In Brief
A 32-year-old woman with stable, well-controlled HIV infection presented for evaluation of subacute progressive dyspnea and evidence of pulmonary hypertension. Chest radiographic imaging revealed mild dependent atelectasis but was otherwise normal. Significant hypoxemia prompted consideration of other causes for the patient’s pulmonary hypertension in addition to HIV infection.
Case Vignette
A 32-year-old obese female with a history of human immunodeficiency virus (HIV) infection, asthma, gastroesophageal reflux, bipolar disorder, and prior tobacco and cocaine use presented to our institution with one year of slowly progressive exertional dyspnea. Two days before presentation, her symptoms worsened to include shortness of breath at rest accompanied by dull substernal chest discomfort, occasional lightheadedness, and a nonproductive cough unrelieved by albuterol. She denied paroxysmal nocturnal dyspnea, orthopnea, fevers, chills, wheezing, or sick contacts.
On initial evaluation, she was afebrile with a blood pressure of 161/94 mm Hg, heart rate of 96 beats/minute, respiratory rate of 22 breaths/minute, and arterial oxygen saturation (SaO2) of 91% while breathing ambient air. With ambulation, her SaO2 dropped to 84% on ambient air and increased to 91–93% when she breathed supplemental oxygen at 4 L/minute by nasal cannula. Her physical examination was notable for a jugular venous pressure of 8 cm H2O, an accentuated P2, a right-sided S3 gallop, coarse breath sounds at the lung bases without wheezing, and an absence of rash or oral ulcers.
Laboratory data revealed an NT-pro brain natriuretic peptide level of 1,751 pg/ml with negative troponin, urine drug screen, and sputum culture. The hemoglobin (Hgb) concentration was 14.9 g/dl and her CD4+ T-cell count measured 1,330 cells/mm3 on a stable regimen of efavirenz, emtricitabine, and tenofovir. Anti-nuclear antibody and liver function tests were normal. Her electrocardiogram demonstrated right axis deviation, an S wave in lead I, and T wave inversion with a Q wave in lead III. Chest X-ray was normal and chest computed tomography (CT) angiography revealed dependent atelectasis without pulmonary embolus.
On echocardiogram, the left ventricular ejection fraction was 70% with mild right atrial (RA) and severe right ventricular (RV) dilation, mildly depressed RV function, and moderate-to-severe tricuspid regurgitation. The estimated pulmonary arterial systolic pressure was 76 mm Hg. Interventricular septal motion was consistent with elevated RV diastolic pressure. A ventilation–perfusion scan was not suggestive of acute or chronic thromboembolic disease. Pulmonary function testing did not reveal any obstructive or restrictive defects.
Because of the findings on echocardiography, right heart catheterization was performed. Results are shown in Table 1.
Table 1.
Hemodynamics measured at initial right heart catheterization
| RA pressure: | 10 mm Hg |
| PAP: | 80/28 mm Hg |
| Mean PAP: | 50 mm Hg |
| Pulmonary artery wedge pressure: | 10 mm Hg |
| Cardiac output: | 4.1 L/min |
| Cardiac index: | 1.9 L/min/m2 |
| PA oxygen saturation: | 61% |
| PVR: | 9.7 Wood units |
| PVRI: | 21 Wood units/m2 |
Definition of abbreviations: PA = pulmonary artery; PAP = pulmonary arterial pressure; PVR = pulmonary vascular resistance; PVRI = pulmonary vascular resistance index; RA = right atrium.
Questions
What is the underlying cause of her pulmonary hypertension?
Does her hypoxemia require further evaluation?
Clinical Reasoning
The patient’s hemodynamics were consistent with pulmonary arterial hypertension. Initial diagnostic evaluation disclosed arterial oxygen saturation values at rest and during ambulation that were below the range of values generally observed in patients with idiopathic or HIV-associated pulmonary arterial hypertension. Chest radiographic imaging did not reveal an explanation for her hypoxemia, prompting measurement of arterial blood gas tensions while the patient breathed 100% oxygen. The calculated cardiopulmonary shunt fraction (s/t) (Equation 1), estimated from these results (Table 2), was abnormally elevated at 27%, where PcO2 is pulmonary end-capillary oxygen partial pressure, Patm is atmospheric pressure, Ph2O is water vapor pressure, PaCO2 is arterial carbon dioxide partial pressure, CcO2 is end-capillary oxygen content, CaO2 is arterial blood oxygen content, CO2 is mixed venous blood oxygen content, s is shunted blood flow, and t is total blood flow.
| (1) |
Table 2.
Arterial and mixed venous blood gas tensions measured while the patient breathed 100% oxygen
| pH | Pco2 | Po2 | O2 saturation | |
|---|---|---|---|---|
| Arterial blood gas tensions | 7.42 | 26 | 71 | 95.5% |
| Mixed venous blood gas tensions | 7.41 | 33 | 38 | 62.4% |
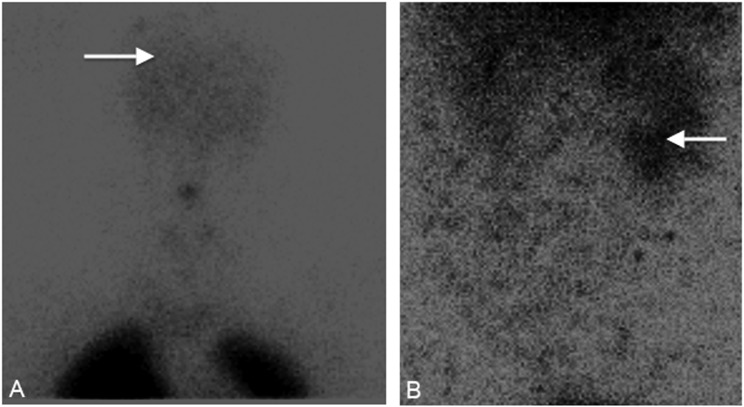
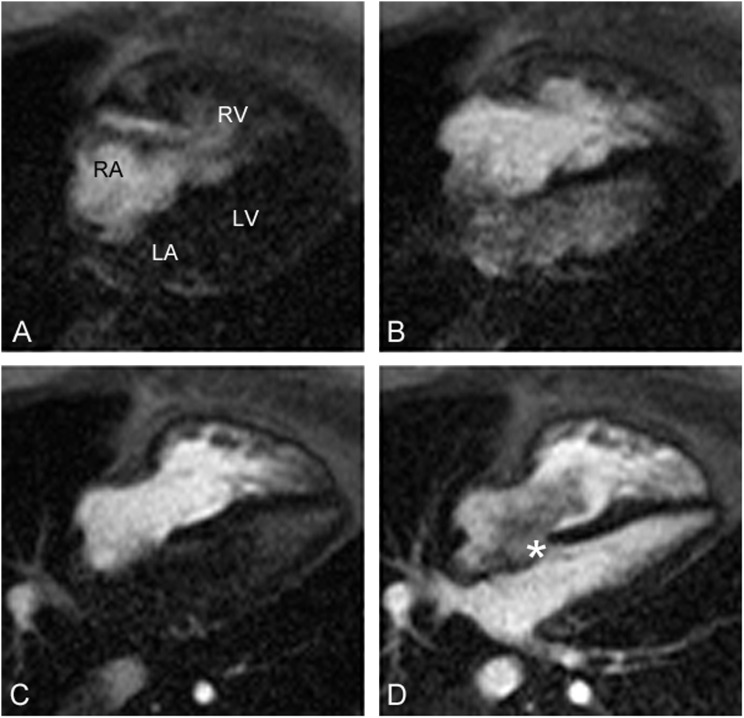
To evaluate the abnormal arterial oxygen tensions, a repeat nuclear medicine lung perfusion scan was requested specifically to evaluate for evidence of a right-to-left shunt. This study revealed 99mTc-labeled macroaggregated albumin accumulation within the brain and kidneys consistent with a right-to-left shunt (Figure 1). Cardiac magnetic resonance imaging (MRI) showed the presence of a large atrial septal defect with bidirectional shunt (Figure 2).
Figure 1.
Nuclear medicine perfusion lung scan demonstrating 99mTc-labeled macroaggregated albumin accumulation (A) within the brain (arrow) and (B) in the right kidney (arrow).
Figure 2.
Cardiac magnetic resonance imaging during injection of contrast. (A) Contrast enters the right atrium (RA) and right ventricle (RV). (B) Contrast enters the left atrium (LA) and left ventricle (LV) before entering the pulmonary veins, indicative of a right-to-left shunt at the atrial level. (C) Contrast clears from the LA and LV because of pressure equalization. (D) Contrast enters the LA and LV from the pulmonary veins. *A turbulent bidirectional shunt is present.
Discussion
Pulmonary hypertension is a clinical syndrome characterized by the presence of a mean pulmonary arterial pressure greater than or equal to 25 mm Hg at rest on right heart catheterization and is further categorized into five groups according to the Nice classification (1). Group 1 encompasses diverse entities that exhibit an obliterative, small-vessel pulmonary arteriopathy, causing an increase in resistance and elevated pulmonary arterial pressure (termed pulmonary arterial hypertension, PAH). In addition to idiopathic disease, PAH is associated with heritable genetic mutations, congenital heart diseases with systemic-to-pulmonary shunting, drugs and toxins, schistosomiasis, portal hypertension, connective tissue diseases, and HIV infection.
Vascular remodeling, inflammation, thrombosis, and an imbalance of vasoconstrictive and vasodilatory mediators contribute to the pathogenesis and histologic changes observed in all forms of PAH (1). HIV-related PAH (HIV-PAH) has an estimated worldwide prevalence of 0.5% among HIV-positive patients and does not definitively correlate with HIV viral load or CD4+ T-cell count (2). A history of intravenous drug abuse is observed with a higher frequency in HIV-PAH compared with those infected with HIV without PAH (3). Limited data suggest a potential role for targeted pulmonary vasoactive therapy with either bosentan or epoprostenol in the treatment of HIV-PAH (2, 4).
Although dyspnea is a cardinal symptom, the degree of hypoxemia in PAH is usually only mild to moderate (5, 6). In the National Institutes of Health primary pulmonary hypertension registry, the mean arterial pressure of oxygen on room air was 70 mm Hg (±13) in males and 72 mm Hg (±16) in females (5). Exertional desaturation greater than or equal to 10 mm Hg can be seen in patients with PAH and is associated with increased mortality (6). Hypoxemia in PAH is multifactorial with contributions from ventilation–perfusion mismatch, reduced diffusing capacity, admixture of mixed venous blood with low oxygen saturation in the setting of decreased cardiac output, or the opening of an intrapulmonary or intracardiac shunt (6, 7).
More severe hypoxemia should prompt evaluation for alternative or additional processes including pulmonary veno-occlusive disease, distal thromboembolic disease, interstitial lung disease, and intracardiac or intrapulmonary shunts. Various forms of congenital heart diseases, such as atrial and ventricular septal defects and patent ductus arteriosus, are associated with initial left-to-right shunting. Over time, ongoing exposure to supranormal pulmonary blood flow can cause remodeling in the pulmonary vascular bed with subsequent development of PAH, rise in pulmonary vascular resistance (PVR), and eventual shunt reversal and hypoxemia (Eisenmenger syndrome). The degree of hypoxemia from shunt-associated congenital heart diseases varies significantly depending on the size, location, direction of flow across the shunt, heterogeneity in pulmonary vascular response to increased pressure/flow, and the coexistence of impaired cardiac or lung function (6). As an example, in an early case series of patients being evaluated for repair of an atrial septal defect, 68 of 128 patients demonstrated an SaO2 less than 95% but only 17 patients had an SaO2 less than 90%; those with higher pulmonary vascular resistance demonstrated more severe hypoxemia (8). Similarly, right-to-left shunting through a patent foramen ovale in some situations can be provoked by exercise, postural changes, or acute changes in ventricular compliance and can present with orthodeoxia that corrects after patent foramen ovale closure (9).
Intrapulmonary shunts can be found in a wide range of disease processes including hereditary hemorrhagic telangiectasia, hepatopulmonary syndrome, or infections such as schistosomiasis or actinomycosis (10, 11). The presence of pulmonary arteriovenous malformations is associated with more severe hypoxemia compared with what is typically seen in PAH, ranging from an SaO2 less than 76% in 32 of 101 patients in one review to a mean PaO2 of 72 mm Hg in one series of patients undergoing pulmonary arteriovenous malformation embolization (10).
If a right-to-left shunt is suspected, the shunt fraction should be calculated. The shunt calculation measures the fraction of total cardiac output (t) that flows through the shunt (s).
CcO2, CaO2, and represent the oxygen content of end-capillary, arterial, and mixed venous serum samples, respectively (12). Oxygen content is given by the general formula:
Arterial blood is sampled at a site distal to the location of the possible shunt, in practice typically from a peripheral artery. For pulmonary end-capillary content, the blood Po2 is estimated according to the alveolar gas equation. When performed on 100% oxygen at sea level, ScO2 = 1 and thus PcO2 = PaO2 = 713 – (PaCO2 × 1.25). The accuracy of this calculation assumes normal diffusion across the alveolar–capillary membrane and therefore may be erroneous in the presence of coexisting intrinsic lung disease. Precise determination of mixed venous content requires oximetric sampling of mixed venous blood and thus the s/t calculation will be most accurate when measured directly at right heart catheterization. A simplified method can be employed, using a single peripheral arterial blood gas sample after breathing 100% oxygen; however, this requires using an assumed value for the arteriovenous O2 difference, which can be problematic in the setting of low cardiac output or abnormal oxygen consumption (12). Multisite oximetric sampling of the right heart chambers can be helpful in elucidating a left-to-right shunt as revealed by a “step up” in measured oxygen saturation; however, in cases of predominantly right-to-left shunting, such a step-up will be absent and thus a defect may be missed if the clinician relies solely on relative differences in right-sided saturations.
If a significant shunt fraction (>5–7%) is calculated, transthoracic contrast echocardiography should be performed, which involves the injection of agitated saline or other echocardiographic contrast material into a peripheral vein. If contrast appears in the left side of the heart within one or two cardiac cycles, then an intracardiac shunt is present; if contrast is visualized within three or four cardiac cycles, an intrapulmonary shunt is implicated (10, 13). Inadequate contrast (e.g., from large body habitus), poor effort during Valsalva maneuver, and predominant left-to-right shunt flow can decrease the sensitivity of detecting a right-to-left shunt on transthoracic contrast echocardiography, which ranges from 22 to 91% (13, 14). The sensitivity and specificity of transthoracic contrast echocardiography for pulmonary arteriovenous malformations are 93% and 52–91%, respectively (10, 11). Detection of 99mTc-labeled macroaggregated albumin in the brain, spleen, and/or kidneys during ventilation–perfusion lung scintigraphy does not distinguish between a right-to-left intracardiac or intrapulmonary shunt, but can be useful in those with nondiagnostic transthoracic contrast echocardiography results. Dose-reduced 99mTc-labeled macroaggregated albumin should be considered in those with severe pulmonary hypertension or a large degree of shunt to minimize occlusion of pulmonary capillaries and embolization of albumin to extrapulmonary sites (11). Transesophageal echocardiography, chest CT, or MRI/magnetic resonance angiography, and cardiac catheterization with multisite oximetry sampling can also be used to diagnose intracardiac or intrapulmonary shunts and identify appropriate operable candidates (9, 11, 13–15).
Treatment of PAH associated with Eisenmenger syndrome involves consideration of advanced PAH therapies such as prostanoids, endothelin receptor antagonists, or phosphodiesterase-5 inhibitors, which have been shown to improve hemodynamics and functional capacity in small trials (16). Shunt closure is contraindicated in patients with significant elevations in PVR and pulmonary artery pressure as it may precipitate RV failure (9). In some cases, targeted PAH therapy may result in sufficient lowering of PVR to allow closure of the defect. Guidelines recommend closure if the PVR index (PVRI) is less than 4 Wood units and to consider closure with PVRI up to 8 Wood units, depending on other factors, such as RV function and relevant comorbidities (1). The decision to proceed with closure is often complex and should involve a comprehensive multidisciplinary evaluation (1, 16).
Percutaneous transcatheter embolization remains the first-line treatment of pulmonary arteriovenous malformations and should be considered in all patients to reduce the risk of embolic stroke, cerebral abscess, or pulmonary hemorrhage. Surgical resection generally is reserved for emergency control of pulmonary hemorrhage (11).
Diagnosis
Pulmonary arterial hypertension associated with large atrial septal defect with Eisenmenger syndrome and HIV infection.
Follow-Up
The patient was not a candidate for closure of her atrial septal defect because of the significantly elevated pulmonary vascular resistance. Given her hemodynamic findings and class IV symptomatology, therapy was initiated with inhaled iloprost (poor social support contraindicated the use of intravenous prostanoids), followed by the addition of ambrisentan. She was discharged on supplemental oxygen (6 L/min). Over the subsequent months, her oxygenation indices improved consistent with reduction in pulmonary vascular resistance and s/t. However, repeat right heart catheterization performed after nearly 2 years of medical therapy revealed persistent PAH with elevated PVR; full right-sided oximetric sampling did not suggest a significant step-up in oxygen saturation at the level of the RA. Her calculated pulmonary-to-systemic flow ratio was 0.6, consistent with a persistent right-to-left shunt.
Insights
-
•
In the absence of an anatomic shunt, pulmonary arterial hypertension is usually associated with mild to moderate hypoxemia. More severe hypoxemia should prompt consideration of an alternative cause of pulmonary hypertension, identification of concomitant pulmonary or cardiac impairment, or evaluation for an intracardiac or intrapulmonary shunt.
-
•
The initial step in the evaluation of suspected shunt-type hypoxemia with clear chest imaging should involve a shunt calculation on 100% fraction of inspired oxygen followed by a transthoracic contrast echocardiogram.
-
•
If a transthoracic contrast echocardiogram is nondiagnostic, a ventilation–perfusion scan with extended imaging to detect extrapulmonary uptake of 99mTc-labeled macroaggregated albumin may imply an intracardiac or intrapulmonary shunt. Oximetric sampling of the right heart chambers may be informative when a significant component of left-to-right shunt is present. Definitive localization of an intracardiac shunt may be achieved with transesophageal echocardiogram or cardiac MRI.
Footnotes
Supported by a National Institutes of Health grant (M.K.P.): T32 HL-007891.
Author Contributions: M.K.P. and J.S.F. contributed equally to the conception and writing of the manuscript.
Author disclosures are available with the text of this occasional essay at www.atsjournals.org.
References
- 1.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25) Suppl:D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Mehta NJ, Khan IA, Mehta RN, Sepkowitz DA. HIV-related pulmonary hypertension: analytic review of 131 cases. Chest. 2000;118:1133–1141. doi: 10.1378/chest.118.4.1133. [DOI] [PubMed] [Google Scholar]
- 3.Sitbon O, Lascoux-Combe C, Delfraissy JF, Yeni PG, Raffi F, De Zuttere D, Gressin V, Clerson P, Sereni D, Simonneau G. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177:108–113. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 4.Sitbon O, Gressin V, Speich R, Macdonald PS, Opravil M, Cooper DA, Fourme T, Humbert M, Delfraissy JF, Simonneau G. Bosentan for the treatment of human immunodeficiency virus–associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;170:1212–1217. doi: 10.1164/rccm.200404-445OC. [DOI] [PubMed] [Google Scholar]
- 5.Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Koerner SK, et al. Primary pulmonary hypertension: a national prospective study. Ann Intern Med. 1987;107:216–223. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 6.Vodoz JF, Cottin V, Glérant JC, Derumeaux G, Khouatra C, Blanchet AS, Mastroïanni B, Bayle JY, Mornex JF, Cordier JF. Right-to-left shunt with hypoxemia in pulmonary hypertension. BMC Cardiovasc Disord. 2009;9:15. doi: 10.1186/1471-2261-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suga K, Tokuda O, Okada M, Koike M, Iwanaga H, Matsunaga N. Assessment of cross-sectional lung ventilation–perfusion imbalance in primary and passive pulmonary hypertension with automated V/Q SPECT. Nucl Med Commun. 2010;31:673–681. doi: 10.1097/MNM.0b013e328339ea9b. [DOI] [PubMed] [Google Scholar]
- 8.Craig RJ, Selzer A. Natural history and prognosis of atrial septal defect. Circulation. 1968;37:805–815. doi: 10.1161/01.cir.37.5.805. [DOI] [PubMed] [Google Scholar]
- 9.Sommer RJ, Hijazi ZM, Rhodes JF., Jr Pathophysiology of congenital heart disease in the adult. I. Shunt lesions. Circulation. 2008;117:1090–1099. doi: 10.1161/CIRCULATIONAHA.107.714402. [DOI] [PubMed] [Google Scholar]
- 10.Gossage JR, Kanj G. Pulmonary arteriovenous malformations: a state of the art review. Am J Respir Crit Care Med. 1998;158:643–661. doi: 10.1164/ajrccm.158.2.9711041. [DOI] [PubMed] [Google Scholar]
- 11.Cartin-Ceba R, Swanson KL, Krowka MJ. Pulmonary arteriovenous malformations. Chest. 2013;144:1033–1044. doi: 10.1378/chest.12-0924. [DOI] [PubMed] [Google Scholar]
- 12.Chiang ST. A nomogram for venous shunt (s – t) calculation. Thorax. 1968;23:563–565. doi: 10.1136/thx.23.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soliman OI, Geleijnse ML, Meijboom FJ, Nemes A, Kamp O, Nihoyannopoulos P, Masani N, Feinstein SB, Ten Cate FJ. The use of contrast echocardiography for the detection of cardiac shunts. Eur J Echocardiogr. 2007;8:S2–S12. doi: 10.1016/j.euje.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Gill EA, Jr, Quaife RA. The echocardiographer and the diagnosis of patent foramen ovale. Cardiol Clin. 2005;23:47–52. doi: 10.1016/j.ccl.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Rajiah P, Kanne JP. Cardiac MRI. 1. Cardiovascular shunts. AJR Am J Roentgenol. 2011;197:W603–W620. doi: 10.2214/AJR.10.7257. [DOI] [PubMed] [Google Scholar]
- 16.Rosenzweig EB, Barst RJ. Congenital heart disease and pulmonary hypertension: pharmacology and feasibility of late surgery. Prog Cardiovasc Dis. 2012;55:128–133. doi: 10.1016/j.pcad.2012.07.004. [DOI] [PubMed] [Google Scholar]