Abstract
A 48-year-old woman was admitted with 15-mo history of abdominal pain, diarrhea and hematochezia, and 5-mo history of defecation difficulty. She had been successively admitted to nine hospitals, with an initial diagnosis of inflammatory bowel disease with stenotic sigmoid colon. Findings from computed tomography virtual colonoscopy, radiography with meglumine diatrizoate, endoscopic balloon dilatation, metallic stent implantation and later overall colonoscopy, coupled with the newfound knowledge of compound Qingdai pill-taking, led to a subsequent diagnosis of ischemic or toxic bowel disease with sigmoid colon stenosis. The patient was successfully treated by laparoscopic sigmoid colectomy, and postoperative pathological examination revealed ischemic or toxic injury of the sigmoid colon, providing a final diagnosis of drug-induced sigmoid colon stenosis. This case highlights that adequate awareness of drug-induced colon stenosis has a decisive role in avoiding misdiagnosis and mistreatment. The diagnostic and therapeutic experiences learnt from this case suggest that endoscopic balloon expansion and colonic metallic stent implantation as bridge treatments were demonstrated as crucial for the differential diagnosis of benign colonic stenosis. Skillful surgical technique and appropriate perioperative management helped to ensure the safety of our patient in subsequent surgery after long-term use of glucocorticoids.
Keywords: Benign colonic stenosis, Compound Qingdai pill, Self-expandable memory metallic stent
Core tip: This case demonstrated that detailed inquiry of medical history and effective auxiliary examinations are important for accurate diagnosis. Endoscopic balloon dilatation and metal stent implantation played key roles in the differential diagnosis and bridge treatment of benign colon stenosis. Finally, adequate awareness of drug-induced sigmoid stenosis is very important for avoiding misdiagnosis and mistreatment.
INTRODUCTION
Drug-induced stenosis of the sigmoid colon is very rare[1]. Benign stricture of the sigmoid colon may be caused by inflammatory bowel disease (IBD) or ischemic bowel disease. IBD, mainly represented by Crohn’s disease (CD) and ulcerative colitis, encompasses a group of nonspecific chronic intestinal diseases of unknown etiology, which typically present as persistent or recurrent mucous and/or bloody stools, abdominal pain and tenesmus accompanied by fever, unintentional weight loss, fatigue and loss of appetite. Ischemic bowel disease is characterized by diffuse mucosal congestion and edema, submucosal hemorrhage and erosion, and longitudinal ulcers. When stenosis occurs with ischemic bowel disease, differentiation from IBD is very difficult. As for the treatment of benign stricture of the sigmoid colon, although glucocorticoids, endoscopic decompression[2], balloon dilatation, metallic stenting[3], and surgery[4] are the options for treatment, the decision is very difficult. Here we report the challenging diagnosis and treatment of a rare case of drug-induced stenosis of the sigmoid colon.
CASE REPORT
A 48-year-old female was admitted to the Beijing Electric Power Hospital on December 7, 2016 with complaints of abdominal pain, diarrhea and hematochezia which had lasted for 15 mo, and difficulty in defecation which had lasted for 5 mo.
Fifteen months prior to the admission, she had self-administered oral compound Qingdai (2 g, t.i.d.) for pityriasis rosea of the abdominal wall and subsequently developed paroxysmal abdominal pain with frequent diarrhea (> 10 times a day), described as watery, yellow, thin stools, without mucus, purulence or blood. The patient reported having no tenesmus, nausea, vomiting or fever. After 3 d, aggravation of the abdominal pain and presence of fresh blood in the stools led the patient to visit a local hospital. Routine hematology tests showed white blood cell (WBC) count of 12.7 × 109/L, neutrophil percentage of 63.4%, and hemoglobin level of 103 g/L. Conventional stool tests showed a brown loose stool upon gross examination, and the presence of 3-5 WBCs/high-power field with a large number of red blood cells (RBCs) upon microscopic examination. The patient was diagnosed with infectious diarrhea. After rehydration and levofloxacin treatment, the symptoms of abdominal pain and diarrhea improved and the patient was discharged from the hospital.
Ten or more days after the patient was discharged, she began re-taking the self-administered compound Qingdai pills. Upon re-taking the pills, the patient again developed abdominal pain, diarrhea, stools with bright red blood and fever (self-reported highest temperature being 38.5 °C), prompting a repeat visit to the local hospital. Rehydration and anti-infection treatment consisting of intravenous levofloxacin and oral berberine hydrochloride did not result in symptom improvement. Upon enhancement of the abdominal pain, the patient was referred to the First Hospital of Shanxi Medical University, where electronic colonoscopy revealed nodular mucosal elevations in the splenic flexure and descending colon, and severe mucosal congestion, erosion and ulcer in the sigmoid colon, as well as mild mucosal congestion, erosion, and ulcer in the rectum. The pathological analysis revealed chronic inflammation associated with necrosis and mild-to-moderate dysplasia in the mucosa of the sigmoid colon, as well as chronic mucosal inflammation and stromal neutrophil/eosinophil infiltration in the transverse colon (Figure 1). The patient’s practice of taking the compound Qingdai pills at home remained undisclosed and a diagnosis of IBD was made[5,6]. Intravenous rehydration and oral mesalazine (1 g, q.i.d.) were administered[7], with dosage reduced to 1 g, t.i.d. 10 d later. After 3 mo of outpatient treatment, the patient’s symptoms improved and she discontinued the hospital-ordered medication herself.
Figure 1.
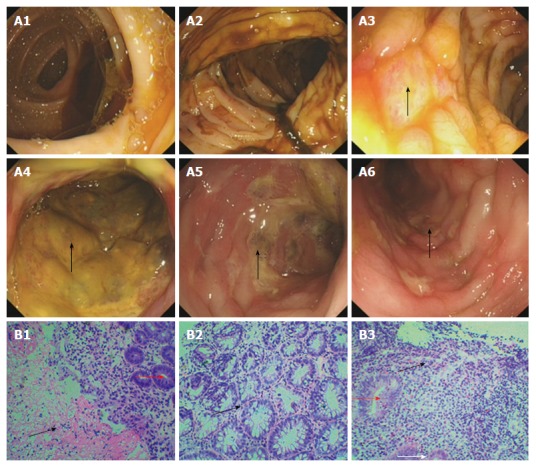
Colonoscopy and biopsy 14 mo ago. Electronic colonoscopy performed 14 mo prior to the ultimate hospital admission revealed smooth mucosa of the ileocecal junction and ascending colon (A1 and A2), nodular mucosal elevations in the transverse colon and descending colon (arrows, A3 and A4), severe mucosal congestion, erosion, and ulcer in the sigmoid colon (arrow, A5), and mild mucosal congestion, erosion and ulcer in the rectum (arrow, A6). Pathological analysis revealed chronic inflammation associated with necrosis (black arrow head, B1) and branching crypts (black arrow head, B2) in the mucosa of the sigmoid colon, as well as chronic mucosal inflammation and cryptitis (red arrow heads, B1 and B3)/eosinophil infiltration (black arrow head, B3) in the transverse colon. Hematoxylin and eosin staining; magnification of × 100 for B1 and B3, and × 200 for B2.
As per self-report, at 5 mo prior to presentation at our hospital, the patient developed difficulty in defecation and pencil-thin stools, needing enema to help achieve defecation. At 4 mo ago, the patient developed abdominal distension and severe pain in the left lower abdomen, with nausea and vomiting, which prompted revisit the local hospital. A diagnosis of intestinal obstruction was made and symptomatic treatment was ordered. Upon symptom improvement, the patient was discharged from the hospital with referral to the General Hospital of Jinan Military Region for electronic colonoscopy. The procedure revealed stenosis of the sigmoid colon (with a diameter of about 0.5 cm, Figure 2A and B); however, mucosal hyperemia and edema in the segment of stenosis blocked the pass-through of the colonoscope. In contrast, the rectal mucosa appeared smooth (Figure 2C). Pathological analysis of the biopsied tissues revealed chronic mucosal inflammation. Thus, a diagnosis of IBD with sigmoid colon stenosis was made. After that, the patient visited the Hospital of Traditional Chinese Medicine of Shanxi Province, where she was diagnosed with sigmoid colon stenosis with ulcer of unclear etiology and administered another course of oral mesalazine. In addition, the patient was administered intravenous dexamethasone (10 mg, q.d.), which was switched to oral prednisone acetate (40 mg, q.d.) 1 wk later.
Figure 2.
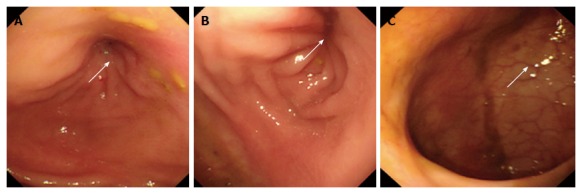
Colonoscopy 4 mo ago. Electronic colonoscopy performed 4 mo prior to the ultimate hospital admission revealed stenosis of the sigmoid colon, with a diameter of about 0.5 cm (arrows, A and B). The colonoscope could not pass through the stenotic segment. The rectal mucosa was smooth, with clear vascular texture (arrow, C).
Three months ago, the patient visited our hospital and underwent a computed tomography virtual colonoscopy (CTVC)[8], and further electronic colonoscopy, which revealed massive colonic effusion, stenosis of the sigmoid colon (with a diameter of 4-5 mm), and smooth rectal mucosa with clear vascular texture (Figure 3A and B). Pathological analysis of the biopsied tissue revealed chronic mucosal inflammation in the sigmoid colon, with gland hyperplasia and stroma edema (Figure 3C). Colonography (performed with 100 mL of 76% compound meglumine diatrizoate injection + 500 mL of 0.9% sodium chloride injection) showed an about 4.3 cm stenosis at the junction between the descending colon and sigmoid colon, with the diameter at the narrowest region measuring approximately 0.5 cm and with slightly rough borders (Figure 4). Inflammatory stenosis of the sigmoid colon was thus suspected, and the patient was referred to Peking University International Hospital to undergo balloon dilatation for stenosis of the sigmoid colon. After admission, the patient’s medical records were reviewed and a detailed inquiry of medical history was performed. At this point, it was noted that the patient had taken compound Qingdai pills for pityriasis rosea before the onset of symptoms and had re-taken the drug before her symptoms were aggravated. This finding, together with the symptoms of abdominal pain, diarrhea, bloody stools and subsequent colon stricture, along with the findings from CTVC and colonoscopy, suggested that the stenosis of sigmoid colon was more likely caused by ischemic bowel disease secondary to the usage of compound Qingdai pills. Given that glucocorticoid treatment is ineffective for ischemic colitis and may exacerbate intestinal ischemia, tapering of the dose of prednisone acetate (40 mg, q.d.) by 5 mg every week was ordered. Subsequently, X-ray-guided colonoscopic balloon dilatation was performed twice at an interval of 3 wk to expand the stenotic intestinal segment to a diameter of 15 mm (Figure 5). After expansion, the endoscope was able to pass through the stenotic segment and the patient’s defecation improved; however, the improvement lasted only 1 wk before the patient redeveloped the stenosis.
Figure 3.
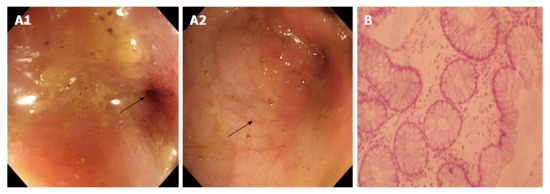
Colonoscopy and biopsy 3 mo ago. Electronic colonoscopy performed 3 mo prior to the ultimate hospital admission revealed stenosis of the sigmoid colon (arrow, A1), with a diameter of 4-5 mm at the site of stenosis. The mucosa was smooth with clear vascular texture (arrow, A2). Pathological analysis of the biopsied tissue revealed mild crypt distortion and stroma edema (B). Hematoxylin and eosin staining; magnification of × 200 for B.
Figure 4.
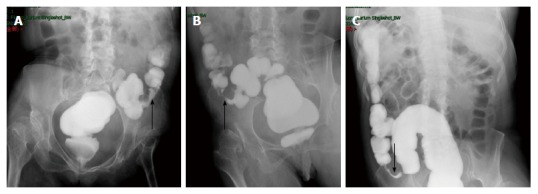
Colonography. Electronic colonoscopy performed 3 mo prior to the ultimate hospital admission revealed stenosis at the junction between the descending colon and sigmoid colon, with the narrowest diameter measuring about 0.5 cm (A, supine position; B, prone position). The stenotic segment was about 4.3 cm long (arrow, C).
Figure 5.
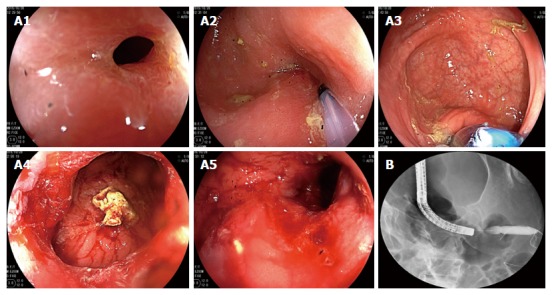
Colonoscopic balloon dilatation 3 mo ago. The patient underwent X-ray-guided colonoscopic balloon dilatation at 3 mo prior to the ultimate hospital admission. Electronic colonoscopy revealed stenosis of the sigmoid colon (A1). A guide wire was inserted through the stenotic segment (A2), and a 15-mm inflatable balloon was introduced via the guide wire (A3) and gradually expanded to a diameter of 15 mm (A4 and A5). After expansion, X-ray revealed that the stenotic segment was well expanded (B).
One month ago, the patient underwent another CTVC at our hospital to investigate whether there were lesions in the proximal colon. The procedure revealed partial wall thickening and luminal stenosis in the sigmoid colon, with smooth mucosa in all other parts of the colon and rectum (Figure 6). The stenosis of the sigmoid colon (about 4-5 mm in diameter) precluded the pass-through of the endoscope; however, there was a slight roughness of the mucosa surrounding the stenotic portion, with smooth rectal mucosa. Serum testing showed negativity for the common immunological indexes for IBD, including antineutrophil cytoplasmic autoantibodies, anti-Saccharomices cerevisiae, and IgG and IgA autoantibodies against goblet cells or targeting the exocrine pancreas. Levels of C3 and C4 were 0.95 g/L and 0.14 g/L, respectively. Serum tumor markers, including carcinoembryonic antigen (4.64 ng/mL), CA19-9 (18.97 U/mL) and CA-125 (15.96 U/mL), were all within normal range. Adrenocortical function tests showed the level of blood cortisol at 7.23 μg/dL, 24-h urinary 17-hydroxyl corticosteroids at 11 nmol, and 24-h urinary free cortisol at 74.06 μg.
Figure 6.
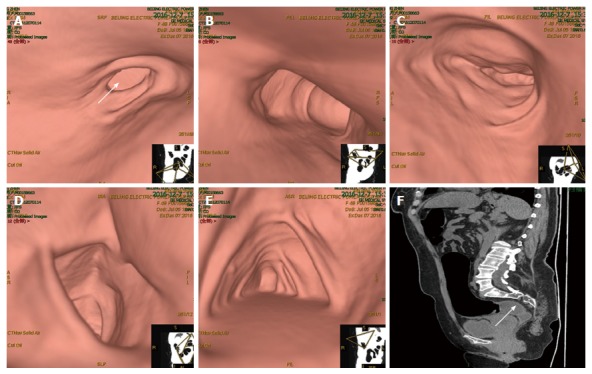
Computed tomography virtual colonoscopy. Computed tomography virtual colonoscopy revealed partial wall thickening and luminal stenosis in the sigmoid colon (arrow, A), as well as unobstructed lumen and smooth mucosa of the descending colon (B), transverse colon (C), ascending colon (D) and cecum (E). Sagittal reconstruction showed stenosis of the sigmoid colon (arrow, F).
On December 12, 2016 (day 5 from admission), the patient underwent stent placement for sigmoid colon stenosis. A guide wire was inserted via electronic colonoscopy to the proximal end of the stenotic segment, and X-ray radiography showed that the stenotic segment was 4 cm long. An 8-cm-long self-expandable memory metallic stent was subsequently placed (Figure 7). Two days after stenting, plain abdominal X-ray showed that the stent was fully expanded, and colonography with meglumine diatrizoate showed that the stenotic sigmoid colon segment was expanded. On day 6, plain abdominal X-ray showed that the stent remained completely expanded and stably positioned. On day 8, electronic colonoscopy showed that the stenotic sigmoid colon segment was successfully expanded by the metallic stent, with local colonic mucosa embedded in stent meshes, with the endoscope passing through the stenotic segment smoothly. Subsequent examination of the colonic mucosa found congestion and edema in the upper edge of the stent. In contrast, the mucosa of the terminal ileum, ileocecal junction, transverse colon, descending colon and rectum was found to be smooth, with a clear vascular texture and no erosion, ulceration or neoplasm (Figure 8).
Figure 7.
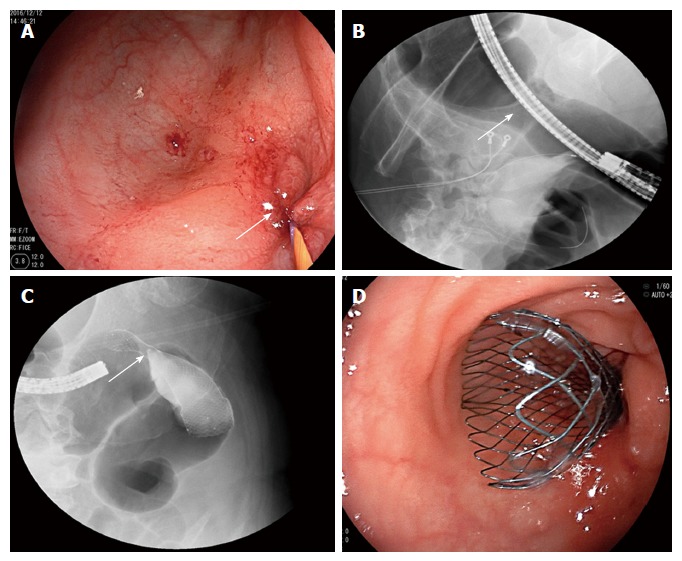
Stent placement for sigmoid colon stenosis. The patient underwent stent placement for sigmoid colon stenosis. A guide wire was inserted via electronic colonoscopy to the proximal end of the stenotic segment (arrow, A). X-ray radiography confirmed that the guide wire had achieved the correct position and showed that the stenotic segment was 4 cm long (arrow, B). Additionally, X-ray showed that an 8-cm-long self-expandable memory metallic stent had been placed at the correct location (arrow, C). Colonoscopy confirmed that the distal end of the stent was at the correct location (D).
Figure 8.
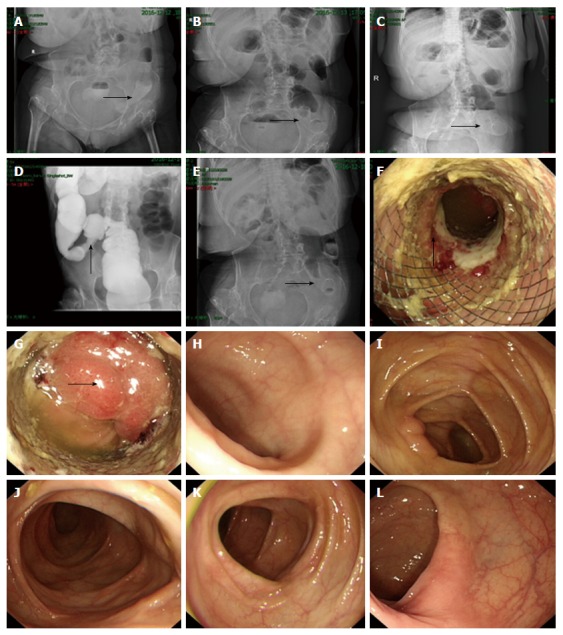
Abdominal X-ray and overall colonoscopy after stent implantation. Plain abdominal X-ray performed on the day of stenting showed that the stent was partially expanded (arrow, A). On post-placement day 1, the stent showed further expansion (arrow, B), and on day 2 showed full expansion (arrow, C). Colonography showed that the stenotic sigmoid colon segment was successfully expanded (arrow, D). On day 6, plain abdominal X-ray showed that the stent remained completely expanded and stably positioned (arrow, E). On day 8, electronic colonoscopy showed the stenotic sigmoid colon segment was expanded by the metallic stent, with local colonic mucosa embedded in the stent meshes, and the colonic mucosa in the upper part of the stent showed congestion, edema, erosion and ulcer (arrow, F). The endoscope was able to pass through the stenotic segment, and the colonic mucosa in the upper edge of the stent showed congestion and edema (arrow, G). The mucosa of the terminal ileum (H), ileocecal junction (I), transverse colon (J), descending colon (K) and rectum (L) was smooth, had clear vascular texture, and showed no erosion, ulceration or neoplasm.
On December 22, 2016 (day 15 from admission), the patient underwent laparoscopic sigmoidectomy. Intraoperatively, sigmoid colon stenosis was visible, as were adhesions between the stenotic segment and the left-side pelvic wall and uterus. After separation of the adhesions, the stenotic segment was dissected at sites about 10 cm beyond both ends, and an end-to-end anastomosis was made between the descending colon and rectum (Figure 9). Pathological analysis of the resected specimen showed multiple ulcers in the mucosa of the sigmoid colon, with the deepest ulcer penetrating through the muscle layer and reaching mesenteric adipose tissue. Fibrous scar tissue was present at the ulcers’ base, but the structure of the mucosa around the ulcers was roughly normal, with no granuloma, vasculitis, lymphatic tissue hyperplasia or full-thickness inflammatory changes. No evidence of IBD was observed. The morphological changes of the sigmoid colon were consistent with ischemic/toxic injury (Figure 10).
Figure 9.
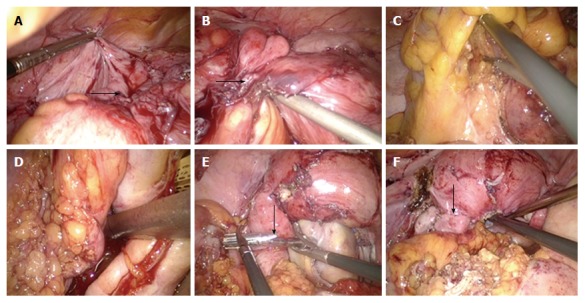
Laparoscopic sigmoidectomy. The patient underwent a laparoscopic sigmoidectomy. Intraoperatively, sigmoid colon stenosis was visible (arrow, A) and the stenotic segment showed adhesions to the left-side pelvic wall and uterus (arrow, B). After separation of the adhesions, the stenotic segment was dissected at sites about 10 cm beyond both ends of the stenotic segment (C and D), and an end-to-end anastomosis was made between the descending colon and rectum (arrows, E and F).
Figure 10.
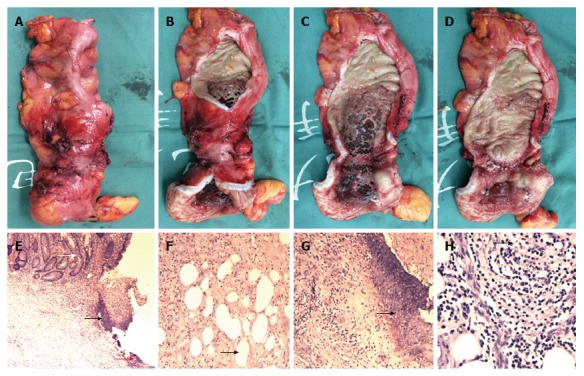
Specimen and pathological analysis. The resected specimen showed stenotic sigmoid colon (A and B), with obvious compression by the metallic stent visible (C and D). Pathological analysis showed multiple ulcers in the mucosa (E, black arrow head), with the deepest ulcer penetrating through the muscle layer and reaching the mesenteric adipose tissue (F and H, black arrow head). Fibrous scar tissue (E, red arrow head) was visible at the ulcer base (G, black arrow head), but the structure of the mucosa around the ulcer was roughly normal (E, white arrow head), with no granuloma, vasculitis, lymphatic tissue hyperplasia, or full-thickness inflammatory changes (E-H). Hematoxylin and eosin staining; magnification of × 40 for E, × 100 for F and G, × 200 for H.
On January 1, 2017 (day 10 after the surgery), the patient was considered recovered and was discharged from the hospital. During the 3 mo of follow-up, the patient reported experiencing no abdominal discomfort and defecation frequency of once every 1-2 d.
DISCUSSION
Compound Qingdai pills are composed of 14 Chinese herbs, including indigo naturalis, dark plum, dandelion, radix arnebiae seu lithospermi, angelica dehurica, salvia miltiorrhiza, cortex dictamni, Jianqu, cyrtomium rhizome, glabrous greenbrier rhizome, purslane, yam rhizome, hawthorn, and fructus schisandrae. Their functions are to reduce heat and remove toxicity, cool and activate the blood, dispel wind and relieve itching, and they are often used for the treatment of advanced psoriasis, pityriasis rosea, and drug eruption.
The patient described herein initially developed abdominal pain, diarrhea and hematochezia, which eventually progressed to benign colonic stenosis. A detailed inquiry about the patient’s medical history, including use of natural supplements or medications taken without physician recommendation or supervision, revealed a history of taking compound Qingdai pills before the onset of the disease. Various examinations made to determine the etiology of colonic stenosis pinpointed the differential diagnosis between ischemic bowel disease[9] and IBD[10]. Although it has been reported that compound Qingdai pills have a therapeutic effect on ulcerative colitis[11,12], colonic mucosal injury caused by compound Qingdai pills has also been reported[13]. The mechanism responsible for such damage involves the direct stimulation of the intestinal mucosa.
Furthermore, compound Qingdai pills are known to cause diarrhea in some sensitive patients. Severe diarrhea can result in reduced blood volume and increased intraluminal pressure, thereby leading to vasospasm and subsequent insufficient blood supply to the bowel wall that eventually causes ischemic changes in the bowel mucosa. The patient described herein developed abdominal pain, diarrhea and subsequent hematochezia after initially taking compound Qingdai pills. She also re-developed these symptoms after re-taking this drug. Review of the symptomology of this case conforms to the process of intestinal mucosal injury induced by compound Qingdai pills. In the process of diagnosis, the patient was diagnosed with IBD and administered mesalazine as well as glucocorticoid treatment at multiple hospitals[14], mainly as a consequence of the lack of detailed inquiry of the patient’s complete medical history and the inadequate awareness of drug-induced colon stenosis[15].
Such misdiagnosis misled the treatment plan, which unfortunately included the use of glucocorticoids and resulted in increased risk for subsequent balloon expansion and surgery for colonic stenosis. For ischemic colonic stenosis, balloon dilatation is the main endoscopic treatment[16], but its requirement for multiple expansions and poor long-term efficacy are key disadvantages. In contrast, surgery is the optimal treatment to achieve a long-term curative effect. For colonic stenosis caused by CD, surgery is often not the first choice because of the possibility of anastomotic fistula. This is also why, in our case, surgery was not adopted initially[17]. Intestinal metallic stenting is associated with a high risk of migration and difficult removal due to embedded growth of granulation tissue. Therefore, metallic stenting is relatively contraindicated in patients with benign stenosis[18-20]. In the present case, however, stent placement was performed for the purpose of preoperative bowel preparation, and it relieved the incomplete intestinal obstruction, reduced the intestinal wall edema, and created the conditions necessary for examining the terminal ileum and the whole colon; ultimately, the stenting helped rule out the possibility of the presence of lesions in other parts of the colon and the possibility of CD preoperatively[21,22], providing a diagnostic basis for the subsequent surgical treatment[9,23,24].
It is well known that long-term use of corticosteroids can affect surgical wound healing and reduce the body’s tolerance to surgical stress[25]. Therefore, proper perioperative management is important for reducing the risk of surgical complications[17]. The present case underwent a gradual tapering of glucocorticoid dosage and was submitted to a detailed assessment of the function of the adrenal axis. Moreover, the patient was given 100 mg of hydrocortisone delivered intravenously for 30 min before the surgery, followed by 5 mg q.d. of intravenous dexamethasone on postoperative days 1 and 2, and somatostatin and growth hormone postoperatively in order to prevent the development of anastomotic fistula[26-28]. The patient did not develop any complication postoperatively, recovered smoothly, and was discharged from the hospital.
The diagnostic and therapeutic experiences learnt from this case suggest that detailed collection of medical history and effective auxiliary examinations play an important role in disease diagnosis[29]. In addition, endoscopic balloon expansion and colonic metallic stent implantation as bridge treatments were demonstrated to be important for the differential diagnosis of benign colonic stenosis. Skillful surgical technique and appropriate perioperative management helped to ensure the safety of our patient in subsequent surgery after long-term use of glucocorticoids. Ultimately, this case should serve as a reminder that adequate understanding of drug-induced colon stenosis has a decisive role in avoiding misdiagnosis and mistreatment[30].
ACKNOWLEDGMENTS
We thank Professor Yu-Min Zhang (Department of Anatomy, Physiology and Genetics, Uniformed Services University of the Health Sciences, United States) for critical reading of the manuscript.
COMMENTS
Case characteristics
A 48-year-old woman was admitted with 15-mo history of abdominal pain, diarrhea and hematochezia, and 5-mo history of defecation difficulty.
Clinical diagnosis
Detailed inquiry of medical history and effective auxiliary examinations are important for accurate diagnosis of drug-induced sigmoid colon stenosis.
Differential diagnosis
An initial diagnosis of inflammatory bowel disease (IBD) with stenotic sigmoid colon and a subsequent diagnosis of ischemic bowel disease with stenotic sigmoid colon.
Laboratory diagnosis
All laboratory tests were within normal limits.
Imaging diagnosis
Findings from computed tomography virtual colonoscopy, radiography with meglumine diatrizoate, endoscopic balloon dilatation, metallic stent implantation and later overall colonoscopy, led to a diagnosis of ischemic bowel disease with sigmoid colon stenosis.
Pathological diagnosis
Ischemic or toxic injury of the sigmoid colon.
Treatment
Laparoscopic sigmoid colectomy.
Related reports
Drug-induced stenosis of the sigmoid colon is very rare. Benign stricture of the sigmoid colon may be caused by IBD or ischemic bowel disease.
Term explanation
Endoscopic balloon dilatation and stent placement to relieve incomplete intestinal obstruction and prepare for examining the whole colon, helping to rule out Crohn’s disease preoperatively and providing a diagnostic basis for surgical treatment.
Experiences and lessons
Adequate awareness of drug-induced sigmoid stenosis is very important for avoiding misdiagnosis and mistreatment. Skillful surgical technique and appropriate perioperative management helped to ensure the safety of the patient in surgery after long-term use of glucocorticoids.
Peer-review
The paper is a very interesting report of a single case of drug-induced stenosis of the sigmoid colon.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of Beijing Electric Power Hospital, State Grid Corporation of China, Capital Medical University, Beijing, China.
Conflict-of-interest statement: The authors declare that there are no conflicts of interest related to this study.
Peer-review started: March 22, 2017
First decision: March 31, 2017
Article in press: May 15, 2017
P- Reviewer: Coelho JC, Dietrich CF, Kapetanos D S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Zhang FF
References
- 1.Ruiz-Tovar J, Candela F, Oliver I, Calpena R. Sigmoid colon stenosis: a long-term sequelae of cocaine-induced ischemic colitis. Am Surg. 2010;76:E178–E179. [PubMed] [Google Scholar]
- 2.Horiuchi A, Nakayama Y, Kajiyama M, Kamijima T, Kato N, Ichise Y, Tanaka N. Endoscopic decompression of benign large bowel obstruction using a transanal drainage tube. Colorectal Dis. 2012;14:623–627. doi: 10.1111/j.1463-1318.2011.02624.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim EJ, Kim YJ. Stents for colorectal obstruction: Past, present, and future. World J Gastroenterol. 2016;22:842–852. doi: 10.3748/wjg.v22.i2.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milone M, Milone F. Segmental left colectomy: a modified caudal-to-cranial approach. Surg Endosc. 2017;31:1487. doi: 10.1007/s00464-016-5100-x. [DOI] [PubMed] [Google Scholar]
- 5.Vegh Z, Kurti Z, Lakatos PL. Epidemiology of inflammatory bowel diseases from west to east. J Dig Dis. 2017;18:92–98. doi: 10.1111/1751-2980.12449. [DOI] [PubMed] [Google Scholar]
- 6.Vuitton L, Peyrin-Biroulet L, Colombel JF, Pariente B, Pineton de Chambrun G, Walsh AJ, Panes J, Travis SP, Mary JY, Marteau P. Defining endoscopic response and remission in ulcerative colitis clinical trials: an international consensus. Aliment Pharmacol Ther. 2017;45:801–813. doi: 10.1111/apt.13948. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Parker CE, Bhanji T, Feagan BG, MacDonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2016;4:CD000543. doi: 10.1002/14651858.CD000543.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roman H, Carilho J, Da Costa C, De Vecchi C, Suaud O, Monroc M, Hochain P, Vassilieff M, Savoye-Collet C, Saint-Ghislain M. Computed tomography-based virtual colonoscopy in the assessment of bowel endometriosis: The surgeon’s point of view. Gynecol Obstet Fertil. 2016;44:3–10. doi: 10.1016/j.gyobfe.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Doulberis M, Panagopoulos P, Scherz S, Dellaporta E, Kouklakis G. Update on ischemic colitis: from etiopathology to treatment including patients of intensive care unit. Scand J Gastroenterol. 2016;51:893–902. doi: 10.3109/00365521.2016.1162325. [DOI] [PubMed] [Google Scholar]
- 10.Monteiro S, Dias de Castro F, Boal Carvalho P, Rosa B, Moreira MJ, Pinho R, Saraiva MM, Cotter J. Essential role of small bowel capsule endoscopy in reclassification of colonic inflammatory bowel disease type unclassified. World J Gastrointest Endosc. 2017;9:34–40. doi: 10.4253/wjge.v9.i1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugimoto S, Naganuma M, Kanai T. Indole compounds may be promising medicines for ulcerative colitis. J Gastroenterol. 2016;51:853–861. doi: 10.1007/s00535-016-1220-2. [DOI] [PubMed] [Google Scholar]
- 12.Sugimoto S, Naganuma M, Kiyohara H, Arai M, Ono K, Mori K, Saigusa K, Nanki K, Takeshita K, Takeshita T, et al. Clinical Efficacy and Safety of Oral Qing-Dai in Patients with Ulcerative Colitis: A Single-Center Open-Label Prospective Study. Digestion. 2016;93:193–201. doi: 10.1159/000444217. [DOI] [PubMed] [Google Scholar]
- 13.Suo BJ, Zhou LY, Ding SG, Lü YM, Gu F, Lin SR, Zheng YA. [The endoscopic and clinical features of Indigo Naturalis-associated ischemic lesions of colonic mucosa] Zhonghua Neike Zazhi. 2011;50:646–649. [PubMed] [Google Scholar]
- 14.Rai T, Choudhury BN, Kedia S, Bopanna S, Venigalla PM, Garg SK, Singla V, Makharia G, Ahuja V. Short-Term Clinical Response to Corticosteroids Can Predict Long-Term Natural History of Ulcerative Colitis: Prospective Study Experience. Dig Dis Sci. 2017;62:1025–1034. doi: 10.1007/s10620-017-4450-0. [DOI] [PubMed] [Google Scholar]
- 15.John ES, Katz K, Saxena M, Chokhavatia S, Katz S. Management of Inflammatory Bowel Disease in the Elderly. Curr Treat Options Gastroenterol. 2016;14:285–304. doi: 10.1007/s11938-016-0099-6. [DOI] [PubMed] [Google Scholar]
- 16.Adler DG. Colonic strictures: dilation and stents. Gastrointest Endosc Clin N Am. 2015;25:359–371. doi: 10.1016/j.giec.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Moszkowicz D, Trésallet C, Mariani A, Lefevre JH, Godiris-Petit G, Noullet S, Rouby JJ, Menegaux F. Ischaemic colitis: indications, extent, and results of standardized emergency surgery. Dig Liver Dis. 2014;46:505–511. doi: 10.1016/j.dld.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Rahimi E, Zhao B, Thosani N. Management of a benign colonic stricture using a through-the-scope fully covered metal stent. Endoscopy. 2016;48 Suppl 1:E138–E139. doi: 10.1055/s-0042-105211. [DOI] [PubMed] [Google Scholar]
- 19.Currie A, Christmas C, Aldean H, Mobasheri M, Bloom IT. Systematic review of self-expanding stents in the management of benign colorectal obstruction. Colorectal Dis. 2014;16:239–245. doi: 10.1111/codi.12389. [DOI] [PubMed] [Google Scholar]
- 20.Bonin EA, Baron TH. Update on the indications and use of colonic stents. Curr Gastroenterol Rep. 2010;12:374–382. doi: 10.1007/s11894-010-0136-x. [DOI] [PubMed] [Google Scholar]
- 21.Boal Carvalho P, Cotter J. Mucosal Healing in Ulcerative Colitis: A Comprehensive Review. Drugs. 2017;77:159–173. doi: 10.1007/s40265-016-0676-y. [DOI] [PubMed] [Google Scholar]
- 22.Khanna R, Zou G, Stitt L, Feagan BG, Sandborn WJ, Rutgeerts P, McDonald JW, Dubcenco E, Fogel R, Panaccione R, et al. Responsiveness of Endoscopic Indices of Disease Activity for Crohn’s Disease. Am J Gastroenterol. 2017 doi: 10.1038/ajg.2016.580. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Trotter JM, Hunt L, Peter MB. Ischaemic colitis. BMJ. 2016;355:i6600. doi: 10.1136/bmj.i6600. [DOI] [PubMed] [Google Scholar]
- 24.Sahara K, Yamada R, Fujiwara T, Koizumi K, Horiguchi S, Hishima T, Yamaguchi T. Idiopathic myointimal hyperplasia of mesenteric veins: Rare case of ischemic colitis mimicking inflammatory bowel disease. Dig Endosc. 2015;27:767–770. doi: 10.1111/den.12470. [DOI] [PubMed] [Google Scholar]
- 25.Rubin DT, Patel H, Shi S, Mody R. Assessment of corticosteroid-related quality of care measures for ulcerative colitis and Crohn’s disease in the United States: a claims data analysis. Curr Med Res Opin. 2017;33:529–536. doi: 10.1080/03007995.2016.1267616. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Ren J, Wang X, Gu J, Jiang J. [Somatostatin and growth hormone promote spontaneous closure of enterocutaneous fistula] Zhonghua Waike Zazhi. 2000;38:447–450. [PubMed] [Google Scholar]
- 27.Donmez R, Oren D, Ozturk G, Kisaoglu A, Ozogul B, Atamanalp SS. The combined effects of glutamine and growth hormone on intestinal anastomosis in the rat intra-abdominal sepsis model. J Surg Res. 2013;182:142–145. doi: 10.1016/j.jss.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 28.Kim JW. Topical Effect of Glutamine for Colorectal Anastomosis. Ann Coloproctol. 2015;31:207–208. doi: 10.3393/ac.2015.31.6.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abe S, Yamaguchi H, Murono K, Kanazawa T, Ishihara S, Sunami E, Watanabe T. Passage of a sigmoid colon cast in a patient with ischemic colitis. Int Surg. 2014;99:500–505. doi: 10.9738/INTSURG-D-14-00066.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reyes-Zamorano J. [Colonic necrosis and stricture due to non-occlusive ischemic colitis. Report of two cases and review of the literature] Cir Cir. 2014;82:442–447. [PubMed] [Google Scholar]


