.2 T-type Ca2+ channel-dependent activation of ERK in paraventricular thalamus modulates acid-induced chronic muscle pain. J Neurosci 2010;30:10360–8. 10.1523/JNEUROSCI.1041-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoeger-Bement MK, Sluka KA. Phosphorylation of CREB and mechanical hyperalgesia is reversed by blockade of the cAMP pathway in a time-dependent manner after repeated intramuscular acid injections. J Neurosci 2003;23:5437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen WH, Cheng SJ, Tzen JT, et al. Probing relevant molecules in modulating the neurite outgrowth of hippocampal neurons on substrates of different stiffness. PLoS One 2013;8:e83394 10.1371/journal.pone.0083394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gandhi R, Ryals JM, Wright DE. Neurotrophin-3 reverses chronic mechanical hyperalgesia induced by intramuscular acid injection. J Neurosci 2004;24:9405–13. 10.1523/JNEUROSCI.0899-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yokoyama T, Maeda Y, Audette KM, et al. Pregabalin reduces muscle and cutaneous hyperalgesia in two models of chronic muscle pain in rats. J Pain 2007;8:422–9. 10.1016/j.jpain.2006.11.007 [DOI] [PubMed] [Google Scholar]
- 44.Nielsen AN, Mathiesen C, Blackburn-Munro G. Pharmacological characterisation of acid-induced muscle allodynia in rats. Eur J Pharmacol 2004;487:93–103. 10.1016/j.ejphar.2004.01.017 [DOI] [PubMed] [Google Scholar]
- 45.Cheng SJ, Chen CC, Yang HW, et al. Role of extracellular signal-regulated kinase in synaptic transmission and plasticity of a nociceptive input on capsular central amygdaloid neurons in normal and acid-induced muscle pain mice. J Neurosci 2011;31:2258–70. 10.1523/JNEUROSCI.5564-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sluka KA, Rohlwing JJ, Bussey RA, et al. Chronic muscle pain induced by repeated acid injection is reversed by spinally administered mu- and delta-, but not kappa-, opioid receptor agonists. J Pharmacol Exp Ther 2002;302:1146–50. 10.1124/jpet.102.033167 [DOI] [PubMed] [Google Scholar]
- 47.Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. Pain 2002;98:69–78. 10.1016/S0304-3959(01)00471-7 [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Zhang Y, Dai J, et al. Electroacupuncture (EA) modulates the expression of NMDA receptors in primary sensory neurons in relation to hyperalgesia in rats. Brain Res 2006;1120:46–53. 10.1016/j.brainres.2006.08.077 [DOI] [PubMed] [Google Scholar]
- 49.Zhang RX, Wang L, Wang X, et al. Electroacupuncture combined with MK-801 prolongs anti-hyperalgesia in rats with peripheral inflammation. Pharmacol Biochem Behav 2005;81:146–51. 10.1016/j.pbb.2005.03.002 [DOI] [PubMed] [Google Scholar]
- 50.Lin JG, Chen WL. Acupuncture analgesia: a review of its mechanisms of actions. Am J Chin Med 2008;36:635–45. 10.1142/S0192415X08006107 [DOI] [PubMed] [Google Scholar]