Abstract
AIM
To assess the prevalence of functional gastrointestinal disorders (FGIDs) in children and adolescents.
METHODS
PubMed, EMBASE, and Scopus databases were searched for original articles from inception to September 2016. The literature search was made in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations. For inclusion, each study had to report epidemiological data on FGIDs in children between 4 and 18 years old and contain standardized outcome based on Rome II, III or IV criteria. The overall quality of included epidemiological studies was evaluated in accordance with Loney’s proposal for prevalence studies of health literature. Two reviewers assessed each study for data inclusion and extraction. Discrepancies were reconciled through discussion with seniors.
RESULTS
A total of 659 articles were identified from the databases and 16 through manual search. A total of 43 articles fulfilled the eligibility criteria for full-text reading, with 26 remaining to be included in the final analysis. All studies were written in English and published between 2005 and 2016. Eight (30.8%) articles were performed in North America, five (19.2%) in Latin America, five (19.2%) in Europe, seven (27%) in Asia, and one (3.8%) in Africa. Sample size varied between 114 and 99416 subjects, totaling 132600 individuals. Fourteen (53.9%) studies recruited their target samples from schools, 11 (42.3%) from healthcare settings and the remaining one (3.8%) from online panel community. The overall FGID prevalence rates for student samples ranged from 9.9% to 29% to as high as 87% in clinical samples. Cyclic vomiting, irritable bowel syndrome and functional constipation were the most researched conditions, with a prevalence ranging from 0.2% to 6.2%, 0% to 45.1% and 0.5% to 86.9%, respectively. The qualitative appraisal revealed that most of the studies showed average or below average generalizability.
CONCLUSION
The heterogeneity of the studies on FGIDs must be improved in order to allow comparison. Improvements should include appropriate sampling of representative population, comparable study setting, and consistent data collection.
Keywords: Functional gastrointestinal disorders, Epidemiology, Prevalence, Children, Adolescents
Core tip: Epidemiological studies on functional gastrointestinal disorders in children and adolescents provide variable prevalence rates in both non-clinical and clinical settings. The scarcity of good quality prevalence data for functional gastrointestinal disorders in light of recent Rome IV criteria reveals an urgent need for more trustworthy information to construct evidence-based health policy. The current literature review suggested higher impact of cyclic vomiting, irritable bowel syndrome and functional constipation in children and adolescents.
INTRODUCTION
Functional gastrointestinal disorders (FGIDs) are considered common, even in children and adolescents. During the last years, the burden of FGIDs is rising[1-4], but no biomarkers[5] or gold standard tests are available to date for diagnosing gastrointestinal (GI) disorders without an established etiology[6].
Pediatric guidelines are dynamic over time and must be driven by evidence-based medicine[7]. The Rome criteria for FGIDs, currently in its 4th edition (Rome IV, May 2016), are guidelines based on a detailed clinical evaluation that must contain complete clinical history, physical examination and growth curves to help clinicians in daily practice[5,8-10].
In the child/adolescent Rome IV chapter, there are two main changes: (1) the term “no evidence for organic disease” was removed from all definitions and replaced by “after appropriate medical evaluation the symptoms cannot be attributed to another medical condition”; and (2) the FGIDs can co-occur with other medical conditions that themselves result in GI symptoms[11].
Table 1 summarizes main Rome IV categories concerning frequency, duration and synonym, subtypes or approximate terms in three broad sections: (H1) nausea and vomiting disorders; (H2) abdominal pain-related disorders; and (H3) defecation disorders.
Table 1.
Classification of functional gastrointestinal disorders in children and adolescents
| Rome IV nomenclature1 | Frequency | Duration | Synonym, subtypes or approximate terms | |
| H1: Functional nausea and vomiting disorders | ||||
| H1a. | Cyclic vomiting syndrome | ≥ 2 periods of intense, unremitting nausea and paroxysmal vomiting | h-d/6 mo | Periodic vomiting |
| H1b1. | Functional nausea | ≥ 2 nausea episodes/wk | ≥ 2 mo | Bothersome nausea |
| H1b2. | Functional vomiting | ≥ 1 vomiting episode/wk | ≥ 2 mo | - |
| H1c. | Rumination syndrome | Repetitive regurgitation and rechewing or expulsion of food | ≥ 2 mo | Adolescent rumination syndrome2; regurgitation, reswallowing, spitting |
| H1d. | Aerophagia | Repetitive belching and/or increased flatus | ≥ 2 mo | - |
| H2: Functional abdominal pain disorders | ||||
| H2a. | Functional dyspepsia | ≥ 1 symptom for ≥ 4 d/mo | ≥ 2 mo | Postprandial distress syndrome; |
| Epigastric pain syndrome | ||||
| H2b. | Irritable bowel syndrome | Abdominal pain for ≥ 4 d/mo | ≥ 2 mo | Abdominal discomfort2; |
| Manning criteria | ||||
| H2c. | Abdominal migraine | ≥ 2 intense abdominal pain episodes | ≥ 1 h/6 mo | Periumbilical pain2 |
| H2d. | Functional abdominal pain - not otherwise specified | ≥ 4 episodic or continuous abdominal pain/mo | ≥ 2 mo | Functional abdominal pain2; |
| Functional abdominal pain syndrome2 | ||||
| H3: Functional defecations disorders | ||||
| H3a. | Functional constipation | ≤ 2 defecations/wk | ≥ 1 mo | - |
| ≥ 1 fecal incontinence/wk | ||||
| H3b. | Nonretentive fecal incontinence | Episodes of fecal loss | ≥ 1 mo | - |
After appropriate evaluation, the symptoms cannot be fully explained by another medical condition;
Rome III nomenclature.
Agreed-upon description of GI syndromes and accurate estimates of FGID prevalence are required for defining the need for treatment in overloaded healthcare settings. Projected proportion of pediatric FGID cases in the community and different levels of healthcare setting obtained through epidemiological studies might help to guide proper allocation of financial support and organize health service delivery.
The aim of this literature review was to critically examine current evidence of knowledge on FGIDs in children and adolescents, through systematic search of frequency or prevalence data on common functional GI problems. Furthermore, we have assessed the quality of existing studies on the target topic.
MATERIALS AND METHODS
Search strategies
A literature search was conducted in the PubMed, EMBASE, and Scopus databases in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)[12]. The search terms were “functional gastrointestinal disorder” OR “functional gastrointestinal symptoms” AND “epidemiology” OR “prevalence” OR “incidence”. In addition, for each of the eleven specific categories of FGIDs in children and adolescents, a new search was performed with the disorder’s nomenclature and equivalent and/or approximate terms. For example, “cyclic vomiting” AND “periodic vomiting” were combined with epidemiological terms (Supplementary Online Content).
There was no language restriction and the period covered was from inception to September 30, 2016. For inclusion, each study had to: (1) contain children and adolescents between 4 and 18 years old; (2) report functional gastrointestinal symptoms and/or disorders according to Rome II, III or IV criteria[13,14] (http://www.romecriteria.org/); (3) design sample from a birth-cohort, population-based, school-based or clinical setting; and (4) report epidemiological outcomes (prevalence, incidence or frequency) for general FGIDs and subtypes. To complete literature investigation, “similar articles” option, manual search of the reference list on reviewed articles, book chapter, and gray literature were accessed. Experts in pediatric gastroenterology were contacted to request full text or unpublished data. Independently, two reviewers (Boronat AC and Ferreira-Maia AP) assessed each study for inclusion and extracted the data. Discrepancies were reconciled through panel discussion with senior authors (Matijasevich A and Wang YP).
Critical literature appraisal
The overall quality of the studies included was evaluated in accordance with Loney’s proposal for prevalence studies in health literature[15]. All studies were scored based on eight criteria: (1) sample size; (2) sampling adequacy; (3) unbiased sampling frame; (4) measures of outcomes; (5) unbiased assessors; (6) response rate with refusals described; (7) prevalence with confidence intervals and by relevant subgroups; and (8) appropriate description of study subjects for the research question. One point was attributed for each met criterion. Higher scores indicate better study quality in a scoring range from zero to eight.
The sample size criterion was not used to exclude studies. However, we considered the sample size to be adequate if it was projected for the study on the basis of local population estimates or if it was higher than 370. This minimum sample size was calculated to allow outcome assessment using simple random sampling, with a conservative estimate of 13.9% for distinct FGIDs in the age bracket of children and adolescents[16], confidence level of 95%, and precision of 1.8%, resulting in a minimum sample size of 370 subjects.
Two reviewers (Boronat AC and Ferreira-Maia AP) performed the evaluation and final results were discussed one by one with senior author (Wang YP).
Methodological issues
For accurate evaluation of the methodological issues on pediatric epidemiological studies, two questions need be highlighted: (1) how representative of the target population are the recruited participants? (2) are the outcome measures reliable and valid?
How representative of the target population are the recruited participants? The most appropriate study design to determine the prevalence of a goal condition (prevalence of FGIDs) is the population-based observational study covering the whole target population, e.g., by census of all subjects between 4-18 years old within a certain area. This is not always possible or feasible as it is a high cost or time-consuming method. Probability sampling, in turn, is essential in prevalence studies to ensure that each potential respondent has an equal chance of selection (non-zero probability), warranting the representativeness of the intended population[15,17].
Convenience sampling provides lower quality epidemiological data than population-based studies. Participants recruited from particular communities (e.g., social network or online panel), schools, primary care and specialty care would result in some types of selection bias. In order to obtain unbiased frequency estimates, all eligible persons susceptible to developing a clinical condition should be included in sampling design, regardless of refusal or reasons of exclusion (i.e., loss to follow-up, incomplete data, and organic exclusion). Otherwise, the rate of disease frequency would be either inflated or reduced.
Assuming that most of children and adolescents are enrolled in schools (except those homeless, correctional institutionalized and hospitalized), conducting a survey in randomly selected schools might be an acceptable alternative. In healthcare treatment settings, the Berkson’s bias may skew the sample characteristics by selecting more symptomatic treatment-seeking individuals.
Sample size is important to ensure measurement precision using confidence limits. Either the confidence interval (CI) or the information needed to calculate CI must be reported to allow quantifying the degree of uncertainty associated with the frequency estimates. Non-representative sampling cannot always be fixed through very large samples. Typically, in case of a high rate of non-response (more than 20%), the socio-demographic characteristics of non-respondent group must be compared with those of respondent group, to evaluate potential selection bias and impact on frequency estimates of target condition[17].
Non-representativeness of recruited participants is a serious threat to external validity by curbing generalization of the results. Hence, effort to fix unequal selection chance is recommended. Weighting procedure and post-stratification adjustment are alternatives to fit the data to target-population structure.
Are the outcome measures reliable and valid? The type of informant and the method of data assessment represent potential sources of error for estimating the prevalence rate of clinical conditions. Standardized data collection methods provide reliable and valid measurement of target outcome.
Expert opinions may diverge on the constellation of signs and symptoms of a functional disorder, as well as the frequency and duration of GI ailments. One of the Rome IV’s goals is to operationalize the construct of FGIDs through reproducible criteria, since to date there has been no gold standard assessment for it. The validity of categories of FGIDs is still a matter of intense research.
In pediatrics, mainly in younger children samples, it is usual to obtain GI information only through parental report. Studies in older children and adolescents have demonstrated that parent-child/adolescent concordance was largely poor[18]. The administration of validated questionnaires like the Questionnaire on Pediatric Gastrointestinal Symptoms (QPGS/QPGS-RIII, parental and/or self-report form)[17,19] is a feasible strategy for ascertaining the symptoms of FGIDs, but the establishment of a case based barely on questionnaire responding may mislead to under or over-estimation of problems in children and adolescents. When objective laboratory measure is lacking, as in the case of FGIDs, multisource informants (parent, children or adolescent) and validated questionnaires plus clinical evaluation may constitute the best strategy for the best possible diagnosis, mitigating information bias.
It is recommended that interviewers are impartial to children’s health status and trained for identification of cases based on external criteria and decision rules for disease diagnosis (FGIDs)[15,17]. Further investigation or therapeutics may confirm or rule out the suspected illness.
Ultimately, the validity and reliability of outcome measures for GI symptoms are intrinsically linked to sensitivity and specificity of the standardized operational procedures, either by independent assessors or assessment tools.
RESULTS
Literature search and general description of included studies
The search flow diagram is displayed in Figure 1. A total of 659 articles were identified through the databases and 16 through the manual search. After removing duplicate records, 149 articles remained for title and abstract reading. Of these, 43 articles fulfilled the eligibility criteria for full-text reading. Finally, 26 articles were included in the present review. The 17 studies excluded were listed in the Supplementary Online Content.
Figure 1.
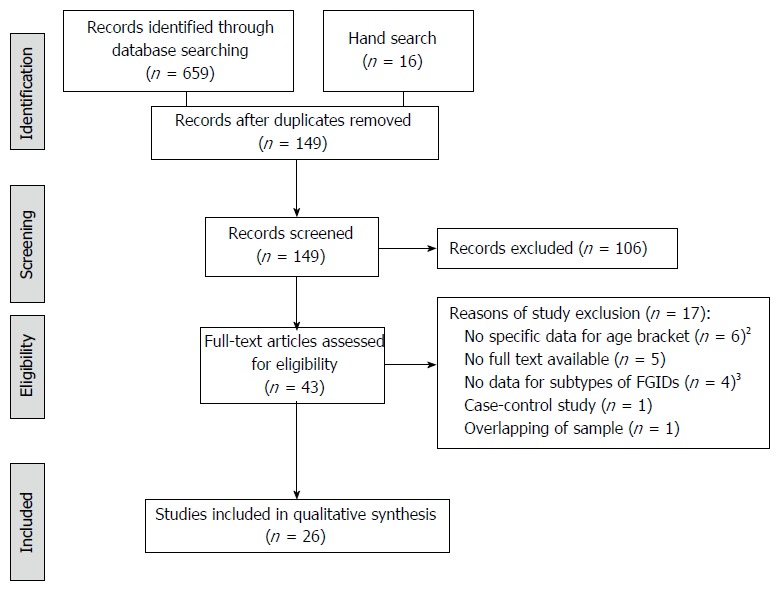
Flow diagram of identifying eligible articles1. 1Flow diagram according to PRISMA (www.prisma-statement.org); 2Age bracket 4-18 years old; 3Prevalence only for general FGIDs. FGIDs: Functional gastrointestinal disorders.
All of the articles included (n = 26) were written in English and published between 2005 and 2016. Eight studies were performed in North America[8,18,20-25], five in Latin America[26-30], five in Europe[31-35], seven in Asia[6,16,36-40], and one in Africa[41]. Five studies were performed by the same Latin American consortium, the Functional International Digestive Epidemiology Research Survey, which adopted a similar methodology, thus allowing comparing the data.
Five articles detailed the distribution of demographic characteristics of the study population. Among the participants, no significant variation for gender[6,20,26,36], age or race[26] was observed.
Concerning FGID outcome criterion, the majority of studies (n = 18) used Rome III criteria to define each specific GI category[6,16,23-30,32-35,38-41]. Five studies used the Rome II criteria to define FGIDs[19,20-22,37], while three others provided comparable data for the versions II and III of Rome criteria[8,31,36]. Until the time of review, no study had reported epidemiological data of FGIDs using Rome IV criteria.
In the great majority of eligible articles, the sample was recruited by convenience (n = 19, 73.1%). Six additional studies described some types of random selection and one study conducted the survey by means of quota sampling. Sample size varied between 114 and 99416 subjects, totaling 132600 individuals. Although most studies (n = 19) recruited participants achieving sufficient sample size, the representativeness of FGID epidemiological data from children and adolescent populations constituted a threat to its external validity.
Regardless of recruitment methods, the sampling setting diverged. Fourteen studies recruited target sample from schools, 11 from healthcare settings, and the remaining from an online panel community. As such, the overall FGID prevalence rates for student samples ranged from 9.9% to 29%[26,41] to as high as 87% in a specialty gastroenterological care service after organic exclusion[35]. This great prevalence variation was reliant on the type of sampling setting.
Seven school-based studies included multiple schools without randomization[6,26-30,34]. Among healthcare settings, most of studies (n = 8) recruited participants from a single tertiary care center[8,19-24,31,33], two from secondary care[32,35], and the remaining one from primary care[22]. As such, the proportion of FGIDs in treated patient samples was much higher than school-based student samples.
Specific categories of FGIDs in half of the articles (n = 13) were exclusively informed by questionnaire, either parental report and/or self-report by children and adolescents[23,25-30,34,36,38-41], while the other half (n = 13) also included clinical evaluation and/or medical records[6,8,16,19,20-22,24,31-33,35,37].
Of interest, the agreement rate between dyads of informants (parents and children) and informant-physician varied greatly in magnitude[19,21,24], within the groups of FGIDs. This non-agreement rate, as expressed through the kappa coefficient, is a serious issue to the prevalence data, as follows.
Functional nausea and vomiting disorders (H1): the parent-children agreement for cyclic vomit was moderate (k = 0.42)[19], and that for aerophagia ranged from no to substantial agreement[19,24].
Functional abdominal pain disorders (H2): the parent-children agreement for dyspepsia was fair to substantial[19,21,24], but this concordance could not replicate for informant-physician dyads (kappa range: 0.02 to -0.06)[24]. Considerable disagreements across all dyads were reported for the irritable bowel syndrome (IBS; kappa range: 0.03 to 0.44) and functional abdominal pain (kappa range: -0.10 to -0.02)[24]. Likewise, the agreement for abdominal migraine ranged from poor to moderate in the parent-children dyad[18,24].
Functional defecation disorders (H3): while the agreement rate for constipation was fair across all dyads[24], no evidence of agreement was reported for fecal retention and nonretentive fecal soiling[19].
Because there is no reliable concordance between dyads, the quality and the magnitude of prevalence data of FGIDs in children and adolescents can be distorted by the type of informant. The observed rate of 7.7% for any FGID among German children cannot be trusted, since the data were solely based on parent report[34].
In terms of outcome criteria, the agreement between Rome II and Rome III to diagnose FIGDs was poor (k = 0.114)[31]. Under more sensitive Rome III criteria, the reported prevalence of FGID might at least double relative to Rome II[8,36]. Since there are no published data based on Rome IV criteria, the effect of this new version on FGID prevalence could not compared.
The appraisal of the 26 included studies indicated that good quality studies reporting the epidemiology of main categories of FGIDs in children and adolescents were scarce, likewise recent reviews of FGIDs in infants and toddlers[42,43].
According to Loney’s proposal[15], a higher score of six was achieved by three school-based studies conducted in Japan[16,40] and China[37]. In general, the studies presented poor quality in half of the retained articles (n = 13), scoring 2 or a maximum of 3 points. By far, the most common problem was prevalence rates without confidence interval and/or no detailed information on subgroup (n = 21), inappropriate sampling frame (n = 21), inadequate sampling method (n = 19), no description of refusers (n = 14) and/or insufficient sample size (n = 7) (Table S1).
Regarding the main epidemiological results on FGIDs, we describe sequentially the groups of H1 (vomiting and aerophagia) in Table 2, H2 (abdominal pain-related functional GI disorders) in Table 3, and H3 (constipation and incontinence) in Table 4. Among the single categories of FGIDs, cyclic vomiting, IBS and constipation were the most researched conditions.
Table 2.
Prevalence or frequency of functional gastrointestinal disorders: Nausea and vomiting problems in children and adolescents
| Author, year, country | Study design, setting | Sample size (participation %) | Age bracket yr | Case definition | Case ascertainment | Score1 |
FGID subtype prevalence % (CI 95%) |
||
| Cyclic vomiting | Aerophagia | Rumination | |||||||
| Bhatia et al[6], 2016, India | Cross-sectional, school-based | 1200 (93.3) | 10-17 | Rome III | Self-reported QPGS-RIII | 5 | 0.2 | 1.5 | 0.3 |
| Medical records | |||||||||
| Physical examination | |||||||||
| Caplan et al[18], 2005, Canada | Cross-sectional, tertiary care | 315 (NR) | 4-18 | Rome II | Self-reported QPGS | 3 | p 4-9 yr = 6.2 | p 4-9 yr = 1.1 | |
| Parental QPGS | p 10-18 yr = 2.2 | p 10-18 yr = 2.2 | |||||||
| Clinical evaluation | a 10-18 yr = 4.3 | a 10-18 yr = 1.4 | |||||||
| Devanarayana et al[36], 2010, Sri Lanka | Cross-sectional, school-based | 464 (92) | 12-16 | Rome II | Self-reported QPGS | 4 | 0.2 | 6.1 | |
| Rome III | 0.5 | 6.3 | 4.0 | ||||||
| Helgeland et al[35], 2009, Norway | Cross-sectional, secondary care | 192 (79.1) | 4-15 | Rome III | Parental QPGS-III | 3 | 6.0 | 15.0 | 2.0 |
| Clinical evaluation | |||||||||
| Medical records | |||||||||
| Physical examination | |||||||||
| Laboratory exams | |||||||||
| Játiva et al[30], 2016, Ecuador | Cross-sectional, school-based | 420 (99.3) | 8-15 | Rome III | Self-reported QPGS-RIII | 3 | 1.0 | 2.6 | 0.7 |
| Parental standard questionnaire | |||||||||
| Lewis et al[25], 2016, United States | Cross-sectional, online panel community | 1447 (NR) | 4-18 | Rome III | Parental QPGS-RIII | 2 | 1.1 | 4.3 | 0.0 |
| PedsQL4.0 | |||||||||
| Lu et al[28], 2016, Panama | Cross-sectional, school-based | 436 (82.8) | 8-14 | Rome III | Self-reported QPGS-RIII | 4 | 0.3 (0.0-0.9) | 0.3 (0.0-0.9) | 0.0 |
| Parental standard questionnaire | |||||||||
| Sagawa et al[16], 2012, Japan | Cross-sectional, school-based | 3976 (NR) | 10-17 | Rome III | Self-reported QPGS-RIII | 6 | 0.2 | 2.0 | 0.1 |
| Self-reported PedsQL4.0 | |||||||||
| Clinical evaluation | |||||||||
| Saps et al[26], 2014, Colombia | Cross-sectional, school-based | 488 (83.2) | 10.0 (mean age) | Rome III | Self-reported QPGS-RIII | 4 | 0.3 (0.0-1.7) | ||
| Parental standard questionnaire | |||||||||
| Uc et al[22], 2006, United States | Cross-sectional, primary care | 243 (100) | 4-17 | Rome II | Parental QPGS | 4 | 0.8 | 2.5 | |
| Clinical evaluation | |||||||||
| van Tilburg et al[24], 2013, United States | Cross-sectional, tertiary care | 135 (NR) | 4-18 | Rome III | Self-reported QPGS-III | 3 | p = 0.8 | p = 0.8 | p = 0.8 |
| Parental QPGS-III | c/a = 5.3 | c/a = 3.5 | c/a = 5.3 | ||||||
| Clinical evaluation | ph = 0 | ph = 0 | ph = 0 | ||||||
| Medical records | |||||||||
| Zablah et al[27], 2015, El Salvador | Cross-sectional, school-based | 434 (NR) | 8-15 | Rome III | Self-reported QPGS-RIII | 3 | 0.5 | 0.2 | |
| Parental standard questionnaire | |||||||||
Score: Methodological strength of study (maximum 8) by Loney’s criteria. NR: Not reported; w: With; p: Parents; c: Children; a: Adolescents; ph: Physician; QPGS-RIII: Questionnaire on Pediatric Gastrointestinal Symptoms - Rome III; QPGS: Questionnaire on Pediatric Gastrointestinal Symptoms - Rome II; PedsQL4.0 Pediatric Quality of Life version Inventory 4.0.
Table 3.
Prevalence or frequency of functional gastrointestinal disorders: Abdominal pain problems in children and adolescents
| Author, year, country | Study design, setting | Sample size (participation %) | Age bracket yr | Case definition | Case ascertainment | Score1 |
FGID subtype prevalence %( 95%CI) |
|||
| Dyspepsia | Irritable bowel | Abdominal migraine | Abdominal pain - NOS | |||||||
| Baber et al[8], 2008, United States | Cross-sectional, tertiary care | 548 (80.1) | 8-17 | Rome II | Parental QPGS | 5 | 19.6 | 44.0 | 5.7 | 2.7 |
| Rome III | Clinical evaluation | 15.2 | 45.1 | 23.1 | 17.4 | |||||
| Medical records | ||||||||||
| Laboratory exams | ||||||||||
| Bhatia et al[6], 2016, India | Cross-sectional, school-based | 1200 (93.3) | 10-17 | Rome III | Self-reported QPGS-RIII | 5 | 2.7 | 1.3 | 1.4 | 0.8 |
| Medical records | ||||||||||
| Physical examination | ||||||||||
| Caplan et al[18], 2005, Canada | Cross-sectional, tertiary care | 315 (NR) | 4-18 | Rome II | Self-reported QPGS | 3 | p 4-9 yr = 13.5 | p 4-9 yr = 22.0 | p 4-9 yr = 0 | p 4-9 yr = 0 |
| Parental QPGS | p 10-18 yr = 14.4 | p 10-18 yr = 23.9 | p 10-18 yr = 0.7 | p 10-18 yr = 2.9 | ||||||
| Clinical evaluation | a 10-18 yr = 10.2 | a 10-18 yr = 35.5 | a 10-18 yr = 2.2 | a 10-18 yr = 2.9 | ||||||
| Cristofori et al[33], 2014, Italy | Cross-sectional, tertiary care | 992 (NR) | 4-16 | Rome III | Clinical evaluation | 4 | 25.7 | 34.5 | 0.0 | 39.8 |
| Medical records | ||||||||||
| Laboratory exams | ||||||||||
| Devanarayana et al[36], 2010, Sri Lanka | Cross-sectional, school-based | 464 (92) | 12-16 | Rome II | Self-reported QPGS | 4 | 1.2 | 2.8 | 0.2 | 1.4 |
| Rome III | 3.5 | 7.0 | 0.2 | 3.0 | ||||||
| Dong et al[37], 2005, China | Cross-sectional, school-based | 5403 (NR) | 6-18 | Rome II | Self-reported standard questionnaire | 6 | 13.2 | |||
| Parental standard questionnaire | ||||||||||
| Medical records | ||||||||||
| Gijsbers et al[32], 2014, the Netherlands | Cross-sectional, secondary care | 220 (NR) | 4-16 | Rome III | Clinical evaluation | 3 | 3.6 | 5.0 | 0.0 | 15.0 |
| Medical records | ||||||||||
| Laboratory exams | ||||||||||
| Gulewitsch et al[34], 2013, Germany | Cross-sectional, school-based | 3658 (43.1) | 5-12 | Rome III | Parental QPGS-RIII | 2 | 0.2 | 4.9 | 1.0 | 3.6 |
| Parental CSI | ||||||||||
| Parental SDQ | ||||||||||
| Helgeland et al[35], 2009, Norway | Cross-sectional, secondary care | 192 (NR) | 4-15 | Rome III | Parental QPGS-III | 3 | 10.0 | 43.0 | 23.0 | 15.0 |
| Clinical evaluation | ||||||||||
| Medical records | ||||||||||
| Physical examination | ||||||||||
| Laboratory exams | ||||||||||
| Játiva et al[30], 2016, Ecuador | Cross-sectional, school-based | 420 (99.3) | 8-15 | Rome III | Self-reported QPGS-RIII | 3 | 0.5 | 4.8 | 2.4 | 3.1 |
| Parental standard questionnaire | ||||||||||
| Lewis et al[25], 2016, United States | Cross-sectional, online painel community | 1447 (NR) | 4-18 | Rome III | Parental QPGS-RIII | 2 | 0.2 | 2.8 | 9.2 | 11.6 |
| PedsQL4.0 | ||||||||||
| Lu et al[29], 2016, Colombia | Cross-sectional, school-based | 4751 (89.8) | 8-18 | Rome III | Self-reported QPGS-RIII | 3 | 4.8 | |||
| Parental standard questionnaire | ||||||||||
| Lu et al[28], 2016, Panama | Cross-sectional, school-based | 436 (82.8) | 8-14 | Rome III | Self-reported QPGS-RIII | 4 | 0.9 (0.0-2.0) | 5.6 (3.1-8.1) | 1.7 (0.2-2.9) | 3.7 (1.7-5.8) |
| Parental standard questionnaire | 0.3 (0.0-0.9) | |||||||||
| Sagawa et al[16], 2012, Japan | Cross-sectional, school-based | 3976 (NR) | 10-17 | Rome III | Self-reported QPGS-RIII | 6 | 0.9 | 5.9 | 1.8 | 4.2 |
| Self-reported PedsQL4.0 | ||||||||||
| Clinical evaluation | ||||||||||
| Saps et al[23], 2012, United States | Cross-sectional, Community | 984 (25) | 4-18 | Rome III | Parental QPGS-III | 2 | 8.1 | |||
| Saps et al[26], 2014, Colombia | Cross-sectional, school-based | 488 (83.2) | 10.0 (mean age) | Rome III | Self-reported QPGS-RIII | 4 | 1.7 (0.8-3.9) | 5.4 (3.9-8.8) | 1.0 (0.3-2.8) | 2.7 (1.6-5.2) |
| Parental standard questionnaire | 0.3 (0.0-1.7) | |||||||||
| Schurman et al[21], 2005, United States | Cross-sectional, tertiary care | 205 (75) | 8-18 | Rome II | Self-reported QPGS | 3 | p = 47 | p = 20; | p < 10 | p < 10 |
| Parental QPGS | c/a = 35 | c/a = 30; | c/a < 10; | c/a < 10 | ||||||
| Clinical evaluation | ph = 57 | ph = 12 | ph < 10 | ph < 10 | ||||||
| Uc et al[22], 2006, United States | Cross-sectional, primary care | 243 (100) | 4-17 | Rome II | Parental QPGS | 4 | 0.8 | 0.0 | 0.4 | 0.4 |
| Clinical evaluation | ||||||||||
| Udoh et al[41], 2016, Nigeria | Cross-sectional, school-based | 856 (NR) | 10-18 | Rome III | Self-reported QPGS-RIII | 4 | 0.4 | 5.6 | 1.8 | 2.6 |
| Standard questionnaires | ||||||||||
| Walker et al[20], 2004, United States | Cross-sectional, tertiary care | 114 (NR) | 4-17 | Rome II | Parental QPGS | 3 | 15.9 | 44.9 | 4.7 | 7.5 |
| Clinical evaluation | ||||||||||
| Parental standard interview | ||||||||||
| Medical records | ||||||||||
| Physical examination | ||||||||||
| Laboratory exams | ||||||||||
| Yamamoto et al[40], 2015, Japan | Cross-sectional, school-based | 99416 (92.2) | 12-18 | Rome III | Self-reported standard questionnaire | 6 | 18.6 (17.9-19.2) | |||
| Zablah et al[27], 2015, El Salvador | Cross-sectional, school-based | 434 (NR) | 8-15 | Rome III | Self-reported QPGS-RIII | 3 | 1.7 | 3.7 | 0.7 | 3.0 |
| Parental standard questionnaire | ||||||||||
| Zhou et al[38], 2011, China | Cross-sectional, school-based | 3671 (NR) | 12-18 | Rome III | Self-reported standard questionnaire | 5 | 19.8 (18.6-21.1) | |||
Score: Methodological strength of study (maximum 8) by Loney’s criteria; NR: Not reported; w: With; p: Parents; c: Children; a: Adolescents; ph: Physician; QPGS-RIII: Questionnaire on Pediatric Gastrointestinal Symptoms - Rome III; QPGS: Questionnaire on Pediatric Gastrointestinal Symptoms - Rome II; PedsQL4.0 Pediatric Quality of Life version Inventory 4.0CSI: Children’s Somatization Inventory; SDQ: Strengths and Difficulties Questionnaire.
Table 4.
Prevalence or frequency of functional gastrointestinal disorders: Defecations problems in children and adolescents
| Author, year, country | Study design, setting | Sample size (participation %) | Age bracket yr | Case definition | Case ascertainment | Score1 |
FGID subtype prevalence % (95%CI) |
|
| Constipation | Nonretentive fecal incontinence | |||||||
| Bhatia et al[6], 2016, India | Cross-sectional, school-based | 1200 (93.3) | 10-17 | Rome III | Self-reported QPGS-RIII | 5 | 0.5 | 0.4 |
| Medical records | ||||||||
| Physical examination | ||||||||
| Burgers et al[31], 2012, Netherlands | Cross-sectional (retrospective), tertiary care | 176 (NR) | 6-18 | Rome II | Clinical evaluation | 3 | 5.7 | |
| Rome III | Medical records | 86.9 | ||||||
| Physical examination | ||||||||
| Caplan et al[18], 2005, Canada | Cross-sectional, tertiary care | 315 (NR) | 4-18 | Rome II | Self-reported QPGS | 3 | p 4-9 yr = 19.2 | p 4-9 yr = 0.6 |
| Parental QPGS | p 10-18 yr = 13.8 | p 10-18 yr = 0.7 | ||||||
| Clinical evaluation | c/a 10-18 yr = 15.2 | c/a 10-18 yr = 0.7 | ||||||
| Devanarayana et al[36], 2010, Sri Lanka | Cross-sectional, school-based | 464 (92) | 12-16 | Rome II | Self-reported QPGS | 4 | 1.4 | 0.2 |
| Rome III | 4.2 | 0.2 | ||||||
| Helgeland et al[35], 2009, Norway | Cross-sectional, tertiary care | 192 (NR) | 4-15 | Rome III | Parental QPGS-III | 3 | 6.0 | |
| Clinical evaluation | ||||||||
| Medical records | ||||||||
| Physical examination | ||||||||
| Laboratory exams | ||||||||
| Játiva et al[30], 2016, Ecuador | Cross-sectional, school-based | 420 (99.3) | 8-15 | Rome III | Self-reported QPGS-RIII | 3 | 11.8 | 0.2 |
| Parental standard questionnaire | ||||||||
| Lewis et al[25], 2016, United States | Cross-sectional, online painel community | 1447 (NR) | 4-18 | Rome III | Parental QPGS-RIII | 2 | 12.9 | 1.8 |
| PedsQL4.0 | ||||||||
| Lu et al[29], 2016, Colombia | Cross-sectional, school-based | 4751 (89.8) | 8-18 | Rome III | Self-reported QPGS-RIII | 3 | 12.7 | |
| Parental standard questionnaire | ||||||||
| Lu et al[28], 2016, Panama | Cross-sectional, school-based | 436 (82.8) | 8-14 | Rome III | Rome III | 4 | 15.9 (11.9-19.9) | 0 (0.0-0.0) |
| Self-reported QPGS-RIII | ||||||||
| Parental standard questionnaire | ||||||||
| Rajindrajith et al[39], 2013, Sri Lanka | Cross-sectional, school-based | 1855 (96.7) | 13-18 | Rome III | Self-reported QPGS-RIII | 5 | 7.7 | |
| Self-reported PedsQL4.0 | ||||||||
| Sagawa et al[16], 2012, Japan | Cross-sectional, school-based | 3976 (NR) | 10-17 | Rome III | Rome III | 6 | 0.3 | 0.2 |
| Self-reported QPGS-RIII | ||||||||
| Self-reported PedsQL4.0 | ||||||||
| Clinical evaluation | ||||||||
| Saps et al[26], 2014, Colombia | Cross-sectional, school-based | 488 (83.2) | 10.0 (mean age) | Rome III | Self-reported QPGS-RIII | 4 | 14.0 (12.0-19.3) | 1.5 (0.7-3.6) |
| Parental standard questionnaire | ||||||||
| Uc et al[22], 2006, United States | Cross-sectional, primary care | 243 (100) | 4-17 | Rome II | Parental QPGS | 4 | 16.1 | 0.4 |
| Clinical evaluation | ||||||||
| Zablah et al[27], 2015, El Salvador | Cross-sectional, school-based | 434 (NR) | 8-15 | Rome III | Self-reported QPGS-RIII | 3 | 10.0 | 0.0 |
| Parental standard questionnaire | ||||||||
| Zhou et al[38], 2011, China | Cross-sectional, school-based | 3671 (NR) | 12-18 | Rome III | Self-reported standard questionnaire | 5 | 24.9 (23.5-26.3) | |
Score: Methodological strength of study (maximum 8) by Loney’s criteria. NR: Not reported; w: With; p: Parents; c: Children; a: Adolescents; ph: Physician; QPGS-RIII: Questionnaire on Pediatric Gastrointestinal Symptoms - Rome III; QPGS: Questionnaire on Pediatric Gastrointestinal Symptoms - Rome II; PedsQL4.0 Pediatric Quality of Life version Inventory 4.0.
Vomiting and aerophagia
There were 12 studies reporting frequency data on vomiting and aerophagia in children and adolescents (Table 2). The choice of the QPGS or parental report to assess the FGID symptoms was the rule. Seven studies were school-based surveys[6,16,26-28,30,36]. Six studies also included external clinical assessment and/or medical records[6,16,19,22,24,35]. For the remaining ones, nine studies used information self-reported by the children or adolescents, while nine used parental report, and six used both types of forms.
Cyclic vomiting and aerophagia were uncommon FGIDs in this age group, although they were the most frequent data collected on the group H1. There were dissimilar rates reported across studies, ranging from 0.2% to 6.2%[6,19] and 0% to 15%[24,35], respectively, for cyclic vomiting and aerophagia. The investigation setting, namely, school-based or healthcare centers, can be considered as influencing factors
Information on rumination was less reported; the rates ranged from 0.3% to 5.3% in nine studies. There were no available data for functional nausea and functional vomiting since these are new categories proposed in the Rome IV criteria.
Abdominal pain-related functional GI disorders
Twenty-three studies addressed this FGID group (H2; Table 3), with IBS being the most reported category across eligible studies. Two large sample studies in China (n = 3671 and 5403) dedicated to explore the prevalence of IBS in school-based settings[37,38]. Data on dyspepsia, abdominal migraine and abdominal pain-not otherwise specified (NOS) were reported in 21 studies. Similarly to the H1 group, QPGS was also the standard assessment tool for reporting the symptoms of abdominal pain-related disorders. School and healthcare setting were the major sources of participant recruitment.
Given its disabling feature, there was a major interest to understand the occurrence and clinical characteristic of IBS. Across all studies on children and adolescents, the rates of IBS ranged from 0% to 45.1%[8,22] according to the setting of recruitment. Possibly, the prevalence rate of IBS would be lower in schools and inflated in healthcare settings due to its disabling condition.
Similarly, the wide prevalence variations of other categories of abdominal pain resulted from the representative sample selection. For instance, the prevalence rate for dyspepsia ranged from 0.2% to 25.7%[25,33,34], abdominal migraine 0% to 23.1%[8,33], and abdominal pain-NOS 0.3% to 39.8%[26,28,33]. Of note, the prevalence rates of the H2 group were much higher than those of the H1 group, suggesting frequent help-seeking behavior and greater burden.
Defecation problems
Table 4 shows 14 epidemiological studies on defecation problems in children and adolescents (H3 group). Twelve studies used self-report form for children and adolescents or parent report with QPGS form, and six studies also included some types of clinical evaluation (physical examination, laboratory examinations, or medical records). Most investigations (n = 9) conducted the study in schools.
Constipation was investigated in all 14 studies and discrepant rates of prevalence ranged from 0.5% to 86.9%[6,31]. School-based studies reported the lowest prevalence and the tertiary care the highest rate. In comparison with the Rome II criteria, the use of broader Rome III also expanded the prevalence rate[31].
Nonretentive fecal incontinence seemed to be a rare disorder, with a prevalence rate ranging from 0% to 1.8%[25,28] in all retained studies (n = 10). Even in non-health settings, a low prevalence of GI disorder was observed, requiring further careful assessment in more representative samples.
DISCUSSION
This study is a systematic review on the epidemiology of FGIDs in children and adolescents. From a total number of 675 identified articles addressing the issue, 26 were included in the final analysis (around 132600 subjects). Search strategies, methodological issues and critical appraisal of literature were systematically presented to summarize the prevalence data on FGIDs in the pediatric population. Cyclic vomiting, IBS and constipation were the most researched conditions, with prevalence ranging from 0.2% to 6.2%, 0% to 45.1% and 0.5% to 86.9%, respectively. This wide variation in prevalence hampers the comparability of epidemiological data, whose reliability needs improvements. The qualitative appraisal revealed that most of the studies showed average or below average generalizability. Several limitations of eligible studies have been acknowledged concerning, e.g., correct sampling of representative population, study setting, and data collection. Future directions in the field of epidemiological studies concerning pediatric FGIDs must follow a more correct methodology, such as appropriate sampling of representative population, comparable study setting, and consistent collection of functional GI symptoms. The scarcity of good quality prevalence data for FGIDs in light of recent Rome IV criteria reveals an urgent need for more trustworthy information to construct evidence-based health policy.
To the best of our knowledge, comprehensive review of prevalence of FGIDs in the age bracket of children and adolescents as a group is lacking. Since the prevalence of a disease can cater for decisions and investments on health policies, good quality epidemiological data are required to understand the neurobiology of the brain-gut axis neurobiology in FGIDs, in view of pursuing applicable treatments in pediatrics.
After reviewing eligible papers on FGIDs, the problem of sample selection must be regarded as the foremost concern. The large variation of the prevalence rates can be attributable to lack of representative children and adolescent sample population. The fact that most of studies in the present review recruited their participants by convenience increases the chance to assess a biased sample with some specific characteristics, mostly in particular schools, chosen by suitability[28,30] or specialized treatment centers[33,35]. Ideally, some types of randomization should be included before the sample recruitment. Clustered and stratified samplings are alternative approaches when a complete list of population is not available[16,37,40,41]. The more the sample resembles the general population, the better is the quality data.
The type of setting also contributes to skewing the sample selection. In the case of children and adolescents aged between 4 and 18 years, school-based sample is a reasonable approach in epidemiological surveys, provided that most of the population in that age bracket are enrolled in a school. Conversely, hospitalized, institutionalized, and homeless populations are not included. On the other hand, only a minor part of the population can be represented in samples drawn from treatment centers, which may exhibit high tendency to help-seeking behavior. Patient samples incur in a double problem, as far as parents interfere with the decision of medical encounter and more symptomatic individuals are recruited into the study. The very large variation reported in the prevalence rates across all retained studies suggests imprecise estimates: while school-based studies may exemplify the closer magnitude of FGID rate[16,40], healthcare centers used to provide inflated rates[8,33,35].
Rome criteria are based on detailed clinical evaluation[5,8-10]. To date, no biomarkers[5] or standard tests are available to diagnose functional disorders[6]. Still, some studies in this review approached the sample only by questionnaires, without clinical assessment[22,27,28,30,36]. The lack of medical evaluation can misdiagnose the complaint of FGID symptoms, leading to non-agreement between informants[19,21,24]. Some evidence of parent-children concordance was described for cyclic vomit, abdominal migraine and constipation. When a high level of disagreement occurs, e.g., IBS and dyspepsia, the type of informant is critical to the quality of the data. Therefore, wider dissemination of clear operationalized criteria, as in the Rome IV criteria, should be recommended for researchers and practicing pediatricians[44].
Limitation
Taking all appraisals into account, conclusive recommendation on the results of the epidemiology of FGIDs should be avoided. There are enormous rate differences and unequivocal methodological limitations across studies. Bearing this in mind, some limitations of the current review need to be discussed. Reporting bias in cross-sectional data is commonly due to publication delay (file drawer bias) and language bias. After trying to contact experts to request non-published data (e.g., non-accessible journals, poster presentation, conference paper) and surveys in other language, we were not able to get access to four studies identified in the initial search. Therefore, it is reasonable to assert that the prevalence heterogeneity of the present review is more attributable to the quality caveats of accessible investigations than to publication bias.
ACKNOWLEDGMENTS
The authors thank Graziela Risolia Gallo for proofreading the original manuscript.
COMMENTS
Background
Functional gastrointestinal disorders (FGIDs) in children and adolescents are mainly a clinical condition and the most common diagnosis in gastroenterology, with a risen burden and, until now, without biomarkers or gold standard diagnosing test available. Furthermore, etiology remains non-established and valid epidemiological data are scarce. The aim of this review was to examine current evidence of knowledge on FGIDs in children and adolescents, through systematic search of frequency or prevalence data on common functional GI problems. The authors also assessed the quality of existing studies on the target topic.
Research frontiers
The validity of explicit diagnostic criteria and the reliability of psychometric tools for FGIDs are still limited. Pediatricians must rely on patient’s symptoms to diagnose and be aware that there are differences between patient and parents reports. Adequate adoption of structured guidelines is useful when replicability is necessary. Reliable data from prospective studies based on structured criteria is necessary to achieve more accurate prevalence data on GI symptoms. Hence, public health decisions can only be established after well-conducted surveys.
Innovations and breakthroughs
FGIDs in children and adolescents seem to be common in clinical and non-clinical settings, mainly cyclic vomiting, irritable bowel syndrome and functional constipation. Conversely, few good quality population-based studies on epidemiology have been conducted so far and good quality epidemiological data to support diagnostic criteria are lacking. As an effort to optimize FGID identification, the use of Rome criteria proved to be a helpful tool. A Rome criteria update, recently launched as Rome IV, merges scientific features and clinical practice, improving the diagnostic classification system. Therefore, its incorporation into epidemiological surveys and clinical practice may increase the pathophysiological comprehension of GI conditions, leading to diagnostic improvement of an important group of functional diseases with a growing burden in the pediatric and adolescent population.
Applications
This review highlights future directions for research: (1) epidemiological, well-designed (sample recruitment, representativeness and clinical assessment) and structured (reproducible) studies shall be conducted among all pediatric levels; (2) classification system on FGIDs must be simple and easy to comprehend, looking for a wider use among pediatricians; and (3) multidimensional approach may bring advances for the Rome criteria symptom-based classification.
Terminology
FGIDs comprise chronic or recurrent symptoms that arise in the absence of anatomic abnormality, inflammation, or tissue damage. The symptoms are variable among children and adolescents.
Peer-review
This systematic review has been presenting a well-designed study on the epidemiology of FGIDs in children and adolescents. Based on the whole data the authors indicate the need for methodology improvement in future epidemiological studies concerning FGIDs.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No potential conflicts of interest exist.
Data sharing statement: No additional data are available.
Peer-review started: February 10, 2017
First decision: March 3, 2017
Article in press: April 12, 2017
P- Reviewer: Contini S, Sun SY, Yucel O S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Zhang FF
References
- 1.Dhroove G, Chogle A, Saps M. A million-dollar work-up for abdominal pain: is it worth it? J Pediatr Gastroenterol Nutr. 2010;51:579–583. doi: 10.1097/MPG.0b013e3181de0639. [DOI] [PubMed] [Google Scholar]
- 2.Hoekman DR, Rutten JM, Vlieger AM, Benninga MA, Dijkgraaf MG. Annual Costs of Care for Pediatric Irritable Bowel Syndrome, Functional Abdominal Pain, and Functional Abdominal Pain Syndrome. J Pediatr. 2015;167:1103–1108.e2. doi: 10.1016/j.jpeds.2015.07.058. [DOI] [PubMed] [Google Scholar]
- 3.Park R, Mikami S, LeClair J, Bollom A, Lembo C, Sethi S, Lembo A, Jones M, Cheng V, Friedlander E, et al. Inpatient burden of childhood functional GI disorders in the USA: an analysis of national trends in the USA from 1997 to 2009. Neurogastroenterol Motil. 2015;27:684–692. doi: 10.1111/nmo.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sommers T, Corban C, Sengupta N, Jones M, Cheng V, Bollom A, Nurko S, Kelley J, Lembo A. Emergency department burden of constipation in the United States from 2006 to 2011. Am J Gastroenterol. 2015;110:572–579. doi: 10.1038/ajg.2015.64. [DOI] [PubMed] [Google Scholar]
- 5.Jung HK. Rome III Criteria for Functional Gastrointestinal Disorders: Is There a Need for a Better Definition? J Neurogastroenterol Motil. 2011;17:211–212. doi: 10.5056/jnm.2011.17.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia V, Deswal S, Seth S, Kapoor A, Sibal A, Gopalan S. Prevalence of functional gastrointestinal disorders among adolescents in Delhi based on Rome III criteria: A school-based survey. Indian J Gastroenterol. 2016;35:294–298. doi: 10.1007/s12664-016-0680-x. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson RM. Pediatrics and evidence-based medicine revisited. J Pediatr. 2007;150:325–326. doi: 10.1016/j.jpeds.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 8.Baber KF, Anderson J, Puzanovova M, Walker LS. Rome II versus Rome III classification of functional gastrointestinal disorders in pediatric chronic abdominal pain. J Pediatr Gastroenterol Nutr. 2008;47:299–302. doi: 10.1097/MPG.0b013e31816c4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mostafa R. Rome III: The functional gastrointestinal disorders, third edition, 2006. World J Gastroenterol. 2008;14:2124–2125. [Google Scholar]
- 10.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, van Tilburg M. Functional Disorders: Children and Adolescents. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.015. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rasquin-Weber A, Hyman PE, Cucchiara S, Fleisher DR, Hyams JS, Milla PJ, Staiano A. Childhood functional gastrointestinal disorders. Gut. 1999;45 Suppl 2:II60–II68. doi: 10.1136/gut.45.2008.ii60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, Walker LS. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527–1537. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loney PL, Chambers LW, Bennett KJ, Roberts JG, Stratford PW. Critical appraisal of the health research literature: prevalence or incidence of a health problem. Chronic Dis Can. 1998;19:170–176. [PubMed] [Google Scholar]
- 16.Sagawa T, Okamura S, Kakizaki S, Zhang Y, Morita K, Mori M. Functional gastrointestinal disorders in adolescents and quality of school life. J Gastroenterol Hepatol. 2013;28:285–290. doi: 10.1111/j.1440-1746.2012.07257.x. [DOI] [PubMed] [Google Scholar]
- 17.Boyle MH. Guidelines for evaluating prevalence studies. Evid Based Ment Health. 1998;1:37–39. [Google Scholar]
- 18.Caplan A, Walker L, Rasquin A. Development and preliminary validation of the questionnaire on pediatric gastrointestinal symptoms to assess functional gastrointestinal disorders in children and adolescents. J Pediatr Gastroenterol Nutr. 2005;41:296–304. doi: 10.1097/01.mpg.0000172748.64103.33. [DOI] [PubMed] [Google Scholar]
- 19.Caplan A, Walker L, Rasquin A. Validation of the pediatric Rome II criteria for functional gastrointestinal disorders using the questionnaire on pediatric gastrointestinal symptoms. J Pediatr Gastroenterol Nutr. 2005;41:305–316. doi: 10.1097/01.mpg.0000172749.71726.13. [DOI] [PubMed] [Google Scholar]
- 20.Walker LS, Lipani TA, Greene JW, Caines K, Stutts J, Polk DB, Caplan A, Rasquin-Weber A. Recurrent abdominal pain: symptom subtypes based on the Rome II Criteria for pediatric functional gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 2004;38:187–191. doi: 10.1097/00005176-200402000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Schurman JV, Friesen CA, Danda CE, Andre L, Welchert E, Lavenbarg T, Cocjin JT, Hyman PE. Diagnosing functional abdominal pain with the Rome II criteria: parent, child, and clinician agreement. J Pediatr Gastroenterol Nutr. 2005;41:291–295. doi: 10.1097/01.mpg.0000178438.64675.c4. [DOI] [PubMed] [Google Scholar]
- 22.Uc A, Hyman PE, Walker LS. Functional gastrointestinal disorders in African American children in primary care. J Pediatr Gastroenterol Nutr. 2006;42:270–274. doi: 10.1097/01.mpg.0000189371.29911.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saps M, Adams P, Bonilla S, Chogle A, Nichols-Vinueza D. Parental report of abdominal pain and abdominal pain-related functional gastrointestinal disorders from a community survey. J Pediatr Gastroenterol Nutr. 2012;55:707–710. doi: 10.1097/MPG.0b013e3182662401. [DOI] [PubMed] [Google Scholar]
- 24.van Tilburg MA, Squires M, Blois-Martin N, Leiby A, Langseder A. Test of the child/adolescent Rome III criteria: agreement with physician diagnosis and daily symptoms. Neurogastroenterol Motil. 2013;25:302–e246. doi: 10.1111/nmo.12056. [DOI] [PubMed] [Google Scholar]
- 25.Lewis ML, Palsson OS, Whitehead WE, van Tilburg MA. Prevalence of Functional Gastrointestinal Disorders in Children and Adolescents. J Pediatr. 2016;177:39–43.e3. doi: 10.1016/j.jpeds.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Saps M, Nichols-Vinueza DX, Rosen JM, Velasco-Benítez CA. Prevalence of functional gastrointestinal disorders in Colombian school children. J Pediatr. 2014;164:542–545.e1. doi: 10.1016/j.jpeds.2013.10.088. [DOI] [PubMed] [Google Scholar]
- 27.Zablah R, Velasco-Benítez CA, Merlos I, Bonilla S, Saps M. Prevalence of functional gastrointestinal disorders in school-aged children in El Salvador. Rev Gastroenterol Mex. 2015;80:186–191. doi: 10.1016/j.rgmx.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Lu PL, Saps M, Chanis RA, Velasco-Benítez CA. The prevalence of functional gastrointestinal disorders in children in Panama: a school-based study. Acta Paediatr. 2016;105:e232–e236. doi: 10.1111/apa.13379. [DOI] [PubMed] [Google Scholar]
- 29.Lu PL, Velasco-Benítez CA, Saps M. Gender, Age, and Prevalence of Pediatric Irritable Bowel Syndrome and Constipation in Colombia: A Population-Based Study. J Pediatr Gastroenterol Nutr. 2016 doi: 10.1097/MPG.0000000000001391. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Játiva E, Velasco-Benítez CA, Koppen IJ, Játiva-Cabezas Z, Saps M. Prevalence of Functional Gastrointestinal Disorders in Schoolchildren in Ecuador. J Pediatr Gastroenterol Nutr. 2016;63:25–28. doi: 10.1097/MPG.0000000000001108. [DOI] [PubMed] [Google Scholar]
- 31.Burgers R, Levin AD, Di Lorenzo C, Dijkgraaf MG, Benninga MA. Functional defecation disorders in children: comparing the Rome II with the Rome III criteria. J Pediatr. 2012;161:615–620.e1. doi: 10.1016/j.jpeds.2012.03.060. [DOI] [PubMed] [Google Scholar]
- 32.Gijsbers CF, Benninga MA, Schweizer JJ, Kneepkens CM, Vergouwe Y, Büller HA. Validation of the Rome III criteria and alarm symptoms for recurrent abdominal pain in children. J Pediatr Gastroenterol Nutr. 2014;58:779–785. doi: 10.1097/MPG.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 33.Cristofori F, Fontana C, Magistà A, Capriati T, Indrio F, Castellaneta S, Cavallo L, Francavilla R. Increased prevalence of celiac disease among pediatric patients with irritable bowel syndrome: a 6-year prospective cohort study. JAMA Pediatr. 2014;168:555–560. doi: 10.1001/jamapediatrics.2013.4984. [DOI] [PubMed] [Google Scholar]
- 34.Gulewitsch MD, Enck P, Schwille-Kiuntke J, Weimer K, Schlarb AA. Rome III criteria in parents’ hands: pain-related functional gastrointestinal disorders in community children and associations with somatic complaints and mental health. Eur J Gastroenterol Hepatol. 2013;25:1223–1229. doi: 10.1097/MEG.0b013e328364b55d. [DOI] [PubMed] [Google Scholar]
- 35.Helgeland H, Flagstad G, Grøtta J, Vandvik PO, Kristensen H, Markestad T. Diagnosing pediatric functional abdominal pain in children (4-15 years old) according to the Rome III Criteria: results from a Norwegian prospective study. J Pediatr Gastroenterol Nutr. 2009;49:309–315. doi: 10.1097/MPG.0b013e31818de3ab. [DOI] [PubMed] [Google Scholar]
- 36.Devanarayana NM, Adhikari C, Pannala W, Rajindrajith S. Prevalence of functional gastrointestinal diseases in a cohort of Sri Lankan adolescents: comparison between Rome II and Rome III criteria. J Trop Pediatr. 2011;57:34–39. doi: 10.1093/tropej/fmq039. [DOI] [PubMed] [Google Scholar]
- 37.Dong L, Dingguo L, Xiaoxing X, Hanming L. An epidemiologic study of irritable bowel syndrome in adolescents and children in China: a school-based study. Pediatrics. 2005;116:e393–e396. doi: 10.1542/peds.2004-2764. [DOI] [PubMed] [Google Scholar]
- 38.Zhou H, Yao M, Cheng GY, Chen YP, Li DG. Prevalence and associated factors of functional gastrointestinal disorders and bowel habits in Chinese adolescents: a school-based study. J Pediatr Gastroenterol Nutr. 2011;53:168–173. doi: 10.1097/MPG.0b013e3182125388. [DOI] [PubMed] [Google Scholar]
- 39.Rajindrajith S, Devanarayana NM, Weerasooriya L, Hathagoda W, Benninga MA. Quality of life and somatic symptoms in children with constipation: a school-based study. J Pediatr. 2013;163:1069–1072.e1. doi: 10.1016/j.jpeds.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto R, Kaneita Y, Osaki Y, Kanda H, Suzuki K, Higuchi S, Ikeda M, Kondo S, Munezawa T, Ohida T. Irritable bowel syndrome among Japanese adolescents: A nationally representative survey. J Gastroenterol Hepatol. 2015;30:1354–1360. doi: 10.1111/jgh.12974. [DOI] [PubMed] [Google Scholar]
- 41.Udoh E, Devanarayana NM, Rajindrajith S, Meremikwu M, Benninga MA. Abdominal Pain-predominant Functional Gastrointestinal Disorders in Adolescent Nigerians. J Pediatr Gastroenterol Nutr. 2016;62:588–593. doi: 10.1097/MPG.0000000000000994. [DOI] [PubMed] [Google Scholar]
- 42.Vandenplas Y, Abkari A, Bellaiche M, Benninga M, Chouraqui JP, Çokura F, Harb T, Hegar B, Lifschitz C, Ludwig T, et al. Prevalence and Health Outcomes of Functional Gastrointestinal Symptoms in Infants From Birth to 12 Months of Age. J Pediatr Gastroenterol Nutr. 2015;61:531–537. doi: 10.1097/MPG.0000000000000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferreira-Maia AP, Matijasevich A, Wang YP. Epidemiology of functional gastrointestinal disorders in infants and toddlers: A systematic review. World J Gastroenterol. 2016;22:6547–6558. doi: 10.3748/wjg.v22.i28.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schurman JV, Hunter HL, Friesen CA. Conceptualization and treatment of chronic abdominal pain in pediatric gastroenterology practice. J Pediatr Gastroenterol Nutr. 2010;50:32–37. doi: 10.1097/MPG.0b013e3181ae3610. [DOI] [PubMed] [Google Scholar]


