Abstract
The bifunctional wax ester synthase/acyl coenzyme A (acyl-CoA):diacylglycerol acyltransferase (WS/DGAT) from Acinetobacter sp. strain ADP1 (formerly Acinetobacter calcoaceticus ADP1) mediating the biosyntheses of wax esters and triacylglycerols was used for the in vivo and in vitro biosynthesis of thio wax esters and dithio wax esters. For in vitro biosynthesis, 5′His6WS/DGAT comprising an N-terminal His6 tag was purified from the soluble protein fraction of Escherichia coli Rosetta(DE3)pLysS (pET23a::5′His6atf). By employing SP-Sepharose high-pressure and Ni-nitrilotriacetic acid fast-protein liquid chromatographies, a 19-fold enrichment with a final specific activity of 165.2 nmol mg of protein−1 min−1 was achieved by using 1-hexadecanol and palmitoyl-CoA as substrates. Incubation of purified 5′His6WS/DGAT with 1-hexadecanethiol and palmitoyl-CoA as substrates resulted in the formation of palmitic acid hexadecyl thio ester (10.4% relative specific activity of a 1-hexadecanol control). Utilization of 1,8-octanedithiol and palmitoyl-CoA as substrates led to the formation of 1-S-monopalmitoyloctanedithiol and minor amounts of 1,8-S-dipalmitoyloctanedithiol (59.3% relative specific activity of a 1-hexadecanol control). The latter dithio wax ester was efficiently produced when 1-S-monopalmitoyloctanedithiol and palmitoyl-CoA were used as substrates (13.4% specific activity relative to that of a 1-hexadecanol control). For the in vivo biosynthesis of thio wax esters, the knockout mutant Acinetobacter sp. strain ADP1acr1ΩKm, which is unable to produce fatty alcohols, was used. Cultivation of Acinetobacter sp. strain ADP1acr1ΩKm in the presence of gluconate, 1-hexadecanethiol, and oleic acid in nitrogen-limited mineral salts medium resulted in the accumulation of unusual thio wax esters that accounted for around 1.19% (wt/wt) of the cellular dry weight and consisted mainly of oleic acid hexadecyl thioester as revealed by gas chromatography-mass spectrometry.
Polyhydroxyalkanoic acids (PHA) in bacteria represent the most abundant group of neutral storage lipids, which serve as intracellular carbon and energy storage compounds (23). In addition, triacylglycerols (TAGs) and wax esters also occur as bacterial storage lipids, though at a much lower frequency than in eukaryotic microorganisms (22). Significant TAG accumulation was found, particularly in species belonging to the class Actinomycetes (1), as was reported for the genera Mycobacterium (3), Nocardia, and Streptomyces (2). TAGs are synthesized by the acyl coenzyme A (acyl-CoA):diacylglycerol acyltransferase (DGAT) (13), which catalyzes the esterification of diacylglycerol with long-chain acyl-CoA. Wax esters are oxoesters of primary long-chain fatty alcohols and long-chain fatty acids. Occurrence of wax esters has been frequently reported for various Acinetobacter species (6). Under growth-limiting conditions, Acinetobacter calcoaceticus accumulates wax esters intracellularly as insoluble inclusions. The chemical structure of the wax esters synthesized by A. calcoaceticus is similar to those of wax esters produced by the jojoba plant (Simmondsia chinensis) and the sperm whale (Physeter macrocephalus), with a carbon chain length of mainly C32 and C34 compounds comprising saturated and unsaturated C16 and C18 fatty acids and fatty alcohol moieties (5).
The biosynthetic pathway of wax esters in Acinetobacter sp. strain ADP1 consists of three different enzymatic steps. Initially, an NADPH-dependent long-chain acyl-CoA reductase catalyzes the reduction of long-chain acyl-CoA to the corresponding fatty aldehyde. These aldehydes are further reduced to fatty alcohols by an NADPH-dependent fatty aldehyde reductase. Finally, fatty alcohol is esterified with acyl-CoA by acyl-CoA:fatty alcohol acyltransferase (wax ester synthase [WS]), forming the wax ester. In addition to wax esters, the Acinetobacter sp. strain also accumulates small amounts of TAGs (18). Recently, we identified a bifunctional enzyme from Acinetobacter sp. strain ADP1 that exhibited WS and DGAT activities at the same time (9). Analysis of a knockout mutant demonstrated the key role of this WS/DGAT for storage lipid accumulation in Acinetobacter sp. strain ADP1, since virtually all of the wax ester and most of the TAG biosynthesis is mediated by this bifunctional enzyme.
The WS/DGAT was biochemically characterized as a rather unspecific enzyme accepting a broad range of various-chain-length saturated and unsaturated acyl-CoAs and fatty alcohols ranging from C12 to C20 as substrates for the WS and/or DGAT reaction (9). The enzyme is also able to use α,ω-alkanediols and monoacylglycerols as alternative acyl acceptors, resulting in an unusual wax diester and in 1,2- and 1,3-diacylglycerols, respectively (10). Due to the apparent broad substrate range of WS/DGAT, it was hypothesized that the substrate spectrum of this enzyme may not be limited only to substrates providing hydroxy functions. In the present study, we therefore investigated whether alkanethiols like 1-hexadecanthiol, 1,8-octanedithiol, and 1-S-monopalmitoyloctanedithiol can serve as alternative acyl acceptors in the WS/DGAT-mediated acyl transfer reaction, resulting in the biosynthesis of unusual thio wax esters. Thioesters are used as activated esters (12, 17, 24) for the synthesis of peptides, macrocyclic antibiotics, and various other pharmaceuticals in organic chemistry and, in particular, in the pharmaceutical industry (4, 7, 11, 14, 16, 19).
MATERIALS AND METHODS
Chemicals.
1-Hexadecanethiol and 1,8-octanedithiol were purchased from Sigma-Aldrich-Fluka (Deisenhofen, Germany), and [1-14C]palmitoyl-CoA was purchased from Hartmann Analytik (Braunschweig, Germany). Hexadecyl thioesters of palmitic and oleic acids were prepared in vacuo by solvent-free, lipase-catalyzed esterification of the corresponding fatty acid with 1-hexadecanethiol as described previously (25, 27). 1-S-Monopalmitoyloctanedithiol and 1,8-S-dipalmitoyloctanedithiol, respectively, were synthesized and purified as described by Weber et al. (28).
Strains, plasmids, media, and growth conditions.
In this study, the acyl-CoA reductase knockout mutant Acinetobacter sp. strain ADP1acr1ΩKm (formerly A. calcoaceticus ADP1acr1ΩKm) (10) and Escherichia coli strain Rosetta(DE3)pLysS (Novagen, Madison, Wis.) were used. The plasmids pBluescript KS− (Stratagene, Heidelberg, Germany) and pET23a (Novagen) were used for cloning purposes.
Cells of Acinetobacter sp. strain ADP1acr1ΩKm were cultivated aerobically in Luria-Bertani (LB) medium (20) in Erlenmeyer flasks at 30°C for 24 h to obtain a high cell density and fast growth. Storage lipid accumulation was induced by cultivating the cells in mineral salts medium (MSM) (21) containing 0.1 g of NH4Cl liter−1 and the indicated carbon sources for another 48 h. These conditions are referred to in this study as storage conditions. For the in vivo production of thio wax esters, cells were cocultivated under storage conditions with 1% (wt/vol) sodium gluconate, 0.3% (vol/vol) 1-hexadecanethiol, and 0.1% (wt/vol) oleic acid. Cells of E. coli Rosetta(DE3)pLysS (pET23a::5′His6atf) were cultivated in LB medium at 37°C. At an optical density (600 nm) of 0.5 to 0.7, isopropyl-β-d-thiogalactopyranoside (IPTG) was added at a final concentration of 1 mM, and cells were harvested after three additional hours of cultivation.
Genetic manipulations.
Standard molecular biological techniques were applied as described by Sambrook et al. (20). For construction of the expression vector pET23a::5′His6atf, the atf gene (for acyltransferase, formerly designated wax/dgat [9]) was amplified from total genomic DNA from Acinetobacter sp. strain ADP1 by tailored PCR with the oligonucleotides 5′-AAGGAGGTATCCACGCTATGCACCACCACCACCACCACCGCCCATTACATCCGATTGAT-3′ (5′ end), introducing six histidine residues at the N terminus of the corresponding gene product, and 5′-TTTGGATCCAGGGCTAATTTAGCCCTTTAGTT-3′ (3′ end). The PCR product was cloned into pBluescript KS−, resulting in pKS::5′His6atf. The modified atf gene was isolated from the hybrid plasmid after restriction with SalI and NotI and was then ligated into the vector pET23a. The resulting expression vector, pET23a::5′His6atf, was used for the transformation of E. coli Rosetta(DE3)pLysS.
Purification of N-terminal His6-tagged WS/DGAT.
Cells from a 5-liter cultivation of E. coli Rosetta(DE3)pLysS (pET23a::5′His6atf) were harvested and washed once with sodium phosphate buffer (125 mM, pH 7.4). The pellet was suspended in sodium phosphate buffer (50 mM, pH 7.4) containing EDTA (1 mM) and glycerol (20%, wt/vol), resulting in a final volume of 42 ml. Cells were disrupted in the presence of 1% (vol/vol) protease inhibitor cocktail (Sigma-Aldrich-Fluka) by ultrasonification, and the soluble protein fraction was obtained by ultracentrifugation at 35,000 × g.
Ion exchange chromatography.
The soluble fraction was applied to a 60-ml SP Sepharose high-pressure (HP) column equilibrated with sodium phosphate buffer (50 mM, pH 7.4) containing EDTA (1 mM) and glycerol (20%, wt/vol) at a flow rate of 2 ml min−1. The bound proteins were washed with 150 ml of sodium phosphate buffer containing 150 mM NaCl. 5′His6WS/DGAT was eluted by an NaCl concentration of 700 mM in 100 ml of sodium phosphate. Fractions were collected and analyzed for WS activity. Fractions with significant WS activity were pooled and charged with protease inhibitor cocktail (1%, vol/vol). The solution was desalted and concentrated with an Amicon (Silver Spring, Md.) ultrafiltration chamber equipped with a YM 30 membrane.
Immobilized metal chelate affinity chromatography.
The concentrate obtained after ion exchange chromatography was diluted with sodium phosphate buffer (50 mM, pH 8) containing 300 mM NaCl, 20% (wt/vol) glycerol, and 5 mM imidazole and was applied to an 8-ml Ni-nitrilotriacetic acid (NTA) Superflow column (QIAGEN GmbH, Hilden, Germany) at a flow rate of 1 ml min−1. Nonbound proteins were removed by washing the column with 50 ml of sodium phosphate buffer (50 mM, pH 8) containing 300 mM NaCl, glycerol (20%, wt/vol), and 5 mM imidazole at a flow rate of 2 ml min−1. Unspecifically bound proteins were removed by a twofold washing step with 50 ml of sodium phosphate buffer containing imidazole at concentrations of 15 and 25 mM, respectively. 5′His6WS/DGAT was eluted with 50 ml of sodium phosphate buffer containing 300 mM imidazole. Protein-containing fractions of the 300 mM imidazole elution step were pooled, charged with protease inhibitors, and concentrated by centrifugation in Vivaspin tubes (cutoff, 10 kDa; Vivascience, Hanover, Germany) at 3,000 × g. The concentrate was radiometrically tested for WS activity and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The purified 5′His6WS/DGAT was used for in vitro enzyme assays.
In vitro enzyme assays with alternative acyl acceptors.
The buffer used for the large-scale in vitro synthesis of various thioesters contained 4.63 mg of bovine serum albumin (BSA) ml−1, 10 mM MgCl2, 200 μM palmitoyl-CoA, 125 mM sodium phosphate buffer (pH 7.4), and 3.75 mM 1,8-octanedithiol in a total volume of at least 2.5 ml. 1,8-Octanedithiol and BSA were applied as a double-concentration stock solution emulsified by ultrasonification. For the reactions, purified 5′His6WS/DGAT was used at a concentration of 0.2 μg ml−1. The assay mixtures were incubated at 35°C for 120 min. The reactions were stopped by extraction with 2 ml of chloroform-methanol (1:1, vol/vol) per ml of assay volume. After centrifugation, the chloroform phase was withdrawn, and thioesters were subsequently extracted as described below.
For determination of the specific enzyme activities of 5′His6WS/DGAT with 1-hexadecanethiol, 1,8-octanedithiol, or 1-S-monopalmitoyloctanedithiol as the acyl acceptor, in vitro enzyme assays were performed as described above but the assay mixtures contained 4.72 μM radiolabeled [1-14C]palmitoyl-CoA (specific activity, 1.961 Bq pmol−1) as the acyl donor in a total volume of 250 μl. These assay mixtures were incubated for 30 min at 35°C. BSA and 1-hexadecanethiol, 1,8-octanedithiol, or 1-S-monopalmitoyloctanedithiol, respectively, were applied as a double-concentration stock solution emulsified by ultrasonification. After extraction with 500 μl of chloroform-methanol (1:1, vol/vol), the chloroform phase was withdrawn, and lipids were separated by thin-layer chromatography (TLC) using hexane-diethyl ether-acetic acid (99:1:1, vol/vol/vol) or hexane-dichloromethane (65:35, vol/vol) as the solvent system for the separation of thio wax esters or monothio- and dithio wax esters, respectively. The radiolabeled reaction products on the TLC plates (Silica Gel 60; Merck, Darmstadt, Germany) were identified by the application of unlabeled synthetic reference substances (palmitic acid hexadecylthiol, 1-S-monopalmitoyloctanedithiol, and 1,8-S-dipalmitoyloctanedithiol) and autoradiography. The activities of the reaction products were measured by scintillation counting using an LS 6500 multipurpose scintillation counter (Beckman Instruments, Fullerton, Calif.).
Extraction of thio wax esters.
Hexadecyl thio wax esters were extracted from lyophilized cells of Acinetobacter sp. strain ADP1 by mixing about 40 mg of dry matter with 0.1 ml of distilled water and extracting this mixture twice with 1.5 ml of dichloromethane-methanol (2:1, vol/vol). i-Hexane (3 ml) was added to the combined extracts, and phases were separated by adding 0.5 to 1 ml of distilled water, followed by centrifugation. The i-hexane layer was removed, concentrated, and used for TLC, gas chromatography (GC), and GC-mass spectrometry (MS) analyses.
Hexadecyl thioesters were extracted from the chloroform extract of in vitro assays by mixing the extract with 1.5 ml of i-hexane per ml, and phases were separated by adding 0.5 to 1 ml of distilled water and then centrifuging. The i-hexane layer was removed, concentrated, and used for TLC, GC, and GC-MS analyses. The chloroform concentrates of in vitro-synthesized 1-S-monopalmitoyloctanedithiol and 1,8-S-dipalmitoyloctanedithiol were dissolved in 150 μl of methyl-tert-butyl ether and used directly for GC and HP liquid chromatography (HPLC) analyses.
TLC.
Aliquots of i-hexane extracts were analyzed by TLC on 0.3-mm-thick layers of Silica Gel H (Merck-VWR International, Darmstadt, Germany) with i-hexane-diethyl ether (99:1, vol/vol) as the solvent system as described previously (26). Similarly, fatty acid hexadecyl thioesters were purified by preparative TLC prior to GC and GC-MS analyses; fatty acid thioesters were eluted from the adsorbent with water-saturated diethyl ether. All lipid fractions were identified by cochromatography with standard substances. Spots were located by iodine staining; alternatively, the TLC plates were sprayed with 30% aqueous sulfuric acid and heated in an oven to 200°C. In addition, the fractions containing thiol groups were detected on TLC plates by spraying with a 0.2% solution of 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) in ethanol-water (2:1, vol/vol).
GC.
Aliquots of lipid extracts were treated with a solution of diazomethane in diethyl ether to convert unreacted or hydrolyzed fatty acids to the corresponding methyl esters. The resulting mixtures of fatty acid methyl esters, fatty acid hexadecyl thioesters or mono- and diacylated 1,8-octanedithiol thioesters, and unreacted 1-hexadecanethiol or 1,8-octanedithiol were analyzed by GC. A Hewlett-Packard (Böblingen, Germany) HP-5890 series II gas chromatograph equipped with a flame ionization detector was used. Separations of hexadecyl thioester extracts were carried out on a Quadrex Corp. (New Haven, Conn.) 400-5HT fused silica capillary column (length, 25 m; inside diameter [i.d.], 0.25 mm; film thickness, 0.1 μm) with hydrogen as the carrier gas (column pressure, 50 kPa). The column temperature was kept at 120°C for 2 min, followed by a linear increase from 120 to 180°C at 5°C min−1 and from 180 to 380°C at 20°C min−1, and was finally kept at 380°C for another 6 min. The split ratio was 1:10. Separations of mono- and diacylated 1,8-octanedithiol thioesters were carried out on a Quadrex 400-1HT fused silica capillary column (length, 15 m; i.d., 0.25 mm; film thickness, 0.1 μm) with hydrogen as the carrier gas (column pressure, 45 kPa). The column temperature was kept at 80°C for 6 min, followed by a linear increase from 80 to 400°C at 20°C min−1, and was finally kept at 400°C for 2 min. Injector and detector temperatures were maintained at 380°C. Peaks in GC were assigned by comparison of their retention times with those of known standards (25, 28). Peak areas and percentages were calculated using Hewlett-Packard 3365 series GC ChemStation software. Stereomutation of oleic to elaidic acid was checked by GC after conversion of purified thioesters to methyl esters by using the GC conditions described above. Separations were carried out on a 60-m-long DB-23 capillary column (0.25-mm i.d., 0.25-μm film thickness; J & W, ASS-Chem, Bad Homburg, Germany) with the following temperature program: from 150°C at 6°C min−1 to 180°C (isothermal for 18 min) and then at 20°C min−1 to 250°C (isothermal for 10 min).
GC-MS.
GC-MS analysis of the hexadecyl thioesters was carried out using the electron ionization mode (70 eV) of a model 5890 series II/5989 A apparatus (Hewlett-Packard) equipped with an HT-5 capillary column (length, 12 m; i.d., 0.22 mm; film thickness, 0.1 μm; SGE, Darmstadt, Germany). Helium at a flow rate of 1 ml min−1 was used as the carrier gas. The column temperature was initially kept at 120°C for 2 min and then programmed to increase from 120 to 200°C at 5°C min−1 and further from 200 to 350°C at 20°C min−1. The final temperature was held for 4.5 min. The split ratio was 1:10 at a temperature of 380°C, an interface temperature of 380°C, and an ion source temperature of 200°C.
HPLC analysis.
Aliquots of in vitro-synthesized mono- and diacylated 1,8-octanedithiol thioesters were dissolved in chloroform and analyzed for their composition by HPLC as follows. The HPLC system consisted of a Merck-Hitachi pump (model L-6200; E. Merck) equipped with a column oven (VDS Optilab, Berlin, Germany) set at 40°C, a Kontron Instruments (Milan, Italy) UV-visible-light HPLC 332 detector (E. Merck) set to a wavelength of 210 nm, and an Alltech (Unterhaching, Germany) evaporative light scattering detector model 500 (thermostat set to 60°C), which were used in series. UV and mass traces were monitored and evaluated in a KromaSystem 2000 data acquisition unit (Kontron Instruments). Mono- and di-S-palmitoyl esters of 1,8-octanedithiol, palmitic acid, or methyl palmitate were separated at 40°C with a mixture of acetonitrile-tetrahydrofurane (THF; 85:15, vol/vol) isocratically until 12 min was reached and then for 8 min by using a gradient (85:15 to 60:40, vol/vol), followed by 5 min isocratically (60:40, vol/vol) and then for 5 min by using a gradient (60:40 to 100% THF, vol/vol), and finally isocratically (100% THF) until 35 min was reached on a LiChrospher RP-18 end-capped column (length, 250 mm; i.d., 4 mm; particle size, 5 μm; Phenomenex, Aschaffenburg, Germany) and a precolumn. The flow rate was set at 0.8 ml min−1. Injections (around 20 to 60 μg) of the reaction mixture in acetonitrile-THF (1:1, vol/vol) were carried out with a Rheodyne 7161 sample injector (Rhonert Park, Calif.) equipped with a 20-μl sample loop. Peak areas and percentages were calculated using KromaSystem 2000 software.
Peaks in HPLC runs were assigned by comparison of their retention times with those of known standards. Response factors of evaporative light scattering detection were determined for 1-S-monopalmitoyloctanedithiol and 1,8-S-dipalmitoyloctanedithiol by using purified compounds as internal standards, which were prepared as described previously (28).
RESULTS
Purification of N-terminal His6-tagged WS/DGAT.
In order to obtain a pure enzyme preparation for enzymatic studies and in vitro synthesis, N-terminally His6-tagged WS/DGAT was heterologously expressed in recombinant E. coli Rosetta(DE3)pLysS to allow its fast and simple enrichment from the soluble protein fraction of the crude extract. However, affinity chromatography on Ni-NTA agarose alone resulted in only poor purity (data not shown). Due to the strong basic character of 5′His6WS/DGAT (theoretical pI of 9.05), cation-exchange chromatography on SP-Sepharose HP plates could be efficiently used to remove large quantities of contaminating proteins. Finally, after two consecutive chromatography steps on SP-Sepharose HP and Ni-NTA agarose gels, 5′His6WS/DGAT was purified to apparent homogeneity as estimated by SDS-PAGE analysis (Fig. 1). The enzyme was enriched 19-fold from the soluble protein fraction with a yield of 13.4% (Table 1) and exhibited a specific activity of 165.2 nmol mg−1 min−1 with 1-hexadecanol as the substrate.
FIG. 1.
SDS-PAGE analysis of the purification of 5′His6WS/DGAT. A total of 30 μg of proteins of the crude extract or of the soluble protein fraction or 10 μg of proteins from each chromatography step was applied to the gel. Lanes: St, standard proteins; 1, crude extract of E. coli Rosetta(DE3)pLysS (pET23a::5′His6atf); 2, soluble fraction of E. coli Rosetta(DE3)pLysS (pET23a::5′His6atf); 3, SP-Sepharose HP concentrate; 4, Ni-NTA concentrate.
TABLE 1.
Chromatographic enrichmenta of 5′His6WS/DGAT from the soluble protein fraction of E. coli Rosetta(DE3)pLysS (pET23a::5′His6atf)
| Purification step | Total protein (mg) | Specific enzyme activity (nmol mg of protein−1 min−1) | Enrichment factor | % Yield |
|---|---|---|---|---|
| Soluble fraction | 1,828.4 | 8.6 | 1 | 100 |
| SP-Sepharose concn | 182.6 | 19.5 | 2.3 | 22.6 |
| Ni-NTA concn | 12.8 | 165.2 | 19.2 | 13.4 |
Values are averages of two independent measurements.
In vitro radiometric enzyme assays with long-chain thiols as alternative substrates.
Previous studies have revealed that WS/DGAT from Acinetobacter sp. strain ADP1 exhibits an unusually broad substrate range (9, 10). Thus, the utilization of long-chain thiols as alternative acyl acceptors for WS/DGAT was considered, too. In addition to the natural acyl acceptor 1-hexadecanol, the sulfur analogues 1-hexadecanethiol, 1,8-octanedithiol, and 1-S-monopalmitoyloctanedithiol were tested as alternative acyl acceptors in the WS/DGAT-mediated acyl transfer reaction. With 1-hexadecanethiol as the acyl acceptor and radiolabeled [1-14C]palmitoyl-CoA as the acyl donor, formation of a radiolabeled C32 thio wax ester (palmitic acid hexadecyl thioester) should be observed (Fig. 2A). With 1,8-octanedithiol as the acyl acceptor, two reaction products could be formed depending on whether one or both thiol groups are esterified. In case only one thiol group is acylated with palmitoyl-CoA, a C24 monothio wax ester (1-S-monopalmitoyloctanedithiol) would result (Fig. 2B). If both thiol groups are esterified with palmitoyl-CoA, a C40 dithio wax ester (1,8-S-dipalmitoyloctanedithiol) would be synthesized (Fig. 2C). Acylation of the free thiol group of 1-S-monopalmitoyloctanedithiol, which is synthesized in vitro by lipase catalysis (28), by palmitoyl-CoA should result in the formation of 1,8-S-dipalmitoyloctanedithiol.
FIG. 2.
Chemical structures of thio wax esters synthesized by WS/DGAT. (A) 9-Octadecenoic acid hexadecyl thioester; (B) 1-S-monopalmitoyloctanedithiol; (C) 1,8-S-dipalmitoyloctanedithiol.
By comparison with synthetic reference substances, all of the putative reaction products mentioned above were identified by autoradiography following TLC separation. The specific activities of 5′His6WS/DGAT with the alternative acyl acceptors were determined by quantification of the radiolabeled products by means of scintillation counting and compared with those obtained when 1-hexadecanol was used as the substrate. With 1-hexadecanethiol as an alternative substrate, 5′His6WS/DGAT exhibited 10.4% of the specific activity demonstrated when 1-hexadecanol was used as the substrate (Table 2). With 1,8-octanedithiol as the substrate, we determined that a total specific activity of 59.3% of the activity exhibited with 1-hexadecanol could be allocated to the activity for the formation of 1-S-monopalmitoyloctanedithiol (55.7%) and for the formation of 1,8-S-dipalmitoyloctanedithiol (3.6%), which was additionally synthesized due to the ability of 1-S-monopalmitoyloctanedithiol to serve also as an acyl acceptor (Table 2). If 1-S-monopalmitoyloctanedithiol, the intermediate of the reaction from 1,8-octanedithiol to 1,8-S-dipalmitoyloctanedithiol, was provided as the sole acyl acceptor together with radiolabeled [1-14C]palmitoyl-CoA, it was converted by 5′His6WS/DGAT into 1,8-S-dipalmitoyloctanedithiol, with 13.4% of the specific activity obtained when 1-hexadecanol was provided as the substrate (Table 2).
TABLE 2.
Specific enzyme activities of 5′His6WS/DGAT with alkanethiols as alternative acyl acceptors
| Acyl acceptor | % Activity with 1-hexadecanol as acyl acceptora |
|---|---|
| 1-Hexadecanol | 100 |
| 1-Hexadecanethiol | 10.4 |
| 1,8-Octanedithiol | 59.3b |
| 1-S-Monopalmitoyloctanedithiol | 13.4 |
Activities were measured in radiometric assays with [1-14C]palmitoyl-CoA as the acyl donor by employing scintillation counting. Values are averages of two independent measurements.
1-S-Monopalmitoyloctanedithiol synthesized from 1,8-octanedithiol can also serve as the acyl acceptor, resulting in the synthesis of 1,8-S-dipalmitoyloctanedithiol (formation of 1-S-monopalmitoyloctanedithiol, 55.7%; formation of 1,8-S-dipalmitoyloctanedithiol, 3.6%).
In vitro thio wax ester biosynthesis.
In order to characterize monothio- and dithio wax esters synthesized by 5′His6WS/DGAT, a large-scale, nonradiometric in vitro enzyme assay was performed. 1,8-Octanedithiol, unlabeled palmitoyl-CoA, and purified 5′His6WS/DGAT from E. coli Rosetta(DE3)pLysS (pET23a::5′His6atf) were employed as described in Materials and Methods. The thio wax esters formed were extracted by using dichloromethane-methanol and i-hexane and separated by preparative TLC. Putative monothio and dithio wax ester fractions were extracted from the silica gel and analyzed by GC and HPLC. In addition to nonconverted 1,8-octanedithiol and trace amounts of palmitic acid resulting from the hydrolysis of palmitoyl-CoA, two reaction products could be detected by GC; their retention times corresponded with those of synthetic 1-S-monopalmitoyloctanedithiol and 1,8-S-dipalmitoyloctanedithiol, respectively, which were used as reference compounds (Fig. 3A). The formation of these products was strictly dependent on the presence of 1,8-octanedithiol in the assay (data not shown). In the in vitro enzyme assay, 46.5 μg of 1-S-monopalmitoyloctanedithiol per ml and 6.4 μg of 1,8-S-dipalmitoyloctanedithiol per ml were synthesized by 5′His6WS/DGAT, as revealed by HPLC analysis. Thus, 64% of palmitoyl-CoA was converted into the mentioned thioester products.
FIG. 3.
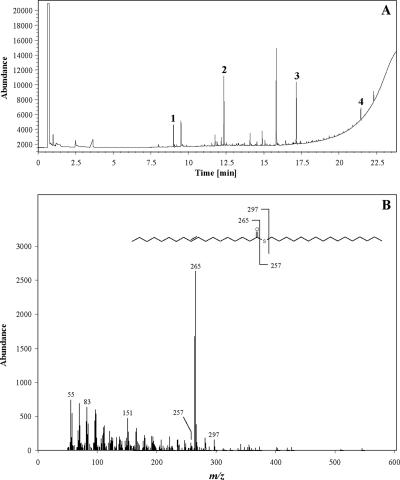
Chemical analyses of thio wax esters. (A) GC analysis of monothio and dithio wax esters synthesized in vitro from 1,8-octanedithiol and palmitoyl-CoA by WS/DGAT. Substances identified are 1,8-octanedithiol (peak 1), palmitic acid methyl ester (peak 2), 1-S-monopalmitoyloctanedithiol (peak 3), and 1,8-S-dipalmitoyloctanedithiol (peak 4). (B) Electron ionization mode mass spectrum of 9-octadecenoic acid hexadecyl thioester isolated from Acinetobacter sp. strain ADP1acr1ΩKm. Thio wax esters were isolated by preparative TLC from cells cultivated in the presence of 1% (wt/vol) sodium gluconate, 0.3% (vol/vol) 1-hexadecanethiol, and 0.1 (wt/vol) oleic acid and analyzed by GC-MS.
These results clearly confirmed that WS/DGAT accepted 1,8-octanedithiol as an alternative acyl acceptor and that both thiol groups were acylated by this bifunctional enzyme with 1-S-monopalmitoyloctanedithiol as the intermediate product and 1,8-S-dipalmitoyloctanedithiol as the final product.
In vivo thio wax ester biosynthesis in Acinetobacter sp. strain ADP1acr1ΩKm.
Due to the dependency on CoA-activated fatty acids as substrates, in vitro production of thio wax ester at a technical scale is not feasible with WS/DGAT. We therefore investigated the possibility of producing thio wax esters in vivo by utilizing living cells as biocatalysts. For this experiment, we used the mutant Acinetobacter sp. strain ADP1acr1ΩKm, which is impaired in fatty alcohol biosynthesis and thus is unable to produce wax esters. However, provision of external long-chain alcohols or thiols, e.g., 1-hexadecanethiol, as alternative substrates may result in the production of wax ester derivatives, as was already demonstrated by wax diester synthesis since the WS/DGAT is still active in this strain (10).
By utilizing the intracellular acyl-CoA pool, the formation of a variety of reaction products is theoretically possible, depending on which fatty acid is incorporated into the thio wax ester. With palmitic and oleic acids being the predominant fatty acids in cells of Acinetobacter sp. strain ADP1acr1ΩKm (10), the formation of C32 and C34 thio wax esters was most likely.
Neither with 1-hexadecanethiol (0.3%, vol/vol) as the sole carbon source nor with sodium gluconate (1%, wt/vol) and 1-hexadecanethiol (0.3%, vol/vol) as cosubstrate cultures did Acinetobacter sp. strain ADP1acr1ΩKm show significant growth, indicating that 1-hexadecanethiol has a growth-inhibitory effect on cells of Acinetobacter sp. strain ADP1 (Fig. 4). Therefore, cells of Acinetobacter sp. strain ADP1acr1ΩKm were first cultivated for 24 h in LB medium to reach high cell density. Cells were then transferred to nitrogen-limited MSM with sodium gluconate (1%, wt/vol), 1-hexadecanethiol (0.3%, vol/vol), and oleic acid (0.1% wt/vol) or with 1-hexadecanethiol (0.3%, vol/vol) and oleic acid (0.1% wt/vol) as carbon sources, respectively, and incubated for 48 h under storage conditions. Total lipids were then extracted from the lyophilized cells of these cultivations and separated by preparative TLC. Possible acyl thioesters were eluted from the silica gel and subjected to chemical analysis by GC and GC-MS.
FIG. 4.
Growth of Acinetobacter sp. strain ADP1acr1ΩKm on different carbon sources. Cells were cultivated at 30°C in MSM containing 1 g of NH4Cl liter−1 with 1% (wt/vol) sodium gluconate (♦), 0.3% (wt/vol) 1-hexadecanethiol and 1% (wt/vol) sodium gluconate (▴), and 0.3% (wt/vol) 1-hexadecanethiol (▪).
The various reaction products were identified by TLC (Fig. 5) and GC-MS showing typical mass fragments of long-chain thioesters such as 9-octadecenoic acid hexadecyl thioester (Fig. 3B) as well as of dihexadecyl disulfide (m/z and relative percentages as follows, respectively: for [M+], 514 and 100%; for [M-C16H32]+, 290 and 40%; for [M-C16H32S]+, 257 and 66%). Moreover, mass spectra of reaction products were identical with those of synthetic standards. Traces of other long-chain thio wax esters such as palmitic acid hexadecyl thioester, most probably formed by the WS/DGAT-catalyzed transthioesterification of internal acyl-CoA esters with 1-hexadecanethiol, were also detected by GC-MS (data not shown).
FIG. 5.
TLC analysis of thio wax esters synthesized in vivo in Acinetobacter sp. strain ADP1acr1ΩKm by utilizing 1-hexadecanethiol as acyl acceptor. Reference substances used were 1-hexadecanethiol (lane 1) and palmitic acid hexadecyl thioester (lane 2). TLC analysis of lipid extracts obtained from lyophilized cells of Acinetobacter sp. strain ADP1acr1ΩKm grown on LB medium for 24 h was followed by 48 h of incubation in MSM under storage conditions with the following carbon sources: sodium gluconate (1%, wt/vol) (lane 3), sodium gluconate (1%, wt/vol) and 1-hexadecanethiol (0.3%, vol/vol) (lane 4), and sodium gluconate (1%, wt/vol), 1-hexadecanethiol (0.3%, vol/vol), and oleic acid (0.1%, wt/vol) (lane 5).
GC analyses revealed long-chain thio esters in cells of both cultures, as was found by comparison of their retention times with those of synthetic standards. Moreover, we identified dihexadecyl disulfide, which was most probably formed by an unspecific reaction of 1-hexadecanethiol with oxygen. Partially thiyl-radical-induced stereomutation of oleic acid (cis-9-octadecenoic acid) to elaidic acid (trans-9-octadecenoic acid) was observed in the isolated thioesters (no quantification performed), whereas addition products of 1-hexadecanethiol and oleic acid, which are the other typical thiyl-radical-induced by-products of the reaction of unsaturated fatty acids with alkanethiols (27), were not formed under the conditions described. 9-Octadecenoylhexadecyl thioesters accumulated by Acinetobacter sp. strain ADP1acr1ΩKm accounted for 0.82% (wt/wt) of the cellular dry weight, whereas palmitoylhexadecyl thioesters were found only in traces. The total thio wax ester content accounted for around 1.19% (wt/wt) of the cellular dry weight, as was calculated by the quantitative evaluation of GC analyses using synthetic thioesters as standards. These results clearly demonstrated the possibility of synthesizing unusual long-chain thio wax esters in vivo in Acinetobacter sp. strain ADP1acr1ΩKm with 1-hexadecanethiol as the acyl acceptor.
DISCUSSION
WS/DGAT from Acinetobacter sp. strain ADP1 is the first characterized member of a new group of bacterial acyl-CoA-dependent long-chain acyltransferases exhibiting no homology to known acyltransferases like members of the DGAT1 or DGAT2 protein families, the WS from jojoba or similar acyltransferases involved in TAGs, or wax ester synthesis in eukaryotes (9). WS/DGAT plays an essential role in the biosynthesis of wax esters and TAGs in Acinetobacter sp. strain ADP1 at the same time. This unique bifunctionality has not yet been reported for other pro- and eukaryotic acyltransferases, indicating that this WS/DGAT lacks specificity to a high degree. In previous studies, this lack of specificity was already demonstrated, since the bifunctional WS/DGAT accepted a broad range of various-chain-length saturated and unsaturated fatty alcohols and acyl-CoAs (9) as well as 1,16-hexadecanediol and 1-monopalmitoylglycerol (10) as substrates. With this study, the range of possible acyl acceptors for WS/DGAT is extended to alkanethiols, such as 1-hexadecanethiol, 1,8-octanedithiol, and 1-S-monopalmitoyloctanedithiol, enabling the in vitro and also in vivo biosynthesis of unusual thio wax esters or dithio wax esters, respectively. This clearly demonstrated that WS/DGAT is capable of catalyzing not only oxo ester bond formation but also thio ester bond formation. In analogy, bacterial PHA synthases catalyzing the biosynthesis of PHA are also capable of utilizing the sulfur analogues of their natural substrates, which are short- and medium-chain-length 3-hydroxyacyl-CoAs, resulting in polythioester biosynthesis (15). Furthermore, it was recently shown that lipases are able to biosynthesize thio wax esters (28) and polythioesters, respectively (8).
For the in vitro biosynthesis of thio wax esters, an N-terminally His6-tagged WS/DGAT was purified to apparent homogeneity in a two-step chromatographic enrichment. Thus, a pure enzyme could be used for in vitro syntheses, resulting in the formation of thio wax esters which were virtually free of by-products that may be produced in the presence of contaminating proteins, e.g., by the release of free fatty acids from acyl-CoA due to thioesterases. This approach will clearly help to identify new products that can be synthesized by WS/DGAT. The in vitro enzyme assays demonstrated that 5′His6WS/DGAT accepted 1-hexadecanethiol as the sulfur analogue of the natural substrate 1-hexadecanol as an alternative acyl acceptor with about 10% of the specific activity. 1,8-Octanedithiol was even much more efficiently utilized as a substrate (59.3% specific activity), which resulted in the formation of 1-S-monopalmitoyloctanedithiol. In addition, minor amounts of 1,8-S-dipalmitoyloctanedithiol were also produced from 1,8-octanedithiol, which is in agreement with the observation that 1-S-monopalmitoyloctanedithiol also serves as an alternative substrate (13.4% specificity).
For the in vivo biosynthesis of thio wax esters, we used the acyl-CoA reductase (acr1) disruption mutant Acinetobacter sp. strain ADP1acr1ΩKm, which is not able to produce fatty aldehydes from acyl-CoA and could therefore not synthesize wax esters (10). In fact, Acinetobacter sp. strain ADP1 is an alkane-degrading bacterium able to grow on long-chain alkanes as the sole carbon source (18). The lack of growth of Acinetobacter sp. strain ADP1acr1ΩKm with 1-hexadecanethiol as the sole carbon source or as a cosubstrate with sodium gluconate indicated the inability of the alkane degradation system to metabolize 1-hexadecanethiol and demonstrated the toxic effect of 1-hexadecanethiol on the cells of Acinetobacter sp. strain ADP1acr1ΩKm. However, 1-hexadecanethiol was obviously transported into the cells. Thus, during cultivation of the cells with gluconate, 1-hexadecanethiol, and oleic acid under storage conditions, the alkanethiol served as a substrate for WS/DGAT. By utilizing the externally supplied oleic acid, mostly C34 thio wax esters were synthesized. This finding demonstrated that thio wax esters were produced intracellularly, although the specific activity of WS/DGAT with 1-hexadecanethiol as the substrate was only 10.4% of that with 1-hexadecanol as the substrate. The recovery of only trace amounts of other acyl hexadecyl thio wax esters may also indicate a putative toxic effect of 1-hexadecanethiol on acyl-CoA or fatty acid de novo biosynthesis and that the intracellular acyl-CoA compounds are only marginally used as substrates by the enzyme under these conditions.
In the presence of oxygen, alkanethiols are unstable, forming thiyl radicals which can cause unspecific reactions, such as the formation of disulfides or thioethers, if they react with unsaturated fatty acids (27). In contrast to the lipase-catalyzed thio wax ester in vitro synthesis (27), no thiyl-radical-induced thioether formation could be observed during in vivo synthesis of thio wax esters by Acinetobacter sp. strain ADP1acr1ΩKm, but major amounts of dihexadecyl disulfides were formed, leading to a decrease of the acyl acceptor concentration and thus most probably lowering the thio wax ester yields. Thioesters are used as activated esters (12, 17, 24) for the synthesis of peptides, for macrocyclic antibiotics, and for various other pharmaceuticals by organic chemists and, in particular, the pharmaceutical industry (4, 7, 11, 14, 16, 19). If the amounts of thio wax ester accumulated by Acinetobacter sp. strain ADP1acr1ΩKm can be enhanced, the in vivo synthesis of long-chain thio wax esters may be an alternative to chemical or lipase-catalyzed synthesis due to a lower by-product content.
The present study demonstrates the extraordinary low specificity of WS/DGAT, providing an impression of the enormous biocatalytic potential of this unusual enzyme. The range of alternative substrates is most probably not limited to the substances tested so far. Therefore, in vivo and in vitro biosyntheses of several other unusual wax ester derivatives and the acylation of other interesting chemical and biological molecules might be possible using WS/DGAT from Acinetobacter sp. strain ADP1.
REFERENCES
- 1.Alvarez, H. M., and A. Steinbüchel. 2002. Triacylglycerols in prokaryotic microorganisms. Appl. Microbiol. Biotechnol. 60:367-376. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez, H. M., R. Kalscheuer, and A. Steinbüchel. 1997. Accumulation of storage lipids in species of Rhodococcus and Nocardia and effects of inhibitors and polyethylene glycol. Fett-Lipid 99:239-246. [Google Scholar]
- 3.Barksdale, L., and K.-S. Kim. 1977. Mycobacterium. Bacteriol. Rev. 41:217-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchi, D., and P. Cesti. 1990. Lipase-catalysed stereoselective thioesterification of mercaptoesters. J. Org. Chem. 55:5657-5659. [Google Scholar]
- 5.Ervin, J. L., J. Geigert, S. L. Neidlerman, and J. Wadsworth. 1984. Substrate-dependent and growth temperature-dependent changes in the wax ester compositions produced by Acinetobacter sp. strain H01-N, p. 217-222. In C. Ratledge, P. Dawson, and L. Rattray (ed.), Biotechnology for the oils and fats industry. American Oil Chemists Society, Champaign, Ill.
- 6.Fixter, L. M., N. M. Nagi, J. G. McCormack, and C. A. Fewson. 1986. Structure, distribution and function of wax esters in Acinetobacter calcoaceticus. J. Gen. Microbiol. 132:3147-3157. [Google Scholar]
- 7.Gauthier, J. Y., F. Bourdon, and R. N. Young. 1986. A mild and efficient synthesis of thiolesters from alcohols. Tetrahedron Lett. 27:15-18. [Google Scholar]
- 8.Iwata, S., K. Toshima, and S. Matsumura. 2003. Enzyme catalyzed preparation of aliphatic polyesters containing thioester linkages. Macromol. Rapid Commun. 24:467-471. [Google Scholar]
- 9.Kalscheuer, R., and A. Steinbüchel. 2003. A novel bifunctional wax ester synthase/acyl-CoA:diacylglycerol acyltransferase mediates wax ester and triacylglycerol biosynthesis in Acinetobacter calcoaceticus ADP1. J. Biol. Chem. 278:8075-8082. [DOI] [PubMed] [Google Scholar]
- 10.Kalscheuer, R., S. Uthoff, H. Luftmann, and A. Steinbüchel. 2003. In vitro and in vivo biosynthesis of wax diesters by an unspecific bifunctional wax ester synthase/acyl-CoA:diacylglycerol acyltransferase from Acinetobacter calcoaceticus ADP1. Eur. J. Lipid Sci. Technol. 105:578-584. [Google Scholar]
- 11.Kunieda, T., Y. Abe, and M. Hirobe. 1981. Efficient conversion of carboxylic acids into thiol esters. Chem. Lett. 10:1427-1428. [Google Scholar]
- 12.Lamberet, G., B. Auberger, and J. L. Bergère. 1997. Aptitude of cheese bacteria for volatile S-methyl thioester synthesis. II. Comparison of coryneform bacteria, Micrococcaceae and some lactic acid bacteria starters. Appl. Microbiol. Biotechnol. 48:393-397. [Google Scholar]
- 13.Lehner, R., and A. Kuksis. 1996. Biosynthesis of triacylglycerols. Prog. Lipid Res. 35:169-201. [DOI] [PubMed] [Google Scholar]
- 14.Li, X., T. Kawakami, and S. Aimoto. 1998. Direct preparation of peptide thioesters using an Fmoc solid-phase method. Tetrahedron Lett. 39:8669-8672. [Google Scholar]
- 15.Lütke-Eversloh, T., and A. Steinbüchel. 2004. Microbial polythioesters. Macromol. Biosci. 4:165-174. [DOI] [PubMed] [Google Scholar]
- 16.Masamune, S., S. Kamata, and W. Schilling. 1975. Syntheses of macrolide antibiotics. III. Direct ester and lactone synthesis from S-tert-butyl thioate (thiol ester). J. Am. Chem. Soc. 97:3515-3516. [DOI] [PubMed] [Google Scholar]
- 17.Nakajima, T., Y. Yabushita, and I. Tabushi. 1975. Amino acid synthesis through biogenetic-type CO2 fixation. Nature 256:60-61. [DOI] [PubMed] [Google Scholar]
- 18.Reiser, S., and C. Somerville. 1997. Isolation of mutants of Acinetobacter calcoaceticus deficient in wax ester synthesis and complementation of one mutation with a gene encoding a fatty acyl coenzyme A reductase. J. Bacteriol. 179:2969-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiβig, H.-U., and B. Scherer. 1980. A simple synthesis of thiol esters from copper-I-mercaptides and acyl chlorides. Tetrahedron Lett. 21:4259-4562. [Google Scholar]
- 20.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 21.Schlegel, H. G., H. Kaltwasser, and G. Gottschalk. 1961. Ein Submersverfahren zur Kultur wasserstoffoxidierender Bakterien: Wachstumsphysiologische Untersuchungen. Arch. Mikrobiol. 38:209-222. [PubMed] [Google Scholar]
- 22.Sorger, D., and G. Daum. 2003. Triacylglycerol biosynthesis in yeast. Appl. Microbiol. Biotechnol. 61:289-299. [DOI] [PubMed] [Google Scholar]
- 23.Steinbüchel, A. 1996. PHB and other polyhydroxyalkanoic acids, p. 403-464. In H. J. Rehm, G. Reed, A. Pühler, and P. Stadler (ed.), Biotechnology, 2nd edition, vol. 6. Wiley-VCH, Weinheim, Germany.
- 24.Taylor, D. C., and N. Weber. 1993. Acyl coenzyme A thioesters, p. 285-320. In K. D. Mukherjee and N. Weber (ed.), CRC handbook of chromatography: analysis of lipids. CRC Press, Boca Raton, Fla.
- 25.Weber, N., E. Klein, and K. D. Mukherjee. 1999. Long-chain acyl thioesters prepared by solvent-free thioesterification and transthioesterification catalysed by microbial lipases. Appl. Microbiol. Biotechnol. 51:401-404. [Google Scholar]
- 26.Weber, N., E. Klein, K. Vosmann, and K. D. Mukherjee. 1998. Preparation of long-chain acyl thioesters—thio wax esters—by the use of lipases. Biotechnol. Lett. 20:687-691. [Google Scholar]
- 27.Weber, N., E. Klein, K. Vosmann, and K. D. Mukherjee. 2000. Antioxidants eliminate stereomutation and thioether formation during lipase-catalyzed thioesterification and transthioesterification for the preparation of uniform cis- and trans-unsaturated thioesters. Chem. Phys. Lipids 105:215-223. [DOI] [PubMed] [Google Scholar]
- 28.Weber, N., E. Klein, K. Vosmann, and K. D. Mukherjee. 2004. Mono-thioesters and di-thioesters by lipase-catalyzed reactions of α,ω-alkanedithiols with palmitic acid or its methyl ester. Appl. Microbiol. Biotechnol. 64:800-805. [DOI] [PubMed] [Google Scholar]