Abstract
Rationale: Vitamin D deficiency, often defined by total serum 25-hydroxyvitamin D (25[OH]D) <20 ng/ml, is common in critically ill patients, with associations with increased mortality and morbidity in the intensive care unit. Correction of vitamin D deficiency in critical illness has been recommended, and ongoing clinical trials are investigating the effect of repletion on patient outcome. The biologically active amount of 25(OH)D depends on the concentration and protein isoform of vitamin D–binding protein (VDBP), which is also an acute-phase reactant affected by inflammation and injury.
Objectives: We performed a secondary analysis of a cohort of critically ill children in which we reported a high rate of vitamin D deficiency, to examine how VDBP level and genotype would impact vitamin D status.
Methods: We prospectively enrolled 511 children admitted to the pediatric intensive care unit over a 12-month period.
Measurements and Main Results: We measured serum VDBP in 479 children. We genotyped single nucleotide polymorphisms rs7041 and rs4588 in the VDBP gene (GC) to determine haplotypes GC1F, GC1S, and GC2 in 178 subjects who consented, then calculated bioavailable 25(OH)D from serum 25(OH)D, VDBP, albumin, and GC haplotype. The median serum VDBP level was 159 μg/ml (interquartile range, 108–221), lower than has been reported in healthy children. Factors predicting lower levels in multivariate analysis included age <1 year, nonwhite race, being previously healthy, 25(OH)D <20 ng/ml and greater illness severity. In the subgroup that was genotyped, GC haplotype had the strongest association with VDBP level; carriage of one additional copy of GC1S was associated with a 37.5% higher level (95% confidence interval, 31.9–44.8; P < 0.001). Bioavailable 25(OH)D was also inversely associated with illness severity (r = −0.24, P < 0.001), and ratio to measured total 25(OH)D was variable and related to haplotype.
Conclusions: Physiologic deficiency of 25(OH)D in critical illness may be more difficult to diagnose, given that lower VDBP levels increase bioavailability. Treatment studies conducted on the basis of total 25(OH)D level, without consideration of VDBP concentration and genotype, may increase the risk of falsely negative results.
Keywords: critical illness, vitamin D, haplotype, bioavailability, vitamin D–binding protein
Low serum concentrations of total 25-hydroxyvitamin D (25[OH]D), widely accepted as indicative of vitamin D status, have been linked to sepsis susceptibility, illness severity, and increased mortality in critically ill adults (1–17). Using the widely accepted cutoff of <20 mg/dl to diagnose 25(OH)D deficiency, we reported recently that >40% of critically ill children in our hospital were deficient. Additional studies of vitamin D deficiency in the pediatric intensive care unit (ICU) (PICU) population have reported a prevalence of between 30 and 86% (18–21). We found lower 25(OH)D levels to be associated with greater illness severity (22); other pediatric critical care studies have confirmed this finding (19, 21, 23, 24), although equal numbers have reported no correlation with illness severity or ICU outcomes (18–20, 25, 26). Despite the rapidly increasing volume of evidence linking 25(OH)D deficiency with ICU morbidity and mortality over the past 10 years (1–4, 6–8, 10, 11, 27, 28), the question of whether vitamin D repletion will improve clinical outcomes in critical illness remains unclear. An intervention trial of calcitriol therapy in critically ill adults with sepsis did not have an impact on the primary outcome, antimicrobial peptide levels (29). A recent trial of high-dose vitamin D3 repletion in deficient critically ill adults did not show an impact on mortality or length of stay, except for subjects with the lowest 25(OH)D levels (<12 ng/ml) (30). The severely 25(OH)D-deficient group that was treated had lower mortality at 28 days after ICU admission, at hospital discharge, and at 6 months later (30).
The majority of 25(OH)D circulates tightly bound to vitamin D–binding protein (VDBP) and more loosely bound to albumin; a much smaller percentage circulates free (31). Bioavailable 25(OH)D (the free plus albumin bound portions) has been proposed as a better indicator of vitamin D activity (32–34), possibly explaining discrepancies between findings of vitamin D effect on health outcomes in studies relying on total 25(OH)D. Several studies have identified a strong relationship between bioavailable vitamin D and indicators of vitamin D status such as bone mineral density and parathyroid hormone (32, 33). Bioavailable 25(OH)D can be calculated using several equations and a mathematical model, which incorporate 25(OH)D, VDBP, albumin levels, and VDBP binding affinity by haplotype to varying degrees (32–34). Recently, an assay of free 25(OH)D has become commercially available, and a comparison of the calculated to measured free 25(OH)D showed that a calculation that does not incorporate VDBP binding affinity overestimates the measured levels (35).
VDBP, originally called group-specific component (Gc), has three major phenotypic variants characterized by isoelectric focusing electrophoresis: Gc1F(fast), Gc1S(slow), and Gc2 (36). Variation in the two most common polymorphisms in the VDBP gene (GC), rs4588 (A vs. C, resulting in lysine vs. threonine at position 420) and rs7041 (T vs. G, resulting in aspartic acid vs. glutamate at position 416), produce the three major GC haplotypes (GC1F, GC1S, and GC2). The protein isoforms corresponding to these haplotypes are thought to have variable binding affinity for vitamin D metabolites, Gc1F having the highest and Gc2 the lowest (37). Carriage of GC1F is more common in African Americans and in groups originating from areas with higher sun exposure (38), whereas carriage of GC1S is more common in those with origins farther from the equator (39, 40). GC2 has been associated repeatedly with lower 25(OH)D levels in genome-wide variation studies (38, 41, 42), as well as in children (43). VDBP plays a role in the immune system, the lung, and scavenging actin. VDBP levels have been shown to be decreased in times of diffuse inflammation, including in sepsis, severe trauma, and after surgery (44–46). In addition, certain disease states, including diffuse inflammation and critical illness, have been shown to be associated with lower VDBP levels (44, 46).
VDBP concentration and haplotype influence the proportion of bioavailable vitamin D (32–34, 47), meaning that the biologic activity of vitamin D could be similar in patients with widely different total 25(OH)D levels. We sought to determine VDBP levels and to identify patient-related factors, including genotype, that may influence these levels in critically ill children. We aimed to identify any association between VDBP level or genotype and diagnosis or reason for PICU admission, specifically, respiratory infections or respiratory failure. In addition, we sought to estimate bioavailable vitamin D levels and to compare them with total 25(OH)D levels in the early days of critical illness, to assess how that would influence categorization of 25(OH)D deficiency.
Methods
We prospectively recruited 520 critically ill children for a study of vitamin D in critical illness, and previously reported the details of recruitment, specimen collection and storage, and measurement of 25(OH)D levels (22). The Boston Children’s Hospital institutional review board approved the study. After giving informed consent, parents or guardians were interviewed, using a questionnaire, about their child’s racial and ethnic background, sun exposure, and intake of vitamin D–containing foods and supplements (48).
We measured VDBP levels in 479 of these children using the monoclonal-based R&D Systems Quantikine ELISA kit (Minneapolis, MN). Repeat VDBP levels were assessed using a polyclonal-based ELISA assay by ALPCO Diagnostics (Salem, NH) in 202 subjects, and albumin by colorimetric reflectance spectrophotometry (bromocresol green) assay (at Fairview Diagnostics Laboratory, St. Paul, MN) in 178 subjects. Both VDBP assays were performed in the University of Minnesota Cytokine Reference Laboratory (Minneapolis, MN).
To determine infection status on admission, patients who had any cultures or viral testing performed on the PICU admission day, or with a diagnosis of a confirmed or suspected infection within 7 days before PICU admission, were reviewed by a critical care physician. Confirmed infection was defined as having a: (1) culture of a pathogenic bacteria from blood, cerebrospinal fluid, or lung, plus receipt of antibiotics; or (2) positive fungal culture plus antifungal treatment; or (3) viral pathogen detected by diagnostic test. Suspected infection included all patients meeting systemic inflammatory response syndrome or community-acquired pneumonia criteria with negative microbial testing who received a course of antibiotic treatment. Those determined to have lower respiratory tract infection (LRTI) included anyone meeting a consensus definition of community-acquired pneumonia (49), as well as any patient who received a clinician diagnosis of LRTI and who required new respiratory support within 72 hours of admission to the PICU. Severity of illness in the first 24 hours was measured using the Pediatric Risk of Mortality-III (PRISM-III) score (50). All data were managed using Research Electronic Data Capture electronic data capture tools hosted at Boston Children’s Hospital (51).
Genotyping Cohort and Bioavailable Vitamin D
Initial informed consent included the option for parents to participate in the genetic analysis section. DNA was available in 207 of 254 subjects who were parent identified as white, without Hispanic ethnicity, to genotype for single nucleotide polymorphisms (SNPs) rs4588 and rs7041. We restricted genetic analysis to these subjects because of insufficient numbers of other racial/ethnic subgroups, to avoid population stratification. Fresh blood was collected and frozen at −80°C, then extracted in batch using the Gentra Puregene Blood Kit (QIAGEN Sciences, Germantown, MD). All samples were genotyped using the TaqMan OpenArray SNP Genotyping Platform (Applied Biosystems, Foster City, CA). Repeat genotyping was performed on 5% of the samples, and negative controls were performed on DNA-free samples, SNPs with success rate <96% were excluded. Haplotypes were assigned on the basis of genotyping results for the SNPs rs4588 and rs7041 (38, 41). Deviations from Hardy-Weinberg were tested using the χ2 test. Bioavailable 25(OH)D levels were calculated using a published method incorporating VDBP genotype, VDBP, albumin, 25(OH)D, and 1,25(OH)2D concentrations. Because 1,25(OH)2D was not measured routinely in this cohort, 0.1 nM was used. Bioavailable 25(OH)D was then calculated using a mathematical model program written in MATLAB (MATLAB and Statistics Toolbox Release 2012b; MathWorks, Inc., Natick, MA), developed by Brad Peercy (34).
Statistical Methods
To allow for the skewed distribution of VDBP levels, we used the Spearman correlation coefficient to assess the association of VDBP with continuous variables, the Mann-Whitney test for dichotomous variables, and the Kruskal-Wallis test for multicategory variables. Patient characteristics associated with VDBP or 25(OH)D in univariate analysis (P ≤ 0.10) were included in the multivariable model. Log VDBP level was used as the outcome in a linear regression model. Fisher’s exact test was used to assess the association between VDBP haplotype and categories of infection. One-way analysis of variance was used to assess the association between VDBP haplotype and the ratio of free to total 25(OH)D level. SAS was used for all computations (version 9.2; SAS Institute, Cary, NC).
Results
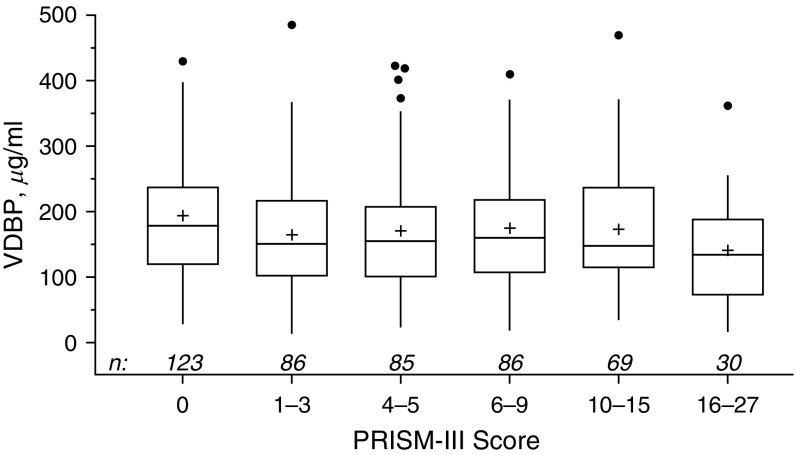
The median VDBP level was 159 μg/ml (interquartile range [IQR], 108–221) in 479 children at PICU admission; baseline characteristics of the sample population are displayed in Table 1. The median age of the subjects was 5.1 years, and VDBP levels were the lowest in infants <1 year old, with no significant difference between the 1- to 4-, 5- to 12-, and 13- to 17-year-old groups, but increased in 18- to 21-year-olds (Table 1). Parental report of race was also significantly associated with VDBP; the highest levels were in white non-Hispanic patients (median, 166 μg/ml), compared with white Hispanic and African-American children (152 and 141 μg/ml, respectively). VDBP was positively correlated with total 25(OH)D level (r = 0.31, P < 0.0001) and inversely correlated with illness severity by PRISM-III score on admission day (r = −0.12, P < 0.01) (Figure 1). There were no significant associations between VDBP and admission diagnosis, including any category of infection at PICU admission, or acute respiratory failure (Table 2).
Table 1.
Demographic and other characteristics of the subjects prior to pediatric intensive care unit admission and their relationship to VDBP levels
| Characteristic | n (%) | VDBP (μg/ml) |
|
|---|---|---|---|
| Median (IQR)* | P Value† | ||
| Total sample | 479 (100) | 159 (108, 221) | |
| Sex | |||
| Female | 238 (50) | 158 (105, 226) | 0.68 |
| Male | 241 (50) | 159 (115, 216) | |
| Age‡ | |||
| <1 yr | 95 (20) | 131 (94, 178) | <0.001 |
| 1–4 yr | 141 (29) | 166 (118, 228) | |
| 5–12 yr | 131 (27) | 159 (88, 216) | |
| 13–17 yr | 87 (18) | 167 (113, 226) | |
| 18–21 yr | 25 (5) | 215 (175, 254) | |
| Race | |||
| White non-Hispanic | 298 (62) | 166 (119, 237) | <0.001 |
| White Hispanic | 53 (11) | 152 (89, 221) | |
| African American | 63 (13) | 141 (61, 178) | |
| Other | 65 (14) | 149 (101, 208) | |
| Insurance | |||
| Private | 192 (40) | 164 (116, 219) | 0.19 |
| Government | 287 (60) | 156 (101, 221) | |
| Season | |||
| Fall or winter | 275 (57) | 165 (109, 229) | 0.25 |
| Spring | 119 (25) | 151 (109, 216) | |
| Summer | 85 (18) | 159 (106, 207) | |
| Supplements | |||
| Vitamin D or multivitamin | 169 (35) | 155 (109, 242) | 0.05 |
| Both | 31 (6) | 189 (130, 279) | |
| None | 279 (58) | 159 (106, 215) | |
| Formula | |||
| Any | 204 (43) | 156 (106, 220) | 0.73 |
| None | 275(57) | 162 (111, 221) | |
| Underlying chronic conditions: | |||
| Any | 403 (84) | 164 (111, 228) | 0.006 |
| None | 76 (16) | 136 (101, 184) | |
Definition of abbreviations: IQR = interquartile range; VDBP = vitamin D–binding protein.
Median (quartile limits).
Testing for association with VDBP level by Mann-Whitney test (dichotomy) or Kruskal-Wallis test (multicategory characteristic).
Spearman correlation between age and VDBP level: 0.135 (P = 0.003).
Figure 1.
VDBP level compared with admission illness severity score (PRISM-III). Box plots represent the median (belt), mean (+), interquartile range (top to bottom), outliers (closed circles; outside box by ≥1.5 × interquartile range), and position of farthest nonoutlier (vertical lines). The two are inversely correlated (r = −0.12, P < 0.01 [Spearman]). PRISM = Pediatric Risk of Mortality; VDBP = vitamin D–binding protein.
Table 2.
Vitamin D–binding protein and bioavailable 25-hydroxyvitamin D levels by diagnosis of respiratory infection and support status on intensive care unit admission
| Condition | VDBP (μg/ml) |
Bioavailable 25(OH)D (ng/ml) |
||||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Median (IQR)* | P Value† | n (%) | Median (IQR)* | P Value† | |||
| Total sample | 479 (100) | 159 (108–221) | |
178 (100) | 4.3 (2.7–6.5) | |
||
| Respiratory Infection |
|
|
||||||
| No infection | 237 (50) | 163 (114–228) | ┐ | 93 (53) | 4.5 (2.7–6.5) | ┐ | ||
| Other infection | 168 (35) | 161 (105–218) | ┤ | 0.28 | 56 (32) | 4.6 (3.0–7.0) | ┤ | 0.25 |
| LRTI | 72 (15) | 142 (103–205) | ┘ | 28 (16) | 3.6 (2.2–6.2) | ┘ | ||
| CAP | 51 (11) | 148 (106–207) | ┐ | 0.21 | 21 (12) | 3.5 (2.1–5.1) | ┐ | 0.16 |
| Other | 21 (4) | 116 (85–169) | ┘ | 7 (4) | 5.8 (2.7–9.2) | ┘ | ||
| Acute Respiratory Failure |
|
|
||||||
| No | 372 (78) | 160 (107–224) | ┐ | 0.66 | 142 (80) | 4.3 (2.8–6.5) | ┐ | 0.91 |
| Yes | 107 (22) | 156 (111–202) | ┘ | 36 (20) | 4.0 (2.6–6.7) | ┘ | ||
Definition of abbreviations: 25(OH)D = 25-hydroxyvitamin D; CAP = community-acquired pneumonia; IQR = interquartile range; LRTI = lower respiratory tract infection; VDBP = vitamin D–binding protein.
Median (quartile limits).
Testing for association of condition with serum level of VDBP or bioavailable 25(OH)D by Kruskal-Wallis test (trichotomy) or Mann-Whitney test (dichotomy).
Factors associated with decreased VDBP in the multivariable regression model were age, race, lack of underlying medical conditions, 25(OH)D <20 ng/ml, and greater illness severity on admission (Table 3). Respiratory infections (LRTIs and community-acquired pneumonia) were not associated with VDBP levels when added to this model. In the 178 subjects with sufficient excess plasma volume to assay albumin, the median level was 3.1 g/dl (IQR, 2.6–3.9), whereas the reference normal range for the assay is 3.9–5.1 g/dl. VDBP was positively correlated with albumin in these subjects (r = 0.49, P < 0.0001).
Table 3.
Joint influence of clinical and demographic factors associated with VDBP levels (model 1), with inclusion of GC haplotype information where available (model 2)
| Predictor | Contrast | % Difference in VDBP Level (95% CI)* | P Value |
|---|---|---|---|
| Model 1† | |||
| Age, yr | 1-17 vs. <1 | 20.9 (6.8 to 37.0) | 0.003 |
| 18-21 vs. <1 | 41.0 (10.9 to 79.4) | 0.005 | |
| 18-21 vs. 1-17 | 16.6 (−6.5 to 45.5) | 0.17 | |
| Race | Non-Hispanic white vs. all others | 27.2 (15.1 to 40.6) | <0.0001 |
| 25(OH)D level, ng/ml | <20 vs. >20 | −22.7 (−30.4 to −14.2) | <0.0001 |
| Medical history | Renal vs. none | 46.8 (12.8 to 91.0) | 0.004 |
| Renal vs. any other | 29.0 (1.7 to 63.4) | 0.04 | |
| Any other vs. none | 13.9 (−0.6 to 30.4) | 0.06 | |
| PRISM-III | Per unit raw score | −1.3 (−2.2 to −0.3) | 0.008 |
| Model 2‡ | |||
| Age, yr | 1-17 vs. <1 | 18.2 (5.2 to 32.8) | 0.005 |
| 18-21 vs. <1 | 44.6 (13.4 to 84.3) | 0.003 | |
| 18-21 vs. 1-17 | 22.3 (−2.5 to 53.5) | 0.08 | |
| 25(OH)D level, ng/ml | <20 vs. >20 | −23.4 (−30.9 to −15.2) | <0.0001 |
| PRISM-III | Per unit raw score | −1.7 (−2.6 to −0.8) | 0.0004 |
| VDBP haplotype | +1 copy GC1s or -1 copy GC1f | 37.5 (31.2 to 44.1) | <0.0001 |
Definition of abbreviations: 25(OH)D = 25-hydroxyvitamin D; CI = confidence interval; GC = VDBP gene; PRISM = Pediatric Risk of Mortality; VDBP = vitamin D–binding protein.
From multiple linear regression analysis of log-transformed VDBP levels. Regression coefficients were retransformed for reporting as percentage difference per indicated contrast or increment in predictor.
n = 477 children with VDBP level and all predictors available. Besides the predictors shown, the model included vitamin D supplementation (P = 0.43).
n = 205 children with VDBP level and all predictors available, including haplotype. Besides the predictors shown, the model included vitamin D supplementation (P = 0.19) and medical history (P = 0.47). Race was omitted because all subjects with haplotype results were non-Hispanic white.
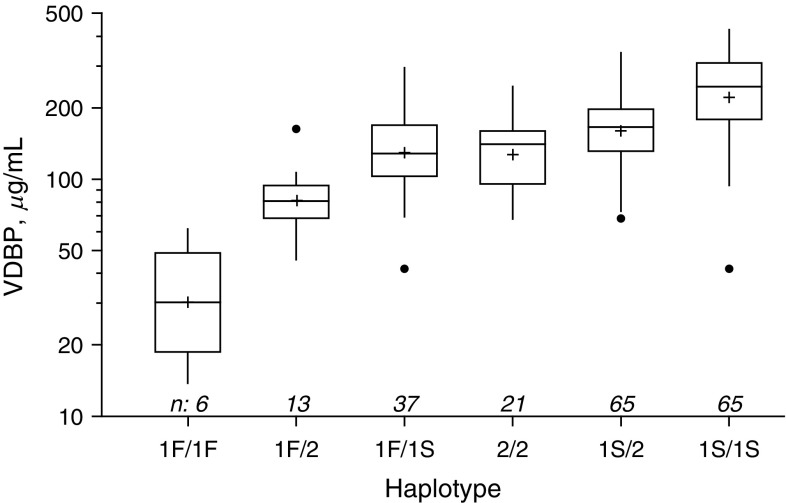
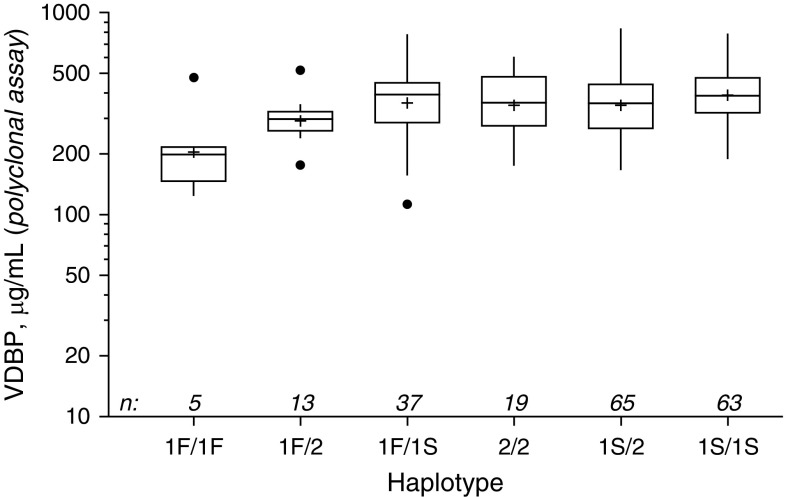
VDBP level varied significantly according to GC haplotype (P < 0.0001) (Figure 1), and the lowest levels were found in the six children carrying two copies of GC1F (median, 32.8 μg/ml), followed by the GC1F-GC2 group (median, 81.0 μg/ml). The majority of subjects who underwent haplotype analysis were either GC1S-GC1S (31.4%) or GC1s-GC2 (31.4%), and they had the highest median VDBP levels (Figure 2) (P < 0.0001). The GC1F-GC1F group also had the lowest 25(OH)D levels (median, 17.6 ng/ml), with GC1S-GC1S and GC1F-GC1S the highest (26.2 and 26.1 ng/ml, respectively, P = 0.02). The two polymorphisms (rs7041 and rs4588) used to determine VDBP haplotype were in Hardy-Weinberg equilibrium (P = 0.73 and 0.32, respectively). VDBP levels were repeated in 202 subjects, using a polyclonal-based assay. Although the values were higher overall (median, 366.0 μg/ml [IQR, 282.9–457.5]), there was a strong correlation with the original assay (r = 0.61, P < 0.0001), with an intercept of 202 μg/ml and a slope of 0.96 compared with the monoclonal assay. When tested against the VDBP haplotype groups, the same significant associations remained (P < 0.001; P = 0.0007) (Figure 3).
Figure 2.
Vitamin D–binding protein (VDBP) levels in the six unique haplotype groups. Sample sizes ranged from 6 to 65. VDBP level increased with each additional copy of G1s and decreased with each added copy of G1f. Box plots represent the median (belt), mean (+), interquartile range (top to bottom), outliers (closed circles; outside box by ≥1.5 × interquartile range), and position of farthest nonoutlier (vertical lines). Differences in levels are statistically significant by Kruskal Wallis, P < 0.0001.
Figure 3.
Results of ALPCO (polyclonal-based) vitamin D binding–protein (VDBP) assay for 202 subjects by VDBP haplotype group. Box plots represent the median (belt), mean (+), interquartile range (top to bottom), outliers (closed circles; outside box by ≥1.5 × interquartile range), and position of farthest nonoutlier (vertical lines). Differences in levels between haplotype groups is significant, P = 0.0007.
GC haplotype was the strongest predictor of VDBP level in the regression model (model 2 in Table 3). Age, PRISM-III score, and 25(OH)D level remained significant predictors of VDBP level, whereas carriage of each additional copy of GC1S or one less copy of GC1F was associated with a 37.5% increase (95% confidence interval, 31.9–44.8; P < 0.0001). VDBP haplotype was not associated with type of infection or presence of lower respiratory infection (P = 0.40).
Bioavailable Vitamin D levels
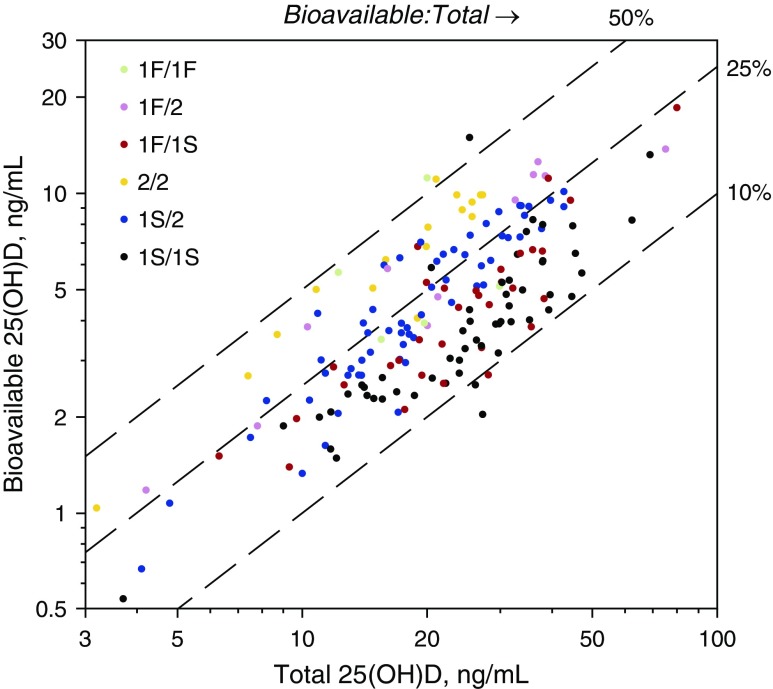
Genotype information was available in 205 subjects and was used to calculate bioavailable 25(OH)D using the published algorithm as described in the Methods (34). The median calculated bioavailable 25(OH)D level was 4.33 ng/ml (range, 0.54–18.54 ng/ml). Bioavailable 25(OH)D was inversely correlated with illness severity, as measured by PRISM-III score (r = −0.24, P = 0.001). As with VDBP, there was no significant relationship between bioavailable 25(OH)D and any respiratory infections on PICU admission. The ratio of bioavailable vitamin D levels to total 25(OH)D levels showed a wide range (Figure 4), varying significantly from approximately 10–50% among the 6 diplotypes (P < 0.0001). Whereas patients with the GC1F/GC1F diplotype had the lowest total 25(OH)D, their calculated bioavailable vitamin D levels were among the highest (Figure 4).
Figure 4.
Calculated bioavailable 25-hydroxyvitamin D (25[OH]D) levels versus measured total 25(OH)D levels for the 207 white non-Hispanic subjects with haplotype data available. Dashed line represents the percentage calculated bioavailable vitamin D of the total measured 25(OH)D.
Discussion
We identified lower VDBP levels (median, 159 μg/ml) in a cohort of critically ill children, especially in infants, nonwhite patients, previously healthy children, and more critically ill children. These are the same risk factors we identified for low total 25(OH)D in the same population of children. Because lower circulating VDBP levels increase the proportion of 25(OH)D that is bioavailable, it follows that the total 25(OH)D may not accurately reflect vitamin D status in this population. We previously recommended targeted screening of critically ill children with risk factors for vitamin D deficiency and implementation of effective repletion strategies, without considering bioavailability (22). We believe these results are an important consideration when interpreting total 25(OH)D levels in critically ill children, especially those at higher risk for low VDBP levels.
Although we did not have a healthy comparison population, reports of average VDBP levels in healthy children range from 232 to 298 μg/ml in pediatric populations of European ancestry using the same assay (52, 53). A mean of 197 μg/ml was reported in young healthy children, 65% Hispanic and 23.4% African American, using a different assay (immunonephelometry) (39), whereas mean levels of 330 to 437 μg/ml have been reported in healthy children using radial immunodiffusion (54, 55). Repeating VDBP levels in our cohort using a polyclonal assay showed a preserved genotype association (Figure 3) with higher levels overall, although questions remain about potential cross-reactivity in this assay (56). VDBP levels in healthy children using this assay have not been reported, but reports in healthy young adults range from 423 to 594 μg/ml, higher than our median of 366 μg/ml (57–59).
We calculated bioavailable 25(OH)D using a method including 25(OH)D, VDBP, albumin, and genotype (34), a very similar method to one used in a cohort of 2,085 adults, in which the predictors associated with VDBP level were similar to those that we report (33). As in that study, we found that VDBP levels are strongly influenced genetically, with carriers of the GC1S haplotype having the highest levels and those carrying GC1F the lowest. We found that GC haplotype explained 37.5% of the VDBP variation, lower than reported in that cohort, possibly related to the effect of critical illness on VDBP level (33). As in that study, we found that subjects heterozygous or homozygous for the GC1F variant had the lowest VDBP levels (33). In contrast, a report of a healthy population of urban children in the United States revealed that the GC2 variants had the lowest levels (39).
The correlation we found between calculated bioavailable 25(OH)D and illness severity (PRISM-III) is of a magnitude similar to that found with total 25(OH)D level (−0.23) (22), although with a significantly smaller sample size (178 vs. 479), and was stronger than the correlation with VBDP (r = −0.12). Although there is a large volume of evidence in both the pediatric and adult ICU populations linking vitamin D deficiency with increased morbidity and mortality, we identified only one study that measured the portion of free or bioavailable 25(OH)D in the ICU (15). This study did not find a difference between total and bioavailable 25(OH)D or 1,25(OH)2D in ability to predict ICU outcomes (15). The ratio of bioavailable to total 25(OH)D in our cohort was 10–50%, largely tracking with VDBP haplotype (see Figure 4). This is a higher range than reported in the adult ICU population, which was 15% overall (15), or in healthy adults, in whom the ratio of bioavailable to total 25(OH)D also varied by race from 12 to 18% (33). Our wide range may be a result of lower overall VDBP and 25(OH)D values in critical illness and the pediatric population, assay variability between populations, or our inclusion of VDBP genotype in bioavailable 25(OH)D calculations.
VDBP has a myriad of effects, including actin scavenging during times of injury (44–46), production of the antimicrobial peptide cathelicidin (48), macrophage activation (especially in the lung), and urinary excretion of 25(OH)D (60), that could influence the severity and outcome of critical illness. GC2 carriers have an increased risk of tuberculosis infection (61) but a decreased risk of chronic obstructive pulmonary disease (62, 63). The Gc2 isoform has decreased conversion to Gc macrophage activating factor, a potent mediator of inflammation. Although we did not find any association among VDBP, genotype, or bioavailable 25(OH)D and respiratory infections in this study, this may be because our measured levels of VDBP were low overall, impacting our ability to identify differences among groups.
Limitations of our study include measurement of VBDP at only one time point during critical illness, limiting our ability to evaluate fluctuations throughout acute illness and during recovery. We also did not have a noncritically ill control group of age and race-matched children for estimating normal VDBP, and the variability in assays in published reports limits our comparison somewhat. To avoid population stratification in a small cohort, we did not genotype the entire population, but limited our analysis to those who self-identified as white. Although this limits the generalizability of the results, we did not have sufficient numbers of African-American patients to perform accurate genotype analysis and calculate bioavailable 25(OH)D levels. Calculated free 25(OH)D has been shown to overestimate measured levels, although those calculations did not incorporate VDBP binding affinity by haplotype (35). To the best of our knowledge, we believe that, despite these limitations, this is the first study to report factors that influence VDBP levels in a relatively large cohort of critically ill children and to attempt to address the issue of 25(OH)D bioavailability in critical illness with some rigor.
Conclusions
Although a number of studies have demonstrated that 25(OH)D levels <20 ng/ml are very common in critically ill patients, there has been little consideration of the influence of critical illness on vitamin D bioavailability. We have demonstrated that VDBP levels are low in pediatric critical illness, and that the strong relationship with GC haplotype is preserved. VDBP is known to be an acute-phase reactant and is lowered in states of acute inflammation and sepsis and after trauma or major surgery (44–46); we now report an inverse relationship with critical illness severity. This may be protective by making a higher proportion of bioavailable 25(OH)D, which is also inversely related to illness severity. Measures of total 25(OH)D may yield an incomplete picture of available active vitamin D. Further research into bioavailable vitamin D in critical illness is needed to clarify this relationship. Using a total 25(OH)D cutoff of 20 mg/ml to diagnose vitamin D deficiency in critically ill patients without considering VDBP level and genotype may result in overtreatment.
Acknowledgments
Acknowledgment
The authors thank the children and parents/guardians who participated in this study. They also acknowledge the staff of the medical and surgical pediatric intensive care units for their support. The Division of Critical Care at Boston Children's Hospital and the Department of Anesthesia at Harvard Medical School supported A.G.R and K.M. throughout the project. The work of Hongyu Jiang, of the Clinical Research Program at Boston Children’s Hospital, was very helpful in the initial stages of the analysis. The genotyping was performed at the Partners HealthCare Center for Personalized Genetic Medicine Research Core. The authors also acknowledge the work of Jillian Adams of the Randolph Laboratory for her work on specimen and data management. We also thank Mike Ehrhardt of the University of Minnesota Cytokine Reference Laboratory.
Footnotes
Supported by a grant from the Clinical Research Center at Boston Children’s Hospital and by National Institutes of Health R01AI084011 (A.G.R.). H.A.F. and K.M. participated with support from Harvard Catalyst (National Institutes of Health Award UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers).
Author Contributions: K.M. and A.G.R. participated in the protocol conception and design, obtaining of funding, enrollment, data collection, management analysis and interpretation, and manuscript preparation; H.A.F. participated in the study design, data analysis and interpretation, manuscript preparation, and critical revision of the article; E.M.S., R.M.S., A.A.A., and S.M.K. participated in the acquisition of data, data management, subject enrollment, laboratory sample management, data analysis, and manuscript revision; R.F.C. participated in calculation of bioavailable 25(OH) vitamin D for this cohort, interpretation of findings, and manuscript revision; A.P.-M. performed the assays for the cohort and assisted with assay interpretation and with writing the manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Braun A, Chang D, Mahadevappa K, Gibbons FK, Liu Y, Giovannucci E, Christopher KB. Association of low serum 25-hydroxyvitamin D levels and mortality in the critically ill. Crit Care Med. 2011;39:671–677. doi: 10.1097/CCM.0b013e318206ccdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braun AB, Gibbons FK, Litonjua AA, Giovannucci E, Christopher KB. Low serum 25-hydroxyvitamin D at critical care initiation is associated with increased mortality. Crit Care Med. 2012;40:63–72. doi: 10.1097/CCM.0b013e31822d74f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ginde AA, Camargo CA, Jr, Shapiro NI. Vitamin D insufficiency and sepsis severity in emergency department patients with suspected infection. Acad Emerg Med. 2011;18:551–554. doi: 10.1111/j.1553-2712.2011.01047.x. [DOI] [PubMed] [Google Scholar]
- 4.McKinney JD, Bailey BA, Garrett LH, Peiris P, Manning T, Peiris AN. Relationship between vitamin D status and ICU outcomes in veterans. J Am Med Dir Assoc. 2011;12:208–211. doi: 10.1016/j.jamda.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Venkatram S, Chilimuri S, Adrish M, Salako A, Patel M, Diaz-Fuentes G. Vitamin D deficiency is associated with mortality in the medical intensive care unit. Crit Care. 2011;15:R292. doi: 10.1186/cc10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnson Y, Gringauz I, Itzhaky D, Amital H. Vitamin d deficiency is associated with poor outcomes and increased mortality in severely ill patients. QJM. 2012;105:633–639. doi: 10.1093/qjmed/hcs014. [DOI] [PubMed] [Google Scholar]
- 7.Flynn L, Zimmerman LH, McNorton K, Dolman M, Tyburski J, Baylor A, Wilson R, Dolman H.Effects of vitamin D deficiency in critically ill surgical patients Am J Surg 2012203379–382.[Discussion, p. 382] [DOI] [PubMed] [Google Scholar]
- 8.Remmelts HH, van de Garde EM, Meijvis SC, Peelen EL, Damoiseaux JG, Grutters JC, Biesma DH, Bos WJ, Rijkers GT. Addition of vitamin D status to prognostic scores improves the prediction of outcome in community-acquired pneumonia. Clin Infect Dis. 2012;55:1488–1494. doi: 10.1093/cid/cis751. [DOI] [PubMed] [Google Scholar]
- 9.Matthews LR, Ahmed Y, Wilson KL, Griggs DD, Danner OK. Worsening severity of vitamin D deficiency is associated with increased length of stay, surgical intensive care unit cost, and mortality rate in surgical intensive care unit patients. Am J Surg. 2012;204:37–43. doi: 10.1016/j.amjsurg.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aygencel G, Turkoglu M, Tuncel AF, Candir BA, Bildaci YD, Pasaoglu H. Is vitamin D insufficiency associated with mortality of critically ill patients? Crit Care Res Pract. 2013;2013:856747. doi: 10.1155/2013/856747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu J, Luo Z, Zhao X, Chen Q, Chen Z, Qin H, Qin Y, Liang X, Suo Y. Changes in the calcium-parathyroid hormone-vitamin d axis and prognosis for critically ill patients: a prospective observational study. PLoS One. 2013;8:e75441. doi: 10.1371/journal.pone.0075441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Türkoğlu M, Aygencel G, Dizbay M, Tuncel AF, Arslan Candır B, Deligöz Bildacı Y, Paşaoğlu H. Is vitamin D deficiency associated with development of Acinetobacter baumannii infections in critically ill patients? J Crit Care. 2013;28:735–740. doi: 10.1016/j.jcrc.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Amrein K, Zajic P, Schnedl C, Waltensdorfer A, Fruhwald S, Holl A, Purkart T, Wünsch G, Valentin T, Grisold A, et al. Vitamin D status and its association with season, hospital and sepsis mortality in critical illness. Crit Care. 2014;18:R47. doi: 10.1186/cc13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moromizato T, Litonjua AA, Braun AB, Gibbons FK, Giovannucci E, Christopher KB. Association of low serum 25-hydroxyvitamin D levels and sepsis in the critically ill. Crit Care Med. 2014;42:97–107. doi: 10.1097/CCM.0b013e31829eb7af. [DOI] [PubMed] [Google Scholar]
- 15.Quraishi SA, Bittner EA, Blum L, McCarthy CM, Bhan I, Camargo CA., Jr Prospective study of vitamin D status at initiation of care in critically ill surgical patients and risk of 90-day mortality. Crit Care Med. 2014;42:1365–1371. doi: 10.1097/CCM.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi A, Bhadade R, Varthakavi PK, DeSouza R, Bhagwat NM, Chadha MD. Vitamin D deficiency is associated with increased mortality in critically ill patients especially in those requiring ventilatory support. Indian J Endocrinol Metab. 2014;18:511–515. doi: 10.4103/2230-8210.137504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rech MA, Hunsaker T, Rodriguez J. Deficiency in 25-hydroxyvitamin D and 30-day mortality in patients with severe sepsis and septic shock. Am J Crit Care. 2014;23:e72–e79. doi: 10.4037/ajcc2014723. [DOI] [PubMed] [Google Scholar]
- 18.Rey C, Sánchez-Arango D, López-Herce J, Martínez-Camblor P, García-Hernández I, Prieto B, Pallavicini Z. Vitamin D deficiency at pediatric intensive care admission. J Pediatr (Rio J) 2014;90:135–142. doi: 10.1016/j.jped.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Rippel C, South M, Butt WW, Shekerdemian LS. Vitamin D status in critically ill children. Intensive Care Med. 2012;38:2055–2062. doi: 10.1007/s00134-012-2718-6. [DOI] [PubMed] [Google Scholar]
- 20.Ayulo M, Jr, Katyal Ch, Agarwal Ch, Sweberg T, Rastogi D, Markowitz M, Ushay HM. The prevalence of vitamin D deficiency and its relationship with disease severity in an urban pediatric critical care unit. Endocr Regul. 2014;48:69–76. doi: 10.4149/endo_2014_02_69. [DOI] [PubMed] [Google Scholar]
- 21.McNally JD, Menon K, Chakraborty P, Fisher L, Williams KA, Al-Dirbashi OY, Girolamo T, Maharajh G, Doherty DR. Impact of anesthesia and surgery for congenital heart disease on the vitamin D status of infants and children: a prospective longitudinal study. Anesthesiology. 2013;119:71–80. doi: 10.1097/ALN.0b013e31828ce817. [DOI] [PubMed] [Google Scholar]
- 22.Madden K, Feldman HA, Smith EM, Gordon CM, Keisling SM, Sullivan RM, Hollis BW, Agan AA, Randolph AG. Vitamin D deficiency in critically ill children. Pediatrics. 2012;130:421–428. doi: 10.1542/peds.2011-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNally JD, Menon K, Chakraborty P, Fisher L, Williams KA, Al-Dirbashi OY, Doherty DR Canadian Critical Care Trials Group. The association of vitamin D status with pediatric critical illness. Pediatrics. 2012;130:429–436. doi: 10.1542/peds.2011-3059. [DOI] [PubMed] [Google Scholar]
- 24.Onwuneme C, Carroll A, Doherty D, Bruell H, Segurado R, Kilbane M, Murphy N, McKenna MJ, Molloy EJ. Inadequate vitamin D levels are associated with culture positive sepsis and poor outcomes in paediatric intensive care. Acta Paediatr. 2015 doi: 10.1111/apa.13090. [DOI] [PubMed] [Google Scholar]
- 25.Hebbar KB, Wittkamp M, Alvarez JA, McCracken CE, Tangpricha V. Vitamin D deficiency in pediatric critical illness. J Clin Transl Endocrinol. 2014;1:170–175. doi: 10.1016/j.jcte.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iqbal S, Mosenkis EV, Jain P, Wiles A, Lerner J, Benton AS, Chamberlain JM, Freishtat RJ, Teach SJ. Vitamin D in pediatric inpatients with respiratory illnesses. Hosp Pediatr. 2013;3:371–376. doi: 10.1542/hpeds.2013-0001. [DOI] [PubMed] [Google Scholar]
- 27.Lucidarme O, Messai E, Mazzoni T, Arcade M, du Cheyron D. Incidence and risk factors of vitamin D deficiency in critically ill patients: results from a prospective observational study. Intensive Care Med. 2010;36:1609–1611. doi: 10.1007/s00134-010-1875-8. [DOI] [PubMed] [Google Scholar]
- 28.Nair P, Lee P, Reynolds C, Nguyen ND, Myburgh J, Eisman JA, Center JR. Significant perturbation of vitamin D-parathyroid-calcium axis and adverse clinical outcomes in critically ill patients. Intensive Care Med. 2013;39:267–274. doi: 10.1007/s00134-012-2713-y. [DOI] [PubMed] [Google Scholar]
- 29.Leaf DE, Raed A, Donnino MW, Ginde AA, Waikar SS. Randomized controlled trial of calcitriol in severe sepsis. Am J Respir Crit Care Med. 2014;190:533–541. doi: 10.1164/rccm.201405-0988OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amrein K, Schnedl C, Holl A, Riedl R, Christopher KB, Pachler C, Urbanic Purkart T, Waltensdorfer A, Münch A, Warnkross H, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA. 2014;312:1520–1530. doi: 10.1001/jama.2014.13204. [DOI] [PubMed] [Google Scholar]
- 31.Bouillon R, Van Assche FA, Van Baelen H, Heyns W, De Moor P. Influence of the vitamin D-binding protein on the serum concentration of 1,25-dihydroxyvitamin D3. Significance of the free 1,25-dihydroxyvitamin D3 concentration. J Clin Invest. 1981;67:589–596. doi: 10.1172/JCI110072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powe CE, Ricciardi C, Berg AH, Erdenesanaa D, Collerone G, Ankers E, Wenger J, Karumanchi SA, Thadhani R, Bhan I. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res. 2011;26:1609–1616. doi: 10.1002/jbmr.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369:1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chun RF, Peercy BE, Adams JS, Hewison M. Vitamin D binding protein and monocyte response to 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D: analysis by mathematical modeling. PLoS One. 2012;7:e30773. doi: 10.1371/journal.pone.0030773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz JB, Lai J, Lizaola B, Kane L, Markova S, Weyland P, Terrault NA, Stotland N, Bikle D. A comparison of measured and calculated free 25(OH) vitamin D levels in clinical populations. J Clin Endocrinol Metab. 2014;99:1631–1637. doi: 10.1210/jc.2013-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christiansen M, Jørgensen CS, Laursen I, Hirschberg D, Højrup P, Houen G. Protein chemical characterization of Gc globulin (vitamin d-binding protein) isoforms; Gc-1f, Gc-1s and Gc-2. Biochim Biophys Acta. 2007;1774:481–492. doi: 10.1016/j.bbapap.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Arnaud J, Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP) Hum Genet. 1993;92:183–188. doi: 10.1007/BF00219689. [DOI] [PubMed] [Google Scholar]
- 38.Engelman CD, Fingerlin TE, Langefeld CD, Hicks PJ, Rich SS, Wagenknecht LE, Bowden DW, Norris JM. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab. 2008;93:3381–3388. doi: 10.1210/jc.2007-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carpenter TO, Zhang JH, Parra E, Ellis BK, Simpson C, Lee WM, Balko J, Fu L, Wong BY, Cole DE. Vitamin D binding protein is a key determinant of 25-hydroxyvitamin D levels in infants and toddlers. J Bone Miner Res. 2013;28:213–221. doi: 10.1002/jbmr.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinotte M, Diorio C, Bérubé S, Pollak M, Brisson J. Genetic polymorphisms of the vitamin D binding protein and plasma concentrations of 25-hydroxyvitamin D in premenopausal women. Am J Clin Nutr. 2009;89:634–640. doi: 10.3945/ajcn.2008.26445. [DOI] [PubMed] [Google Scholar]
- 41.Gozdzik A, Zhu J, Wong BY, Fu L, Cole DE, Parra EJ. Association of vitamin D binding protein (VDBP) polymorphisms and serum 25(OH)D concentrations in a sample of young Canadian adults of different ancestry. J Steroid Biochem Mol Biol. 2011;127:405–412. doi: 10.1016/j.jsbmb.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 42.McGrath JJ, Saha S, Burne TH, Eyles DW. A systematic review of the association between common single nucleotide polymorphisms and 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol. 2010;121:471–477. doi: 10.1016/j.jsbmb.2010.03.073. [DOI] [PubMed] [Google Scholar]
- 43.Santos BR, Mascarenhas LP, Boguszewski MC, Spritzer PM. Variations in the vitamin D-binding protein (DBP) gene are related to lower 25-hydroxyvitamin D levels in healthy girls: a cross-sectional study. Horm Res Paediatr. 2013;79:162–168. doi: 10.1159/000348847. [DOI] [PubMed] [Google Scholar]
- 44.Dahl B, Schiødt FV, Nielsen M, Kiaer T, Williams JG, Ott P. Admission level of Gc-globulin predicts outcome after multiple trauma. Injury. 1999;30:275–281. doi: 10.1016/s0020-1383(99)00080-7. [DOI] [PubMed] [Google Scholar]
- 45.Dahl B, Schiødt FV, Ott P, Wians F, Lee WM, Balko J, O’Keefe GE. Plasma concentration of Gc-globulin is associated with organ dysfunction and sepsis after injury. Crit Care Med. 2003;31:152–156. doi: 10.1097/00003246-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 46.Dahl B, Schiodt FV, Gehrchen PM, Ramlau J, Kiaer T, Ott P. Gc-globulin is an acute phase reactant and an indicator of muscle injury after spinal surgery. Inflamm Res. 2001;50:39–43. doi: 10.1007/s000110050722. [DOI] [PubMed] [Google Scholar]
- 47.Chun RF, Lauridsen AL, Suon L, Zella LA, Pike JW, Modlin RL, Martineau AR, Wilkinson RJ, Adams J, Hewison M. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab. 2010;95:3368–3376. doi: 10.1210/jc.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon CM, Feldman HA, Sinclair L, Williams AL, Kleinman PK, Perez-Rossello J, Cox JE. Prevalence of vitamin D deficiency among healthy infants and toddlers. Arch Pediatr Adolesc Med. 2008;162:505–512. doi: 10.1001/archpedi.162.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Langley JM, Bradley JS. Defining pneumonia in critically ill infants and children. Pediatr Crit Care Med. 2005;6:S9–S13. doi: 10.1097/01.PCC.0000161932.73262.D7. [DOI] [PubMed] [Google Scholar]
- 50.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated pediatric risk of mortality score. Crit Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ashraf AP, Huisingh C, Alvarez JA, Wang X, Gower BA. Insulin resistance indices are inversely associated with vitamin D binding protein concentrations. J Clin Endocrinol Metab. 2014;99:178–183. doi: 10.1210/jc.2013-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adams LA, White SW, Marsh JA, Lye SJ, Connor KL, Maganga R, Ayonrinde OT, Olynyk JK, Mori TA, Beilin LJ, et al. Association between liver-specific gene polymorphisms and their expression levels with nonalcoholic fatty liver disease. Hepatology. 2013;57:590–600. doi: 10.1002/hep.26184. [DOI] [PubMed] [Google Scholar]
- 54.Chen WT, Yamaoka K, Nakajima S, Tanaka Y, Yamamoto T, Satomura K, Okada S, Seino Y. Evaluation of vitamin D-binding protein and vitamin D metabolite loss in children on continuous ambulatory peritoneal dialysis. Bone Miner. 1992;17:389–398. doi: 10.1016/0169-6009(92)90788-f. [DOI] [PubMed] [Google Scholar]
- 55.Specker BL, Tsang RC, Ho M, Buckley D. Seasonal differences in serum vitamin D binding protein in exclusively breast-fed infants: negative relationship to sunshine exposure and 25-hydroxyvitamin D. J Pediatr Gastroenterol Nutr. 1986;5:290–294. [PubMed] [Google Scholar]
- 56.Powe CE, Karumanchi SA, Thadhani R. Vitamin D-binding protein and vitamin D in blacks and whites. N Engl J Med. 2014;370:880–881. doi: 10.1056/NEJMc1315850. [DOI] [PubMed] [Google Scholar]
- 57.Blanton D, Han Z, Bierschenk L, Linga-Reddy MV, Wang H, Clare-Salzler M, Haller M, Schatz D, Myhr C, She JX, et al. Reduced serum vitamin D-binding protein levels are associated with type 1 diabetes. Diabetes. 2011;60:2566–2570. doi: 10.2337/db11-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winters SJ, Chennubhatla R, Wang C, Miller JJ. Influence of obesity on vitamin D-binding protein and 25-hydroxy vitamin D levels in African American and white women. Metabolism. 2009;58:438–442. doi: 10.1016/j.metabol.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 59.Thrailkill KM, Jo CH, Cockrell GE, Moreau CS, Fowlkes JL. Enhanced excretion of vitamin D binding protein in type 1 diabetes: a role in vitamin D deficiency? J Clin Endocrinol Metab. 2011;96:142–149. doi: 10.1210/jc.2010-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 61.Martineau AR, Leandro AC, Anderson ST, Newton SM, Wilkinson KA, Nicol MP, Pienaar SM, Skolimowska KH, Rocha MA, Rolla VC, et al. Association between Gc genotype and susceptibility to TB is dependent on vitamin D status. Eur Respir J. 2010;35:1106–1112. doi: 10.1183/09031936.00087009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen LH, Zhang XM, Su DJ, Yao SP, Yu BQ, Wang HW, Lu FZ. Association of vitamin D binding protein variants with susceptibility to chronic obstructive pulmonary disease. J Int Med Res. 2010;38:1093–1098. doi: 10.1177/147323001003800337. [DOI] [PubMed] [Google Scholar]
- 63.Wood AM, Bassford C, Webster D, Newby P, Rajesh P, Stockley RA, Thickett DR. Vitamin D-binding protein contributes to COPD by activation of alveolar macrophages. Thorax. 2011;66:205–210. doi: 10.1136/thx.2010.140921. [DOI] [PubMed] [Google Scholar]