Abstract
Rationale: Bronchial thermoplasty is an alternative treatment for patients with severe, uncontrolled asthma in which the airway smooth muscle is eliminated using radioablation. Although this emerging therapy shows promising outcomes, little is known about its effects on airway inflammation.
Objectives: We examined the presence of bronchoalveolar lavage cytokines and expression of smooth muscle actin in patients with severe asthma before and in the weeks after bronchial thermoplasty.
Methods: Endobronchial biopsies and bronchoalveolar lavage samples from 11 patients with severe asthma were collected from the right lower lobe before and 3 and 6 weeks after initial bronchial thermoplasty. Samples were analyzed for cell proportions and cytokine concentrations in bronchoalveolar lavage and for the presence of α-SMA in endobronchial biopsies.
Measurements and Main Results: α-SMA expression was decreased in endobronchial biopsies of 7 of 11 subjects by Week 6. In bronchoalveolar lavage fluid, both transforming growth factor-β1 and regulated upon activation, normal T-cell expressed and secreted (RANTES)/CCL5 were substantially decreased 3 and 6 weeks post bronchial thermoplasty in all patients. The cytokine tumor-necrosis-factor–related apoptosis-inducing ligand (TRAIL), which induces apoptosis in several cell types, was increased in concentration both 3 and 6 weeks post bronchial thermoplasty.
Conclusions: Clinical improvement and reduction in α-SMA after bronchial thermoplasty in severe, uncontrolled asthma is associated with substantial changes in key mediators of inflammation. These data confirm the substantial elimination of airway smooth muscle post thermoplasty in the human asthmatic airway and represent the first characterization of significant changes in airway inflammation in the first weeks after thermoplasty.
Keywords: airway smooth muscle, pulmonary disease, asthma, airway inflammation
Asthma continues to be one of the most prevalent health conditions, affecting 25 million individuals in the United States and more than 200 million worldwide (1). Although only 10% of patients have severe, uncontrolled, persistent asthma, these patients account for an estimated 80% of the economic burden of healthcare costs attributed to this disease (2, 3). One key pathogenic feature is chronic airway inflammation stemming from the infiltration of eosinophils and Th2 cells into the airways (4) that contribute significantly to the immune response in asthma (5, 6). Recent studies suggest that Th17 cells, induced by cytokines such as transforming growth factor-beta (TGF-β), also may worsen airway inflammation (7–9). Th17 cells produce a signature of cytokines, including IL-17A, IL-17F, IL-21, and IL-22 (10), which in turn stimulate the production of proinflammatory cytokines and chemokines, such as IL-6, IL-1β, tumor necrosis factor (TNF)-α, and granulocyte-macrophage colony-stimulating factor (GM-CSF) (11). These cytokines, chemokines, and adaptive immunity cells together with the response of structural cells within the airway generate the ongoing airway inflammation that is the hallmark of severe asthma.
Severe asthma is defined by the requirement for guidelines-suggested medications for Global Initiative for Asthma steps 4 to 5 asthma, including high-dose inhaled corticosteroids and long-acting β-adrenergic agonist or leukotriene blocker for the previous year or the use of systemic corticosteroid for greater than 50% of the previous year to prevent loss of control, or is uncontrolled despite addition of such therapies (12). Uncontrolled asthma, in which poor symptom control, frequent serious exacerbations, and severe airflow limitation with an FEV1 less than 80% predicted after correction of adherence and other factors that limit treatment, remains a substantial issue in severe asthma (12). Recent addition of monoclonal antibody therapy directed against immunoglobulin E has also emerged as a potential treatment (13) but may also not control severe asthma. New therapies may extend the use of monoclonal antibodies against cytokines such as IL-5 or IL-13 (14) that are believed to have a key role in airway inflammation in asthma, but these therapies are not yet in clinical use (15).
Bronchial thermoplasty (BT) offers an alternative therapeutic for patients with severe, uncontrolled asthma that targets airway smooth muscle, a key component of bronchoconstriction and the elevated airway resistance seen in severe asthma, especially during asthma exacerbations (16). BT is performed by the delivery of controlled, therapeutic radiofrequency energy to the visible, accessible airways via bronchoscopy. BT is delivered in three sessions each separated by 3 weeks. The benefits of BT as demonstrated in clinical trials include reduced asthma symptoms, fewer acute exacerbations, and improved quality of life (16–23). The mechanism is believed to be radioablation of airway smooth muscle mass within the central airways with consequent reduction in bronchial reactivity (19, 20). Changes in muscle mass have been demonstrated in canine models (24) and in normal human airways in the setting of resection surgery (25). One recently published preliminary study suggests that BT decreases airway smooth muscle mass in asthmatic airways (26). However, to date there have been no studies that examine changes in airway inflammation, including inflammatory cells and local mediators, in the airway in the immediate period after BT.
Here, we present findings from a single-center case series to examine local airway changes and inflammation in patients with severe, persistent asthma who met the clinical criteria for performance of BT.
Methods
Subjects
This was a prospective, observational clinical study. Adult subjects with severe, uncontrolled asthma were sequentially recruited from the Refractory Obstructive Lung Disease Clinic at the University of Chicago. Subjects met the criteria for step 4 through step 6 asthma as defined by the Expert Panel Report 3 Guidelines on Asthma (27). Confirmation of airway reactivity in subjects with asthma was done using methacholine challenge when baseline FEV1 was greater than or equal to 60% of predicted value; when this could not be done, measurement of reversibility before and after treatment with nebulized 0.083% albuterol (minimum 12% improvement in FEV1) was done per guidelines of the American Thoracic Society (28).
Approval for the use of samples generated from these subjects was obtained from the Institutional Review Board at the University of Chicago (IRB 12-1739). All subjects provided written, informed consent for the BT procedure that reflected the severe nature of their disease, published guidelines for the procedure, and modifications to the guidelines based on their pulmonary function. Only after that consent was obtained were patients recruited for the research protocol, for which a separate written, informed consent for the collection of research samples, including endobronchial biopsies, was obtained.
All patients were treated with 50 mg prednisone 3 days before, the day of, and 1 day after each BT in addition to any oral or inhaled corticosteroid therapy required for asthma control before the procedure. Bronchoscopy was done using standard methods and sedation. Bronchoalveolar lavage (BAL) was performed before biopsy by instillation of 120 ml saline into the medial segment (RB7) of the right lower lobe followed by in-line trap suction recovery. Airway biopsies, four to six total, were collected at right lower lobe airway carinas using Precisor Forceps (ConMed, New York, NY) as previously described (29). Samples were collected from the same location before the first BT and then 3 and 6 weeks later. No samples were collected from any other segments of the lung. After this, BT then was performed at the appropriate locations using the standard clinical protocol (16) using the Alair Bronchial Thermoplasty System (Boston Scientific, Inc., Natick, MA), which includes the Alair RF controller and Alair RF catheter. All subjects tolerated bronchoscopy and thermoplasty without severe adverse events or complications. All subjects were monitored appropriately by clinical staff, and treatment with oral corticosteroids and short-acting β-agonists was provided as required to manage asthma exacerbations between BT procedures.
Sample Analysis
BAL was kept at 4°C during all handling. Lavage fluid was centrifuged at 1,500 × 10 min; cells were collected for count and differential, and lavage supernatant then was stored at −80°C until use. For Bio-Plex analysis, 10 ml of BAL fluid was concentrated to 500 μl at 4°C using Amicon Ultra-15 Centrifugal Filter Units (Millipore, UFC900308). These samples then were analyzed for cytokine/chemokine concentrations using Milliplex multiplex Assay kits (EMD Millipore, Darmstadt, Germany) using Luminex according to manufacturer’s protocol. Endobronchial biopsies collected from the same airway in each BT procedure (RB7) were paraffin embedded, after which 5-μm sequential slices were stained with hematoxylin and eosin or were labeled with an antibody directed against α-smooth muscle actin (α-SMA) (Sigma, Inc., St. Louis, MO) using a standard immunoperoxidase protocol.
Data Analysis
As this was a clinical series and patients were recruited for the research protocol after a clinical decision had been made to perform BT, no subjects were recruited for a “placebo” or sham-bronchoscopy trial. Data from the two time points after first BT were compared in a paired manner to data generated at the first BT for each patient.
For airway smooth muscle mass analysis, total area and α-SMA–positive area were measured from three endobronchial biopsy sections at each time point for each patient using ImageJ (30). Samples that were considered inadequate were excluded from analysis. Data are presented as the percentage of α-SMA–positive tissue related to the total tissue area. For cytokine/chemokine expression, analytes were measured according to manufacturer’s protocol. Observed concentrations were determined by comparison to known standards analyzed at the same time.
All data are expressed as the mean ± SEM. Statistical analysis of clinical and demographic data was done using paired t tests or by F tests followed by t tests with correction using the Bonferroni method as appropriate. Statistical analyses were conducted in STATA (version 13.1; StataCorp, College Station, TX), or R (version 3.0.2; http://www.r-project.org) as required.
Results
Eleven adult patients (Table 1) with severe, uncontrolled, persistent asthma underwent BT per a standard protocol as described previously (16). Three men and eight women ranged in age from 19 to 56 years old. At the time of the first BT procedure, all 11 patients were receiving corticosteroids. Of these, eight (73%) were treated with oral corticosteroids: one patient received 10 mg prednisone daily and seven patients received 50 mg prednisone daily. All 11 patients received 50 mg oral corticosteroids 3 days before and 1 day after each BT. Patients taking oral corticosteroids for asthma treatment remained on oral corticosteroids during the days between BT (Table 2). Of the 11 patients, 8 (73%) also received combination inhaled corticosteroid/long-acting β-agonists in a combination inhaler device, and 3 (27%) received inhaled corticosteroid alone. All 11 patients also received short-acting β-agonist treatment as required for symptom control, and 2 (18%) were treated with other asthma medications (Table 1).
Table 1.
Patient demographics
| Patients (n = 11) | |
|---|---|
| Sex, male/female | 3/8 |
| Age, mean ± SE, yr | 40 ± 4 |
| Race, white/other | 10/1 |
| Medication use, % (n/total) | |
| Oral corticosteroids | 73 (8/11) |
| Inhaled corticosteroids/long-acting β-agonists | 73 (8/11) |
| Inhaled corticosteroids | 27 (3/11) |
| Short-acting β-agonists | 27 (3/11) |
| Other | 18 (2/11) |
Table 2.
Oral corticosteroid use before and during bronchial thermoplasty
| Patient | Pre-BT (mg) | BT1–BT2 (mg) | BT2–BT3 (mg) |
|---|---|---|---|
| 1 | None | None | None |
| 2 | None | None | None |
| 3 | 50 | 50 | 50 |
| 4 | None | None | None |
| 5 | 10 | 10 | 10 |
| 6 | 50 | 50 | 50 |
| 7 | 50 | 50 | 50 |
| 8 | 50 | 50 | 50 |
| 9 | 50 | 50 | 50 |
| 10 | 50 | 50 | 50 |
| 11 | 50 | 50 | 50 |
Definition of abbreviation: BT = bronchial thermoplasty.
Patients underwent spirometry at each time point just before BT. Eight of the 11 patients (73%) had an FEV1 < 60% predicted before the first BT (Table 3). By 3 weeks, five of these eight (63%) patients had improved to an FEV1 above 80% predicted (P < 0.001). This increased FEV1 was maintained at 6 weeks post BT (P < 0.001). Three patients (27%) with an initial FEV1 greater than 60% predicted remained unchanged at both 3 and 6 weeks (Table 3). Three patients (27%) with FEV1 < 60% predicted did not improve after BT.
Table 3.
Spirometry before and after bronchial thermoplasty
| Week 0 | Week 3 | Week 6 | |
|---|---|---|---|
| FEV1 % predicted, mean ± SE | 59.1 ± 7.9 | 63.7 ± 6.5 | 61.3 ± 7.3 |
| FEV1 < 60% predicted, n | 8 | 3* | 3* |
P < 0.01 by paired t test and Bonferroni correction as appropriate.
Endobronchial biopsies and BAL fluid were collected during each procedure. Differential BAL cell counts were determined at each time point (Table 4). The percentage of eosinophils decreased from 4 ± 1% to 1 ± 0% by week 3 and remained low 6 weeks after the initial BT (P < 0.001), and macrophage percentages did not significantly change at either time point.
Table 4.
Cellular composition of bronchoalveolar lavage fluid before and after bronchial thermoplasty
| BAL cellular content (%)* | Week 0 | Week 3 | Week 6 |
|---|---|---|---|
| Eosinophils | 4 ± 1 | 1 ± 0† | 1 ± 0† |
| Macrophages | 92 ± 2 | 94 ± 1 | 92 ± 1 |
| Lymphocytes | 3 ± 2 | 4 ± 1 | 6 ± 2 |
Definition of abbreviation: BAL = bronchoalveolar lavage.
Data presented as mean ± SE.
Proportions of neutrophils and epithelial cells were <1% in the majority of samples counted.
P < 0.01 by repeated measures F test followed by paired t test and Bonferroni correction as appropriate.
We measured the expression of α-SMA in endobronchial biopsies collected at each time point (Figure 1). α-SMA expression, measured as a percentage of total tissue area, decreased, though not significantly, from 38 ± 5% from Week 0 (Figures 1A–1C) to 29 ± 4% at Week 3. By Week 6 (Figures 1A, 1B, and 1D), SMA expression had significantly decreased to 16 ± 5% (P < 0.001). These data suggest a time course over which smooth muscle presence decreased after the initial BT procedure to this airway.
Figure 1.
Smooth muscle actin (SMA) expression decreases after treatment with bronchial thermoplasty. (A, B) Percentage of α-SMA abundance within endobronchial biopsies at Week 0, 3, and 6 after bronchial thermoplasty. *P < 0.05 by repeated measures F test followed by paired t test and Bonferroni correction as appropriate. (C, D) Representative images of endobronchial biopsies from a single patient stained for α-SMA, as demonstrated by immunoperoxidase (brown) stain, at week 0 (C) and week 6 (D). The blue dot in A indicates an individual data point outside the bulk of the group.
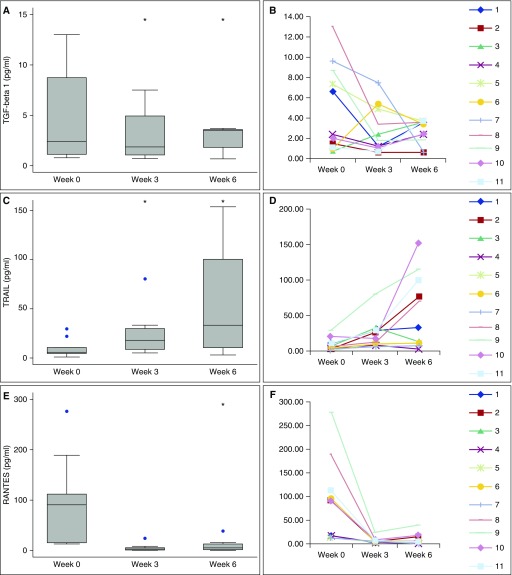
The presence of key cytokines and chemokines believed to have a potential role in asthmatic airway inflammation was measured in the BAL samples collected at each time point using EMD Millipore Milliplex assay microplates. Results for each cytokine and chemokine measured are summarized in Table 5. Three of these cytokines/chemokines were observed to have significant changes in concentration over time after BT (Figure 2). Three weeks post BT, TGF-β1 concentration decreased more than twofold from 4.9 ± 1.3 pg/ml to 2.3 ± 0.9 pg/ml (P = 0.04) (Figures 2A and 2B). At 6 weeks post initial BT; the average TGF-β1 concentration had decreased further to 1.2 ± 0.7 pg/ml (P = 0.03). A second mediator, TNF-related apoptosis-inducing ligand (TRAIL) was dramatically increased in concentration at both 3 and 6 weeks post BT from 9.1 ± 2.7 pg/ml before BT to 21.7 ± 6.5 pg/ml and 56.4 ± 15.2 pg/ml (P < 0.05) (Figures 2C and 2D). Regulated upon activation, normal T-cell expressed and secreted (RANTES)/CCL5 decreased in concentration from 84.7 ± 25.8 pg/ml at Week 0 to 5.2 ± 2.0 pg/ml and 8.93 ± 3.30 pg/ml at 3 and 6 weeks (P < 0.05) (Figures 2E and 2F). These data demonstrate clear changes in select, key mediators that may modulate airway inflammation and fibrosis in the early weeks after BT.
Table 5.
Cytokine/chemokine concentrations in bronchoalveolar lavage fluid before and after bronchial thermoplasty
| Analyte | Week 0 | Week 3 | Week 6 |
|---|---|---|---|
| EGF | 27.2 ± 4.0 | 22.1 ± 6.0 | 23.7 ± 7.8 |
| Eotaxin | 26.2 ± 4.3 | 27.2 ± 4.9 | 29.0 ± 5.3 |
| IL-6 | 0.68 ± 0.26 | 0.64 ± 0.16 | 1.43 ± 0.79 |
| IL-10 | 0.7 ± 0.2 | 0.5 ± 0.1 | 0.6 ± 0.1 |
| IL-12 | 0.13 ± 0.02 | 0.16 ± 0.3 | 0.17 ± 0.04 |
| IL-13 | 0.26 ± 0.03 | 0.31 ± 0.06 | 0.32 ± 0.05 |
| IL-15 | 1.49 ± 0.4 | 1.75 ± 0.42 | 1.32 ± 0.39 |
| IL-21 | 0.18 ± 0.03 | 0.2 ± 0.03 | 0.2 ± 0.04 |
| IL-33 | 1.36 ± 0.91 | 0.5 ± 0.2 | 0.5 ± 0.26 |
| MIP-3α | 3.1 ± 1.7 | 3.8 ± 1.5 | 9.1 ± 5.1 |
| RANTES | 84.7 ± 25.8 | 5.2 ± 2.0* | 8.9 ± 3.3* |
| TGF-α | 256.3 ± 51.9 | 191.0 ± 47.8 | 207.2 ± 58.0 |
| TGF-β1 | 4.9 ± 1.3 | 2.3 ± 0.7* | 1.2 ± 0.4* |
| TNF-α | 0.25 ± 0.06 | 0.13 ± 0.03 | 0.17 ± 0.04 |
| TRAIL | 9.1 ± 2.7 | 21.7 ± 6.5* | 56.4 ± 15.2* |
Definition of abbreviations: EGF = epidermal growth factor; MIP = macrophage inflammatory protein; RANTES = regulated upon activation, normal T-cell expressed and secreted; TGF = transforming growth factor; TNF = tumor necrosis factor; TRAIL = tumor necrosis factor–related apoptosis-inducing ligand.
Undetected analytes: granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-1β, IL-4, IL-5, IL-9, IL-17A, IL-17E, IL-17F, IL-22, IL-23, IL-27, IL-28A, IL-31, IFN-γ, TNF-β.
P < 0.05 by repeated measures F test followed by paired t test and Bonferroni correction as appropriate.
Figure 2.
Cytokine/chemokine expression before and after bronchial thermoplasty. Box plots representing the expression levels of transforming growth factor (TGF)-β1 (A, B), tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) (C, D), and regulated upon activation, normal T-cell expressed and secreted (RANTES)/CCL5 (E, F), as demonstrated by Bio-Plex assay. *P < 0.05 by repeated measures F test followed by paired t test and Bonferroni correction as appropriate. The blue dots indicate individual data points outside the bulk of the group.
Discussion
In this study we analyzed pulmonary function, BAL cellular content and cytokine/chemokine concentration, and α-SMA abundance in a single asthmatic airway immediately before and both 3 and 6 weeks after BT. Our study design thus used each patient as her/his own control in a before-and-after examination of airway inflammation and airway smooth muscle mass. We demonstrate that airway obstruction as measured by FEV1 predicted improved significantly by Week 3 after the initial BT, and this improvement correlated with significant changes in both airway smooth muscle mass and airway inflammation.
Airway smooth muscle mass is in increased abundance in patients with severe asthma (31, 32) and is an active participant in the pathophysiology of asthma. This increase in airway smooth muscle exacerbates airway constriction through hypertrophy and hyperplasia of airway smooth muscle cells (33) and contributes to the inflammatory response through the production of cytokines and chemokines (34). BT was designed to target airway smooth muscle directly. We show here that airway smooth muscle mass within the airway is significantly reduced, as demonstrated by labeling for smooth muscle–specific actin, 3 and 6 weeks after the initial BT. A reduction in airway smooth muscle mass was previously demonstrated in the first trial of BT in humans without asthma performed by Miller and colleagues (25). Very recently, a clinical trial of 10 subjects conducted in France demonstrated reduction in airway smooth muscle mass in Paris, France in airways treated with BT at about 3 months after the procedure (26). Our study thus confirms the reduction in airway smooth muscle mass within asthmatic airways after BT and further demonstrates that this reduction occurs early, within 6 weeks of the procedure.
Our study is the first to examine local inflammatory events in the airways affected by BT in the weeks after the procedure. A striking finding is the substantial change in select cytokines involved in asthma-associated airway inflammation. One of these, TGF-β, has a complex role in the pathogenesis of severe asthma (35). It is produced by several cell types, including epithelial cells, eosinophils, macrophages, fibroblasts, and helper T cells (36, 37), and is involved in epithelial transformation, subepithelial fibrosis, airway smooth muscle remodeling, microvascular changes, mucus production, and both suppressing and activating inflammatory cytokines (38–41). TGF-β1 is a major regulator as well as effector in the immune response. TGF-β1 expression is markedly increased in asthmatic airways (42, 43) and further augmented by infiltrating inflammatory cells. The clear reduction in TGF-β1 concentration suggests the potential to down-regulate inflammation and fibrosis in the first weeks after BT. Although it is tempting to suggest that the reduction in eosinophil proportion is responsible for the decreased TGF-β1 concentration in BAL fluid, we recognize the other cell contributors to the total pool of TGF-β1 and thus suggest caution as to which cells were responsible.
RANTES/CCL5 is a chemoattractant that recruits eosinophils that has been shown to account for 80% of TGF-β expression in asthma (44, 45). Even in low concentrations, BAL RANTES incites eosinophil attraction and has been shown to correlate with the proportion of BAL eosinophils (46). Our data demonstrate a reduction in both TGF-β1 and RANTES in BAL fluid in the weeks after BT applied to a single lobe, which in turn correlated with a decrease in BAL eosinophil proportion throughout the time period of BT treatment. It is interesting in this context that eotaxin, another cytokine expressed by epithelial cells that can elicit eosinophil recruitment into airways, did not change significantly in the three measurements. This suggests that eotaxin had no significant influence on the change in eosinophil proportion and that RANTES/CCL5 was more responsible.
Interestingly, the concentration of the chemokine TRAIL was significantly increased in the BAL fluid of our cohort after BT. Apoptosis has been implicated in the resolution of inflammatory processes and reestablishment of tissue homeostasis, and TRAIL signaling is reported to have beneficial effects in several disease states (47–50). In a mouse model of asthma, increased expression of TRAIL was shown to be responsible for increased apoptosis of airway leukocytes and associated with the resolution of allergy through a reduction in Th2 production of IL-5 (51). In human asthma, a higher BAL eosinophil count is associated with a decreased expression of the canonical TRAIL death receptors (52). Conversely, TRAIL signaling has also been linked to nonapoptotic and proliferative events (52–54), thus indicating that further investigation into the role of TRAIL after BT is necessary to establish its function.
Surprisingly, we detected no significant changes in select key asthma cytokines, including IL-4, IL-5, IL-13, and IL-17. We suspect this may be due to the nature of our study design. In the days before each BT procedure, patients received high doses of oral prednisone per the clinical protocol for purposes of safety, which very likely altered cytokine expression. Additionally, the times at which our patient samples were collected may limit our ability to observe significant changes in these inflammatory mediators.
Previous human studies of BT have proven the safety and long-term efficacy of BT on patients with both mild and severe asthma (17–23). However, sustained improvement in pulmonary function, as measured by FEV1 or FVC, generally has not been demonstrated after BT in these studies. Our data demonstrate that airway obstruction is improved early after BT, as indicated by an improvement in FEV1 at both time points after the first procedure. This correlates with a significant decrease in the percentage of eosinophils in BAL in the majority of patients in our cohort. We interpret this finding cautiously and note that improvement in spirometric values in our subjects may not differ long term compared with pre-BT determinations, as has generally been seen in prior studies.
We also note that several of the patients in our study had an FEV1 as percent predicted significantly lower than the cut-off used in previous trials such as the Asthma Intervention Research 2 trial (55). We have recently demonstrated in a case series that BT can be done with no increase in serious adverse events in patients with FEV1 in the range of 40 to 60% predicted (23). As such, patients with FEV1 values in this range were not excluded from our study.
Our data offer insight into how the airways of patients with severe asthma respond to BT in the first few weeks of the procedure. However, it is important to note the limitations of our study. Our study is small and reflects recruitment from a single center in the local community. Thus, our data do not take into account the several phenotypes of severe asthma, the potential influences of geography, or household and environment exposures. In addition, although all subjects received a standard protocol of oral corticosteroids shortly before and after each BT procedure, it is unknown how these and inhaled corticosteroids, long-acting β-agonists, and other agents influence the airway before BT and thus how each alone or in combination might affect the outcomes in terms of airway inflammation and smooth muscle mass in the weeks after the procedure.
Finally, the follow-up time in our study subjects was 6 weeks. We took advantage of the need to perform bronchoscopy at the stated intervals for the BT procedure to collect samples from the right lower lung lobe treated in the first procedure. We were not able to perform bronchoscopy at time points beyond 6 weeks; therefore, the changes in airway inflammation, remodeling, and smooth muscle mass beyond that point are not known. We further note that we do not have data concerning ultimate clinical status and thus cannot, in this small study, correlate changes in airway inflammation and smooth muscle mass to (for example) clinical asthma symptoms, exacerbations, and quality of life in the months after the procedure.
In summary, we demonstrate clear changes in the airway smooth muscle mass and inflammatory mediators after bronchial thermoplasty in patients with severe asthma. This study sets the stage for future trials that will aid in more clearly understanding the role of BT and the changes in airway inflammation in the early weeks to months after BT in severe, persistent asthma.
Acknowledgments
Acknowledgment
The authors thank Stephany Contrella, M.S., Jerrica Hill, Kathy Reilly, R.N., and Cynthia Warnes, R.N., in the Asthma Clinical Research Center, University of Chicago, for their assistance in patient recruitment and evaluation. We thank Jyotsna Sudi, M.S., Randi Stern, M.S., and Bharathi Laxman, Ph.D., Section of Pulmonary and Critical Care Medicine, University of Chicago, for their technical assistance.
Footnotes
Supported by the National Heart, Lung and Blood Institute grant T32-HL007605, the National Center for Advancing Translational Sciences grant UL1-TR000430, and the Institute for Translational Medicine of the University of Chicago.
Author Contributions: Conception and design: D.C.D., E.T.N., and S.R.W. Data collection: D.R.D., D.C.D., and D.K.H. Data analysis and interpretation: D.R.D., D.C.D., K.D., and S.R.W. Statistical analysis: D.R.D. and S.R.W. Drafting and editing manuscript for important intellectual content: D.R.D., E.T.N., and S.R.W.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bell MC, Busse WW. Severe asthma: an expanding and mounting clinical challenge. J Allergy Clin Immunol Pract. 2013;1:110–121. [Quiz, p. 122.]. doi: 10.1016/j.jaip.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett SB, Nurmagambetov TA. Costs of asthma in the United States: 2002-2007. J Allergy Clin Immunol. 2011;127:145–152. doi: 10.1016/j.jaci.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Smith DH, Malone DC, Lawson KA, Okamoto LJ, Battista C, Saunders WB. A national estimate of the economic costs of asthma. Am J Respir Crit Care Med. 1997;156:787–793. doi: 10.1164/ajrccm.156.3.9611072. [DOI] [PubMed] [Google Scholar]
- 4.Morishima Y, Ano S, Ishii Y, Ohtsuka S, Matsuyama M, Kawaguchi M, Hizawa N. Th17-associated cytokines as a therapeutic target for steroid-insensitive asthma. Clin Dev Immunol. 2013;2013:609395. doi: 10.1155/2013/609395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ray A, Cohn L. Altering the Th1/Th2 balance as a therapeutic strategy in asthmatic diseases. Curr Opin Investig Drugs. 2000;1:442–448. [PubMed] [Google Scholar]
- 6.Barnes PJ. Th2 cytokines and asthma: an introduction. Respir Res. 2001;2:64–65. doi: 10.1186/rr39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ivanov II, II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 8.Zhao J, Lloyd CM, Noble A. Th17 responses in chronic allergic airway inflammation abrogate regulatory T-cell-mediated tolerance and contribute to airway remodeling. Mucosal Immunol. 2013;6:335–346. doi: 10.1038/mi.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosmi L, Liotta F, Maggi E, Romagnani S, Annunziato F. Th17 cells: new players in asthma pathogenesis. Allergy. 2011;66:989–998. doi: 10.1111/j.1398-9995.2011.02576.x. [DOI] [PubMed] [Google Scholar]
- 10.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 11.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 13.Bonini M, Di Maria G, Paggiaro P, Rossi A, Senna G, Triggiani M, Canonica GW. Potential benefit of omalizumab in respiratory diseases. Ann Allergy Asthma Immunol. 2014;113:513–519. doi: 10.1016/j.anai.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 14.Hilvering B, Pavord ID. What goes up must come down: biomarkers and novel biologicals in severe asthma. Clin Exp Allergy. 2015;45:1162–1169. doi: 10.1111/cea.12500. [DOI] [PubMed] [Google Scholar]
- 15.Bice JB, Leechawengwongs E, Montanaro A. Biologic targeted therapy in allergic asthma. Ann Allergy Asthma Immunol. 2014;112:108–115. doi: 10.1016/j.anai.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Sheshadri A, McKenzie M, Castro M. Critical review of bronchial thermoplasty: where should it fit into asthma therapy? Curr Allergy Asthma Rep. 2014;14:470. doi: 10.1007/s11882-014-0470-4. [DOI] [PubMed] [Google Scholar]
- 17.Castro M, Rubin AS, Laviolette M, Fiterman J, De Andrade Lima M, Shah PL, Fiss E, Olivenstein R, Thomson NC, Niven RM, et al. AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181:116–124. doi: 10.1164/rccm.200903-0354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox G, Miller JD, McWilliams A, Fitzgerald JM, Lam S. Bronchial thermoplasty for asthma. Am J Respir Crit Care Med. 2006;173:965–969. doi: 10.1164/rccm.200507-1162OC. [DOI] [PubMed] [Google Scholar]
- 19.Cox G, Thomson NC, Rubin AS, Niven RM, Corris PA, Siersted HC, Olivenstein R, Pavord ID, McCormack D, Chaudhuri R, et al. AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327–1337. doi: 10.1056/NEJMoa064707. [DOI] [PubMed] [Google Scholar]
- 20.Pavord ID, Cox G, Thomson NC, Rubin AS, Corris PA, Niven RM, Chung KF, Laviolette M RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176:1185–1191. doi: 10.1164/rccm.200704-571OC. [DOI] [PubMed] [Google Scholar]
- 21.Pavord ID, Thomson NC, Niven RM, Corris PA, Chung KF, Cox G, Armstrong B, Shargill NS, Laviolette M Research in Severe Asthma Trial Study Group. Safety of bronchial thermoplasty in patients with severe refractory asthma. Ann Allergy Asthma Immunol. 2013;111:402–407. doi: 10.1016/j.anai.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler ME, Laviolette M, Rubin AS, Fiterman J, Lapa e Silva JR, Shah PL, Fiss E, Olivenstein R, Thomson NC, Niven RM, et al. Asthma Intervention Research 2 Trial Study Group. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132:1295–1302. doi: 10.1016/j.jaci.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doeing DC, Mahajan AK, White SR, Naureckas ET, Krishnan JA, Hogarth DK. Safety and feasibility of bronchial thermoplasty in asthma patients with very severe fixed airflow obstruction: a case series. J Asthma. 2013;50:215–218. doi: 10.3109/02770903.2012.751997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang H, Rao K, Halayko AJ, Kepron W, Stephens NL. Bronchial smooth muscle mechanics of a canine model of allergic airway hyperresponsiveness. J Appl Physiol (1985) 1992;72:39–45. doi: 10.1152/jappl.1992.72.1.39. [DOI] [PubMed] [Google Scholar]
- 25.Miller JD, Cox G, Vincic L, Lombard CM, Loomas BE, Danek CJ. A prospective feasibility study of bronchial thermoplasty in the human airway. Chest. 2005;127:1999–2006. doi: 10.1378/chest.127.6.1999. [DOI] [PubMed] [Google Scholar]
- 26.Pretolani M, Dombret MC, Thabut G, Knap D, Hamidi F, Debray MP, Taille C, Chanez P, Aubier M. Reduction of airway smooth muscle mass by bronchial thermoplasty in patients with severe asthma. Am J Respir Crit Care Med. 2014;190:1452–1454. doi: 10.1164/rccm.201407-1374LE. [DOI] [PubMed] [Google Scholar]
- 27.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 28.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, et al. Disordered microbial communities in asthmatic airways. Plos One. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon IO, Husain AN, Charbeneau J, Krishnan JA, Hogarth DK. Endobronchial biopsy: a guide for asthma therapy selection in the era of bronchial thermoplasty. J Asthma. 2013;50:634–641. doi: 10.3109/02770903.2013.794239. [DOI] [PubMed] [Google Scholar]
- 30.Raman B, Raman R, Raman L, Beaulieu CF. Radiology on handheld devices: image display, manipulation, and PACS integration issues. Radiographics. 2004;24:299–310. doi: 10.1148/rg.241035127. [DOI] [PubMed] [Google Scholar]
- 31.Erle DJ, Sheppard D. The cell biology of asthma. J Cell Biol. 2014;205:621–631. doi: 10.1083/jcb.201401050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dowell ML, Lavoie TL, Solway J, Krishnan R. Airway smooth muscle: a potential target for asthma therapy. Curr Opin Pulm Med. 2014;20:66–72. doi: 10.1097/MCP.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 33.James AL, Elliot JG, Jones RL, Carroll ML, Mauad T, Bai TR, Abramson MJ, McKay KO, Green FH. Airway smooth muscle hypertrophy and hyperplasia in asthma. Am J Respir Crit Care Med. 2012;185:1058–1064. doi: 10.1164/rccm.201110-1849OC. [DOI] [PubMed] [Google Scholar]
- 34.Panettieri RA, Jr, Kotlikoff MI, Gerthoffer WT, Hershenson MB, Woodruff PG, Hall IP, Banks-Schlegel S National Heart, Lung, and Blood Institute. Airway smooth muscle in bronchial tone, inflammation, and remodeling: basic knowledge to clinical relevance. Am J Respir Crit Care Med. 2008;177:248–252. doi: 10.1164/rccm.200708-1217PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Alawi M, Hassan T, Chotirmall SH. Transforming growth factor β and severe asthma: a perfect storm. Respir Med. 2014;108:1409–1423. doi: 10.1016/j.rmed.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Coker RK, Laurent GJ, Shahzeidi S, Hernández-Rodríguez NA, Pantelidis P, du Bois RM, Jeffery PK, McAnulty RJ. Diverse cellular TGF-beta 1 and TGF-beta 3 gene expression in normal human and murine lung. Eur Respir J. 1996;9:2501–2507. doi: 10.1183/09031936.96.09122501. [DOI] [PubMed] [Google Scholar]
- 37.Lee KY, Ho SC, Lin HC, Lin SM, Liu CY, Huang CD, Wang CH, Chung KF, Kuo HP. Neutrophil-derived elastase induces TGF-beta1 secretion in human airway smooth muscle via NF-kappaB pathway. Am J Respir Cell Mol Biol. 2006;35:407–414. doi: 10.1165/rcmb.2006-0012OC. [DOI] [PubMed] [Google Scholar]
- 38.Halwani R, Al-Muhsen S, Al-Jahdali H, Hamid Q. Role of transforming growth factor-β in airway remodeling in asthma. Am J Respir Cell Mol Biol. 2011;44:127–133. doi: 10.1165/rcmb.2010-0027TR. [DOI] [PubMed] [Google Scholar]
- 39.Michalik M, Pierzchalska M, Legutko A, Ura M, Ostaszewska A, Soja J, Sanak M. Asthmatic bronchial fibroblasts demonstrate enhanced potential to differentiate into myofibroblasts in culture. Med Sci Monit. 2009;15:BR194–BR201. [PubMed] [Google Scholar]
- 40.Ohbayashi H, Shimokata K. Matrix metalloproteinase-9 and airway remodeling in asthma. Curr Drug Targets Inflamm Allergy. 2005;4:177–181. doi: 10.2174/1568010053586246. [DOI] [PubMed] [Google Scholar]
- 41.Watelet JB, Bachert C, Claeys C, Van Cauwenberge P. Matrix metalloproteinases MMP-7, MMP-9 and their tissue inhibitor TIMP-1: expression in chronic sinusitis vs nasal polyposis. Allergy. 2004;59:54–60. doi: 10.1046/j.1398-9995.2003.00364.x. [DOI] [PubMed] [Google Scholar]
- 42.Howell JE, McAnulty RJ. TGF-beta: its role in asthma and therapeutic potential. Curr Drug Targets. 2006;7:547–565. doi: 10.2174/138945006776818692. [DOI] [PubMed] [Google Scholar]
- 43.Duvernelle C, Freund V, Frossard N. Transforming growth factor-beta and its role in asthma. Pulm Pharmacol Ther. 2003;16:181–196. doi: 10.1016/S1094-5539(03)00051-8. [DOI] [PubMed] [Google Scholar]
- 44.Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, Barnes N, Robinson D, Kay AB. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vignola AM, Chanez P, Chiappara G, Merendino A, Pace E, Rizzo A, la Rocca AM, Bellia V, Bonsignore G, Bousquet J. Transforming growth factor-beta expression in mucosal biopsies in asthma and chronic bronchitis. Am J Respir Crit Care Med. 1997;156:591–599. doi: 10.1164/ajrccm.156.2.9609066. [DOI] [PubMed] [Google Scholar]
- 46.Teran LM, Seminario MC, Shute JK, Papi A, Compton SJ, Low JL, Gleich GJ, Johnston SL. RANTES, macrophage-inhibitory protein 1alpha, and the eosinophil product major basic protein are released into upper respiratory secretions during virus-induced asthma exacerbations in children. J Infect Dis. 1999;179:677–681. doi: 10.1086/314618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grosse-Wilde A, Voloshanenko O, Bailey SL, Longton GM, Schaefer U, Csernok AI, Schütz G, Greiner EF, Kemp CJ, Walczak H. TRAIL-R deficiency in mice enhances lymph node metastasis without affecting primary tumor development. J Clin Invest. 2008;118:100–110. doi: 10.1172/JCI33061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffmann O, Priller J, Prozorovski T, Schulze-Topphoff U, Baeva N, Lunemann JD, Aktas O, Mahrhofer C, Stricker S, Zipp F, et al. TRAIL limits excessive host immune responses in bacterial meningitis. J Clin Invest. 2007;117:2004–2013. doi: 10.1172/JCI30356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cretney E, McQualter JL, Kayagaki N, Yagita H, Bernard CC, Grewal IS, Ashkenazi A, Smyth MJ. TNF-related apoptosis-inducing ligand (TRAIL)/Apo2L suppresses experimental autoimmune encephalomyelitis in mice. Immunol Cell Biol. 2005;83:511–519. doi: 10.1111/j.1440-1711.2005.01358.x. [DOI] [PubMed] [Google Scholar]
- 50.McGrath EE, Marriott HM, Lawrie A, Francis SE, Sabroe I, Renshaw SA, Dockrell DH, Whyte MK. TNF-related apoptosis-inducing ligand (TRAIL) regulates inflammatory neutrophil apoptosis and enhances resolution of inflammation. J Leukoc Biol. 2011;90:855–865. doi: 10.1189/jlb.0211062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faustino L, Fonseca DM, Florsheim EB, Resende RR, Lepique AP, Faquim-Mauro E, Gomes E, Silva JS, Yagita H, Russo M. Tumor necrosis factor-related apoptosis-inducing ligand mediates the resolution of allergic airway inflammation induced by chronic allergen inhalation. Mucosal Immunol. 2014;7:1199–1208. doi: 10.1038/mi.2014.9. [DOI] [PubMed] [Google Scholar]
- 52.Robertson NM, Zangrilli JG, Steplewski A, Hastie A, Lindemeyer RG, Planeta MA, Smith MK, Innocent N, Musani A, Pascual R, et al. Differential expression of TRAIL and TRAIL receptors in allergic asthmatics following segmental antigen challenge: evidence for a role of TRAIL in eosinophil survival. J Immunol. 2002;169:5986–5996. doi: 10.4049/jimmunol.169.10.5986. [DOI] [PubMed] [Google Scholar]
- 53.Lorz C, Benito A, Ucero AC, Santamaría B, Ortiz A. Trail and kidney disease. Front Biosci (Landmark Ed) 2009;14:3740–3749. doi: 10.2741/3485. [DOI] [PubMed] [Google Scholar]
- 54.Collison A, Hatchwell L, Verrills N, Wark PA, de Siqueira AP, Tooze M, Carpenter H, Don AS, Morris JC, Zimmermann N, et al. The E3 ubiquitin ligase midline 1 promotes allergen and rhinovirus-induced asthma by inhibiting protein phosphatase 2A activity. Nat Med. 2013;19:232–237. doi: 10.1038/nm.3049. [DOI] [PubMed] [Google Scholar]
- 55.Castro M, Cox G. Asthma outcomes from bronchial thermoplasty in the AIR2 trial. Am J Respir Crit Care Med. 2011;184:743–744. doi: 10.1164/ajrccm.184.6.743. [DOI] [PubMed] [Google Scholar]