Abstract
Asthma exacerbations are an important cause of asthma morbidity. Although viral infection of the upper airway is a common cause of asthma exacerbations, the reasons why some patients with asthma are exacerbation prone and others are exacerbation resistant are not fully understood. In this review, we examine whether Type 2 inflammation modifies airway function to make patients more susceptible to asthma exacerbations. The best data supporting a role for Type 2 inflammation in asthma exacerbations come from clinical trials of inhibitors of Type 2 inflammation in asthma. These trials include studies with omalizumab (an inhibitor of IgE) and others with inhibitors of Type 2 cytokines (IL-4, IL-5, and IL-13). All of these trials consistently show that inhibiting the Type 2 pathway causes a clinically significant reduction in asthma exacerbations. Thus, it is now clear that Type 2 inflammation is an important mechanism of susceptibility to asthma exacerbation.
Keywords: asthma, exacerbation, IgE, IL-13, Type 2 inflammation
Asthma affects 235 million people worldwide and represents a growing public health problem (1). In the United States, 1 in 12 people (approximately 25 million) had asthma in 2009, compared with 1 in 14 (approximately 20 million) in 2001 (2). A significant proportion of morbidity and public health expenditure related to asthma results from acute exacerbations of asthma. For example, although only 20% of patients with asthma experience an asthma exacerbation annually, the cost of treating exacerbations accounts for more than 80% of the total direct costs of asthma (3).
Type 2 immune responses in the airway involve accumulation of eosinophils, mast cells, basophils, Th2 cells, Type 2 innate lymphoid cells (ILC2s) and IgE-producing B cells (4), and Type 2 mediators and cytokines (IL-4, IL-5, and IL-13) have been implicated in the pathogenesis of asthma exacerbations (5). The purpose of this review is to describe the epidemiology of exacerbation-prone asthma, summarize our understanding of the biology of Type 2 inflammation in asthma, and explore the role of Type 2 inflammation in mediating susceptibility to asthma exacerbations.
Asthma Exacerbations
Overview
Asthma exacerbations describe acute or subacute episodes of progressively worsening shortness of breath, cough, wheeze, and chest tightness or some combination of these symptoms, along with decreases in expiratory airflow as measured with a peak flow meter or spirometry (6). Rates of exacerbations are comparable across the seasons in adults (7, 8), whereas children tend to display a seasonal pattern of exacerbation, with rates lowest in the summer and highest in the fall (9). This seasonal variation in children has been attributed to increased frequency of rhinovirus (RV) infection coinciding with return to school. Some strains of human rhinovirus (HRV) are more associated with exacerbation than others, with HRV-C and HRV-A strains detected most frequently in children experiencing an exacerbation (10).
Exacerbations of asthma impact quality of life (11) and long-term decline in lung function (12) and can cause death in rare instances (8). The effect of exacerbations on health care costs arising from health care use, medication use, loss of productivity, and premature death are substantial. Compared with patients without exacerbations, patients with one or more exacerbation per year have higher annual total health care costs ($9,223 versus $5,011) and more asthma-related health care use ($1,740 versus $847) (13). The total economic effects of exacerbation are even higher when work loss and lost productivity costs are taken into account.
In 2010, there were 14.2 million physician office visits, 1.8 million emergency department visits, and 439,400 hospitalizations for management of asthma exacerbations the United States (14). Among children under the age of 15 years, asthma represented the third leading cause of hospitalization (15). The annual direct health care cost of asthma in the United States is estimated to be $50.1 billion, with indirect costs (e.g., lost productivity) adding another $5.9 billion (14). Hospitalizations for acute asthma constitute one-third of the annual health care costs in the United States, and the treatment of exacerbations accounts for more than 80% of the total direct costs of asthma (3).
Exacerbation-prone Asthma Phenotype
Because exacerbations of asthma requiring acute medical intervention occur mostly in response to a specific environmental exposure (e.g., a respiratory virus), all patients with asthma would be expected to be equally susceptible to exacerbation. It is clear, however, that not all patients with asthma are equally susceptible to exacerbation; some patients with asthma experience exacerbations frequently, whereas others rarely if ever experience an exacerbation (16). For example, 73% of 3,151 patients presenting to 83 U.S. emergency departments with acute asthma gave a history of at least one visit for asthma within the previous year, and 21% reported six or more visits (17). Risk factors for frequent exacerbations included nonwhite race, type of medical insurance, and markers of asthma severity. Data derived from the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program show that the proportion of patients with asthma with three or more exacerbations per year increases as disease severity worsens. Specifically, the Severe Asthma Research Program investigators have shown that 5% of patients with mild asthma have experienced three or more exacerbations within the past year, compared with 13% of patients with moderate asthma and 54% of patients with severe asthma (18). Consistent with this finding are data showing that only 5–10% of the 25 million patients with asthma in the United States have severe disease but that this subgroup represents over half the annual costs of asthma (19).
Recognition that some patients with asthma are exacerbation prone but others remain relatively exacerbation resistant (5) has resulted in increased efforts to understand in a systematic way the biology underlying heterogeneity in asthma. Epidemiologically, the factors that are important predictors of future exacerbation include an exacerbation within the past year, degree of asthma control, asthma severity, and Type 2 inflammation (18, 20–22).
Type 2 Inflammation
Overview
Type 2 inflammation describes an inflammatory pathway involving a subpopulation of CD4+ T cells known as Th2 cells that secrete IL-4, IL-5, and IL-13 and stimulate Type 2 immunity, which is characterized by high IgE antibody titers and eosinophilia (23). Type 2 immune responses in the airway are mediated mainly by eosinophils, mast cells, basophils, Th2 cells, ILC2s, and IgE-producing B cells (24). Upstream events in the airway epithelium involving master regulators such as IL-33, IL-25, or thymic stromal lymphopoietin regulate maturation of CD4+ T cells into Th2 cells and overproduction of Type 2 cytokines (IL-4, IL-5, and IL-13). Type 2 cytokines drive a cascade of downstream events, including activation of airway epithelial cells, chemoattraction of effector cells (mast cells, eosinophils, and basophils), and remodeling of the epithelium and subepithelial matrix. Together, these inflammatory and pathologic changes in the airway predispose an individual to exaggerated responses to inhaled exacerbants (23). The characteristics associated with an exaggerated response to an exacerbation are airway eosinophilia and airway remodeling. The relevant remodeling changes are smooth muscle cell changes (hyperplasia and hypertrophy), mucus cell changes (hyperplasia, metaplasia), and vascular remodeling (more vessels and more leaky vessels) that together predispose the airway mucosa to an exaggerated response to inhaled allergens or inhaled environmental stimuli such as viruses and oxidizing stimuli (cigarette smoke and other air pollutants).
Th2-high and Th2-low Asthma Endotypes
Although Type 2 inflammation plays an important role in asthma pathophysiology, not all patients with asthma have Type 2 inflammation in their airways. Gene expression levels of Type 2 cytokines (IL-4, IL-5, and IL-13) and of epithelial cell genes that are upregulated by IL-13 (periostin, CLCA1, and SERPINB2) range from low to high in airway biospecimens from patients with asthma (25, 26), and there is a threshold level of expression in the airway that, when exceeded, defines a distinct, “Th2-high” endotype of asthma. Th2-high asthma is characterized by greater airway hyperresponsiveness, airway and systemic eosinophilia, and responsiveness to glucocorticoids and inhibitors of Type 2 inflammation. Only 50% patients with asthma have the Th2-high endotype (25, 26), and alternative mechanisms must operate to drive disease in “Th2-low” asthma. The pathways operating in Th2-low asthma have not been elucidated and are a subject of ongoing research.
Does Type 2 Inflammation Promote Susceptibility to Asthma Exacerbation?
Evidence that Type 2 inflammation is a risk for exacerbation proneness comes from genetic studies, clinical studies, and studies of treatment trials targeting Type 2 inflammation. Genetic studies in two independent cohorts have shown that the IL-4 receptor gene IL4Rα is associated with a history of severe asthma exacerbations and lower lung function (27). The polymorphisms were more prevalent in African Americans but conferred similar risk in both African Americans and whites. One of the polymorphisms was also associated with higher tissue mast cells and higher levels of IgE bound to mast cells.
The most convincing evidence for the role of Type 2 inflammation in asthma exacerbation comes from the effects of its suppression by Type 2–directed therapies. With growing understanding of the pathways of Type 2 inflammation, multiple potential therapeutic targets for Type 2 inhibition have been identified, including IgE, IL-5, and IL-13.
Inhibiting IgE
For more than 30 years, IgE has been recognized to play a key role in asthma. IgE antibodies attach to mast cells, basophils, and dendritic cells (DCs) through high-affinity IgE receptors (FcεRIα) that prime these cells for activation during subsequent allergen exposure (28, 29). Upon antigen binding, adjacent IgE receptors on mast cells and basophils become cross-linked to trigger release of preformed proinflammatory autacoids that mediate early- and late-phase responses to inhaled aeroallergens (30). Omalizumab is a recombinant, humanized monoclonal antibody specific to IgE that blocks interaction between IgE and FcεRIα. Its biologic effects are to deplete IgE, disarm mast cells and basophils, and block the effect of IgE on DCs. Clinical trials of omalizumab have consistently shown a 50% reduction in the number of asthma exacerbations (31, 32), and omalizumab has been shown to nearly eliminate seasonal peaks in exacerbations when used in children (31–33). Other benefits seen include a reduction in maintenance dose inhaled corticosteroids, lower symptom scores, lower rescue medication use, and a modest but statistically significant increase in FEV1. Multiple postregistration studies have replicated the finding that omalizumab reduces exacerbation rates by approximately 50%, even in patients on high-dose inhaled corticosteroids, despite having only a modest effect on lung function and airway hyperresponsiveness (31, 32, 34).
Inhibiting IL-5
IL-5 is produced by CD4+T helper cells (Th2 cells), mast cells, basophils, and ILC2s (35). IL-5 is a major regulator of eosinophil accumulation in tissue and eosinophil function at every stage of maturation. It mediates its response through interaction with its receptor (IL-5Rα), which is present on eosinophils and some basophils. Because levels of sputum eosinophils are predictive of increased exacerbation risk, targeting eosinophils is a rational strategy to reduce exacerbations, with IL-5 being the logical pathway (36). Mepolizumab is a humanized monoclonal antibody that binds to IL-5 and prevents its interaction with the IL-5Rα (37). By blocking IL-5, mepolizumab selectively inhibits eosinophilic inflammation. The initial multicenter study of the effect of mepolizumab failed to show an improvement in asthma control, including exacerbation rates (38). Subsequent studies of mepolizumab in selected patients (subjects with persistent eosinophilia despite steroid treatment and history of exacerbation) showed a 50% reduction in rate of exacerbation and significant improvements in asthma control (39–41). Similar effects of IL-5 inhibition on asthma exacerbations have been shown with other IL-5 inhibitors. For example, treatment with reslizumab (a humanized monoclonal antibody against IL-5) is associated with a 50% reduction in exacerbation rate (42), and a phase IIB dose-ranging study with benralizumab (a recombinant IgG1 antibody that binds to IL-5Rα) has shown a similar effect on exacerbations in patients with uncontrolled eosinophilic asthma (43).
Inhibiting IL-13
IL-13 is a central mediator of asthma with roles in regulation of eosinophilic inflammation, airway hyperresponsiveness, mucus secretion, and airway remodeling (44). IL-13 induces bronchial epithelial cells to secrete large quantities of periostin, a matricellular protein with effects on both epithelial and fibroblast function (45). IL-13 signals through two receptors, the first a heterodimer composed of IL-13 receptor α1 and IL-4 receptor α (IL-4Rα) and the second a high-affinity monomeric receptor, IL-13 receptor α2 (46). Currently, two monoclonal antibodies that inhibit binding of IL-13 to its receptors have been studied in phase III clinical trials in asthma. Lebrikizumab is an IgG4 humanized monoclonal antibody that binds to IL-13 to block its binding to IL-4Rα. Lebrikizumab treatment in asthma is associated with improvements in lung function, asthma control, and exacerbation rates, especially in the subgroup with high periostin levels (47). Dupilumab is a humanized monoclonal antibody that binds to IL-4Rα to block IL-4 and IL-13 activity. Blockade of these pathways in subjects with persistent eosinophilia is associated with a large reduction in exacerbations (87%) and improvements in lung function and asthma control (48).
Lessons learned from Type 2 inhibition
Although inhibiting Type 2 inflammation has limited effects on baseline measures of asthma activity such as airway hyperresponsiveness or lung function, it has large effects on exacerbation rate and asthma control. These findings are consistent with the conceptualization of asthma as a disease with a core abnormality in smooth airway muscle function that can be modified by Type 2 inflammation to worsen asthma control and promote susceptibility to asthma exacerbations. A major lesson derived from the advent of specific treatment for Type 2 inflammation is that treatment of Type 2 inflammation significantly reduces the frequency of asthma exacerbations.
Type 2 Inflammation and Susceptibility to Asthma Exacerbation
Airway remodeling
One possible explanation for how Type 2 inflammation promotes susceptibility to asthma exacerbation is through its remodeling effects on the airway. When activated, Type 2 inflammatory cells migrate to the airway epithelium and subepithelial mucosa and secrete IL-5 and IL-13 to mediate inflammatory and remodeling changes in the airway. Pathological changes of smooth muscle hypertrophy, goblet cell metaplasia and hyperplasia, and subepithelial fibrosis combine to narrow airway caliber at baseline and alter structural elements of the airway, predisposing to an exaggerated response to inhaled antigen (23). Exacerbations then further activate multiple pathways that may have cumulative remodeling effects. In this way, asthma exacerbation may become a self-perpetuating cycle, with one exacerbation causing changes in lung function that promote a subsequent exacerbation. The interrelationship between exacerbation and airway remodeling is a potential explanation for the susceptibility to exacerbation seen in the subgroup of exacerbation-prone patients with asthma (5).
Increased susceptibility to respiratory viruses
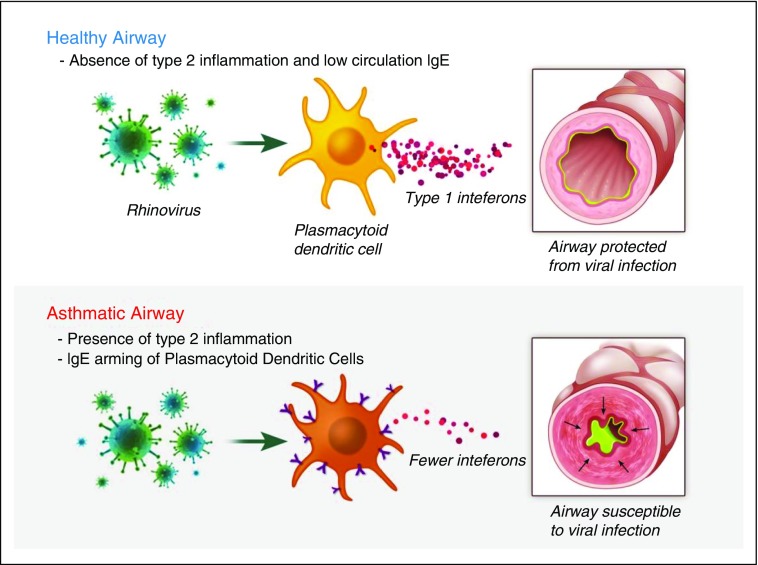
The majority of asthma exacerbations are associated with upper respiratory viral infection (7). The mechanism responsible for increased susceptibility to developing virus-induced asthma exacerbations is poorly understood but likely relates to impairment of antiviral responses in the asthmatic airway. DCs are antigen-presenting cells that have the capacity to bridge innate and adaptive responses through induction of both primary immune response and long-term immune tolerance. Plasmacytoid dendritic cells (pDCs) are a subset of blood DCs that are recruited to the airways during an acute viral infection (49). pDCs express Toll-like receptor (TLR)7 and TLR9, which recognize single-stranded RNA and unmethylated CpG motifs in DNA, respectively, and initiate signaling to produce large concentrations of Type I IFN in response to viral stimulation (50). They also express FcεRIα and have been implicated in a range of allergic diseases, including allergic asthma and atopic dermatitis (29). Patients with allergic asthma express increased surface FcεRIα on pDCs, which is associated with reduced IFN-α secretion in response to influenza viruses. Likewise, cross-linking of FcεRIα in response to IgE is associated with decreased expression of TLR7 and TLR9 and reduces influenza-induced secretion of IFN-α by pDCs (51–53). This supports a biological mechanism for IgE in the pathogenesis of asthma exacerbations whereby IgE cross-linking impairs virus recognition and IFN-α production by pDCs (Figure 1). On the basis of this mechanism, the mechanism by which omalizumab decreases asthma exacerbations is through disarming pDCs of IgE and restoration of the antiviral activity of pDCs.
Figure 1.
Plasmacytoid dendritic cells (pDCs) secrete IFN-α to activate the innate immune response to virus in the airway. pDCs in healthy airways secrete large amounts of IFN-α in response to viruses. The presence of Type 2 inflammation in asthmatic airways is associated with increased expression of high-affinity IgE receptor FcεRI on the surface of pDCs, which causes a blunted IFN-α response to airway viral infection.
Other deficiencies in innate immune response to viral infections have also been described in asthma. Suboptimal production of IFN-β in response to RV is seen in primary bronchial epithelial cells cultured from patients with asthma, and it results in greater viral replication and shedding and impaired apoptosis (54). Treatment of these infected cells with exogenous IFN-β restored apoptosis and reduced virus replication. In a randomized controlled trial of inhaled IFN-β in subjects with mild to moderate asthma and a history of cold-induced exacerbations, IFN-β resulted in a marked decrease in asthma symptoms (as measured with the six-item Juniper Asthma Control Questionnaire) and improvements in peak expiratory flow in the subgroup with more severe disease (55). The authors concluded that future studies of the utility of IFN-β should target patients with more severe asthma. Because total IgE levels are inversely associated with RV-induced IFN production (51, 53), it is plausible that patients with Type 2 inflammation and moderate to severe disease may benefit from inhaled IFN-β.
Conclusions
Asthma exacerbations are an important cause of asthma morbidity and a key driver of the economic burden associated with asthma. Not all persons with asthma are equally susceptible to exacerbations; some are exacerbation prone, whereas others are exacerbation resistant. Mechanisms of susceptibility to exacerbation include Type 2 immune pathways, including arming of pDCs with IgE and increased accumulation of eosinophils in the airway. Treatments that target IgE and Type 2 cytokines are proving effective in reducing susceptibility to asthma exacerbations.
Footnotes
Supported by National Institutes of Health Grants HL080414, HL107202, and HL109146.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.World Health Organization. Fact sheet number 307: asthma [updated 2013; accessed 2015 Sep 13] http://www.who.int/mediacentre/factsheets/fs307/en/
- 2.Centers for Disease Control and Prevention (CDC) Vital signs: asthma prevalence, disease characteristics, and self-management education: United States, 2001–2009. MMWR Morb Mortal Wkly Rep. 2011;60:547–552. [PubMed] [Google Scholar]
- 3.Rodrigo GJ, Rodrigo C, Hall JB. Acute asthma in adults: a review. Chest. 2004;125:1081–1102. doi: 10.1378/chest.125.3.1081. [DOI] [PubMed] [Google Scholar]
- 4.Oliphant CJ, Barlow JL, McKenzie AN. Insights into the initiation of type 2 immune responses. Immunology. 2011;134:378–385. doi: 10.1111/j.1365-2567.2011.03499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dougherty RH, Fahy JV. Acute exacerbations of asthma: epidemiology, biology and the exacerbation-prone phenotype. Clin Exp Allergy. 2009;39:193–202. doi: 10.1111/j.1365-2222.2008.03157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma–Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [Published erratum appears inJ Allergy Clin Immunol 2008;121:1330.] [DOI] [PubMed] [Google Scholar]
- 7.Johnston NW, Sears MR. Asthma exacerbations. 1: epidemiology. Thorax. 2006;61:722–728. doi: 10.1136/thx.2005.045161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnan V, Diette GB, Rand CS, Bilderback AL, Merriman B, Hansel NN, Krishnan JA. Mortality in patients hospitalized for asthma exacerbations in the United States. Am J Respir Crit Care Med. 2006;174:633–638. doi: 10.1164/rccm.200601-007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teach SJ, Gergen PJ, Szefler SJ, Mitchell HE, Calatroni A, Wildfire J, Bloomberg GR, Kercsmar CM, Liu AH, Makhija MM, et al. Seasonal risk factors for asthma exacerbations among inner-city children. J Allergy Clin Immunol. 2015;135:1465–1473.e5. doi: 10.1016/j.jaci.2014.12.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khetsuriani N, Lu X, Teague WG, Kazerouni N, Anderson LJ, Erdman DD. Novel human rhinoviruses and exacerbation of asthma in children. Emerg Infect Dis. 2008;14:1793–1796. doi: 10.3201/eid1411.080386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J. 2007;16:22–27. doi: 10.3132/pcrj.2007.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai TR, Vonk JM, Postma DS, Boezen HM. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J. 2007;30:452–456. doi: 10.1183/09031936.00165106. [DOI] [PubMed] [Google Scholar]
- 13.Ivanova JI, Bergman R, Birnbaum HG, Colice GL, Silverman RA, McLaurin K. Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. J Allergy Clin Immunol. 2012;129:1229–1235. doi: 10.1016/j.jaci.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2013. Asthma facts: CDC’s National Asthma Control Program grantees. [accessed 2015 Sep 13]. http://www.cdc.gov/asthma/pdfs/asthma_facts_program_grantees.pdf. [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC); National Center for Health Statistics. National Hospital Discharge Survey, 1995–2010. Analysis by the American Lung Association Research and Health Education Division. [accessed 2015 Sep 13]. http://www.cdc.gov/nchs/nhds.htm.
- 16.Innes AL, McGrath KW, Dougherty RH, McCulloch CE, Woodruff PG, Seibold MA, Okamoto KS, Ingmundson KJ, Solon MC, Carrington SD, et al. The H antigen at epithelial surfaces is associated with susceptibility to asthma exacerbation. Am J Respir Crit Care Med. 2011;183:189–194. doi: 10.1164/rccm.201003-0488OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griswold SK, Nordstrom CR, Clark S, Gaeta TJ, Price ML, Camargo CA., Jr Asthma exacerbations in North American adults: who are the “frequent fliers” in the emergency department? Chest. 2005;127:1579–1586. doi: 10.1378/chest.127.5.1579. [DOI] [PubMed] [Google Scholar]
- 18.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, et al. National Heart, Lung, Blood Institute’s Severe Asthma Research Program. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serra-Batlles J, Plaza V, Morejón E, Comella A, Brugués J. Costs of asthma according to the degree of severity. Eur Respir J. 1998;12:1322–1326. doi: 10.1183/09031936.98.12061322. [DOI] [PubMed] [Google Scholar]
- 20.ten Brinke A, Sterk PJ, Masclee AA, Spinhoven P, Schmidt JT, Zwinderman AH, Rabe KF, Bel EH. Risk factors of frequent exacerbations in difficult-to-treat asthma. Eur Respir J. 2005;26:812–818. doi: 10.1183/09031936.05.00037905. [DOI] [PubMed] [Google Scholar]
- 21.Miller MK, Lee JH, Miller DP, Wenzel SE TENOR Study Group. Recent asthma exacerbations: a key predictor of future exacerbations. Respir Med. 2007;101:481–489. doi: 10.1016/j.rmed.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan SD, Wenzel SE, Bresnahan BW, Zheng B, Lee JH, Pritchard M, Kamath TV, Weiss ST TENOR Study Group. Association of control and risk of severe asthma-related events in severe or difficult-to-treat asthma patients. Allergy. 2007;62:655–660. doi: 10.1111/j.1398-9995.2007.01383.x. [DOI] [PubMed] [Google Scholar]
- 23.Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15:57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Locksley RM. Asthma and allergic inflammation. Cell. 2010;140:777–783. doi: 10.1016/j.cell.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, Ellwanger A, Sidhu SS, Dao-Pick TP, Pantoja C, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci USA. 2007;104:15858–15863. doi: 10.1073/pnas.0707413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, Arron JR, Fahy JV. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wenzel SE, Balzar S, Ampleford E, Hawkins GA, Busse WW, Calhoun WJ, Castro M, Chung KF, Erzurum S, Gaston B, et al. IL4Rα mutations are associated with asthma exacerbations and mast cell/IgE expression. Am J Respir Crit Care Med. 2007;175:570–576. doi: 10.1164/rccm.200607-909OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Presta L, Shields R, O’Connell L, Lahr S, Porter J, Gorman C, Jardieu P. The binding site on human immunoglobulin E for its high affinity receptor. J Biol Chem. 1994;269:26368–26373. [PubMed] [Google Scholar]
- 29.Foster B, Metcalfe DD, Prussin C. Human dendritic cell 1 and dendritic cell 2 subsets express FcεRI: correlation with serum IgE and allergic asthma. J Allergy Clin Immunol. 2003;112:1132–1138. doi: 10.1016/j.jaci.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Turner H, Kinet JP. Signalling through the high-affinity IgE receptor FcεRI. Nature. 1999;402(6760 Suppl):B24–B30. doi: 10.1038/35037021. [DOI] [PubMed] [Google Scholar]
- 31.Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, van As A, Gupta N. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184–190. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 32.Solèr M, Matz J, Townley R, Buhl R, O’Brien J, Fox H, Thirlwell J, Gupta N, Della Cioppa G. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–261. doi: 10.1183/09031936.01.00092101. [DOI] [PubMed] [Google Scholar]
- 33.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, Gruchalla RS, Kattan M, Teach SJ, Pongracic JA, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milgrom H, Fick RB, Jr, Su JQ, Reimann JD, Bush RK, Watrous ML, Metzger WJ rhuMAb-E25 Study Group. Treatment of allergic asthma with monoclonal anti-IgE antibody. N Engl J Med. 1999;341:1966–1973. doi: 10.1056/NEJM199912233412603. [DOI] [PubMed] [Google Scholar]
- 35.Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009;21:1303–1309. doi: 10.1093/intimm/dxp102. [DOI] [PubMed] [Google Scholar]
- 36.Belda J, Giner J, Casan P, Sanchis J. Mild exacerbations and eosinophilic inflammation in patients with stable, well-controlled asthma after 1 year of follow-up. Chest. 2001;119:1011–1017. doi: 10.1378/chest.119.4.1011. [DOI] [PubMed] [Google Scholar]
- 37.Abonia JP, Putnam PE. Mepolizumab in eosinophilic disorders. Expert Rev Clin Immunol. 2011;7:411–417. doi: 10.1586/eci.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flood-Page P, Swenson C, Faiferman I, Matthews J, Williams M, Brannick L, Robinson D, Wenzel S, Busse W, Hansel TT, et al. International Mepolizumab Study Group. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. 2007;176:1062–1071. doi: 10.1164/rccm.200701-085OC. [DOI] [PubMed] [Google Scholar]
- 39.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, Ortega HG, Pavord ID SIRIUS Investigators. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189–1197. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- 41.Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, Humbert M, Katz LE, Keene ON, Yancey SW, et al. MENSA Investigators. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 42.Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, Murphy K, Maspero JF, O’Brien C, Korn S. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:355–366. doi: 10.1016/S2213-2600(15)00042-9. [DOI] [PubMed] [Google Scholar]
- 43.Castro M, Wenzel SE, Bleecker ER, Pizzichini E, Kuna P, Busse WW, Gossage DL, Ward CK, Wu Y, Wang B, et al. Benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med. 2014;2:879–890. doi: 10.1016/S2213-2600(14)70201-2. [DOI] [PubMed] [Google Scholar]
- 44.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 45.Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, Muller SJ, Fahy JV. Roles of epithelial cell-derived periostin in TGF-β activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci USA. 2010;107:14170–14175. doi: 10.1073/pnas.1009426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tabata Y, Khurana Hershey GK. IL-13 receptor isoforms: breaking through the complexity. Curr Allergy Asthma Rep. 2007;7:338–345. doi: 10.1007/s11882-007-0051-x. [DOI] [PubMed] [Google Scholar]
- 47.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 48.Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, Wang L, Kirkesseli S, Rocklin R, Bock B, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368:2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 49.Gill MA, Palucka AK, Barton T, Ghaffar F, Jafri H, Banchereau J, Ramilo O. Mobilization of plasmacytoid and myeloid dendritic cells to mucosal sites in children with respiratory syncytial virus and other viral respiratory infections. J Infect Dis. 2005;191:1105–1115. doi: 10.1086/428589. [DOI] [PubMed] [Google Scholar]
- 50.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 51.Gill MA, Bajwa G, George TA, Dong CC, Dougherty II, Jiang N, Gan VN, Gruchalla RS. Counterregulation between the FcεRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol. 2010;184:5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schroeder JT, Bieneman AP, Xiao H, Chichester KL, Vasagar K, Saini S, Liu MC. TLR9- and FcεRI-mediated responses oppose one another in plasmacytoid dendritic cells by down-regulating receptor expression. J Immunol. 2005;175:5724–5731. doi: 10.4049/jimmunol.175.9.5724. [DOI] [PubMed] [Google Scholar]
- 53.Durrani SR, Montville DJ, Pratt AS, Sahu S, DeVries MK, Rajamanickam V, Gangnon RE, Gill MA, Gern JE, Lemanske RF, Jr, et al. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol. 2012;130:489–495. doi: 10.1016/j.jaci.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Djukanović R, Harrison T, Johnston SL, Gabbay F, Wark P, Thomson NC, Niven R, Singh D, Reddel HK, Davies DE, et al. INTERCIA Study Group. The effect of inhaled IFN-β on worsening of asthma symptoms caused by viral infections: a randomized trial. Am J Respir Crit Care Med. 2014;190:145–154. doi: 10.1164/rccm.201312-2235OC. [DOI] [PMC free article] [PubMed] [Google Scholar]