Abstract
Background
Recent clinical and experimental studies have confirmed the effects of Xinfuli Granule (XG), a compound Chinese medicine in the prevention and treatment of heart failure (HF). This study aimed to investigate the effects and the mechanisms of XG on ventricular reconstruction in rats with acute myocardial infarction (AMI).
Methods
Sprague-Dawley rats were subjected to left anterior descending branch ligation. The rats that survived 24 h were randomly assigned to five groups: medium-dose of XG group (MI+XGM), high-dose of XG group (MI+XGH), carvedilol group (MI+C), medium-dose of XG + carvedilol group (MI+C+XGM). Fourteen rats underwent identical surgical procedures without artery ligation, serving as sham controls. At 28 days, left ventricular weight to body weight (LVW/BW) and heart weight to body weight (HW/BW) were calculated; left ventricular ejection fraction (LVEF), left ventricular shortening fraction (LVFS), left ventricular internal diameter at systole (LVIDS) were measured by ultrasound; HE staining, Masson staining, and Sirius red staining were used to assess the myocardial pathological and physiological changes as well as myocardial fibrosis area and non-infarct zone I/III collagen ratio. Expression of Smad3 were detected and analyzed by Western blot, immunohistochemistry and immunofluorescence. P-Smad3, Smad2 and Smad7 in the TGF-β/Smads signaling pathway were also analyzed by Western blot.
Results
The LVIDS (P < 0.01), HW/BW (P < 0.05), type I/III collagen ratio (P < 0.01) and myocardial collagen (P < 0.01) decreased significantly while the LVW/BW, LVFS (P < 0.05) increased significantly in MI+XGM group as compared with those in other groups. The expression of key signal molecules of the TGF-β/Smads signaling pathway, including Smad3, P-Smad3 and Smad2 protein were decreased, while the expression of Smad7 increased in both XG and carvedilol treatment groups as compared to those of the MI group (all P < 0.01). Immunohistochemistry and immunofluorescence further confirmed the down-regulated Smad3 expression.
Conclusion
XG can improve ventricular reconstruction and inhibit myocardial fibrosis in rats with AMI by regulating TGF-β/Smads signaling pathway.
Keywords: Acute myocardial infarction, Myocardial fibrosis, TGF-β/Smads signaling pathway, Ventricular remodeling, Wnt/β-catenin signaling pathway, Xinfuli Granule
1. Introduction
The prognosis and outcome after acute myocardial infarction (AMI) are related to multiple processes including myocyte hypertrophy, necrosis and apoptosis as well as ventricular reconstruction and fibrosis.[1] Myocardial ischemic injury causes myocardial hypertrophy, reactive hypertrophy and elongation of infarct ventricular wall and deformation accompanied with cardiac function. Hyperplasia of fibroblasts and vascular structure changes are to a great extent found in myocardial interstitial reconstruction.[2] Myocardial fibrosis leads to excessive deposition of extracellular matrix with significantly increased concentration and volume of various collagens. The TGF-β/Smads signaling pathway is recognized as the most critical signaling pathways of myocardial fibrosis.[3] Xinfuli Granule (XG) is a compound traditional Chinese medicine which has been used in Fuwai Hospital (Beijing, China) for the treatment of heart failure (HF). Previous experimental studies showed that XG had certain protective effects on myocardial cells of rats with HF,[4],[5] but the effects and underlying molecular mechanism of XG in preventing or reversing ventricular reconstruction and myocardial fibrosis remained unclear.
This study aimed to investigate the effects and the mechanisms of XG on ventricular reconstruction in rats with AMI.
2. Methods
2.1. Animal model and grouping
Sprague-Dawley male rats (SPF) weighting 200 ± 20 g provided by Beijing Vital River Laboratory Animal Technology Co., Ltd. were prepared for AMI models. Ultrasonic echocardiography was used immediately after modeling, and successful modeling was judge by left ventricular ejection fraction (LVEF < 60%). By confirming successful modeling, the rats were randomly divided into six groups: medium-dose of XG group (MI+XGM, n = 13), high-dose of XG group (MI+XGH, n = 12), carvedilol control group (MI+C, n = 12), medium-dose of XG group + carvedilol (MI+C+XGM, n = 13), sham group (n = 14) and MI group (n = 11).
2.2. Establishment of AMI models in rats
Rats were given 10% chloral hydrate (i.p.) for anesthesia. The left chests of the rats were prepared, and given endotracheal intubation in supine position. The rats were connected with breathing ventilators for assisted respiration under ECG monitoring. The skin, subcutaneous tissue and muscles were cut. The catheter entered the chest from the 4th intercostal space. The level of the lower edge of left atrial appendage was positioned as the mark to ligature the left anterior descending branch, with the local color of the anterior cardiac wall changing to pale. ECG showed ST-T changes after ligation. When the color of the anterior wall of left ventricle changed to pale, and the ventricular wall motion decreased, the model was confirmed successfully established. The sham group was only given threading instead of ligation. Ultrasonic echocardiography screening was used after successful modeling. The model inclusion criteria were: (1) objective indicators, LVEF < 60% recorded as a baseline; and (2) subjective indicators, obvious abnormal contraction of anterior ventricular wall in at least one level judged by the ultrasonic practitioner.
2.3. Experimental drug dosage
According to related literature[6] and the rat conversion coefficient (6.3) of the human clinical dosage from experimental zoology, the adult clinical dosage of XG is 15 g/d, so the lavage dose of the medium-dose group was 1.50 mg/kg per day and of the high-dose group was 3.00 mg/kg per day. The rats were given lavage once every morning, for 28 consecutive days. The rats were given carvedilol 10 mg/kg per day; all the required doses were prepared in physiological saline (10 mL/kg) and then administrated via lavage at a fixed time. The sham group and the model groups were given equivalent normal saline, 24 h after successful modeling.
2.4. Hemodynamics and cardiac function parameters
The rats were put in the supine position after weighted and anesthetized, with chest hair removed. M type Doppler ultrasound was used to monitor the cardiac function changes about the standard short-axis plane of left ventricular papillary muscles and the long-axis plane of left ventricle. For each raw data, an average value of three consecutive cardiac cycles was selected. The single blind method was adopted to record LVEF, left ventricular shortening fraction (LVFS), and left ventricular internal diameter at systole (LVIDS). Rats hearts were taken out, and the blood was discarded; the hearts were flushed with pre-cooled saline, and dried by filter paper. Heart weight (HW) was recorded. The left ventricle was cut completely and weighted to obtain the left ventricular weight (LVW). The LVW/body weight (BW) and HW/BW were calculated, respectively.
2.5. Tissue sample preparation
The great vessels and left and right auricle were removed. The heart was transected along the left ventricular infarction. HE staining was used to observe the pathological physiology changes of myocardial tissues. Masson staining was used to observe the myocardial interstitial fibrosis, and to calculate the collagen fraction, the percentage of the collagen fiber accounting for the total infarction area under 100 × vision. The ratio of collagen I/III in the non-infarction was calculated by Sirius red staining. Parts of the myocardium were fixed by myocardial liquid, and preserved under −80°C. Western blot analysis was used to detect the expressions of Smad3, P-Smad3, Smad2 and Smad7, the key signaling molecule proteins of TGF-β/Smads signal pathways. Immunohistochemical staining and immunofluorescence were also used to verify the expressions of Smad3.
2.6. Statistical analysis
All data were presented as mean ± SD. Homogeneity tests of variance were carried out on the sample mean values. Least significant difference (LSD) was used on the sample mean values with equal variance. For sample mean values with unequal variance, the Kruskal-Wallis method of multiple rank-sum inspection was used. Rank-based variance analysis for pair-wise comparison was also used. Statistical analysis was performed with SPSS 16.0 (Santa Cruz, California, USA). A P value of less than 0.05 was considered as statistically significant.
3. Results
3.1. Gross observation of the condition before and after treatment
Among the 120 successful modeling rats, 79 survived, with an overall survival rate of 65.8%. Rats of the sham group were sensitive in response, quick in action, with luster fur. Rats of all the groups decreased in activity in the first week of lavage. A few rats were extremely weak, three rats developed concurrently with ascites and death; the rats started to improve in spirit after 1–2 weeks and symptoms basically disappeared on the 18th day, and BWs were increased. One rat died due to various reasons. After 28-day intervention, a total of 75 rats survived: 13 rats in the MI+XGM group, 12 rats in the MI+XGH group, 12 rats in the MI+C group, 13 rats in the MI+C+XGM group, 14 rats in the sham group and 11 in the MI group.
Rat hearts of each modeling group were slightly enlarged during sampling and severely adhered to the pleural cavity. The overall ventricle of the infarction area was yellow-white, dry and lackluster. The left ventricular chamber wall often collapses, with interaction of infarct and non-infarct myocardium. The heart was cut longitudinally, and different degrees of thinning in the infarction ventricular wall, with necrosis involving layers of ventricular wall, were found.
3.2. Changes of ventricular reconstruction hemodynamics and cardiac function parameters
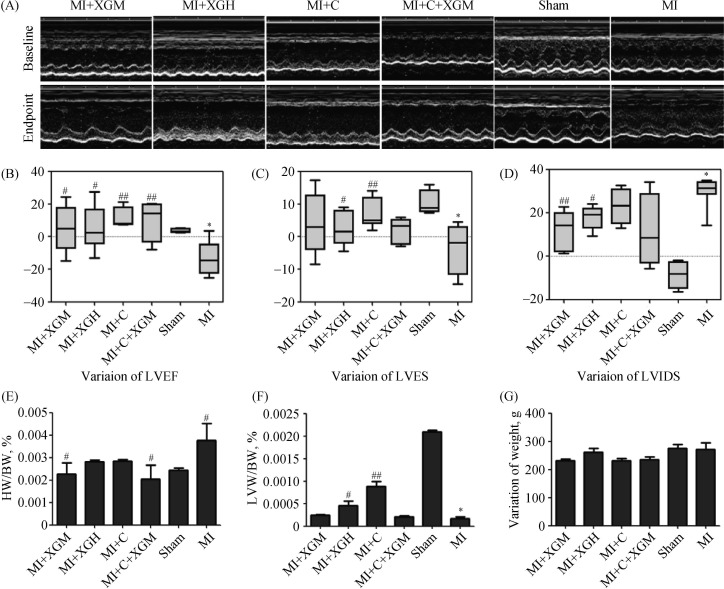
As shown in Figure 1, compared with the sham group, LVEF and LVFS were obviously decreased in the MI group (P < 0.01), and LVIDS was increased significantly (P < 0.01). Compared with the MI group, the LVEF of the MI+XGM and MI+XG groups increased (P < 0.05), a more significantly increased were found in the MI+C and MI+C+XGM groups; LVFS was increased in the MI+C group (P < 0.01) while LVIDS was significantly decreased in the MI+XGM group (P < 0.01); both LVFS and LVIDS were changed in the MI+XGH group (P < 0.05).
Figure 1. Changes of hemodynamics and cardiac function parameters in rats of each group after AMI.
(A): ECG of ventricular wall motion of each group at baseline and after treatments; (B–D): changes of LVEF, LVFS and LVIDS before and after the treatments; (E–F): comparison on HW/BW and LVW/BW in each group; (G): changes of BW before and after treatments. *P < 0.05, compared with the Sham group; #P < 0.05 and ##P < 0.01 compared with the MI group. AMI: acute myocardial infarction; BW: body weight; LVW: left ventricular weight MI+C: carvedilol group; MI+C+XGM: medium-dose of XG + carvedilol group; MI+XGH: high-dose of XG group; MI+XGM: medium-dose of XG group; XG: Xinfuli granule group.
There were no significant differences in BW between each group before (baseline) and after the treatments (28th day). Compared with the sham group, HW/BW was higher in the MI group (P < 0.05), and LVW/BW was at a low level (P < 0.01). Compared with the MI group, HW/BW was decreased in the MI+XGM and MI+C+XGM groups (P < 0.05); LVW/BW was increased in the MI+XGM group, and significantly increased in MI+C group (P < 0.01).
3.3. Histopathological changes in the structure of the infarction area
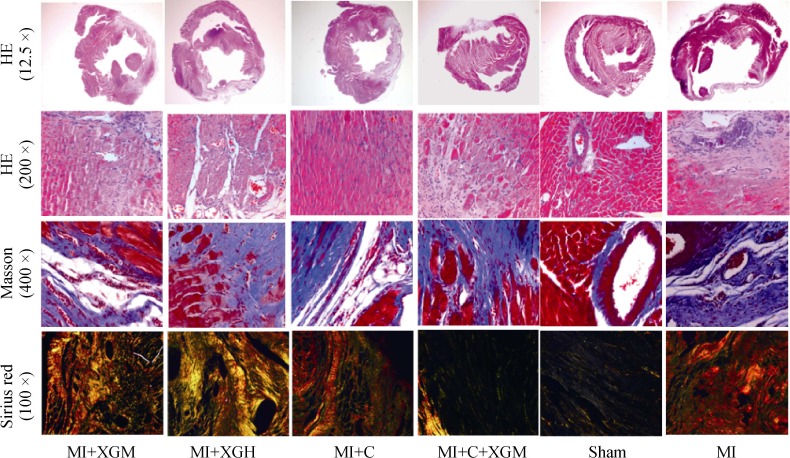
HE staining results showed that there were significant left ventricular pathophysiology changes in rats of the model groups compared with the sham group; part of the myocytes at the infarction edge had hypertrophy and elongation, and part of the left ventricular wall had myocardial coagulation necrosis. Cytoplasm was deeply stained by eosin; and some parts appeared loosening or edema, enlargement, degeneration, dissolved or necrosis of myocardial cells, myofibril twist, inflammatory cells infiltration, myocardial fiber rupture, and extensive necrotic lesions. Most lesions were absorbed in the treatment groups; individuals still had a small amount of inflammatory cells infiltration, hyperplasia of fibrous tissue, and myofibril dissolution (Figure 2); Masson staining results showed that compared with the sham group, the myocardial collagen fibers were obviously increased in the MI group, with fibrous scar formation and larger myocardial collagen area. Compared with the MI group, there was a certain degree of alleviation in the MI+C+XGM (P < 0.05); the MI+XGH and MI+C groups were most obvious (P < 0.01), but there were no significant statistical differences between the two groups (P > 0.05) (Table 1). Sirius red staining results showed that type I collagen and type III collagen were significantly increased in the infarction area in the MI group (P < 0.01) compared with the sham group. There were obvious deposition of type I and III collagen in each group, including smaller areas of myocardial collagen in the MI+C group, and the lower ratio of type I/III collagen (P < 0.01), compared with the MI Group (Table 1).
Figure 2. Pathological changes of the infarction area in rats with AMI of each group under HE staining (12.5 ×/200 ×), Masson staining (400 ×), and Sirius red staining (100 ×).
AMI: acute myocardial infarction; MI+C: carvedilol group; MI+C+XGM: medium-dose of XG + carvedilol group; MI+XGH: high-dose of XG group; MI+XGM: medium-dose of XG group; XG: Xinfuli granule group.
Table 1. Changes of ratio of collagen area and type I/III collagen in the infarct border zone in rats with AMI of each group.
| Groups | n | Collagen area in infarction area (Masson 100 ×) | Type I/III collagen (Sirius Red 100 ×) |
| MI+XGM | 13 | 47.88 ± 6.23 | 2.25 ± 0.26 |
| MI+XGH | 12 | 25.72 ± 4.18## | 4.87 ± 0.31 |
| MI+C | 12 | 28.61 ± 10.38## | 0.61 ± 0.39## |
| MI+C+XGM | 13 | 30.01 ± 8.34# | 4.81 ± 0.24 |
| Sham | 14 | 8.42 ± 9.70 | 0.85 ± 0.25 |
| MI | 11 | 44.54 ± 10.91* | 1.63 ± 0.16* |
Data are presented as mean ± SD. *P < 0.05 compared with the Sham group; #P < 0.05 and ##P < 0.01 compared with the MI group. AMI: acute myocardial infarction; MI+C: carvedilol group; MI+C+XGM: medium-dose of XG + carvedilol group; MI+XGH: high-dose of XG group; MI+XGM: medium-dose of XG group; XG: Xinfuli granule group.
3.4. Expressions of TGF-β/Smads signal pathways
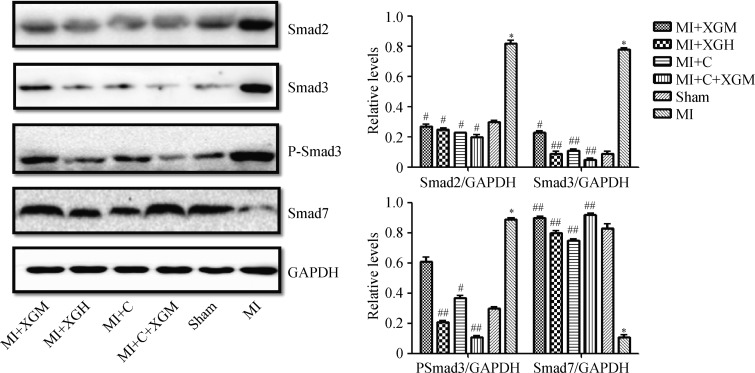
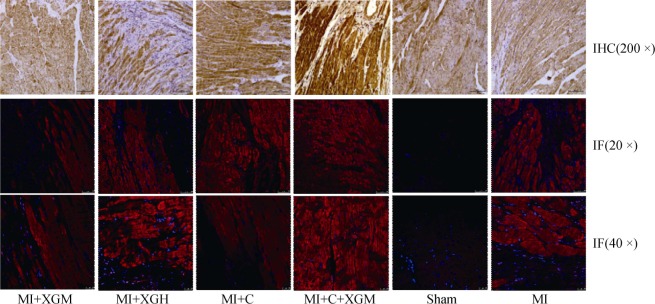
Western blot was used to analyze and detect the key signal molecules including Smad3, P-Smad3, Smad2 and Smad7 in TGF-β/Smads pathways in the infarction area in rats with AMI of each group (Figure 3). Smad3, P-Smad3 and Smad2 in the MI group showed a high expression level while the expression of Smad7 in the MI Group was significantly down-regulated (P < 0.01), compared with the Sham group. The expressions of key signal molecules including Smad3, P-Smad3 and Smad2 proteins in TGF-β/Smads pathways of each group were down-regulated at different levels and the expression of Smad7 was up-regulated (P < 0.01), compared with the MI Group. Immunohistochemical staining and immunofluorescence were used to respectively verify the expressions of Smad3 in groups, which were generally the same with that of Western blot (Figure 4).
Figure 3. Expression of key proteins in TGF-β/Smads signaling pathway in the infarction area in rats with AMI.
*P < 0.05 compared with the Sham group; #P < 0.05 and ##P < 0.01 compared with the MI group. AMI: acute myocardial infarction; MI+C: carvedilol group; MI+C+XGM: medium-dose of XG + carvedilol group; MI+XGH: high-dose of XG group; MI+XGM: medium-dose of XG group; XG: Xinfuli granule group.
Figure 4. Protein expressions of Smad3 detected by IHC staining and IM in each group.
IF: immunofluorescence; IHC: immunohistochemical; MI: acute myocardial infarction; MI+C: carvedilol group; MI+C+XGM: medium-dose of XG + carvedilol group; MI+XGH: high-dose of XG group; MI+XGM: medium-dose of XG group; XG: Xinfuli granule group.
4. Discussion
In the present study, we showed that XG can improve ventricular reconstruction and inhibit myocardial fibrosis in rats with AMI. This effect may related to the regulation of TGF-β/Smads signaling pathway. High dose of XG has a more significant effect over medium dose.
Changes of ventricular walls in the infarction area of rats with AMI in each group reflected the generation of ventricular remodeling, including a series of processes such as myocardial thinning and elongation of ventricular wall in infarction area, progressive expansion of the left ventricle and deformation with decreasing cardiac function caused by infarct expansion and the reactive hypertrophy and elongation of myocardium of ventricular wall in the non-infarction area.[7]
The very complicated formation mechanism of myocardial fibrosis is the result of collagen synthesis metabolism and degradation mechanism imbalance.[8] For instance, much of the myocardial collagen was deposited due to changes of ratio of type I and III collagen contents caused by the loss of myocardial cells and myocardial interstitial cells in the case of HF, and the activation of myocardial fibroblasts including proliferation and phenotype shift as well as changes of collagen components through the synthesis and secretion of myocardial fibroblasts. In addition to the down-regulated expression and decreased activity of matrix metalloproteinase promoting collagen degradation, the synthesis of collagen was greatly increased and the degradation of collagen was greatly decreased, which led to the occurrence of myocardial fibrosis, which was a process of continuous changes.[9] Mesenchyme outside myocardial cells was mainly a co-adjacent and complicated three-dimensional spatial network structure formed by collagen, fibronectin and so on. Its main components were type I and III collagen. The increasing expression of the two and inconstant increased rate were reasons for left ventricular dysfunction.[10] This research found that myocardial collagen fibers in the infarction area were increased significantly. Fibrotic scars were formed. The area of myocardial collagen was large; the deposition of type I and III collagen was obvious; type I collagen played a supporting role and maintained normal movement; type III collagen determined the diameter and flexibility of collagen fibers.[11],[12] In the model groups, much of collagen was deposited, among which type I collagen was in the majority and led to the poor elasticity of ventricular wall. After the intervention of XG, collagen fibers were significantly decreased and the proportion of type I and III collagen was reduced. Ventricular wall had certain ability in compensatory movement.
Several previous studies had investigated effects of Chinese medicine on the myocardial fibrosis of ventricular remodeling in rats with AMI.[13]–[15] Our present study showed that XG significantly increased LVEF, LVFS and LVW/ BW and was able to improve the ventricular remodeling of experimental rats with AMI and restrain myocardial fibrosis. It had similar effect with carvedilol. It could be concluded that the high dose of XG has a more significant effect over medium dose. Carvedilol is a third generation β receptor blocker and the first one approved to treat HF after MI by FDA. In recent years, clinical researches have showed that carvedilol can not only reduce the mortality rate of patients with HF and improve prognosis, but also prevent, and even reverse, the left ventricular remodeling of patients with ischemic HF.[16] In this research, the combination of medium-dose XG and carvedilol was not superior to the single application of carvedilol. This remains to be further studied.
TGF-β/Smads signal transduction pathways were closely related to cardiovascular diseases.[17] The activated transforming growth factor β could promote the transformation and proliferation of myocardial fibroblasts through phosphorylation of the induced Smads proteins. Smads proteins were the downstream signal molecules of silk/threonine kinase receptors and also the only substrate of intracellular kinase in TGF-β receptor and possessed the effect of transcriptional activation. The research results were in line with expectations. Expressions of Smad2, Smad3 and PSmad3 were up-regulated in rats with AMI, restraining the reduction of protein Smad7. This indicated that TGF-β/Smads signal transduction pathways participated in the ventricular remodeling process of experimental AMI rats. XG could significantly down-regulate the expressions of Smad2, Smad3 and PSmad3 proteins caused by AMI in the left ventricle and restrain the elevation of protein Smad7. It gave a hint that medication might intervene with the ventricular remodeling process of experimental AMI rats through regulating TGF-β/Smads signal transduction pathways. Immunohistochemical staining and immunofluorescence were used to confirm the expression of Smad3 in each group. Although it is not flawless, it was consistent with Western blot.
Acknowledgments
This study was supported by the grants from the “Ten Chinese Medicine for Ten Diseases” Project of Beijing, China (SBSY2013-005), National Science Foundation of China (81541010) and Capital Medical Development Scientific Research Fund (2014-4-4035).
References
- 1.Weisman HF, Bush DE, Mannisi JA, et al. Global cardiac remodeling after acute myocardial infarction: a study in the rat model. J Am Coll Cardiol. 1985;5:1355–1362. doi: 10.1016/s0735-1097(85)80348-x. [DOI] [PubMed] [Google Scholar]
- 2.Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 2010;122:2727–2735. doi: 10.1161/CIRCULATIONAHA.110.942268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zelarayan LC, Noack C, Zafiriou MP, et al. Wnt signaling molecules in left ventricular remodeling: focus on dishevelled 1. Hypertension. 2010;55:852–854. doi: 10.1161/HYPERTENSIONAHA.109.143297. [DOI] [PubMed] [Google Scholar]
- 4.Lu PP, Ma J, Liang XP, et al. Xinfuli improves cardiac function, histopathological changes and attenuate cardiomyocyte apoptosis in rats with doxorubicin-induced cardiotoxicity. J Geriatr Cardiol. 2016;13:968–972. doi: 10.11909/j.issn.1671-5411.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang WH, Huang Y. Cardiotonic modulation in heart failure: insights from traditional Chinese medicine. J Am Coll Cardiol. 2013;62:1073–1074. doi: 10.1016/j.jacc.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maekawa Y, Anzai T, Yoshikawa T, et al. Effect of granulocyte-macrophage colony-stimulating factor inducer on left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol. 2004;44:1510–1520. doi: 10.1016/j.jacc.2004.05.083. [DOI] [PubMed] [Google Scholar]
- 7.Sun L, Cui M, Wang Z, et al. Mesenchymal stem cells modified with angiopoietin-1 improve remodeling in a rat model of acute myocardial infarction. Biochem Biophys Res Commun. 2007;357:779–784. doi: 10.1016/j.bbrc.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Abbate A, Bussani R, Sinagra G, et al. Right ventricular cardiomyocyte apoptosis in patientswith acute myocardial infarction of the left ventricular wall. Am J Cardiol. 2008;102:658–662. doi: 10.1016/j.amjcard.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu M, Wang XR, Wang C, et al. Panax quinquefolium saponin attenuates ventricular remodeling after acute myocardial infarction by inhibiting chop-mediated apoptosis. Shock. 2013;40:339–344. doi: 10.1097/SHK.0b013e3182a3f9e5. [DOI] [PubMed] [Google Scholar]
- 10.Mckay RG, Pfeffer MA, Pasternak RC, et al. Left ventricular remodeling after myocardial infarction: a corollary to infarct expansion. Circulation. 1986;74:693–702. doi: 10.1161/01.cir.74.4.693. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y, Jia ZM, Wang W, et al. Protective effect of spironolactone onventricular remodeling after acute myocardial infarction in rats. J Chin Med Univ. 2006;35:487–490. [Article in Chinese] [Google Scholar]
- 12.Ertl G, Gaudron P, Hu K. Ventricular remodeling after myocardial infarction. Experimental and clinical studies. Basic Res Cardiol. 1993;88(Suppl 1):S125–S137. doi: 10.1007/978-3-642-72497-8_9. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Peter K, Shi D, et al. Traditional formula, modern application: chinese medicine formula sini tang improves early ventricular remodeling and cardiac function after myocardial infarction in rats. Evid Based Complement Alternat Med. 2014;2014:141938. doi: 10.1155/2014/141938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Xu L, Qiao Z, et al. YiXin-Shu, a Sheng Mai-San based traditional Chinese medicine formula, attenuates myocardial ischemia/reperfusion injury by suppressing mitochondrial mediated apoptosis and upregulating liver-X-receptor alpha. Sci Rep. 2016;6:23025. doi: 10.1038/srep23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tong JY, Xu YJ, Bian YP, et al. Effect and mechanism of Qishen Yiqi Pills on adriamycin-induced cardiomyopathy in mice. Chin J Nat Med. 2013;11:514–518. doi: 10.1016/S1875-5364(13)60093-X. [DOI] [PubMed] [Google Scholar]
- 16.Yaoita H. Different effects of carvedilol, metoprolol, and propranolol on left ventricular remodeling after coronary stenosis or after permanent coronary occlusion in rats. Circulation. 2002;105:975–980. doi: 10.1161/hc0802.104503. [DOI] [PubMed] [Google Scholar]
- 17.Zelarayan LC, Noack C, Zafiriou MP, et al. Wnt signaling molecules in left ventricular remodeling: focus on dishevelled 1. Hypertension. 2010;55:852–854. doi: 10.1161/HYPERTENSIONAHA.109.143297. [DOI] [PubMed] [Google Scholar]