Abstract
Background
Active myofascial trigger points (MTrPs) are major pain generators in myofascial pain syndrome. Dry needling (DN) is an effective method for the treatment of MTrPs.
Objective
To assess the immediate neurophysiological and clinical effects of DN in patients with upper trapezius MTrPs.
Methods
This was a prospective, clinical trial study of 20 patients with upper trapezius MTrPs and 20 healthy volunteers (matched for height, weight, body mass index and age), all of whom received one session of DN. Primary outcome measures were neuromuscular junction response (NMJR) and sympathetic skin response (SSR). Secondary outcomes were pain intensity (PI) and pressure pain threshold (PPT). Data were collected at baseline and immediately post-intervention.
Results
At baseline, SSR amplitude was higher in patients versus healthy volunteers (p<0.003). With respect to NMJR, a clinically abnormal increment and normal reduction was observed in patients and healthy volunteers, respectively. Moreover, PPT of patients was less than healthy volunteers (p<0.0001). After DN, SSR amplitude decreased significantly in patients (p<0.01), but did not change in healthy volunteers. A clinically important reduction in the NMJR of patients and increment in healthy volunteers was demonstrated after DN. PPT increased after DN in patients, but decreased in healthy volunteers (p<0.0001). PI improved after DN in patients (p<0.001).
Conclusions
The results of this study showed that one session of DN targeting active MTrPs appears to reduce hyperactivity of the sympathetic nervous system and irritability of the motor endplate. DN seems effective at improving symptoms and deactivating active MTrPs, although further research is needed.
Trial registration number
IRCT20130316128.
Keywords: MYOFASCIAL PAIN, NEUROPHYSIOLOGY
Introduction
Myofascial pain syndrome, associated with myofascial trigger points (MTrPs), is a musculoskeletal disorder that is characterised by the presence of palpable taut bands and highly irritable points in the skeletal muscles. MTrPs can be active or latent. Active MTrPs produce spontaneous pain and symptoms, while latent MTrPs do not produce any symptoms unless pressure is applied.1 2 MTrPs more commonly affect postural muscles including the trapezius.3 4 Formation of MTrPs can cause pain (sensory component), motor dysfunction (motor component) and autonomic reactions (autonomic component) in the affected muscles.2
Simons et al2 hypothesised that palpable taut bands in the affected muscles are due to excessive acetylcholine release at the neuromuscular junction (motor endplate). In this situation, continuous contraction of the muscle fibres, which is accompanied by increased metabolism and local ischaemia, leads to increased secretion of sensitising substances and can subsequently cause pain and autonomic reactions such as increased sweating, vasoconstriction or vasodilation, and pilomotor activity in the muscle.5 6 Although it is speculated that such motor and autonomic changes occur in patients with MTrPs, to our knowledge no previous human study has evaluated the extent of such reactions in these patients after MTrP dry needling (DN). The sympathetic skin reaction (SSR), which is commonly used to evaluate functions of the autonomic nervous system7 and neuromuscular junction response (NMJR) to repetitive nerve stimulation (RNS), which is frequently used for the assessment of motor endplate dysfunction,8 can be used to objectively detect autonomic changes and motor dysfunction in patients with MTrPs.
Various invasive and non-invasive procedures are available to help manage MTrPs. DN is a minimally invasive method that has recently gained popularity for the treatment of MTrPs.9 DN involves inserting an acupuncture needle directly into an MTrP without injection of material.10 The effectiveness and safety of this technique has been confirmed by several studies.11–13 DN of upper trapezius MTrPs is increasingly documented. Previous studies assessing the effect of DN on MTrPs of the upper trapezius muscle have demonstrated reduced pain and pain pressure threshold (PPT), increased local blood flow,14 a restored range of motion at the neck,15 16 and improved quality of life.17–19 To our knowledge, there are no existing data on the neurophysiological effects of DN (including changes in NMJR and SSR) in patients with upper trapezius MTrPs. The aims of this study were: (1) to examine for differences in SSR, NMJR and PPT between patients with active MTrPs and healthy volunteers; and (2) to assess the neurophysiological and clinical effects of DN on sensory, motor and autonomic components of MTrPs in the trapezius muscle. We hypothesised that DN stimulation of MTrPs would relieve pain, decrease sympathetic outflow and improve the NMJR.
Methods
Study design
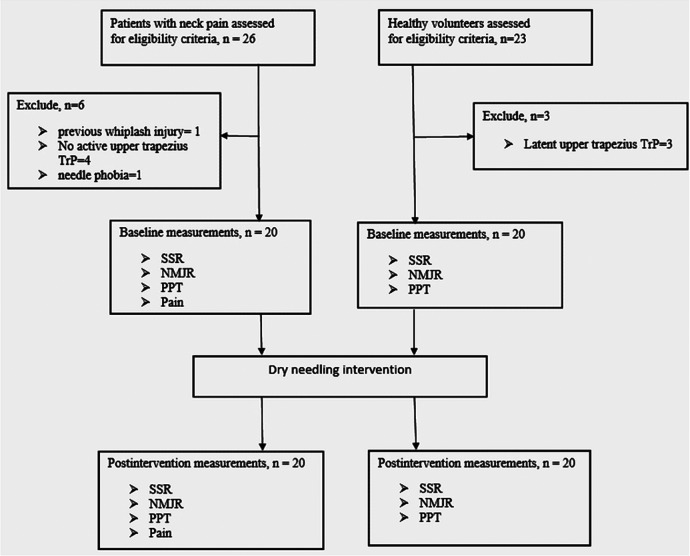
Forty participants comprising 20 patients (aged 31.7±10.8 years) and 20 matched healthy volunteers (aged 30.4±5.6 years) were included in this study (figure 1). The patients were recruited from the orthopaedic and physiotherapy clinics of Tehran University of Medical Sciences (TUMS) and were deemed eligible if they had experienced persistent neck pain for >6 months and were found to have an active MTrP at the standardised location ‘TrP2’ with spontaneous pain in the right (dominant) upper trapezius muscle based on the following definition of an active MTrP:1 2 presence of a palpable, discrete nodule within a taut band of skeletal muscle with reproduction of pain on palpation.5 Patients with whiplash injury, previous cervical or shoulder surgery, systemic disorders, neurological deficits, use of sedative or anticoagulant drugs, epilepsy, pregnancy, needle phobia, skin lesions or evidence of infection at the MTrP site were excluded. Healthy volunteers (with no history of neck pain) were matched to the patients based on height, weight, body mass index (BMI) and age. Key inclusion criteria for the healthy volunteers were good general health and lack of latent MTrPs within the upper trapezius muscle. They reported no pain or discomfort on palpation of the upper trapezius in the TrP2 region. All participants gave written informed consent before entering the study, which was approved by the Ethical Committee of TUMS (reference no. 2185).
Figure 1.
Flow diagram of the study. DN, dry needling; NMJR, neuromuscular junction response; PPT, pressure pain thresholds; SSR, sympathetic skin response; TrP, trigger point.
Interventions
The treatment protocol for this study was as previously described.20 Participants were asked to rest in a supine position on the treatment table in a silent, semi-dark room for 10 min before the experiment began. A comfortable room temperature of 24°C was maintained for standardisation. Then, baseline measurements of SSR, NMJR, pain intensity (PI) and PPT were recorded by a trained physiotherapist. Subsequently, all participants received one session of DN that included insertion of an acupuncture needle of 0.30 mm diameter and 50 mm length (Seirin J, Japan) into the identified active MTrP of the upper trapezius muscle in the same supine position (figure 2A). The needle was partially moved up and down 3–5 times and then removed irrespective of whether a twitch response was elicited or not. The same procedure was performed on the healthy individuals; however, the needle was inserted into the middle of the almost horizontal fibres of the right upper trapezius at a location similar to that of the upper trapezius TrP2 of the patients.2 Thus, the needle was inserted into the TrP2 region in the patients and a corresponding point in the healthy volunteers. Before the intervention and immediately afterwards, outcome measures (SSR, NMJR, PI and PPT) were recorded by the physiotherapist performing the intervention.
Figure 2.
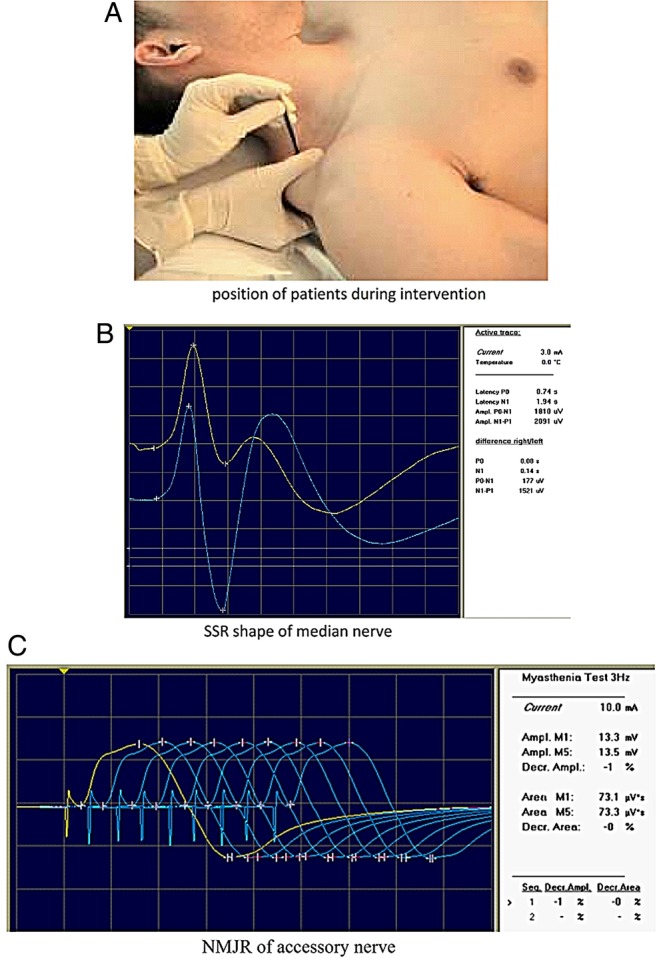
(A) Positioning of participant for dry needling intervention. (B) Sympathetic skin response (SSR) of the median nerve. (C) Neuromuscular junction response (NMJR) of the accessory nerve.
Measurements
Primary and secondary outcome measures were recorded at baseline and immediately after DN. The primary outcome measures were SSR and NMJR. SSR, which is expressed as a potential generated in the sweat glands of the skin, was assessed by Toennies Neuroscreen Plus (Toennies, Germany), an electromyography (EMG) instrument used to calculate electrodiagnostic parameters measured via surface electrodes. SSR was measured by electrical stimulation of the median nerve at the wrist with a sensitivity of 500 μV/div and sweep speed of 1000 ms/div. SSR signals were filtered at a frequency of 0.08–20 Hz. The participants rested in a supine position on a treatment table in a semi-dark and silent room for 10 min before EMG evaluation. The room temperature was maintained at 24°C. The surface recording electrodes were applied bilaterally to the palmar and dorsal aspects of both hands, 2 cm from the medial border. A grounding electrode was fastened on the wrist. Electrical stimulation was applied to the proximal part of the volar wrist in the distribution of the median nerve only on the affected side, while simultaneous recordings were made bilaterally. Three repetitive stimulations of the median nerve were delivered at 1-min intervals. The mean score of SSR latency and amplitude of the three trials were calculated for each side20 (figure 2B). The other primary outcome, NMJR, was evaluated using an RNS technique. A 3 Hz RNS was applied using trains of nine supramaximal electrical stimulations of the spinal accessory motor nerve with a recording electrode over the upper trapezius and the EMG instrument (Toennies Neuroscreen Plus, Germany) with a sensitivity of 5 mV/div, sweep speed of 5 ms/div and filter of 5 Hz–5 KHz. Surface stimulating electrodes were placed on the posterior border of the sternocleidomastoid muscle at the level of the upper border of the thyroid cartilage, while surface recording electrodes were located over the upper trapezius muscle 5 cm from the C7 spinous process. The spinal accessory nerve was subsequently stimulated and compound muscle action potentials (CMAPs) were recorded. To characterise NMJR, the first and fifth CMAPs were compared and the resultant decrement or increment of the amplitude was represented as a percentage. A decrement of up to 8–10% in the amplitude of the CMAP was considered normal, while a decrement of >10% or any increment of the tested muscle was considered to be an abnormal NMJR (figure 2C).
The secondary outcome measures were PI and PPT. PI was assessed using a 0–10 numerical rating scale (NRS), with 0 representing no pain and 10 representing worst imaginable pain. PPT was measured relative to the perpendicular pressure from the metal rod of an algometer (Digital Instrument, Lutron, Taiwan) applied over TrP2 of the upper trapezius muscle in patients and the corresponding point in healthy volunteers in a supine position at 1 kg/cm.2 The pressure was removed when the patient reported an increase in PI or discomfort. For the healthy volunteer, pressure by the algometer was removed when they felt pain rather than pressure. Measurements were repeated three times at 40 s intervals and averaged.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) V.17 (SPSS Inc, Chicago, IL, USA) was used to analyse the data. A sample size calculation was performed based on a pilot study with the following assumptions: effect size 0.8 for primary outcome measures SSR and NMJR; α=0.05; and power=80%. The Kolmogorov-Smirnov test was used to examine the normality of distribution for quantitative data (p>0.05). Baseline measurements were compared between the two groups using the independent t-test. The Wilcoxon signed rank test was used to assess changes in PI before and after the intervention within each group. A two-by-two mixed design analysis of variance (ANOVA) with time (pre-intervention vs post-intervention) as the within-subject factor and group (patients vs healthy volunteers, each receiving DN) as the between-subject factor, was used to determine the effects of the intervention on SSR and PPT. The output of interest was the group-by-time interaction at α=0.05.
Results
Seventeen of 20 patients and 16 of 20 healthy volunteers were women. The right trapezius was the affected side in all of the patients. By design, there were no statistically significant differences in age, weight, height and BMI between the two groups (table 1). There were also no significant differences in SSR latency on the affected or unaffected sides between the two groups; however, patients with active trigger points had a significantly higher mean amplitude of SSR on both sides compared to healthy volunteers. With respect to NMJR, a clinically abnormal increment percentage response and a normal reduction were seen in the patients and healthy volunteers, respectively. Before the intervention, PPT in patients with MTrPs was significantly less than healthy volunteers (table 2).
Table 1.
Baseline characteristics
| MTrP patients (n=20) | Healthy volunteers (n=20) | p Value | |
|---|---|---|---|
| Age (years) | 31.7±10.9 | 30.4±5.6 | 0.6 |
| Weight (kg) | 63.4±9.5 | 61.0±15.9 | 0.6 |
| Height (cm) | 164±6 | 165±9 | 0.6 |
| BMI (kg/m2) | 23.3±2.5 | 21.9±3.2 | 0.1 |
Data are presented as mean±SD.
BMI, body mass index.
Table 2.
Comparisons of the SSR, PPT and NMJR between the two groups at baseline
| Variable | Patients (n=20) | Healthy volunteers (n=20) | t | p Value |
|---|---|---|---|---|
| Ipsilateral SSR latency (s) | 1.1±0.3 | 1.1±0.2 | 0.3 | 0.7 |
| Ipsilateral SSR amplitude (mV) | 2.5±1.4 | 1.4±0.7 | 3.2 | 0.003 |
| Contralateral SSR latency (s) | 1.2±0.3 | 1.1±0.3 | 0.8 | 0.4 |
| Contralateral SSR amplitude (mv) | 2.0±1.4 | 1.1±1.0 | 2.1 | 0.03 |
| PPT (kg/cm2) | 1.1±0.5 | 2.4±1.3 | −3.8 | 0.0001 |
| NMJR (%) | 5.6±26.7 | −0.9±10.9 | ||
p value<0.05 is significant.
Data are presented as mean±SD.
NMJR, neuromuscular junction response; PPT, pressure pain threshold; SSR, skin sympathetic response.
Primary outcome measure
The mixed-model ANOVA did not indicate a statistically significant time-by-group interaction for SSR latencies on the affected and opposite sides. However, there was a significant effect of time, with both groups experiencing similar increases in SSR latencies. The other parameter of SSR, amplitude, showed a statistically significant time-by-group interaction on both sides. Patients experienced a decrease in amplitude. With respect to NMJR, it is agreed that a decrement of up to 8–10% in the CMAP of the upper trapezius muscle as a response to RNS can be considered to be a clinically normal response.21 As shown in table 3, the mean NMJR among the patients was +5.6% and thus outside of the normal range. By comparison, the NMJR of the healthy group (−0.9%) was within the normal range. Immediately following DN, the abnormal incremental response of NMJR of patients at baseline was decreased to a value within the normal range (5.6% to −2.9%; table 3).
Table 3.
Comparison of the final values of SSR, PPT and NMJR between the two groups
| Variable | Patients (n=20) |
Healthy volunteers (n=20) |
Time |
Time×group interaction |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-DN | Post-DN | Pre-DN | Post-DN | F | p Value | Effect size | F | p Value | Effect size | |
| Ipsilateral SSR latency (s) | 1.15±0.38 | 1.34±0.29 | 1.11±0.28 | 1.35±0.48 | 16 | 0.0001 | 0.3 | 0.2 | 0.7 | 0.005 |
| Ipsilateral SSR amplitude (mV) | 2.5±1.4 | 1.3±1.0 | 1.4±0.7 | 1.4±1.1 | 7.3 | 0.01 | 0.2 | 6.9 | 0.01 | 0.15 |
| Contralateral SSR latency (s) | 1.2±0.37 | 1.32±0.34 | 1.11±0.33 | 1.28±0.37 | 8.9 | 0.005 | 0.2 | 0.3 | 0.6 | 0.008 |
| Contralateral SSR amplitude (mV) | 2.0±1.4 | 1.2±1.3 | 1.2±1.1 | 1.5±1.6 | 0.5 | 0.5 | 0.1 | 4.2 | 0.04 | 0.1 |
| PPT (kg/cm2) | 1.15±0.50 | 1.52±0.59 | 2.42±1.38 | 1.71±1.16 | 2.8 | 0.1 | 0.07 | 28 | 0.0001 | 0.4 |
| NMJR (%) | 5.6±26.7 | −2.9±13.8 | −0.9±10.9 | 0.9±6.8 | ||||||
| PI (NRS score) | 5 (4–7) | 2 (1–3.8) | z=−3.9, p= 0.0001 | |||||||
p value<0.05 is significant.
Data are presented as mean±SD or median (IQR).
DN, dry needling; NMJR, neuromuscular junction response; NRS, numerical rating scale; PI, pain intensity; PPT, pressure pain threshold; SSR, skin sympathetic response.
Secondary outcome measure
The two-by-two mixed-model ANOVA revealed a significant time-by-group interaction for PPT; patients receiving DN experienced an increase in PPT whereas the healthy control group showed a decrease in PPT after DN. The result of the Wilcoxon rank test indicated a significant decrease in PI of patients after DN (table 3).
Discussion
In the current study, we considered the neurophysiological and clinical changes induced by DN including SSR, NMJR, PPT and PI in patients with active MTrPs in the trapezius muscle. Our findings indicated a significant decrease in SSR amplitude and NMJR after a single session of DN. Indeed, there was significant improvement in PI and PPT after DN.
Effect of DN on SSR
Evaluation of the SSR can be used for the diagnosis of autonomic disorders.22 However, there is still no consensus on how to process quantitative evaluation of the SSR. Some researchers have used the latency parameter,23 24 while other investigators have advocated using the amplitude parameter because they believe that it is difficult to measure the exact onset of deflection from the baseline to assess changes in latency.7 25 Accordingly, we focused on changes in amplitude. In this study, the mean amplitude of SSR recorded from the patient's hands (on the affected and unaffected sides) was higher than that of the healthy volunteer control group at baseline. The increased SSR amplitude in individuals with MTrPs may be related to increased psychological stress in these patients.2 Constant stress has been shown to cause functional changes in the autonomic nervous system, such as increased SSR amplitude and decreased latency.26 Therefore, in accordance with other studies,27 28 we believe that increased SSR amplitude, as observed in the present study, is an indication of sympathetic system dysfunction in patients with MTrPs.
After unilateral DN of the trapezius muscle, the SSR amplitude on both the affected and unaffected sides decreased significantly in the participants with MTrPs but did not change in the healthy volunteers. This observation implies, firstly, that DN improves global sympathetic function via central (spinal or supraspinal) mechanisms rather than local effects (as measured changes were symmetrical) and, secondly, that DN impacts sympathetic function only in patients. Mechanical stimulation such as DN has been shown to activate afferent Aβ and Aδ fibres.29 In our study, subjective sensation due to needle penetration differed between patients and healthy volunteers. Patients reported pain following needle stimulation, while the healthy volunteers denied any pain or discomfort. It is possible that these differences in sensation lead to differential effects on the sympathetic nervous system in the two groups. Excitation of pain due to needle stimulation via Aδ afferent fibres in patients may have suppressed the sympathetic nervous system in this study. Previous studies have reported that acupuncture stimulation activates various brain regions including the insula, anterior cingulate cortex, prefrontal cortex, visual cortex and cerebellar cortex.30 31 Since almost all cortical brain areas (including the prefrontal cortex, anterior cingulate cortex, sensorimotor cortex, inferior parietal lobule, lingual and fusiform gyri, temporal cortex, insular and extrastriate visual cortices and the cerebellar cortex) are involved in autonomic control,32–34 we hypothesise that needle stimulation of Aδ afferent fibres modulates higher brain centres to induce inhibitory effects on the autonomic nervous system. At the very least, these findings suggest that DN might relieve chronic pain through its effects on the autonomic nervous system.
Effect of DN on NMJR
In this study, motor endplate activity following the RNS test on the trapezius muscle in patients with MTrPs demonstrated an abnormal increment compared to healthy volunteers at baseline; a decrement of up to 8–10% in the amplitude of the CMAP after RNS is considered normal.21 Abnormal NMJR values after the RNS test, noted in the patients with MTrPs in the current study, support the hypothesis put forward by Simons et al2 that hyperactive motor endplates may contribute to MTrP formation in the muscle. Many other studies have also reported hyperactivity of the motor endplate, measured via spontaneous endplate activity (SEA) in single-fibre EMG (SFEMG) studies.35–38 The presence of SEA is an indication of spontaneous release of acetylcholine (ACh) at the neuromuscular junction (NMJ).1 Our study is consistent with the work of Simons et al, given that we exhibited NMJ dysfunction in MTrP patients.
After DN, the percentage changes in NMJR among patients decreased back to the normal range, while it increased into the abnormal range in the healthy volunteers. Decreased irritability of the motor endplate after DN has been shown previously via the SFEMG technique.39 40 The lack of previous studies using the RNS technique make it difficult to compare the findings of this study with other published trials. It seems that the elevated NMJR is related to increased concentration of biochemicals such as substance P and calcitonin gene-related peptide (CGRP) in the vicinity of active MTrPs. It has been shown that the levels of such biochemical irritants drop immediately after DN.14 41 42 Moreover, CGRP can increase the release of ACh from the motor endplate and decrease the effectiveness of acetylcholinesterase in the synaptic cleft and enhance ACh receptor efficiency at the same time.17 Therefore, DN, by modulating the biochemical milieu of MTrPs, can lead to reduction of ACh efficacy and consequently decrease the irritability of the motor endplate.
Effect of DN on PI and PPT
This study, based on NRS evaluation, has shown that DN is effective at decreasing PI, consistent with previous research.10 43–45 A ‘gate control’ mechanism likely underlies the alleviation of pain after DN,29 although its analgesic effects are unlikely to be fully explained by one single mechanism. Analgesia may also be related to the endogenous opioid system including β-endorphin and enkephalins.29 Furthermore, the PPT values of the trapezius muscle were significantly lower in patients versus healthy volunteers at baseline, which may reflect greater sensitisation of MTrP regions, as previously shown.37 46 47 PPT is a valid clinical method of assessing MTrP sensitivity.48 In our study, DN decreased PPT in the normal subjects but increased it in patients. The observed decrease in PPT value among normal subjects might have been due to muscle damage by needle penetration. While this was also likely to have occurred in patients, pain from this type of minor injury may have been mitigated by other mechanisms such as neuronal (spinal or supraspinal) effects or biochemical changes at the MTrP site following DN.49
Advantages and limitations of the study
To the best of our knowledge, this study is the first clinical investigation to examine the immediate effects of DN on sympathetic and motor responses, as measured by SSR and surface EMG, respectively. One major limitation is that the therapist applying the intervention and the investigator collecting the data were not blind to the treatment group. Moreover, this study did not measure patients' functional abilities or the long-term effects of DN.
Conclusion
In summary, one session of DN increased PPT and decreased pain, SSR and NMJR in patients with upper trapezius MTrPs. Therefore, DN may play an important role in treating active MTrPs via inhibition of sympathetic nervous activity and reduction of NMJ hyperactivity.
Acknowledgments
We would like to express our appreciation to the Tehran University of Medical Sciences for supporting our study. We would also like to thank all the individuals who participated.
Footnotes
Contributors: MA-A was involved in the design of the work, delivered the intervention, collected and analysed the data and drafted the paper. NNA contributed to the design of the work, monitored data collection and revised the paper. SN designed the data collection tools and monitored data collection. GO contributed to the design of the work and data collection tools. MRN wrote the statistical analysis plan and revised the paper. All authors approved the final accepted version.
Funding: This research was supported by the Tehran University of Medical Sciences.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The Ethical Committee of Tehran University of Medical Sciences approved this study.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Simons DG. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J Electromyogr Kinesiol 2004;14:95–107. 10.1016/j.jelekin.2003.09.018 [DOI] [PubMed] [Google Scholar]
- 2.Simons DG , Travell JG, Simons LS. Travell & Simons’ myofascial pain and dysfunction the trigger point manual. Baltimore: Williams & Wilkins, 1999. [Google Scholar]
- 3.Chang CW, Chang KY, Chen YR, et al. Electrophysiologic evidence of spinal accessory neuropathy in patients with cervical myofascial pain syndrome. Arch Phys Med Rehabil 2011;92:935–40. 10.1016/j.apmr.2011.01.010 [DOI] [PubMed] [Google Scholar]
- 4.Ustun N, Arslan F, Mansuroglu A, et al. Efficacy of EMLA cream phonophoresis comparison with ultrasound therapy on myofascial pain syndrome of the trapezius: a single-blind, randomized clinical study. Rheumatol Int 2014;34:453–7. 10.1007/s00296-013-2881-2 [DOI] [PubMed] [Google Scholar]
- 5.Dommerholt J HP. Myofascial Trigger points, Pathophysiology and Evidence… Informed Diagnosis and Management. Jones and Bartlett Publisher, 2010. [Google Scholar]
- 6.Ge HY, Fernández-de-las-Peñas C, Arendt-Nielsen L. Sympathetic facilitation of hyperalgesia evoked from myofascial tender and trigger points in patients with unilateral shoulder pain. Clin Neurophysiol 2006;117:1545–50. 10.1016/j.clinph.2006.03.026 [DOI] [PubMed] [Google Scholar]
- 7.Muslumanoglu L, Akyuz G, Aki S, et al. Evaluation of autonomic nervous system functions in post-stroke patients. Am J Phys Med Rehabil 2002;81:721–5. 10.1097/00002060-200210000-00001 [DOI] [PubMed] [Google Scholar]
- 8.Tidswell T, Pitt MC. A new analytical method to diagnose congenital myasthenia with stimulated single-fiber electromyography. Muscle Nerve 2007;35:107–10. 10.1002/mus.20637 [DOI] [PubMed] [Google Scholar]
- 9.Kalichman L, Vulfsons S. Dry needling in the management of musculoskeletal pain. J Am Board Fam Med 2010;23:640–6. 10.3122/jabfm.2010.05.090296 [DOI] [PubMed] [Google Scholar]
- 10.Tekin L, Akarsu S, Durmus O, et al. The effect of dry needling in the treatment of myofascial pain syndrome: a randomized double-blinded placebo-controlled trial. Clin Rheumatol 2013;32:309–15. 10.1007/s10067-012-2112-3 [DOI] [PubMed] [Google Scholar]
- 11.Vulfsons S, Ratmansky M, Kalichman L. Trigger point needling: techniques and outcome. Curr Pain Headache Rep 2012;16:407–12. 10.1007/s11916-012-0279-6 [DOI] [PubMed] [Google Scholar]
- 12.Kietrys DM, Palombaro KM, Azzaretto E, et al. Effectiveness of dry needling for upper-quarter myofascial pain: a systematic review and meta-analysis. J Orthop Sports Phys Ther 2013;43:620–34. 10.2519/jospt.2013.4668 [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Huang QM, Liu QG, et al. Effectiveness of dry needling for myofascial trigger points associated with neck and shoulder pain: a systematic review and meta-analysis. Arch Phys Med Rehabil 2015;96:944–55. 10.1016/j.apmr.2014.12.015 [DOI] [PubMed] [Google Scholar]
- 14.Shah JP, Gilliams EA. Uncovering the biochemical milieu of myofascial trigger points using in vivo microdialysis: an application of muscle pain concepts to myofascial pain syndrome. J Bodyw Mov Ther 2008;12:371–84. 10.1016/j.jbmt.2008.06.006 [DOI] [PubMed] [Google Scholar]
- 15.Ga H, Choi JH, Park CH, et al. Acupuncture needling versus lidocaine injection of trigger points in myofascial pain syndrome in elderly patients--a randomised trial. Acupunct Med 2007;25:130–6. 10.1136/aim.25.4.130 [DOI] [PubMed] [Google Scholar]
- 16.Tsai CT, Hsieh LF, Kuan TS, et al. Remote effects of dry needling on the irritability of the myofascial trigger point in the upper trapezius muscle. Am J Phys Med Rehabil 2010;89:133–40. 10.1097/PHM.0b013e3181a5b1bc [DOI] [PubMed] [Google Scholar]
- 17.Gerber NL SS, Hammond J, Shah J. A brief overview and update of myofascial pain syndrome and myofascial trigger points. J Spinal Res Found 2011;6:55–64. [Google Scholar]
- 18.Mejuto-Vazquez MJ, Salom-Moreno J, Ortega-Santiago R, et al. Short-term changes in neck pain, widespread pressure pain sensitivity, and cervical range of motion after the application of trigger point dry needling in patients with acute mechanical neck pain: a randomized clinical trial. J Orthop Sports Phys Ther 2014;44:252–60. [DOI] [PubMed] [Google Scholar]
- 19.Rayegani SM, Bayat M, Bahrami MH, et al. Comparison of dry needling and physiotherapy in treatment of myofascial pain syndrome. Clin Rheumatol 2014;33:859–64. 10.1007/s10067-013-2448-3 [DOI] [PubMed] [Google Scholar]
- 20.Abbaszadeh-Amirdehi M, Ansari NN, Naghdi S, et al. The neurophysiological effects of dry needling in patients with upper trapezius myofascial trigger points: study protocol of a controlled clinical trial. BMJ Open 2013;3:pii: e002825 10.1136/bmjopen-2013-002825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh SJ. Electromyography: neuromuscular transmission studies. Baltimore: Williams & Wilkins, 1988. [Google Scholar]
- 22.Kucera P, Goldenberg Z, Kurca E. Sympathetic skin response: review of the method and its clinical use. Bratisl Lek Listy 2004;105:108–16. [PubMed] [Google Scholar]
- 23.Denislic M, Meh D. Sympathetic skin response in parkinsonian patients. Electromyogr Clin Neurophysiol 1996;36:231–5. [PubMed] [Google Scholar]
- 24.Karatas GK OM, Meray J. Autonomic nervous system involvement in Bechet's disease. Rheumatol Int 2002;22:155–59. [DOI] [PubMed] [Google Scholar]
- 25.Emad MR PK, Sedaghat P. Evaluation of sympathetic skin response in old-polio patients. Iranian Red Crescent Med J 2011;13:829–31. [PMC free article] [PubMed] [Google Scholar]
- 26.Mohan A, Sharma R, Bijlani RL. Effect of meditation on stress-induced changes in cognitive functions. J Altern Complement Med 2011;17:207–12. 10.1089/acm.2010.0142 [DOI] [PubMed] [Google Scholar]
- 27.Chung JW, Ohrbach R, McCall WD Jr. Effect of increased sympathetic activity on electrical activity from myofascial painful areas. Am J Phys Med Rehabil 2004;63:842–50. [DOI] [PubMed] [Google Scholar]
- 28.Kimura Y, Ge HY, Zhang Y, et al. Evaluation of sympathetic vasoconstrictor response following nociceptive stimulation of latent myofascial trigger points in humans. Acta Physiol 2009;196:411–17. 10.1111/j.1748-1716.2009.01960.x [DOI] [PubMed] [Google Scholar]
- 29.Cagnie B, Dewitte V, Barbe T, et al. Physiologic effects of dry needling. Curr Pain Headache Rep 2013;17:348 10.1007/s11916-013-0348-5 [DOI] [PubMed] [Google Scholar]
- 30.Biella G, Sotgiu ML, Pellegata G, et al. Acupuncture produces central activations in pain regions. Neuroimage 2001;14(Pt 1):60–6. 10.1006/nimg.2001.0798 [DOI] [PubMed] [Google Scholar]
- 31.Pariente J, White P, Frackowiak RS, et al. Expectancy and belief modulate the neuronal substrates of pain treated by acupuncture. Neuroimage 2005;25:1161–7. 10.1016/j.neuroimage.2005.01.016 [DOI] [PubMed] [Google Scholar]
- 32.Gianaros PJ, Van Der Veen FM, Jennings JR. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology 2004;41:521–30. 10.1111/1469-8986.2004.00179.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakai S, Hori E, Umeno K, et al. Specific acupuncture sensation correlates with EEGs and autonomic changes in human subjects. Auton Neurosci 2007;133:158–69. 10.1016/j.autneu.2007.01.001 [DOI] [PubMed] [Google Scholar]
- 34.Viltart O, Mullier O, Bernet F, et al. Motor cortical control of cardiovascular bulbar neurones projecting to spinal autonomic areas. J Neurosci Res 2003;73:122–35. 10.1002/jnr.10598 [DOI] [PubMed] [Google Scholar]
- 35.Chou LW, Hsieh YL, Kao MJ, et al. Remote influences of acupuncture on the pain intensity and the amplitude changes of endplate noise in the myofascial trigger point of the upper trapezius muscle. Arch Phys Med Rehabil 2009;90:905–12. 10.1016/j.apmr.2008.12.020 [DOI] [PubMed] [Google Scholar]
- 36.Ge HY, Arendt-Nielsen L. Latent myofascial trigger points. Curr Pain Headache Rep 2011;15:386–92. 10.1007/s11916-011-0210-6 [DOI] [PubMed] [Google Scholar]
- 37.Ge HY, Nie H, Madeleine P, et al. Contribution of the local and referred pain from active myofascial trigger points in fibromyalgia syndrome. Pain 2009;147:233–40. 10.1016/j.pain.2009.09.019 [DOI] [PubMed] [Google Scholar]
- 38.Simons DG, Hong CZ, Simons LS. Endplate potentials are common to midfiber myofacial trigger points. Am J Phys Med Rehabil 2002;81:212–22. 10.1097/00002060-200203000-00010 [DOI] [PubMed] [Google Scholar]
- 39.Chen JT, Chung KC, Hou CR, et al. Inhibitory effect of dry needling on the spontaneous electrical activity recorded from myofascial trigger spots of rabbit skeletal muscle. Am J Phys Med Rehabil 2001;80:729–35. 10.1097/00002060-200110000-00004 [DOI] [PubMed] [Google Scholar]
- 40.Hsieh YL, Chou LW, Joe YS, et al. Spinal cord mechanism involving the remote effects of dry needling on the irritability of myofascial trigger spots in rabbit skeletal muscle. Arch Phys Med Rehabil 2011;92:1098–105. 10.1016/j.apmr.2010.11.018 [DOI] [PubMed] [Google Scholar]
- 41.Shah JP, Danoff JV, Desai MJ, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil 2008;89:16–23. 10.1016/j.apmr.2007.10.018 [DOI] [PubMed] [Google Scholar]
- 42.Shah JP HJ. New frontiers in the pathophysiology of myofascial pain. Pain Practitioner 2012;22:26–33. [Google Scholar]
- 43.Cummings TM, White AR. Needling therapies in the management of myofascial trigger point pain: a systematic review. Arch Phys Med Rehabil 2001;82:986–92. 10.1053/apmr.2001.24023 [DOI] [PubMed] [Google Scholar]
- 44.Kietrys DM, Palombaro KM, Mannheimer JS. Dry needling for management of pain in the upper quarter and craniofacial region. Curr Pain Headache Rep 2014;18:437 10.1007/s11916-014-0437-0 [DOI] [PubMed] [Google Scholar]
- 45.Tough EA, White AR, Cummings TM, et al. Acupuncture and dry needling in the management of myofascial trigger point pain: a systematic review and meta-analysis of randomised controlled trials. Eur J Pain 2009;13:3–10. 10.1016/j.ejpain.2008.02.006 [DOI] [PubMed] [Google Scholar]
- 46.Li LT, Ge HY, Yue SW, et al. Nociceptive and non-nociceptive hypersensitivity at latent myofascial trigger points. Clin J Pain 2009;25:132–7. 10.1097/AJP.0b013e3181878f87 [DOI] [PubMed] [Google Scholar]
- 47.Wang YH, Ding XL, Zhang Y, et al. Ischemic compression block attenuates mechanical hyperalgesia evoked from latent myofascial trigger points. Exp Brain Res 2010;202:265–70. 10.1007/s00221-009-2129-2 [DOI] [PubMed] [Google Scholar]
- 48.Wang G, Gao Q, Hou J, et al. Effects of temperature on chronic trapezius myofascial pain syndrome during dry needling therapy. Evid Based Complement Alternat Med 2014;2014:638268 10.1155/2014/638268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Srbely JZ, Dickey JP, Lee D, et al. Dry needle stimulation of myofascial trigger points evokes segmental anti-nociceptive effects. J Rehabil Med 2010;42:463–8. 10.2340/16501977-0535 [DOI] [PubMed] [Google Scholar]