Abstract
The randomized phase 3 study ENDEAVOR demonstrated a statistically significant and clinically meaningful improvement in progression-free survival (PFS) for carfilzomib and dexamethasone (Kd) vs bortezomib and dexamethasone (Vd) in relapsed or refractory multiple myeloma (MM). We conducted a preplanned subgroup analysis of ENDEAVOR to evaluate Kd vs Vd by cytogenetic risk. Of 785 patients with known cytogenetics, 210 (27%) had high-risk cytogenetics (Kd, n=97 (25%); Vd, n=113 (28%)) and 575 (73%) had standard-risk cytogenetics (Kd, n=284 (75%); Vd, n=291 (72%)). Median PFS in the high-risk group was 8.8 months for Kd vs 6.0 months for Vd (hazard ratio (HR), 0.65; 95% confidence interval (CI), 0.45–0.92; P=0.0075). Median PFS in the standard-risk group was not estimable for Kd vs 10.2 months for Vd (HR, 0.44; 95% CI, 0.33–0.58; P<0.0001). Overall response rates were 72.2% (Kd) vs 58.4% (Vd) in the high-risk group and 79.2% (Kd) vs 66.0% (Vd) in the standard-risk group. In the high-risk group, 15.5% (Kd) vs 4.4% (Vd) achieved a complete response (CR) or better. In the standard-risk group, 13.0% (Kd) vs 7.9% (Vd) achieved ⩾CR. This preplanned subgroup analysis found that Kd was superior to Vd in relapsed or refractory MM, regardless of cytogenetic risk.
Introduction
Certain chromosomal abnormalities, such as t(4;14) and del(17p), are not uncommon in patients with multiple myeloma (MM) (occurring in ~11% and 14% of MM patients, respectively) and are associated with poor outcomes, including in those patients with relapsed or refractory disease.1, 2, 3, 4 A retrospective analysis found that in patients with relapsed or refractory MM who were treated with lenalidomide plus dexamethasone (Rd; n=207), the presence of t(4;14) was predictive of shorter overall survival (median 9.4 vs 15.4 months; P=0.005).5 Similar results have also been observed for the presence of del(17p), with the presence of this abnormality found to be independently associated with shorter survival in patients with recurrent MM who were treated with Rd.6
Although the presence of the aforementioned cytogenetic abnormalities has been shown to affect treatment response and survival, there are limited data on the ability of novel agents to improve or overcome the adverse prognosis of high-risk cytogenetics. Although the first-generation proteasome inhibitor bortezomib has been shown to be beneficial in patients with high-risk cytogenetics, there have been conflicting reports regarding its benefit associated with t(4;14) or del(17p) cytogenetic abnormalities.7, 8, 9, 10 Based on review of the literature, the International Myeloma Working Group (IMWG) recently stated that bortezomib partly overcomes the adverse effect of t(4;14) and possibly del(17p) on survival.11 Given the prognostic importance of high-risk cytogenetics in relapsed/refractory MM, there is an unmet need to understand the interaction between the efficacy of novel treatments and baseline cytogenetic risk status. Accordingly, the IMWG has stated that the analysis of cytogenetic subgroups in trials comparing different therapies is an important goal.11
Carfilzomib is a second-generation proteasome inhibitor that is approved in the United States in relapsed or refractory MM when used as a single agent for the treatment of patients who have received one or more lines of therapy and when used in combination with dexamethasone or lenalidomide plus dexamethasone for the treatment of patients who have received one to three prior lines of therapy. The combinations of carfilzomib with dexamethasone or lenalidomide plus dexamethasone are also approved in the European Union for the treatment of patients with MM who have received at least one prior therapy. The approval of carfilzomib plus dexamethasone (Kd) was based on interim results from the randomized, phase 3 ENDEAVOR study that compared Kd with bortezomib and dexamethasone (Vd) in patients with relapsed or refractory MM.12 Treatment with Kd led to a clinically meaningful and statistically significant two-fold improvement in median progression-free survival (PFS) when compared with Vd (18.7 vs 9.4 months; hazard ratio (HR), 0.53; 95% confidence interval (CI), 0.44–0.65, P<0.0001) at the interim analysis. Patients in the Kd group had a significantly higher overall response rate (ORR, 77% vs 63% odds ratio, 2.03; 95% CI, 1.52–2.72; P<0.0001) than patients in the Vd group. Overall survival data were immature at the time of the interim analysis with 75 deaths in the Kd group and 88 deaths in the Vd group (HR=0.79).
Herein we present results of a preplanned subgroup analysis of the ENDEAVOR study at the interim analysis that was conducted to evaluate Kd vs Vd based on baseline cytogenetic risk status.
Subjects and methods
Study design and participants
ENDEAVOR was a randomized, open-label, multicenter, phase 3 study that evaluated Kd vs Vd in patients with relapsed or refractory MM, for which the study design has been described previously.12 The primary end point was PFS (that is, the time from randomization until disease progression or death due to any cause, whichever occurred first) as determined by an Independent Review Committee. Secondary end points included overall survival, ORR, duration of response (DOR; that is, the time from first evidence of a partial response (PR) or better to confirmation of disease progression or death from any cause), grade ⩾2 peripheral neuropathy rate, and safety.
Adult patients with relapsed or refractory MM (1–3 prior lines of therapy) were eligible for enrollment. Patients previously exposed to carfilzomib or bortezomib were eligible provided that they achieved at least a PR to prior treatment with a ⩾6-month proteasome inhibitor treatment-free interval and did not discontinue due to toxicity. Patients were randomly assigned (1:1 ratio) to receive Kd or Vd in 28-day or 21-day cycles, respectively, until withdrawal of consent, physician's decision, death, disease progression or unacceptable toxicity. Randomization was stratified by prior proteasome inhibitor therapy, prior lines of treatment, International Staging System (ISS) stage, and route of bortezomib administration (if randomized to the Vd group). All patients received dexamethasone (20 mg; oral or intravenous) on days 1, 2, 8, 9, 15, 16, 22 and 23 in the Kd arm and on days 1, 2, 4, 5, 8, 9, 11 and 12 in the Vd arm. Patients in the Kd group received carfilzomib (20 mg/m2 (days 1 and 2 of cycle 1); 56 mg/m2 thereafter) as a 30-min intravenous infusion on days 1, 2, 8, 9, 15 and 16 of 28 cycles. Patients in the Vd group received bortezomib (1.3 mg/m2) as an intravenous bolus or subcutaneous injection on days 1, 4, 8 and 11 of 21-day cycles.
Assessments
Bone marrow aspirate and biopsy were collected at baseline to quantify percent myeloma cell involvement and for fluorescent in situ hybridization (FISH). The FISH analyses were performed at a central laboratory and used to assess cytogenetic risk status. Of note, FISH results were not available before randomization and cytogenetic risk status was not a stratification factor.
Patients were classified into cytogenetic subgroups. The high-risk group consisted of patients with the genetic subtype t(4;14) or t(14;16) in ⩾10% of screened plasma cells or with del(17p) in ⩾20% of screened plasma cells. The standard-risk group consisted of all other patients with available and known baseline cytogenetics. The unknown/missing cytogenetics subgroup included patients who had a FISH assessment, but were either not analyzable or did not yield a definitive result.
Statistical analysis
Efficacy analyses were based on the intention-to-treat (ITT) population, including all randomized patients, whereas the safety population included those who received at least one dose of study treatment. The per-arm distribution of PFS, including the median, was estimated for each cytogenetic subgroup using the Kaplan–Meier method. The 95% CI for the median PFS was estimated using the method by Klein and Moeschberger13 with log–log transformation. HRs were estimated from a Cox proportional hazards model.
The ORRs for each treatment arm and cytogenetic subgroup were calculated as the proportion of patients who had a best overall response of a PR or better. The 95% CI for the ORR was estimated using the Clopper–Pearson interval. The per-arm distribution of DOR, including the median, was estimated for each cytogenetic subgroup using the Kaplan–Meier method. Treatment effects in the subgroups for ORR were represented by an odds ratio and its corresponding 95% CI.
Reported P-values for this subgroup analysis are one-sided, unadjusted for multiple comparisons, and are descriptive in nature.
Results
Patients and enrollment
Between June 2012 and June 2014, 929 patients from North America, Europe and the Asia-Pacific region were enrolled and randomized (Kd, n=464; Vd, n=465), for which patient characteristics, efficacy and safety have been reported previously.12 The cutoff date for the interim analysis was 10 November 2014.
A total of 785 patients (Kd, n=381; Vd, n=404), representing 84.5% of the study population, had known cytogenetic risk status. Of these, 210 patients (27%) had high-risk cytogenetics (Kd, n=97 (25%); Vd, n=113 (28%)) and 575 patients (73%) had standard-risk cytogenetics (Table 1; Kd, n=284 (75%); Vd, n=291 (72%)). The remaining 144 patients had missing (Kd, n=28; Vd, n=31) or unknown (Kd, n=55; Vd, n=30) cytogenetics. Among patients with known cytogenetics, 111 (14%) had the t(4;14) abnormality in ⩾10% of screened plasma cells (Kd, n=50 (13%); Vd, n=61 (15%)), 19 (2%) had the t(14;16) abnormality in ⩾10% of screened plasma cells (Kd, n=10 (3%); Vd, n=9 (2%)), and 92 (12%) had the del(17p) abnormality in ⩾20% of screened plasma cells (Kd, n=40 (10%); Vd, n=52 (13%)).
Table 1. Baseline cytogenetics among patients with known cytogenetics.
| Cytogenetics, n (%) | Kd (n=381) | Vd (n=404) |
|---|---|---|
| High risk | 97 (25.5) | 113 (28.0) |
| t(4;14) | 50 (13.1) | 61 (15.1) |
| t(14;16) | 10 (2.6) | 9 (2.2) |
| del(17p) | 40 (10.5) | 52 (12.9) |
| Standard risk | 284 (74.5) | 291 (72.0) |
Abbreviations: Kd, carfilzomib and dexamethasone; Vd, bortezomib and dexamethasone.
Overall, baseline patient characteristics were generally balanced across cytogenetic subgroups (Table 2; Supplementary Table S1). High-risk patients in both arms had higher rates of ISS stage 2 or 3 disease.
Table 2. Prior treatment (ITT population).
| High risk | Standard risk | Unknown/missing | ||||
|---|---|---|---|---|---|---|
| Kd (n=97) | Vd (n=113) | Kd (n=284) | Vd (n=291) | Kd (n=83) | Vd (n=61) | |
| Number of prior regimens,a n (%) | ||||||
| 1 | 44 (45.4) | 53 (46.9) | 149 (52.5) | 144 (49.5) | 39 (47.0) | 35 (57.4) |
| 2 | 37 (38.1) | 37 (32.7) | 86 (30.3) | 95 (32.6) | 34 (41.0) | 13 (21.3) |
| 3 | 16 (16.5) | 22 (19.5) | 49 (17.3) | 52 (17.9) | 10 (12.0) | 13 (21.3) |
| Prior therapy, n (%) | ||||||
| Bortezomib | 54 (55.7) | 61 (54.0) | 150 (52.8) | 158 (54.3) | 46 (55.4) | 33 (54.1) |
| Carfilzomib | 0 | 0 | 1 (0.4) | 0 | 1 (1.2) | 1 (1.6) |
| Lenalidomide | 43 (44.3) | 51 (45.1) | 100 (35.2) | 104 (35.7) | 34 (41.0) | 22 (36.1) |
| Thalidomide | 47 (48.5) | 62 (54.9) | 126 (44.4) | 152 (52.2) | 38 (45.8) | 33 (54.1) |
Abbreviations: ITT, intention-to-treat; Kd, carfilzomib and dexamethasone; Vd, bortezomib and dexamethasone.
One patient in the bortezomib group (high risk) had four prior regimens.
Efficacy by cytogenetic subgroup
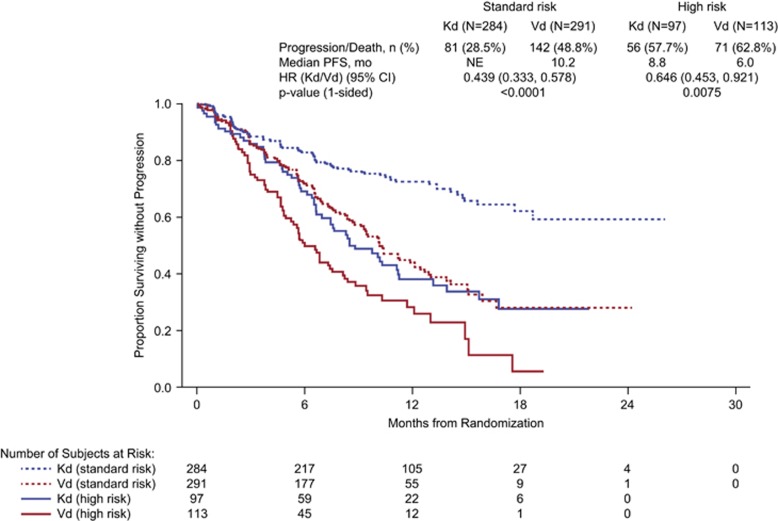
Consistent with the overall study results,12 Kd reduced the risk of disease progression or death relative to Vd in the high-risk and standard-risk cytogenetic subgroups (Figure 1). In the high-risk group, patients receiving Kd had a median PFS of 8.8 months compared with 6.0 months in those receiving Vd (HR, 0.646; 95% CI, 0.453–0.921; P=0.0075). In the standard-risk group, the median PFS was not estimable in patients receiving Kd and was 10.2 months in those receiving Vd (HR, 0.439; 95% CI, 0.333–0.578; P<0.0001); in the relatively small subgroup of patients with unknown/missing cytogenetics status (15.5% of the study population), the corresponding durations were 15.4 months and 12.2 months, respectively (HR, 0.673; 95% CI, 0.410–1.106; P=0.058).
Figure 1.
Kaplan–Meier estimate of progression-free survival by cytogenetic risk status. CI, confidence interval; Kd, carfilzomib and dexamethasone; NE, not estimable; PFS, progression-free survival; Vd, bortezomib and dexamethasone.
Kd was also more effective than Vd with respect to the rate, depth and DOR regardless of cytogenetic risk status (Table 3). In the high-risk cytogenetic subgroup, the ORR was 72.2% with Kd vs 58.4% with Vd (odds ratio, 1.85; 95% CI, 1.03–3.30; P=0.019), with complete responses (CRs) or better in 15.5% and 4.4% of patients, respectively. In the standard-risk subgroup, corresponding ORRs were 79.2% and 66.0%, respectively (odds ratio, 1.97; 95% CI, 1.35–2.86; P=0.0002), including CR or better rates of 13.0% and 7.9%, respectively. Median DOR was 10.2 months for Kd vs 8.3 months for Vd in the high-risk subgroup and not estimable vs 11.7 months, respectively, in the standard-risk subgroup.
Table 3. Response by cytogenetic risk status at baseline (ITT population).
|
High risk |
Standard risk |
Unknown/missing |
||||
|---|---|---|---|---|---|---|
| Kd (n=97) | Vd (n=113) | Kd (n=284) | Vd (n=291) | Kd (n=83) | Vd (n=61) | |
| Overall response rate, n (%)a | 70 (72.2) | 66 (58.4) | 225 (79.2) | 192 (66.0) | 62 (74.7) | 33 (54.1) |
| 95% CIb | 62.1–80.8% | 48.8–67.6% | 74.0–83.8% | 60.2–71.4% | 64.0–83.6% | 40.8–66.9% |
| Odds ratio for Kd vs Vd (95% CI)c | 1.85 (1.03–3.30) | 1.97 (1.35–2.86) | 2.51 (1.24–5.08) | |||
| One-sided P-valued | 0.0190 | 0.0002 | 0.0051 | |||
| Best overall response, n (%)a | ||||||
| Complete response or better | 15 (15.5) | 5 (4.4) | 37 (13.0) | 23 (7.9) | 6 (7.2) | 1 (1.6) |
| Stringent complete response | 2 (2.1) | 3 (2.7) | 6 (2.1) | 6 (2.1) | 0 | 0 |
| Complete response | 13 (13.4) | 2 (1.8) | 31 (10.9) | 17 (5.8) | 6 (7.2) | 1 (1.6) |
| Very good partial response | 30 (30.9) | 29 (25.7) | 130 (45.8) | 63 (21.6) | 34 (41.0) | 12 (19.7) |
| Partial response | 25 (25.8) | 32 (28.3) | 57 (20.1) | 105 (36.1) | 22 (26.5) | 20 (32.8) |
| Minimal response | 8 (8.2) | 11 (9.7) | 12 (4.2) | 36 (12.4) | 4 (4.8) | 6 (9.8) |
| Stable disease | 9 (9.3) | 17 (15.0) | 21 (7.4) | 28 (9.6) | 10 (12.0) | 8 (13.1) |
| Progressive disease | 6 (6.2) | 10 (8.8) | 15 (5.3) | 16 (5.5) | 4 (4.8) | 5 (8.2) |
| Not evaluable | 4 (4.1) | 9 (8.0) | 12 (4.2) | 20 (6.9) | 3 (3.6) | 9 (14.8) |
| Duration of response, median months | 10.2 | 8.3 | NE | 11.7 | 21.3 | 11.7 |
| 95% CI | 7.4–15.8 | 5.0–12.2 | NE | 9.3–14.9 | 10.7–NE | 8.3–NE |
Abbreviations: CI, confidence interval; ITT, intention-to-treat; Kd, carfilzomib and dexamethasone; NE, not estimable; Vd, bortezomib and dexamethasone.
Determined by Independent Review Committee according to the International Myeloma Working Group Uniform Response Criteria. Patients evaluated for overall response rate had a best overall response of partial response or better.
Clopper−Pearson interval.
The odds ratio and 95% CI were calculated using 2x2 tables.
The P-values were calculated using chi-square tests.
PFS HRs and response rates also favored the Kd group in the small subgroups of patients with specific cytogenetic abnormalities (Table 4). For patients with del(17p), patients treated with Kd had a median PFS of 7.6 months compared with 4.9 months for Vd (HR, 0.73; 95% CI, 0.42–1.27; P=0.13). For patients with t(4;14), patients treated with Kd had a median PFS of 10.1 months compared with 6.8 months for Vd (HR, 0.63; 95% CI, 0.38–1.02; P=0.03). There were too few patients with t(14;16) to perform an informative analysis and results are therefore not reported for this subgroup.
Table 4. Efficacy by specific cytogenetic abnormalities (ITT population).
|
High risk |
||||
|---|---|---|---|---|
|
del(17p) |
t(4;14) |
|||
| Kd (n=40) | Vd (n=52) | Kd (n=50) | Vd (n=61) | |
| PFS, median months (95% CI) | 7.6 (5.6–11.2) | 4.9 (3.9–7.5) | 10.1 (6.9–NE) | 6.8 (5.6–9.4) |
| Hazard ratio (95% CI) | 0.73 (0.42–1.27) | 0.63 (0.38–1.02) | ||
| One-sided P-value | 0.13 | 0.03 | ||
| ORR,a % (95% CI) | 62.5 (45.8–77.3) | 50.0 (35.8–64.2) | 78.0 (64.0–88.5) | 65.6 (52.3–77.3) |
| Odds ratio (95% CI) | 1.67 (0.72–3.86) | 1.86 (0.79–4.37) | ||
| One-sided P-value | 0.12 | 0.08 | ||
Abbreviations: CI, confidence interval; ITT, intention-to-treat; Kd, carfilzomib and dexamethasone; NE, not estimable; ORR, overall response rate; PFS, progression-free survival; Vd, bortezomib and dexamethasone.
Determined by Independent Review Committee according to the International Myeloma Working Group Uniform Response Criteria. Patients evaluated for overall response rate had a best overall response of partial response or better.
In patients with high-risk cytogenetics, the median PFS was longer for patients receiving Kd than for those receiving Vd irrespective of the number of prior lines of therapy or prior exposure to bortezomib (Supplementary Tables S2 and S3). The ORRs in the high-risk cytogenetics subgroup were higher with Kd than with Vd, irrespective of the number of prior lines of therapy (Supplementary Table S2) or prior exposure to bortezomib (Supplementary Table S3).
Safety
The safety and tolerability profiles with Kd and Vd in the settings of high- and standard-risk cytogenetics were consistent with previously published data for the overall population.12 Kd was associated with a slightly higher rate of grade ⩾3 treatment-emergent adverse events (TEAEs; 70–75% across groups vs 63–68% with Vd) but not of TEAE-related treatment discontinuations (19–22% across groups with both Kd and Vd), despite longer median treatment durations for Kd vs Vd (Table 5). Grade ⩾2 peripheral neuropathy was reported at lower rates with Kd vs Vd in the high-risk group (3.1% vs 35.1% odds ratio, 0.059; 95% CI, 0.018–0.198) and also in the standard-risk group (6.4% vs 33.4% odds ratio, 0.135; 95% CI, 0.079–0.231).
Table 5. Adverse events, treatment discontinuations and deaths (safety population).
| Preferred term |
High risk |
Standard risk |
Unknown/missing |
|||
|---|---|---|---|---|---|---|
| Kd (n=97) | Vd (n=111) | Kd (n=283) | Vd (n=287) | Kd (n=83) | Vd (n=58) | |
| Treatment duration, median weeks (range) | 30.3 (1.0–93.1) | 22.0 (1.0–85.0) | 40.9 (2.0–108.1) | 28.0 (1.0–106.1) | 36.9 (1.0–104.0) | 21.3 (1.0–70.0) |
| Grade ⩾3 treatment-emergent AE, n (%) | 68 (70.1) | 70 (63.1) | 209 (73.9) | 196 (68.3) | 62 (74.7) | 39 (67.2) |
| Patients with at least one treatment-emergent AE leading to study treatment discontinuation, n (%) | 18 (18.6) | 22 (19.8) | 56 (19.8) | 62 (21.6) | 18 (21.7) | 11 (19.0) |
| Deaths within 30 days of last dose of any study drug, n (%)a | 8 (8.2) | 5 (4.4) | 9 (3.2) | 14 (4.8) | 5 (6.0) | 2 (3.3) |
| Hematologic grade ⩾3 AEs,b n (%) | ||||||
| Anemia | 14 (14.4) | 14 (12.6) | 44 (15.5) | 23 (8.0) | 9 (10.8) | 8 (13.8) |
| Thrombocytopenia | 7 (7.2) | 14 (12.6) | 23 (8.1) | 21 (7.3) | 9 (10.8) | 8 (13.8) |
| Decreased platelet count | 2 (2.1) | 6 (5.4) | 11 (3.9) | 16 (5.6) | 4 (4.8) | 2 (3.4) |
| Decreased lymphocyte count | 3 (3.1) | 2 (1.8) | 21 (7.4) | 6 (2.1) | 2 (2.4) | 0 |
| Lymphopenia | 5 (5.2) | 1 (0.9) | 10 (3.5) | 6 (2.1) | 5 (6.0) | 5 (8.6) |
| Nonhematologic grade ⩾3 AEs,b n (%) | ||||||
| Pneumonia | 8 (8.2) | 10 (9.0) | 19 (6.7) | 18 (6.3) | 5 (6.0) | 8 (13.8) |
| Fatigue | 5 (5.2) | 7 (6.3) | 18 (6.4) | 20 (7.0) | 2 (2.4) | 5 (8.6) |
| Asthenia | 4 (4.1) | 3 (2.7) | 11 (3.9) | 8 (2.8) | 1 (1.2) | 3 (5.2) |
| Hypertension | 6 (6.2) | 4 (3.6) | 30 (10.6) | 8 (2.8) | 5 (6.0) | 0 |
| Diarrhea | 4 (4.1) | 9 (8.1) | 10 (3.5) | 23 (8.0) | 2 (2.4) | 2 (3.4) |
| Peripheral neuropathy | 1 (1.0) | 4 (3.6) | 4 (1.4) | 20 (7.0) | 1 (1.2) | 0 |
| Dyspnea | 5 (5.2) | 1 (0.9) | 16 (5.7) | 6 (2.1) | 4 (4.8) | 3 (5.2) |
| Back pain | 1 (1.0) | 0 | 4 (1.4) | 9 (3.1) | 3 (3.6) | 3 (5.2) |
| Bone pain | 1 (1.0) | 0 | 4 (1.4) | 4 (1.4) | 5 (6.0) | 2 (3.4) |
| Syncope | 0 | 1 (0.9) | 0 | 8 (2.8) | 0 | 3 (5.2) |
Abbreviations: AE, adverse event; Kd, carfilzomib and dexamethasone; Vd, bortezomib and dexamethasone.
Percentage based on the intention-to-treat population of 97 (Kd) and 113 (Vd) high-risk patients; 284 (Kd) and 291 (Vd) standard-risk patients; and 83 (Kd) and 61 (Vd) unknown/missing cytogenetics patients.
Grade ⩾3 adverse events reported in ⩾5% of patients in any subgroup.
Discussion
Per this preplanned cytogenetic subgroup analysis of the ENDEAVOR study, Kd improved PFS relative to Vd in patients with both high- and standard-risk cytogenetics. For patients with standard-risk cytogenetics, treatment with Kd vs Vd resulted in a longer PFS (median, NE vs 10.2 months; HR, 0.439; P<0.0001) and higher responses rates (ORR, 79.2% vs 66.0% ⩾CR rate, 13.0% vs 7.9%). For patients with high-risk cytogenetics, treatment with Kd vs Vd also resulted in a longer PFS (8.8 vs 6.0 months; HR, 0.646; P=0.0075) and higher response rates (ORR, 72.2% vs 58.4% ⩾CR rate, 15.5% vs 4.4%). However, it should be noted that although the magnitude of the ORR benefit for Kd vs Vd was similar between the standard- and high-risk groups, the magnitude of the PFS benefit for Kd vs Vd was lower in the high-risk group compared with the standard-risk group. The efficacy results from this subgroup analysis are consistent with those reported in the overall ITT population, where Kd was shown to be superior to Vd in terms of PFS and treatment response.12 From a safety standpoint, despite the longer durations of treatment with Kd than with Vd, no safety signals emerged in any cytogenetics subgroup and the safety profile was consistent between high- and standard-risk subgroups.
A consistency of benefit was observed for Kd vs Vd, irrespective of the number of prior lines of therapy, prior exposure to bortezomib, and the presence of specific cytogenetic abnormalities; however, the number of patients within each of these subgroups was small. In this analysis, the median PFS durations in the Vd and Kd group were lower for patients with these cytogenetic abnormalities compared with the overall cohorts (t(4;14), 6.8 months; del(17p), 4.9 months; overall, 9.4 months for the Vd population and t(4;14), 10.1 months; del(17p), 7.6 months; overall, 18.7 months for the Kd population).
Overall, about 84% of the ENDEAVOR population had known cytogenetics, which compares favorably to other cytogenetic studies.1, 14 Nonetheless, a potential limitation of the current analysis is that 16% of patients had unknown or missing cytogenetics. However, baseline patient and disease characteristics were generally similar between patients with known and unknown/missing cytogenetics, with no apparent differences with respect to efficacy and safety outcomes in the unknown/missing subgroup relative to the ITT population.
The findings from this subgroup analysis build upon previous studies of the effect of carfilzomib by cytogenetic risk status. In a subgroup analysis of the PX-171–003-A1 study evaluating single-agent carfilzomib in patients with relapsed and refractory MM, the ORR was similar between patients with high- and standard-risk cytogenetics (25.8% vs 24.6% P=0.85). However, time-to-event outcomes were shorter in patients with high-risk cytogenetics than in those with standard-risk cytogenetics.15 Carfilzomib combined with thalidomide and dexamethasone, cyclophosphamide and dexamethasone, or lenalidomide and dexamethasone has demonstrated similar response rates between cytogenetic risk groups in patients with newly diagnosed MM.16, 17, 18, 19 Of note, data are also available from a cytogenetics subanalysis of the ASPIRE phase 3 trial,14 which compared carfilzomib, lenalidomide and dexamethasone (KRd) versus lenalidomide and dexamethasone (Rd) in a similar patient population of patients who received one to three prior lines of therapy.20 The ASPIRE cytogenetics subanalysis found longer median PFS and higher ORRs in high-risk patients receiving KRd vs Rd (23.1 vs 13.9 months and 79.2% vs 59.6%, respectively), as well as standard-risk patients (29.6 vs 19.5 months, and 91.2% versus 73.5%, respectively).14 Collectively, the results of these studies have demonstrated that carfilzomib-based therapies carry a favorable benefit-risk profile in the treatment of MM across cytogenetic risk categories.
Determining the impact of newer agents on the outcomes of patients with high-risk MM has been of interest, and an increasing number of studies are finding that they are efficacious in patients with relapsed/refractory MM who harbor cytogenetic aberrations. In phase 2 and phase 3 studies, pomalidomide plus low-dose dexamethasone has demonstrated efficacy in patients with relapsed/refractory MM who harbor the del(17p) and/or t(4;14) cytogenetic abnormalities.1, 21 In the randomized, phase 3 TOURMALINE-MM1 study, the median PFS observed with ixazomib, lenalidomide, and dexamethasone was similar among patients with del(17p), those with t(4;14), and those without these cytogenetic abnormalities.22 Monoclonal antibodies have also shown activity in patients with high-risk cytogenetics.23 In the randomized, phase 3 ELOQUENT-2 study evaluating elotuzumab, lenalidomide and dexamethasone (ERd) vs Rd in relapsed or refractory MM, HRs for PFS favored the ERd group in patients with del(17p) and also in those with t(4;14).24 Along with the results from this subgroup analysis of the ENDEAVOR study, these findings suggest that modern therapies are effective in patients with historically high-risk disease.
Notably, the cutoff value for the proportion of plasma cells with del(17p) used to define adverse cytogenetics has varied between studies. The ENDEAVOR study used a cutoff value of 20%, whereas the ASPIRE study used a cutoff value of 60% other studies have also used the presence of any detectable mutation to indicate a positive result.1, 20 It is presently not clear which cutoff value for del(17p) is optimal and whether the cutoff value should vary by treatment and disease stage; additional studies are ongoing to address these issues.11
Although this subgroup analysis found evidence of benefit for Kd vs Vd, irrespective of cytogenetic risk, there are potential limitations of the analysis. The first was that this was an open-label study design which has the potential to introduce bias. Another limitation was that this subanalysis used the genetic subtypes t(4;14), t(14;16) and del(17p) to risk-classify patients and did not incorporate other factors or abnormalities which may represent high-risk patients.
In conclusion, this preplanned subgroup analysis of the ENDEAVOR study found that Kd was superior to Vd in patients with relapsed or refractory MM, regardless of baseline cytogenetic risk status. Across cytogenetic risk subgroups, Kd should be considered in patients with relapsed or refractory MM for whom Vd is a potential treatment option. As patients with high-risk cytogenetics still have poor outcomes after frontline treatment, including therapies incorporating novel agents,11 the benefit-risk profile of carfilzomib-based regimens by cytogenetic risk status merits further evaluation in the upfront setting.
Acknowledgments
We thank all of the patients, families, caregivers, research nurses, study coordinators, and support staff who contributed to this study. This study was supported by Onyx Pharmaceuticals, Inc., an Amgen subsidiary. Medical writing and editorial assistance was provided by BlueMomentum, an Ashfield Company, part of UDG Healthcare PLC, and funded by Amgen Inc.
Footnotes
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
The ENDEAVOR study was supported by Onyx Pharmaceuticals, Inc., an Amgen subsidiary. HG played a consulting or advisory role in Bristol-Myers Squibb, Celgene Corporation, Janssen Pharmaceuticals, Millennium Pharmaceuticals, Novartis and Onyx Pharmaceuticals; received honoraria from Celgene Corporation, Chugai, Janssen Pharmaceuticals, Millennium Pharmaceuticals, Novartis and Onyx Pharmaceuticals and received research funding from Bristol-Myers Squibb, Celgene Corporation, Chugai, Janssen Pharmaceuticals and Novartis. MAD received honoraria from Celgene and Onyx and played a consulting or advisory role in Celgene and Onyx. PM played a consulting or advisory role in Celgene, Janssen, Millennium, Novartis and Onyx, and received honoraria from Celgene, Janssen, Millennium, Novartis and Onyx. DJ participated in a Speakers' bureau for Amgen. W-JC received honoraria from Celgene, Janssen, Novartis and Takeda and received research funding from Celgene, Novartis and Merck. AP is an employee of Takeda; has received honoraria from and served as consultant for Amgen, Novartis, Bristol-Myers Squibb, Genmab A/S, Celgene, Janssen-Cilag, Takeda, Sanofi Aventis and Merck; has received research funding from Amgen, Novartis, Bristol-Myers Squibb, Genmab A/S, Celgene, Janssen-Cilag, Takeda, Sanofi Aventis, Merck and Binding Site; and participated in a speakers' bureau for Bristol-Myers Squibb. TF has served on a advisor committee for Onyx/Amgen. HL participated in a Speakers' bureau for Amgen, Bristol-Myers Squibb, Celgene, Novartis and Takeda and received study support from Takeda. RN played a consulting or advisory role in Celgene, Millennium and Onyx and participated in a Speakers' bureau for Celgene, Millennium and Onyx. AO played a consulting or advisory role in Amgen, Celgene and Janssen and participated in a Speakers' bureau for Amgen, Celgene and Janssen. LR received honoraria from Amgen, Celgene and Janssen. GG received honoraria from Amgen, Roche, Janssen, Gilead, Karyopharm and Morphosys, and played a consulting or advisory role in Amgen, Roche, Janssen, Gilead, Karyopharm and Morphosys. KW received honoraria from Amgen, Bristol-Myers Squibb, Celgene, Janssen, Novartis, Onyx and Takeda and played a consulting or advisory role in Amgen, Bristol-Myers Squibb, Celgene, Janssen, Novartis, Onyx and Takeda. HHG and NM were formerly employed by Onyx Pharmaceuticals, an Amgen subsidiary. SA and SF are employees of Amgen. RH received honoraria from Celgene, Janssen, Merck, Onyx and Amgen. The remaining authors declare no conflict of interest.
Supplementary Material
References
- Dimopoulos MA, Weisel KC, Song KW, Delforge M, Karlin L, Goldschmidt H et al. Cytogenetics and long-term survival of patients with refractory or relapsed and refractory multiple myeloma treated with pomalidomide and low-dose dexamethasone. Haematologica 2015; 100: 1327–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myélome. Blood 2007; 109: 3489–3495. [DOI] [PubMed] [Google Scholar]
- Avet-Loiseau H, Hulin C, Campion L, Rodon P, Marit G, Attal M et al. Chromosomal abnormalities are major prognostic factors in elderly patients with multiple myeloma: the Intergroupe Francophone du Myélome experience. J Clin Oncol 2013; 31: 2806–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chng WJ, Dispenzieri A, Chim CS, Fonseca R, Goldschmidt H, Lentzsch S et alIMWG consensus on risk stratification in multiple myeloma. Leukemia 2014; 28: 269–277. [DOI] [PubMed] [Google Scholar]
- Avet-Loiseau H, Soulier J, Fermand JP, Yakoub-Agha I, Attal M, Hulin C et al. Impact of high-risk cytogenetics and prior therapy on outcomes in patients with advanced relapsed or refractory multiple myeloma treated with lenalidomide plus dexamethasone. Leukemia 2010; 24: 623–628. [DOI] [PubMed] [Google Scholar]
- Klein U, Jauch A, Hielscher T, Hillengass J, Raab MS, Seckinger A et al. Chromosomal aberrations +1q21 and del(17p13) predict survival in patients with recurrent multiple myeloma treated with lenalidomide and dexamethasone. Cancer 2011; 117: 2136–2144. [DOI] [PubMed] [Google Scholar]
- Neben K, Lokhorst HM, Jauch A, Bertsch U, Hielscher T, van der Holt B et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood 2012; 119: 940–948. [DOI] [PubMed] [Google Scholar]
- Chang H, Trieu Y, Qi X, Xu W, Stewart KA, Reece D. Bortezomib therapy response is independent of cytogenetic abnormalities in relapsed/refractory multiple myeloma. Leuk Res 2007; 31: 779–782. [DOI] [PubMed] [Google Scholar]
- Avet-Loiseau H, Leleu X, Roussel M, Moreau P, Guerin-Charbonnel C, Caillot D et al. Bortezomib plus dexamethasone induction improves outcome of patients with t(4;14) myeloma but not outcome of patients with del(17p). J Clin Oncol 2010; 28: 4630–4634. [DOI] [PubMed] [Google Scholar]
- Cavo M, Tacchetti P, Patriarca F, Petrucci MT, Pantani L, Galli M et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet 2010; 376: 2075–2085. [DOI] [PubMed] [Google Scholar]
- Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood 2016; 127: 2955–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hajaek R et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol 2016; 17: 27–38. [DOI] [PubMed] [Google Scholar]
- Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data, Springer-Verlag: New York, NY, USA, 1997.
- Avet-Loiseau H, Fonesca R, Siegel D, Dimopoulos MA, Spicka I, Masszi T et al. Efficacy and safety of carfilzomib, lenalidomide, and dexamethasone vs lenalidomide and dexamethasone in patients with relapsed multiple myeloma based on cytogenetic risk status: subgroup analysis from the phase 3 study ASPIRE (NCT01080391). Blood 2015; 126: 731. [Google Scholar]
- Jakubowiak AJ, Siegel DS, Martin T, Wang M, Vij R, Lonial S et al. Treatment outcomes in patients with relapsed and refractory multiple myeloma and high-risk cytogenetics receiving single agent carfilzomib in the PX-171-003-A1 study. Leukemia 2013; 27: 2351–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringhen S, Petrucci MT, Larocca A, Conticello C, Rossi D, Magarotto V et al. Carfilzomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed multiple myeloma: a multicenter, phase 2 study. Blood 2014; 124: 63–69. [DOI] [PubMed] [Google Scholar]
- Sonneveld P, Asselbergs E, Zweegman S, van der Holt B, Kersten MJ, Vellenga E et al. Phase 2 study of carfilzomib, thalidomide, and dexamethasone as induction/consolidation therapy for newly diagnosed multiple myeloma. Blood 2015; 125: 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korde N, Roschewski M, Zingone A, Kwok M, Manasanch EE, Bhutani M et al. Treatment with carfilzomib-lenalidomide-dexamethasone with lenalidomide extension in patients with smoldering or newly diagnosed multiple myeloma. JAMA Oncol 2015; 1: 746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowiak AJ, Dytfeld D, Griffith KA, Lebovic D, Vesole DH, Jagannath S et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood 2012; 120: 1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Spicka I, Oriol A et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med 2015; 372: 142–152. [DOI] [PubMed] [Google Scholar]
- Leleu X, Karlin L, Macro M, Hulin C, Garderet L, Roussel M et al. Pomalidomide plus low-dose dexamethasone in multiple myeloma with deletion 17p and/or translocation (4;14): IFM 2010-02 trial results. Blood 2015; 125: 1411–1417. [DOI] [PubMed] [Google Scholar]
- Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 2016; 374: 1621–1634. [DOI] [PubMed] [Google Scholar]
- van de Donk NW, Moreau P, Plesner T, Palumbo A, Gay F, Laubach JP et al. Clinical efficacy and management of monoclonal antibodies targeting CD38 and SLAMF7 in multiple myeloma. Blood 2016; 127: 681–695. [DOI] [PubMed] [Google Scholar]
- Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med 2015; 373: 621–631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.