Abstract
Rationale: Testing for underlying etiology is a key part of bronchiectasis management, but it is unclear whether the same extent of testing is required across the spectrum of disease severity.
Objectives: The aim of the present study was to identify the etiology of bronchiectasis across European cohorts and according to different levels of disease severity.
Methods: We conducted an analysis of seven databases of adult outpatients with bronchiectasis prospectively enrolled at the bronchiectasis clinics of university teaching hospitals in Monza, Italy; Dundee and Newcastle, United Kingdom; Leuven, Belgium; Barcelona, Spain; Athens, Greece; and Galway, Ireland. All the patients at every site underwent the same comprehensive diagnostic workup as suggested by the British Thoracic Society.
Measurements and Main Results: Among the 1,258 patients enrolled, an etiology of bronchiectasis was determined in 60%, including postinfective (20%), chronic obstructive pulmonary disease related (15%), connective tissue disease related (10%), immunodeficiency related (5.8%), and asthma related (3.3%). An etiology leading to a change in patient’s management was identified in 13% of the cases. No significant differences in the etiology of bronchiectasis were present across different levels of disease severity, with the exception of a higher prevalence of chronic obstructive pulmonary disease–related bronchiectasis (P < 0.001) and a lower prevalence of idiopathic bronchiectasis (P = 0.029) in patients with severe disease.
Conclusions: Physicians should not be guided by disease severity in suspecting specific etiologies in patients with bronchiectasis, although idiopathic bronchiectasis appears to be less common in patients with the most severe disease.
Keywords: bronchiectasis, etiology, severity of illness index
Non–cystic fibrosis (non-CF) bronchiectasis (henceforth referred to as bronchiectasis) is a progressive respiratory disease characterized by a permanent dilation of bronchi, retention of mucus, and ciliary clearance impairment. Clinical features include chronic daily sputum, cough, shortness of breath, and recurrent respiratory infections, with consequent increased morbidity and worsened quality of life (1). One of the cornerstones in the management of bronchiectasis is the identification and treatment of underlying causes.
Bronchiectasis may be caused by several factors, including previous severe respiratory infections (e.g., bacterial pneumonia or tuberculosis), allergic bronchopulmonary aspergillosis (ABPA), impairment of ciliary clearance (e.g., primary ciliary dyskinesia), and primary or secondary immunodeficiency, and it may also be associated with other diseases, such as chronic obstructive pulmonary disease (COPD) and severe asthma.
The first recommendations for the evaluation of the etiology of bronchiectasis were published by the British Thoracic Society (BTS) in 2010. They included a comprehensive laboratory and instrumental workup as well as respiratory function and microbiological evaluation (2). Notably, a wide range of bronchiectasis etiologies may exist, and their prevalence may vary among different patient cohorts. No data outside the United Kingdom have been published on the evaluation of the BTS recommendations for the investigation of bronchiectasis. Furthermore, in the majority of previously published studies, researchers investigated the etiology of bronchiectasis in patients with medium to high severity of disease and predominantly in tertiary referral centers; the role of a comprehensive workup to define etiologies in patients with mild to moderate disease is still unknown.
The aim of our present study was to evaluate the etiology of bronchiectasis in seven European cohorts according to different levels of disease severity. We also aimed to understand how often such etiological categorization could lead to changes in case management.
Methods
Study Population
We conducted an analysis of seven databases of outpatients with bronchiectasis prospectively enrolled between 2009 and 2013 at the bronchiectasis clinics of university teaching hospitals in Monza, Italy; Dundee and Newcastle, United Kingdom; Leuven, Belgium; Barcelona, Spain; Athens, Greece; and Galway, Ireland. Consecutive adult patients in stable state with a diagnosis of bronchiectasis based on high-resolution computed tomographic scans were enrolled at each site. Patients with CF or traction bronchiectasis due to pulmonary fibrosis were excluded. Cohort building was approved at each individual center by the local ethics committee or institutional review board.
Demographics, comorbidities, and previous medical history (including exacerbations during the previous year), radiological features of bronchiectasis, respiratory symptoms, and laboratory and microbiological tests during stable state were recorded at each site using the same data-reporting spreadsheet and then centralized in Monza, Italy, for complete analysis.
An exacerbation of bronchiectasis was defined as a clinical diagnosis of exacerbation by the physician for which antibiotics were prescribed in the presence of at least one (and usually more than one) of the following symptoms: increasing cough, increasing sputum volume, worsening sputum purulence, worsening dyspnea, increased fatigue and/or malaise, fever, and hemoptysis (2). A severe exacerbation was defined as an exacerbation requiring hospitalization.
Workup to Define Bronchiectasis Etiology
All participating sites agreed to employ the recommendations for diagnostic evaluation of patients with bronchiectasis suggested in the 2010 BTS guidelines for non-CF bronchiectasis, including complete blood count; serum electrophoresis; serum IgG, IgA, IgM, total IgE, specific IgE, and precipitins for Aspergillus fumigatus; and a pulmonary function test (2).
In cases of clinical suspicion of ciliary dyskinesia, such as recurrent sinusitis and/or chronic otitis media, nasal mucociliary clearance was measured by the saccharin test and nasal nitric oxide or referral to a specialized primary ciliary dyskinesia center. α1-Antitrypsin was evaluated in the presence of emphysema affecting lower lobes based on high-resolution computed tomographic scans and/or in the presence of a significant family history. Autoimmunity testing, including antinucleolar antibodies, extractable nuclear antigens, antineutrophil cytoplasmic antibodies, rheumatoid factor, and anticitrullinated protein antibody, was requested if a rheumatologic disease was clinically suspected. Sweat tests and cystic fibrosis transmembrane conductance regulator genetic testing were requested if signs and symptoms suggestive of CF were present, as suggested by the BTS guidelines (2).
Process to Define Bronchiectasis Etiology
As the first step, an evaluation of CT images and testing results was performed to identify those patients with one of the following etiologies of bronchiectasis: congenital abnormalities of the bronchial tree, postobstructive bronchiectasis, primary or secondary immunodeficiency, α1-antitrypsin deficiency, or ABPA.
As second step, in the presence of negative results of the above tests, a careful evaluation of prior episodes of severe respiratory infections (including previous infection with nontuberculous mycobacteria) was conducted to define a postinfective etiology of bronchiectasis.
As a third step, in the absence of a positive history of previous severe respiratory infection, an association between bronchiectasis and other diseases, including COPD, asthma, inflammatory bowel disease (IBD), esophageal reflux, and connective tissue disease, was investigated to define a link between these diseases and the development of bronchiectasis.
Finally, if tests were negative and no association with either prior severe respiratory infections or other diseases was found, a diagnosis of idiopathic bronchiectasis was made.
Definitions of Etiologies of Bronchiectasis
ABPA was diagnosed in the presence of serum IgE greater than 1000 international units per milliliter and A. fumigatus precipitins greater than 10 international units per milliliter or raised specific IgE to Aspergillus plus sputum and/or peripheral blood eosinophilia (>500/mm3) and central bronchiectasis (3).
A diagnosis of postinfective bronchiectasis was made if the patient reported a history of symptoms due to bronchiectasis with an onset after a severe respiratory infection, such as pneumonia or tuberculosis, according to clinical judgment and regardless of the latency between the event and the occurrence of symptoms of bronchiectasis.
Bronchiectasis associated with COPD was diagnosed in the presence of a smoking history of at least 10 pack-years with airflow obstruction (FEV1/FVC ratio <0.7) according to the Global Initiative for Chronic Obstructive Lung Disease (4).
Bronchiectasis associated with asthma was diagnosed in patients without postinfective bronchiectasis and with normal or negative results of blood investigations and who had severe asthma according to Global Initiative for Asthma guidelines (5).
Bronchiectasis associated with IBD was diagnosed if patients had ulcerative colitis or Crohn’s disease and no other suggested etiology for bronchiectasis.
In the presence of a diagnosis of both bronchiectasis and connective tissue disease, including rheumatoid arthritis, Sjögren syndrome, and systemic sclerosis, a diagnosis of connective tissue disease–associated bronchiectasis was made.
Yellow nail syndrome was diagnosed when examination showed yellow discoloration of dystrophic nails together with bronchiectasis and sinusitis, regardless of whether patients had other features of the syndrome.
The etiologies of bronchiectasis that were regarded as leading to a specific change in patient management were ABPA, immunodeficiency, aspiration, α1-antitrypsin deficiency, and obstruction (e.g., carcinoid tumor), and ciliary dysfunction was also included as it often prompts wider case management (e.g., familial screening).
Microbiological Evaluation
Sputum cultures for both bacteria and mycobacteria were performed for every patient with productive cough. At least one bronchoscopy for microbiological examination was performed in patients without productive cough but with other respiratory symptoms, including exacerbation. Microbiological examinations were performed on sputum, bronchial aspiration, or bronchoalveolar lavage during stable state. Identification of microorganisms and susceptibility testing were performed according to standard methods (6). A patient was considered colonized if the same pathogen was found in at least three different cultures during the previous 3 months or in two cultures 3 months apart during the last year with the patient in stable state (2).
Severity Evaluation
The severity of bronchiectasis was evaluated according to the Bronchiectasis Severity Index (BSI) (7). Patients were classified into mild (BSI score 0–4), moderate (BSI score 5–8), and severe (BSI score ≥9) disease categories.
Statistical Analysis
All statistical analyses were run using the IBM SPSS version 21.0 for Windows software platform (IBM, Armonk, NY). Continuous data were presented as median values with 25th–75th interquartile ranges. Categorical data were presented as absolute numbers and percentages. Data among groups were compared using a Kruskal–Wallis test if continuous and a χ2 test or Fisher’s exact test if categorical. A P value less than 0.05 was considered statistically significant.
Results
Study Population
A total of 1,258 patients were enrolled within the seven centers (median age, 67 yr; age range, 18–94 yr; 40% males), comprising 286 patients in Dundee, 253 in Leuven, 208 in Galway, 205 in Monza, 113 in Athens, 110 in Newcastle, and 83 in Barcelona. Demographic data; clinical, functional, and radiological status; microbiology; and disease severity of the entire study population are depicted in Table 1. A detailed overview of the seven cohorts is provided in Table E1 in the online supplement.
Table 1.
Demographics, clinical and functional status, and severity of the disease of the study population
| Study Population | |
|---|---|
| Total, n (%) | 1,258 (100) |
| Demographics | |
| Age, yr, median (IQR) | 67 (58–75) |
| Age <50 yr, n (%) | 167 (13) |
| Age 50–65 yr, n (%) | 379 (30) |
| Age 66–75 yr, n (%) | 419 (34) |
| Age 76+ yr, n (%) | 293 (23) |
| Male, n (%) | 506 (40) |
| BMI, kg/m2, median (IQR) | 25 (21–28) |
| Smokers and ex-smokers, n (%) | 457 (36) |
| Clinical status, n (%) | |
| Daily cough | 938 (75) |
| Daily sputum | 775 (62) |
| Prior history of hemoptysis | 190 (15) |
| Exacerbations in previous year, median (IQR) | 2 (1–3) |
| ≥3 exacerbations in previous year, n (%) | 436 (36) |
| Functional and radiological status | |
| FEV1 % predicted, median (IQR) | 73 (51–93) |
| FEV1 % predicted, <50%, n (%) | 270 (22) |
| FEV1 % predicted, <30%, n (%) | 61 (4.8) |
| LTOT, n (%) | 92 (7.3) |
| Reiff score ≥3, n (%) | 693 (55) |
| Microbiology | |
| Colonized patients | 485 (39) |
| Pseudomonas aeruginosa | 189 (15) |
| Other pathogens | 296 (24) |
| Severity of the disease | |
| BSI score, median (IQR) | 6 (4–9) |
| Study groups | |
| Mild BSI score (0–4), n (%) | 394 (31) |
| Moderate BSI score (5–8), n (%) | 506 (40) |
| Severe BSI score (≥9), n (%) | 358 (29) |
Definition of abbreviations: BMI = body mass index; BSI = Bronchiectasis Severity Index; IQR = 25th–75th interquartile range; LTOT = long-term oxygen therapy.
Etiology of Bronchiectasis
Among the entire study population, the etiology of bronchiectasis was identified in 60% of patients (range among cohorts, 46–82%). Excluding idiopathic bronchiectasis, the first five most commonly identified etiologies were postinfective (20%), COPD related (15%), connective tissue disease related (10%), immunodeficiency related (5.8%), and asthma related (3.3%) (see Table 2).
Table 2.
Etiology of bronchiectasis among the entire population and according to severity of disease
| Entire Study Population | Mild Disease | Moderate Disease | Severe Disease | P Value* | |
|---|---|---|---|---|---|
| 1,258 (100) | 394 (100) | 506 (100) | 358 (100) | ||
| Postinfective | 257 (20) | 88 (22) | 104 (21) | 65 (18) | 0.364 |
| COPD | 129 (15) | 11 (2.8) | 41 (8.1) | 77 (22) | <0.001 |
| Connective tissue disease | 128 (10) | 35 (8.9) | 62 (12) | 31 (8.7) | 0.131 |
| Immunodeficiency | 73 (5.8) | 26 (6.6) | 32 (6.3) | 15 (4.2) | 0.299 |
| Asthma | 41 (3.3) | 12 (3) | 22 (4.3) | 7 (2) | 0.143 |
| ABPA | 56 (4.5) | 13 (3.3) | 23 (4.5) | 20 (5.6) | 0.313 |
| Ciliary dysfunction | 21 (1.7) | 11 (2.8) | 8 (1.6) | 2 (0.6) | 0.567 |
| Inflammatory bowel disease | 24 (1.9) | 14 (3.6) | 5 (1) | 5 (1.4) | 0.014 |
| Aspiration/esophageal reflux | 8 (0.6) | 1 (0.3) | 1 (0.2) | 6 (1.7) | 0.014 |
| Congenital malformation | 7 (0.6) | 2 (0.5) | 3 (0.6) | 2 (0.6) | 0.986 |
| α1-Antitrypsin deficiency | 8 (0.6) | 3 (0.8) | 3 (0.6) | 2 (0.6) | 0.929 |
| Obstructive | 2 (0.2) | 1 (0.3) | 1 (0.2) | 0 (0) | 0.657 |
| Pink disease | 1 (0.1) | 0 (0) | 1 (0.2) | 0 (0) | 0.475 |
| Yellow nail syndrome | 1 (0.1) | 0 (0) | 0 (0) | 1 (0.3) | 0.284 |
| Idiopathic | 502 (40) | 176 (45) | 200 (40) | 126 (35) | 0.029 |
Definition of abbreviations: ABPA = allergic bronchopulmonary aspergillosis; BSI = Bronchiectasis Severity Index; COPD = chronic obstructive pulmonary disease.
Among groups: mild = BSI score 0–4; moderate = BSI score 5–8; severe = BSI score ≥9.
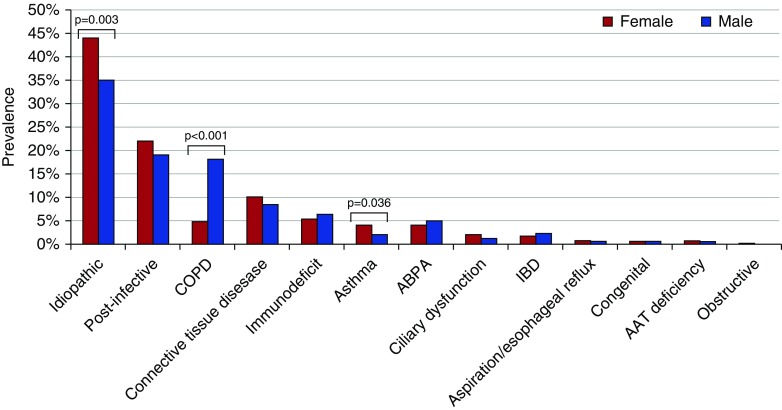
Among all the evaluated etiologies of bronchiectasis, three showed a significantly different prevalence between male and female patients: COPD related was more common in males (18% vs. 5%; P < 0.001), whereas asthma related (2% vs. 4%; P = 0.036) and idiopathic bronchiectasis (35% vs. 44%; P = 0.003) were more common in females (Figure 1).
Figure 1.
Etiology of bronchiectasis according to sex. AAT = alpha-1 antitrypsin; ABPA = allergic bronchopulmonary aspergillosis; COPD = chronic obstructive pulmonary disease; IBD = inflammatory bowel disease.
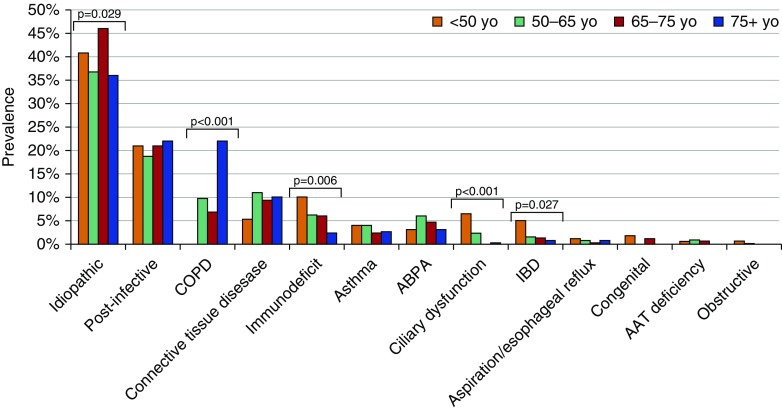
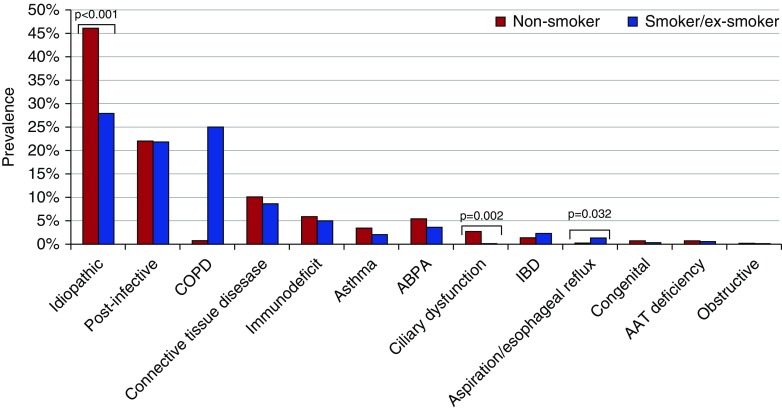
Etiologies of bronchiectasis due to ciliary dysfunction, immunodeficiency, and etiologies related to IBD were significantly more common in patients younger than 50 years of age, whereas COPD-associated bronchiectasis was significantly more common among those 75 years of age or older (Figure 2). Among all the etiologies, three showed a significantly different prevalence between smokers/former smokers and nonsmokers: aspiration/esophageal reflux-related (0.3% vs. 1.4%; P = 0.032), ciliary dysfunction (0.2% vs. 2.7%; P = 0.002), and idiopathic (28% vs. 46%; P < 0.001) (Figure 3).
Figure 2.
Etiology of bronchiectasis according to age. AAT = alpha-1 antitrypsin; ABPA = allergic bronchopulmonary aspergillosis; COPD = chronic obstructive pulmonary disease; IBD = inflammatory bowel disease.
Figure 3.
Etiology of bronchiectasis according to smoking status. AAT = alpha-1 antitrypsin; ABPA = allergic bronchopulmonary aspergillosis; COPD = chronic obstructive pulmonary disease; IBD = inflammatory bowel diseases.
We evaluated whether different etiologies had different microbiological patterns. No significant differences in terms of prevalence of each etiology of bronchiectasis were detected between patients colonized with Pseudomonas and those without this infection or between patients colonized with pathogens other than Pseudomonas and noncolonized patients (Tables E2 and E3). Table E4 summarizes the etiological breakdown within the different cohorts.
Etiology of Bronchiectasis According to Disease Severity
Among the study population, 394 patients (31%) were classified as having mild disease, 506 (40%) as having moderate disease, and 358 (29%) as having severe disease. Differences among these groups (in terms of demographics; clinical, functional, and radiological status; and microbiology) are depicted in Table 3.
Table 3.
Demographics, clinical status, type of bronchiectasis, functional status, and severity of disease, according to the three study groups
| Mild Disease | Moderate Disease | Severe Disease | P Value* | |
|---|---|---|---|---|
| Total, n (%) | 394 (100) | 506 (100) | 358 (100) | |
| Demographics | ||||
| Age, yr, median (IQR) | 58 (46–66) | 71 (63–77) | 73 (65–79) | <0.001 |
| Male, n (%) | 140 (36) | 200 (40) | 166 (46) | 0.009 |
| BMI, kg/m2, median (IQR) | 25 (21–27) | 25 (22–29) | 25 (21–28) | 0.055 |
| Smokers and ex-smokers, n (%) | 129 (33) | 175 (35) | 153 (43) | 0.012 |
| Clinical status, n (%) | ||||
| Daily cough | 274 (70) | 375 (74) | 289 (81) | 0.002 |
| Daily sputum | 204 (52) | 289 (57) | 282 (79) | <0.001 |
| Hemoptysis | 57 (15) | 76 (15) | 57 (16) | 0.855 |
| Exacerbations in previous year, median (IQR) | 1 (0–2) | 2 (1–3) | 3 (2–4) | <0.001 |
| ≥3 exacerbations in previous year, n (%) | 54 (15) | 176 (36) | 206 (59) | <0.001 |
| Functional and radiological status | ||||
| FEV1 % predicted, median (IQR) | 90 (77–101) | 72 (55–90) | 49 (36–68) | <0.001 |
| FEV1 % predicted, <50%, n (%) | 8 (2.2) | 88 (19) | 174 (50) | <0.001 |
| FEV1 % predicted, <30%, n (%) | 0 (0) | 15 (3.2) | 46 (13) | <0.001 |
| LTOT, n (%) | 0 (0) | 21 (4.2) | 71 (21) | <0.001 |
| Reiff score ≥3, n (%) | 142 (40) | 292 (63) | 259 (81) | <0.001 |
| Microbiology | ||||
| Colonized patients | 75 (19) | 178 (35) | 232 (65) | <0.001 |
| Pseudomonas aeruginosa | 3 (0.8) | 42 (8.3) | 144 (40) | <0.001 |
| Other pathogens | 72 (18) | 136 (27) | 88 (25) | 0.009 |
Definition of abbreviations: BMI = body mass index; BSI = Bronchiectasis Severity Index; IQR = 25th–75th interquartile range; LTOT = long-term oxygen therapy.
Mild vs. moderate vs. severe disease: mild = BSI score 0–4; moderate = BSI score 5–8; severe = BSI score ≥9.
The etiologies of bronchiectasis according to disease severity are shown in Table 2. A significantly higher prevalence of COPD-related bronchiectasis was detected in patients with severe disease (22%) in comparison to those with mild disease (2.8%) and moderate disease (8.1%), according to BSI categories (P < 0.001). In contrast, a lower prevalence of idiopathic bronchiectasis was present in the severe category (35%) in comparison to the mild (45%) and moderate (40%) categories (P = 0.029).
An etiology of bronchiectasis that had the potential to alter management or requiring genetic screening was identified in 166 patients (13%), 45 with severe disease, 67 with moderate disease, and 54 with mild disease (P = 0.490). Fifty-six cases were due to ABPA, 8 to aspiration, and 73 to immunodeficiency. Eight cases of alpha-1 antitrypsin deficiency and 21 of ciliary dysfunction were identified that prompted clinicians to prescribe intravenous therapy or undertake familial screening.
Discussion
This large European cohort study indicates that, following current guideline recommendations, an etiology of bronchiectasis was identified in 60% of the patients, and an etiology of bronchiectasis leading to a change in patient management was identified in 13% of cases. No clinically important differences in etiology of bronchiectasis were present across different levels of disease severity, with the exception of a higher prevalence of COPD-related bronchiectasis in patients with severe disease. According to these findings, we suggest that all symptomatic patients, regardless of severity, should undergo detailed comprehensive testing to detect treatable causes. Early treatment of bronchiectasis in this population could prevent disease progression, perhaps even in patients with low-severity disease.
This study is important because our current testing strategy for etiology is based on low-quality evidence and expert opinion. Data published so far have involved predominantly patients with severe disease treated in expert tertiary referral centers, so the value of testing for etiology across the spectrum of bronchiectasis disease severity has not been established. Our study supports the view that all patients with bronchiectasis should undergo testing for etiology, because a similar frequency of underlying causes was found across the spectrum of disease. Nevertheless, we show that the yield increases as severity increases, so testing should be mandatory in patients with severe disease.
Our results are in agreement with previous experiences in that the etiology of bronchiectasis remained unidentified in the majority of patients, ranging from 26% to 74% (8). We found a prevalence of 40% for idiopathic bronchiectasis, and these patients were most frequently in the mild to moderate severity category. This calls for urgent basic and translational research to improve understanding of the mechanisms that cause bronchiectasis in these patients.
Postinfective bronchiectasis was the most frequent etiology in our population. It should be acknowledged that, although we used a protocolized definition of postinfective bronchiectasis in the present study, the relationship between previous respiratory infections and the occurrence of bronchiectasis is difficult to prove in daily clinical practice. The severity of the initial infectious event, the temporal relationship between the original event and bronchiectasis, and patients’ recall bias might represent some of the major confounders for this association.
After postinfective bronchiectasis, the second most prevalent cause in our cohort was COPD. This finding is in line with previously published data by Anwar and coworkers, who also identified COPD as the second leading cause of bronchiectasis after a postinfective etiology (8). We also found a higher prevalence of COPD-related bronchiectasis among patients with severe disease in comparison to the other groups, likely in light of the older age in the severe disease group. Recent studies demonstrated earlier functional decline and higher mortality in patients affected by both COPD and bronchiectasis (9, 10). According to these findings, experts tend to consider patients with COPD and bronchiectasis as a clinical independent phenotype and suggest basic and/or translational research to better investigate this relationship (11, 12).
A significant higher prevalence of esophageal reflux as an etiology of bronchiectasis was identified in patients with severe disease in comparison to those with mild to moderate disease, although the relatively small number of patients means we cannot draw any strong conclusions. Although it could be argued that the definition of esophageal reflux–associated bronchiectasis is difficult to achieve and is not yet standardized, our data seem to be in line with recent studies demonstrating that hiatal hernia and esophageal reflux are associated with more severe disease and higher mortality in this population (13).
Notably, the percentage of patients with an etiology that could potentially lead to a change in management of their disease was 13% in our study, in line with the 7% published by Anwar and coworkers (8).
Our study has some limitations, including the absence of data on timing from diagnosis of bronchiectasis to patient enrollment in the study and estimation of the long-term impact of the etiology on both functional decline and clinical outcome. Longitudinal data are needed to evaluate disease progression, quality of life, and adverse outcome, including exacerbations, hospitalizations, and mortality, associated with different etiologies. A systematic approach in the evaluation of the etiology of bronchiectasis following the 2010 BTS guidelines represents a strength of this study, along with evaluation at different European sites in the largest cohort of patients with bronchiectasis published to date. Further strengths of our study are the comprehensive evaluation of disease severity through a widely validated score—the BSI—and the use of a specific flowchart to classify etiology, along with specific definitions prospectively evaluated.
In conclusion, we present the first pan-European overview of the etiology of bronchiectasis in adult patients. Our results indicate that following recommendations suggested in the 2010 BTS guidelines, an etiology was identified in 60% of patients and that disease severity might not be an adequate marker for physicians to suspect specific etiologies.
Acknowledgments
Acknowledgment
The authors acknowledge the support of Thomas C Fardon, M.D.; Sara Marshall, Ph.D.; and Elizabeth Furrie, M.D.
Footnotes
This study was supported by the European Bronchiectasis Network (EMBARC), a European Respiratory Society Clinical Research Collaboration (www.bronchiectasis.eu). J.D.C. acknowledges fellowship support from the Medical Research Council (MRC) and the Wellcome Trust. M.J.M. acknowledges fellowship support from the European Respiratory Society/European Lung Foundation and the Health Research Board, Ireland. A.D.S. acknowledges a Higher Education Funding Council for England senior lectureship, support from the National Institute for Health Research Biomedical Research Centre, and MRC funding for a U.K. multicenter registry (BRONCH-UK). M.I.R.’s time is partially protected by award K23HL096054 from the NHLBI. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the National Institutes of Health. The funding agencies had no role in the preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Author Contributions: study concept and design: S.A., S.L., and J.D.C.; acquisition of data: S.L., S.A., J.D.C., P.C.G., M.J.M., K.D., A.D.S., E.P., C.V.d.K., R.R., J.D., and E.R.; analysis and interpretation of data: S.A., S.L., J.D.C., P.C.G., M.J.M., K.D., A.D.S., E.P., M.I.R., A.P., and A.T.; drafting of the manuscript: S.L., S.A., and J.D.C.; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: S.L. and S.A.; study supervision: S.A. and J.D.C.; and read and approved the final manuscript: all authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Keistinen T, Säynäjäkangas O, Tuuponen T, Kivelä SL. Bronchiectasis: an orphan disease with a poorly-understood prognosis. Eur Respir J. 1997;10:2784–2787. doi: 10.1183/09031936.97.10122784. [DOI] [PubMed] [Google Scholar]
- 2.Pasteur MC, Bilton D, Hill AT British Thoracic Society Bronchiectasis non-CF Guideline Group. British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65(Suppl 1):i1–i58. doi: 10.1136/thx.2010.136119. [DOI] [PubMed] [Google Scholar]
- 3.Shoemark A, Ozerovitch L, Wilson R. Aetiology in adult patients with bronchiectasis. Respir Med. 2007;101:1163–1170. doi: 10.1016/j.rmed.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease [updated 2014; accessed 2015 Oct 16]. http://www.goldcopd.org/uploads/users/files/GOLD_Report2014_Feb07.pdf.
- 5.Global Initiative for Asthma. Global strategy for asthma management and prevention [updated 2012; accessed 2015 Oct 16]. http://www.ginasthma.org/local/uploads/files/GINA_Report_March13_1.pdf.
- 6.Clinical and Laboratory Standards Institute (CLSI) Wayne, PA: Clinical and Laboratory Standards Institute; Performance standards for antimicrobial susceptibility testing: 14th informational supplement [CLSI document M100-S14] January 2004. [Google Scholar]
- 7.Chalmers JD, Goeminne P, Aliberti S, McDonnell MJ, Lonni S, Davidson J, Poppelwell L, Salih W, Pesci A, Dupont LJ, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med. 2014;189:576–585. doi: 10.1164/rccm.201309-1575OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anwar GA, McDonnell MJ, Worthy SA, Bourke SC, Afolabi G, Lordan J, Corris PA, DeSoyza A, Middleton P, Ward C, et al. Phenotyping adults with non-cystic fibrosis bronchiectasis: a prospective observational cohort study. Respir Med. 2013;107:1001–1007. doi: 10.1016/j.rmed.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Patel IS, Vlahos I, Wilkinson TM, Lloyd-Owen SJ, Donaldson GC, Wilks M, Reznek RH, Wedzicha JA. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:400–407. doi: 10.1164/rccm.200305-648OC. [DOI] [PubMed] [Google Scholar]
- 10.Gatheral T, Kumar N, Sansom B, Lai D, Nair A, Vlahos I, Baker EH. COPD-related bronchiectasis: independent impact on disease course and outcomes. COPD. 2014;11:605–614. doi: 10.3109/15412555.2014.922174. [DOI] [PubMed] [Google Scholar]
- 11.O’Donnell AE. Bronchiectasis in patients with COPD: a distinct COPD phenotype? Chest. 2011;140:1107–1108. doi: 10.1378/chest.11-1484. [DOI] [PubMed] [Google Scholar]
- 12.Hurst JR, Elborn JS, De Soyza A BRONCH-UK Consortium. COPD–bronchiectasis overlap syndrome. Eur Respir J. 2015;45:310–313. doi: 10.1183/09031936.00170014. [DOI] [PubMed] [Google Scholar]
- 13.McDonnell MJ, Ahmed M, Das J, Ward C, Mokoka M, Breen DP, O’Regan A, Gilmartin JJ, Bruzzi J, Rutherford RM. Hiatal hernias are correlated with increased severity of non-cystic fibrosis bronchiectasis. Respirology. 2015;20:749–757. doi: 10.1111/resp.12522. 10.1111/resp.12522 [DOI] [PubMed] [Google Scholar]