Abstract
Despite significant advances in treatment strategies targeting the underlying defect in cystic fibrosis (CF), airway infection remains an important cause of lung disease. In this two-part series, we review recent evidence related to the complexity of CF airway infection, explore data suggesting the relevance of individual microbial species, and discuss current and future treatment options. In Part I, the evidence with respect to the spectrum of bacteria present in the CF airway, known as the lung microbiome is discussed. Subsequently, the current approach to treat methicillin-resistant Staphylococcus aureus, gram-negative bacteria, as well as multiple coinfections is reviewed. Newer molecular techniques have demonstrated that the airway microbiome consists of a large number of microbes, and the balance between microbes, rather than the mere presence of a single species, may be relevant for disease pathophysiology. A better understanding of this complex environment could help define optimal treatment regimens that target pathogens without affecting others. Although relevance of these organisms is unclear, the pathologic consequences of methicillin-resistant S. aureus infection in patients with CF have been recently determined. New strategies for eradication and treatment of both acute and chronic infections are discussed. Pseudomonas aeruginosa plays a prominent role in CF lung disease, but many other nonfermenting gram-negative bacteria are also found in the CF airway. Many new inhaled antibiotics specifically targeting P. aeruginosa have become available with the hope that they will improve the quality of life for patients. Part I concludes with a discussion of how best to treat patients with multiple coinfections.
Keywords: Burkholderia cepacia, methicillin-resistant Staphylococcus aureus, microbiome, Pseudomonas aeruginosa, Stenotrophomonas maltophilia
Cystic fibrosis (CF) lung disease is characterized by airway obstruction, chronic bacterial infection, and a vigorous host inflammatory response (1). Antibiotic therapy of bacterial lung infections has tremendously contributed to the increased survival in CF (2). However, many bacteria form biofilms in the CF lung that make their eradication difficult (3). In addition, it has also become clear that only a small fraction of the microbes present in the CF airway are being identified with routine laboratory techniques (4, 5), and both extended culture methods and molecular techniques have identified organisms that previously were not routinely cultured (6). Traditional antibiotic susceptibility testing performed on planktonic bacteria has been found to be of limited clinical use in chronic airway infection as most bacteria in the CF lung exist in biofilms (7). Although it has long been recognized that patients clinically respond even when their infecting organisms are pan-resistant, 25% of patients do not reach preexacerbation values in lung function measures despite aggressive treatment for their bacterial lung infections (8), demonstrating that current treatment is inadequate when addressing the complexity of airway infection. In addition to bacteria identified by routine sputum culture methods, clinicians are often faced with an array of multidrug-resistant organisms that are difficult to treat.
In this article and its companion article, we provide a summary of current aspects of airway infection in CF. These manuscripts are derived from a symposium organized by the Scientific Assemblies on Pediatrics and Clinical Problems and presented at the 2013 American Thoracic Society (ATS) International Conference in Philadelphia, Pennsylvania. In Part I, we discuss the lung microbiome in CF, methicillin-resistant Staphylococcus aureus (MRSA), gram-negative bacteria, and approaches to treating multiple infections. In Part II, we discuss nontuberculous mycobacteria, anaerobic bacteria, and fungi. The current evidence for treatment of these lung infections in CF, which we summarized, is limited. Within these documents, we also provide a pragmatic approach as to how one might treat these infections. However, it is important to note that these manuscripts are not meant to represent definitive treatment guidelines or consensus recommendations. For available guideline recommendations, the reader is referred elsewhere in the published literature (9–13).
The Lung Microbiome in CF
The conventional view of CF airway microbiology has been based on the recovery in culture of a suite of bacterial pathogens, including S. aureus and opportunists such as Pseudomonas aeruginosa, Burkholderia cepacia complex, Achromobacter spp., and Stenotrophomonas maltophilia. The Human Microbiome Project, a National Institutes of Health–sponsored initiative launched in 2007, applying culture-independent methods to assess bacterial ecology, has significantly broadened this view. Numerous studies now provide compelling evidence that the airways of persons with CF may be inhabited by diverse bacterial communities composed of dozens of species (14–23). In addition to “typical” CF pathogens, “nonpathogenic oral bacteria,” including many obligate and facultative anaerobic species, are often present in densities that well exceed those of the traditional opportunists associated with CF (4). Although most of these studies analyzed expectorated sputum samples that would be expected to be “contaminated” with bacteria residing in the nonsterile oropharynx during expectoration, several lines of evidence indicate that this has only a marginal impact on measures of airway microbiota (17, 24, 25).
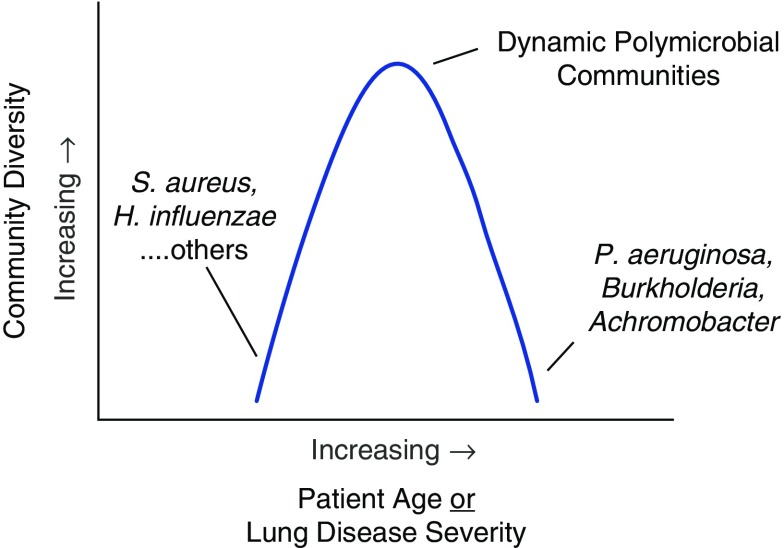
Limited studies of children with CF indicate that bacterial community diversity (a measure of both the number and relative abundances of the species present) increases with age (14, 26). In contrast, several cross-sectional and longitudinal studies of adults have shown that diversity decreases with age and declining lung function (14, 18, 22, 23). Analyses of respiratory specimens from persons with end-stage lung disease or from lung explants show very constrained communities, often limited to a single dominant species (23, 27). These observations suggest that after an initial increase during childhood, airway bacterial diversity peaks in young adulthood and then declines with advancing age and disease progression (Figure 1). Antibiotic use may be the primary driver of decreasing diversity with advancing disease (23).
Figure 1.
Schematic representation of airway bacterial community diversity versus patient age or lung disease severity. Available data suggest that after an initial increase during childhood, airway bacterial diversity peaks in young adulthood and then declines with advancing age and lung disease progression. At end-stage disease, bacterial communities may be dominated by a single species, most often a “typical” cystic fibrosis opportunistic pathogen. H. influenza = Haemophilus influenzae; P. aeruginosa = Pseudomonas aeruginosa; S. aureus = Staphylococcus aureus.
The change in the airway microbiota around the time of exacerbations of pulmonary symptoms is an area of intense interest. Fodor and colleagues (15) showed that bacterial community richness decreased transiently with antibiotic therapy but rebounded quickly thereafter. Zhao and colleagues (23) similarly showed a significant decrease in diversity with antibiotic therapy of exacerbation but did not find significant changes in diversity when comparing samples from periods of clinical stability to those taken at the onset of exacerbation symptoms, a finding that was subsequently confirmed in a larger study (28). Interestingly, this latter study showed a decrease in the relative and absolute abundance of P. aeruginosa in communities dominated by this species at the onset of symptoms leading to exacerbation.
Thus, our traditional view of CF airway microbiology is changing. Although bacterial communities likely become more diverse during childhood, they become increasing confined with advancing lung disease and antibiotic treatment in adulthood. Eventually, a single species representing one of the traditional CF pathogens (S. aureus, P. aeruginosa, B. cepacia complex, or Achromobacter spp.) dominates the community. Despite this dramatic decrease in diversity, total bacterial density appears to remain rather constant. The dynamics of bacterial communities around the time of exacerbation suggest that the dominant pathogen in the community may decrease with onset of symptoms. As our understanding of the dynamics of airway bacterial ecology continues to expand, so too will the opportunities to develop novel strategies to better manage airway infection in CF.
Methicillin-resistant Staphylococcus aureus
The prevalence of MRSA has increased dramatically over the last decade and now is detected in the respiratory tract of greater than 25% of patients with CF in the United States (29). The prevalence of MRSA in Canada and Europe is lower, ranging from 3 to 11% of patients with CF (30). Chronic MRSA infection in patients with CF is associated with increased rate of lung function decline, failure to recover lung function after a pulmonary exacerbation, and decreased survival (8, 31, 32). Currently, there are no conclusive studies demonstrating an effective and safe treatment protocol for MRSA respiratory infection in CF (33). Here, we provide a practical framework on how to treat MRSA infection in different clinical scenarios by first describing antibiotic choices and then subsequently provide guidance for defining patients that require treatment.
In patients with CF infected with MRSA who are experiencing an acute pulmonary exacerbation, vancomycin and linezolid are the first-line antimicrobial choices (34, 35). Dosing of vancomycin is based on the patient’s weight and creatinine clearance. A trough concentration of 15 to 20 μg/ml, which is the practice of most CF physicians based on a recent survey (36), should be the considered target. Linezolid dosing may need adjustment in children with CF (37) but not adults (38). Because approximately 80% of patients with CF with chronic MRSA may also be infected with P. aeruginosa, linezolid, rather than vancomycin with its associated renal effects, may be a good option in these patients who require antipseudomonal treatment with other nephrotoxic drugs, such as aminoglycosides. As depression is detected in greater than 20% of adults with CF (29), it is important to note that linezolid can be associated with the serotonin syndrome in patients with CF taking serotonergic psychiatric drugs (39). Chronic linezolid use may also be associated with the development of potentially irreversible peripheral and optic neuropathies and linezolid-resistant MRSA (40). In patients with allergies or contraindications to the above medications, alternative antibiotics include rifampin, Fucidin (not available in the United States), ceftaroline, tigecycline, chloramphenicol, and/or clindamycin.
The approach to patients with CF with MRSA infection seen in the outpatient clinic has recently been reviewed (30). Doe and colleagues (41) reported that patient segregation and aggressive antibiotic eradication therapy can achieve eradication in the majority of patients with CF. Numerous antibiotic regimens were used; however, the most successful were those regimens that included two oral antibiotics (one of which was rifampin) and nebulized vancomycin. Rifampin has been a component of successful MRSA eradication protocols due to its high mucosal concentrations and activity against biofilms, but it should be used in combination with another antibiotic as resistance develops quickly with monotherapy (30). Rifampin can also be associated with worsening gastroesophageal reflux and decreased efficacy of oral contraceptives (42).
Inhaled antibiotics have been used as a treatment for CF respiratory tract infections since penicillin first became available (43). Fosfomycin tobramycin (FTI) for inhalation has activity against anaerobic, gram-negative, and gram-positive bacteria, including MRSA. In a clinical trial of FTI in patients with CF with P. aeruginosa infection, 29 patients with MRSA at baseline had significant decreases in the concentration of MRSA after 28 days of FTI (n = 19) compared with placebo (n = 10) (44). Current published experience with aerosolized vancomycin suggests that it is safe and well tolerated (45, 46). There are two ongoing studies investigating the use of inhaled vancomycin, including one assessing a novel dry powder formulation (47, 48). The results of these trials will help to delineate the risks and benefits of treating chronic MRSA infection.
Because there are no definitive studies to guide the decision to treat (or not to treat) MRSA infections in CF, the following approach is based on uncontrolled studies and anecdote. The MRSA population can be split into four groups: (1) new MRSA infection in an asymptomatic patient, (2) new MRSA infection in a symptomatic patient, (3) chronic MRSA infection in an asymptomatic patient, and (4) chronic MRSA infection in a symptomatic patient. There is no standard definition for chronic MRSA infection, but previous and ongoing studies typically have defined chronic infection as having at least three MRSA-positive cultures within the previous 6 to 12 months (32, 47, 48). The most straightforward decision for treatment occurs in those patients in whom MRSA is cultured from the respiratory tract and who are also experiencing an acute pulmonary exacerbation. Ninety-eight percent of CF providers in the United States who responded to a survey regarding MRSA treatment stated they would give oral or intravenous antibiotics in this situation (36). The advantages and disadvantages of various treatment regimens are detailed in the above paragraphs.
Many CF providers have wondered if there is a role for eradication of respiratory tract MRSA infection. Arguments for recommending and withholding systemic therapy can be made for eradication of a new MRSA infection. Previous studies have suggested that one-third of new MRSA infections may subsequently clear, suggesting that the risks of treatment may not outweigh the benefits (32). However, the easiest time to eradicate MRSA is most likely when it is first cultured, before it becomes entrenched in the lung. Unfortunately, at the time of first culture, it is not possible to determine which patients will clear spontaneously and which will progress to chronic MRSA infection. For these reasons, one approach to a new MRSA infection is to perform an eradication attempt with oral antibiotics. Because MRSA is often found outside of the respiratory tract, an eradication attempt may also include treatment with nasal mupirocin and chlorhexidine or bleach baths. Interestingly, in contrast to P. aeruginosa, there is some evidence that chronic MRSA may be eradicated from the respiratory tract (41). Given that oral antibiotics alone may not be enough to eradicate chronic MRSA, the addition of inhaled antibiotics targeting MRSA also may be considered.
The most difficult-to-treat patients with MRSA are those who are chronically infected and who do not have enough symptoms to trigger the administration of intravenous antibiotics but who have persistent respiratory symptoms. These patients may respond temporarily to repeated courses of oral antibiotics, but eventually this treatment may become associated with decreased efficacy, resistance, and/or side effects. One suggested approach is to administer 250 mg of the intravenous formulation of vancomycin reconstituted in 5 ml of sterile water via nebulization mist treatment (48). The patient inhales the medication twice daily for 28 days. Albuterol is often inhaled before the administration of the antibiotic, although a pilot study did not demonstrate that bronchospasm was a significant issue (46). At the conclusion of the 28-day treatment period, a repeat culture is obtained to determine if MRSA can still be detected in the respiratory tract. If the patient becomes symptomatic when not taking inhaled vancomycin and has not eradicated MRSA, then suppressive treatment with either every other month or continuous inhaled vancomycin may be given. Again, these are potential options for patients with new or chronic MRSA infection while we are awaiting the results of ongoing clinical trials that will further inform treatment decisions (47–49).
Gram-Negative Bacteria
As individuals with CF age, their airways become more frequently infected with gram-negative bacteria. In the United States, the overall prevalence of pulmonary infection with multidrug-resistant P. aeruginosa, S. maltophilia, and B. cepacia complex in patients with CF is 9, 14, and 3%, respectively (29). Infection with these gram-negative organisms is associated with poorer clinical outcomes, such as rapid lung function decline, increased risk of pulmonary exacerbation, and greater rates of mortality or need for lung transplantation (50–55).
P. aeruginosa, B. cepacia complex, and S. maltophilia are found in the environment and have consequently developed ways of surviving in harsh milieus with exposure to naturally occurring antimicrobials. Treatment is thus difficult due to their impressive array of antimicrobial resistance mechanisms, which include efflux pumps, chromosomally encoded β-lactamases, decreased outer membrane permeability, and biofilm formation (56). Given these numerous mechanisms of antimicrobial resistance, these bacteria are deemed resistant to drugs such as aminoglycosides, β-lactams, and fluoroquinolones by in vitro testing according to Clinical Laboratory Standards Institute guidelines (57). However, aerosolized antibiotics can yield higher sputum concentrations, in areas of the lung that remain well ventilated, through direct delivery to the site of infection (Table 1) (58–65). There is a relationship between the maximal drug concentration achieved and the minimum inhibitory concentration (MIC) required to inhibit bacterial growth, with higher ratios associated with greater reduction in bacterial density (66). Therefore, newer inhaled antibiotics, herein discussed, have the potential to be used as chronic suppressive treatment for pathogens traditionally considered resistant to these agents.
Table 1.
Serum and sputum antibiotic concentrations
| Drug | Mean Peak Serum Concentrations (μg/ml) | Mean Peak Sputum Concentrations (μg/g) |
|---|---|---|
| Tobramycin | ||
| Intravenous, 8 mg/kg/d (range) | 29.4 (23.1–35.5) | 3.88 (1.8–5.7) |
| Aerosolized | ||
| Solution, 300 mg, ±SD | 1.04 ± 0.58 | 737 ± 1,028 |
| Powder, 112 mg, ±SD | 1.02 ± 0.53 | 1,048 ± 1,080 |
| Amikacin | ||
| Intravenous, 35 mg/kg, ±SD | 121.4 ± 37.3 | 10.95 ± 7.55 |
| Aerosolized, 560 mg, ±SD | 1.29 ± 0.77 | 2,286 (11.6–11,220) |
| Levofloxacin | ||
| Oral, 500 mg | 6.5 | 5.1 |
| Aerosolized, 240 mg, ±SD | 1.71 ± 0.62 | 4,691 ± 4,516 |
| Aztreonam | ||
| Intravenous, 2 g | 80.1 | 5.2 |
| Aerosolized, 75 mg, range | 0.622 (0.31–1.7) | 537 (0.2–3,010) |
| Colistimethate (colistin) | ||
| Intravenous, 7 mg/kg/d, ±SD | 23 ± 6 | N/A |
| Aerosolized, 2 million units, ±SEM | 0.178 ± 0.018 | 40 ± 5 |
One of the new inhalational antibiotics available is tobramycin inhalation powder (TIP) delivered by the podhaler device. TIP has been shown to result in comparable increases in FEV1 and decreases in hospitalization as tobramycin inhalation solution (TIS) in the treatment of chronic P. aeruginosa in patients with CF (67). However, TIP can achieve up to 1.5- to 2-fold higher sputum tobramycin concentrations (up to 2,000 μg/g) than TIS. In vitro studies of 180 B. cepacia complex and 103 S. maltophilia isolates demonstrated a minimum inhibitory concentration at which 50% of isolates were susceptible (MIC50) of 100 μg/ml, tested by planktonic and biofilm growth (68). This suggests that a maximum serum concentration/MIC ratio of up to 20 may be achievable with TIP treatment of these pathogens. Clinical trials of TIP in patients with CF with B. cepacia complex and S. maltophilia infection to decrease sputum bacterial density are planned.
Inhaled aztreonam solution is another aerosolized antimicrobial for the treatment of chronic P. aeruginosa in CF. Noninferiority studies have shown that it is comparable, if not superior, to TIS in non–treatment-naive individuals with respect to increases in lung function (69). When used in trials for patients with CF with chronic B. cepacia complex infection, however, inhaled aztreonam did not result in any statistically significant improvement in FEV1 or decreases in sputum bacterial density compared with placebo (70). The ability of β-lactam antibiotics to function in the CF lung could be limited by the slow, anaerobic biofilm growth of organisms (71). In vitro studies of biofilm growth of P. aeruginosa on CF airway cells have demonstrated little additional benefit of aztreonam in combination with tobramycin, likely due to bacterial exopolysaccharide production causing tolerance to aztreonam (72). In addition, in an ongoing clinical trial of biofilm susceptibility testing of more than 1,000 clinical P. aeruginosa CF isolates, the percentage of β-lactam–susceptible isolates was reduced when grown as a biofilm compared with planktonically. These data suggest that, despite the known limitations of antimicrobial susceptibility testing in CF, this class of antimicrobials may be less effective in this context (73).
Finally, studies of aerosolized levofloxacin have demonstrated improvements in lung function (8.7% increase in FEV1 vs. placebo) and decreases in bacterial pulmonary burden (0.96 log difference in density vs. placebo) in P. aeruginosa–infected patients with CF (74). Levofloxacin is a second-generation fluoroquinolone that in addition to having anti–P. aeruginosa effects has activity against S. maltophilia (56). In an in vitro study of a large number of clinical S. maltophilia CF isolates, levofloxacin, at levels achievable by inhalation, was the most active antibiotic alone and in combination against S. maltophilia grown as a biofilm or planktonically (75). In addition to achieving high levels of drug in the lung (4,000 μg/g), levofloxacin also has antiinflammatory effects (58, 76). Inhaled levofloxacin may thus be an effective chronic suppressive antimicrobial therapy in patients with CF with chronic S. maltophilia infection and warrants further investigation as it may have usefulness beyond the treatment of P. aeruginosa infection.
The treatment of multidrug-resistant gram-negative bacteria in patients with CF with advanced lung disease is challenging given the intrinsic resistance of these organisms to antimicrobials of several different classes. Engaging a microbiologist and/or infectious disease expert in a discussion about potential therapeutic options for these patients may thus be fruitful.
Treating Multiple Infections
The recognition that there is a diverse microbiota in sputum samples from people with CF raises questions about how we approach antibiotic therapy. Conventional bacterial culture in aerobic conditions allows isolation of a limited number of organisms. Extended culture methods identify a much wider range of bacteria, which include more difficult to culture bacteria, such as anaerobic bacteria (4, 15, 23). At present, there is no readily available methodology to identify all of these organisms in a way that makes this information valuable for clinical treatment (77). Studies are under way to develop technologies to allow molecular identification without prior culture (78). The choice of antibiotics for pulmonary exacerbations associated with multiple bacteria is an area that has not been extensively studied. A number of different oral and intravenous antibiotics may be combined to tailor antibiotic therapy as best possible to particular combinations of positive bacterial culture results. Antimicrobial susceptibility testing with single agents or synergy protocols for P. aeruginosa and B. cepacia complex organisms are not helpful as they do not predict response to treatment (79–81). However, treatment with antibiotics for a pulmonary exacerbation to which the main bacterial species is resistant is associated with treatment failure (82). These studies have been observational, and there are few randomized controlled trials to help in choosing antibiotics for pulmonary exacerbations. The choice is largely empirical and based on the experience of the physician, patient, and previous occurrence of drug allergy. In addition, there are no data to suggest that this also applies to other bacteria cultured in CF sputum. The dosages and side effects of common antibiotics used to treat MRSA and gram-negative bacteria are provided in Table 2.
Table 2.
Empiric antibiotic therapy for the treatment of difficult pulmonary bacterial infections in cystic fibrosis
| Organism | Antibiotic | Pediatric Dose | Adult Dose | Side Effects |
|---|---|---|---|---|
| Gram-positive organisms | ||||
| Methicillin-resistant Staphylococcus aureus | Vancomycin | 15 mg/kg Intravenously every 6 h | 1 g intravenously every 12 h | Oto/nephrotoxicity, red man syndrome |
| OR Linezolid | If < 11 yr: 10 mg/kg intravenously or orally every 8 h | 600 mg intravenously or orally every 12 h | Optic/peripheral neuropathy, myelosuppression | |
| If > 11 yr: 10 mg/kg intravenously or orally every 12 h | ||||
| Gram-negative organisms | ||||
| Pseudomonas aeruginosa | Tobramycin* | 10 mg/kg intravenously every 24 h | 10 mg/kg intravenously every 24 h | Ototoxicity, nephrotoxicity |
| OR Amikacin† | 30 mg/kg intravenously every 24 h | 30 mg/kg intravenously every 24 h | ||
| OR Colistin (colistimethate sodium) | 8 mg/kg/d intravenously divided every 8 h | 8 mg/kg/d intravenously divided every 8 h (max 480 mg/d) | Nephrotoxicity, neurotoxicity | |
| PLUS (choose one): Ticarcillin/clavulanate | 100 mg/kg of ticarcillin component intravenously every 6 h | 3 g of ticarcillin component intravenously every 6 h | GI, rash, hepatitis, neutropenia | |
| Ceftazidime | 50 mg/kg intravenously every 6 h‡ | 2 g intravenously every 8 h‡ | GI, rash | |
| Meropenem | 40 mg/kg intravenously every 8 h | 2 g intravenously every 8 h | GI, rash, hepatitis, neutropenia | |
| Ciprofloxacin | 15 mg/kg intravenously or 20 mg/kg orally every 12 h | 400 mg intravenously or 750 mg orally every 12 h | GI, rare seizure, tendinopathy | |
| Burkholderia cepacia complex | Meropenem | 40 mg/kg intravenously every 8 h | 2 g intravenously every 8 h | GI, rash, hepatitis, neutropenia |
| PLUS (choose 1): Ceftazidime | 50 mg/kg intravenously every 6 h | 2 g intravenously every 8 h | GI, rash | |
| Chloramphenicol§ | 15-20 mg/kg intravenously every 6 h | 1 g intravenously every 6 h | Bone marrow suppression/failure | |
| Trimethoprim/sulfamethoxazole | 4–5 mg/kg (max 240 mg) of trimethoprim component intravenously or orally every 12 h | 4–5 mg/kg (max 240 mg) of trimethoprim component intravenously or orally every 12 h | GI, hypersensitivity neutropenia, serum sickness | |
| Aztreonam | 50 mg/kg intravenously every 8 h | 2 g intravenously every 8 h | GI, rash | |
| Stenotrophomonas maltophilia | Trimethoprim/sulfamethoxazole | 4–5 mg/kg (max 240 mg) of trimethoprim component intravenously or orally every 12 h | 4–5 mg/kg (max 240 mg) of trimethoprim component intravenously or orally every 12 h | GI, hypersensitivity neutropenia, serum sickness |
| PLUS (choose 1): Ticarcillin/clavulanate | 100 mg/kg of ticarcillin component intravenously every 6 h | 3 g of ticarcillin component intravenously every 6 h | GI, rash, hepatitis, neutropenia | |
| Levofloxacin | If < 5 yr: 10 mg/kg intravenously or orally every 12 h | 500–750 mg intravenously or orally once daily | GI, rarely seizure, tendinopathy | |
| If > 5 yr: 10 mg/kg intravenously or orally once daily | ||||
| Doxycycline|| | 2 mg/kg intravenously or orally every 12 h | 100 mg intravenously or orally every 12 h | GI, photosensitivity | |
| Tigecycline | 1.2 mg/kg intravenously every 12 h | 50 mg intravenously every 12 h | GI, cholestasis | |
| Achromobacter species | Meropenem | 40 mg/kg intravenously every 8 h | 2 g intravenously every 8 h | GI, rash, hepatitis |
| OR Imipenem | 15–25 mg/kg intravenously every 6 h | 500 mg–1 g intravenously every 6 h | GI, rarely seizures | |
| PLUS (choose 1): Trimethoprim/sulfamethoxazole | 4–5 mg/kg (max 240 mg) of trimethoprim component intravenously or orally every 12 h | 4–5 mg/kg (max 240 mg) of trimethoprim component intravenously or orally every 12 h | GI, hypersensitivity neutropenia, serum sickness | |
| Ciprofloxacin | 15 mg/kg intravenously or 20 mg/kg orally every 12 h | 400 mg intravenously or 750 mg orally every 12 h | GI, rarely seizure, tendinopathy | |
| Minocycline|| | 2 mg/kg intravenously or orally every 12 h | 100 mg orally every 12 h | GI, photosensitivity | |
Definition of abbreviations: Cmax = maximum serum concentration; Cmin = minimum serum concentration; GI = gastrointestinal.
The antibiotic doses given in this table come from a compilation of sources and practice patterns including commonly prescribed off-label doses and uses. Sources include the pharmacy formulary of The Hospital for Sick Children in Toronto, Ontario, which is based on product inserts and the published literature. The doses given are general guidelines and may vary somewhat between institutions. It is recommended that the clinician consult his/her institution's pharmacy, product inserts, and published literature before prescribing these drugs.
Serum concentrations should be monitored and aim for a Cmax in the range of 20–40 mg/L with a Cmin of <1 mg/L.
Serum concentrations should be monitored and aim for a Cmax in the range of 80–120 mg/L with a Cmin of <1 mg/L.
Continuous infusion of ceftazidime may be considered in cases of clinical failure or for the treatment of multidrug-resistant Pseudomonas aeruginosa to maximize the time above the minimum inhibitory concentration.
Serum concentrations should be monitored; the peak concentration ranges from 15–25 μg/ml and the trough from 5–15 μg/ml.
Should not be given to children < 8 yr of age.
Combinations of organisms that are commonly encountered with P. aeruginosa are S. aureus, Haemophilus influenzae, S. maltophilia, B. cepacia complex, and Achromobacter spp. Other combinations can occur, and studies describing the airway microbiome indicate that coinfection is common and often complex. Two or more organisms may be cultured in approximately 25% of sputum samples. Table 3 and 4 indicate the susceptibility of these bacteria to antibiotics used to treat P. aeruginosa. These susceptibilities are a guide to consider combinations of intravenous and oral antibiotics for pulmonary exacerbations where multiple bacteria are present to maximize the appropriateness of antibiotic choice against the organisms isolated. As molecular diagnostics for a wider range of bacteria in the CF airway microbiome become available, clinical trials will be needed to better inform the choice of antibiotics for long-term bacterial suppression and treatment (6).
Table 3.
Typical susceptibilities to commonly used antipseudomonal antibiotics of bacteria frequently cultured from the cystic fibrosis airway
| Bacteria | Antibiotic |
|||||
|---|---|---|---|---|---|---|
| CAZ | PIP/TAZ | MER | AZT | TOB | COL | |
| Stenotrophomonas maltophilia | +/− | — | — | — | — | +/− |
| Achromobacter spp. | +/− | ✓ | +/− | — | — | ✓ |
| BCC | +/− | +/− | +/− | — | — | — |
| MSSA | +/− | ✓ | ✓ | — | ✓ | — |
| MRSA | — | — | ✓ | — | — | — |
| Streptococci | +/− | ✓ | ✓ | — | — | — |
| Haemophilus influenzae | ✓ | ✓ | ✓ | ✓ | ✓ | — |
| Pseudomonas aeruginosa | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Anaerobes | — | ✓ | ✓ | — | — | — |
Definition of abbreviations: AZT = aztreonam; BCC = Burkholderia cepacia complex; CAZ = ceftazidime; COL = colistimethate; MER = meropenem; MRSA = methicillin-resistant Staphylococcus aureus; MSSA = methicillin-sensitive Staphylococcus aureus; PIP/TAZ = piperacillin-tazobactam; TOB = tobramycin.
✓ Indicates in vitro susceptibility; +/− indicates borderline susceptibility; — indicates resistant or resistance not known.
Table 4.
Typical susceptibilities to less commonly used antipseudomonal antibiotics of bacteria frequently cultured from the cystic fibrosis airway
| Bacteria | Antibiotic |
||||
|---|---|---|---|---|---|
| CO-T | DOX | CHL | FOS | TIG | |
| Stenotrophomonas maltophilia | ✓ | +/− | ✓ | — | ✓ |
| Achromobacter spp. | — | — | +/− | — | — |
| BCC | +/− | +/− | +/− | +/− | +/− |
| MSSA | ✓ | +/− | +/− | ✓ | ✓ |
| MRSA | ✓ | +/− | — | ✓ | ✓ |
| Streptococci | ✓ | +/− | ✓ | — | ✓ |
| Haemophilus influenzae | +/− | +/− | ✓ | — | ✓ |
| Pseudomonas. aeruginosa | — | — | — | +/− | — |
| Anaerobes | — | +/− | ✓ | — | ✓ |
Definition of abbreviations: BCC = Burkholderia cepacia complex; CO-T = cotrimoxazole; CHL = chloramphenicol; DOX = doxycycline; FOS = fosfomycin; MRSA = methicillin-resistant Staphylococcus aureus; MSSA = methicillin-sensitive Staphylococcus aureus; TIG = tigecycline.
✓ Indicates in vitro susceptibility; +/− indicates borderline susceptibility; — indicates resistant or resistance not known.
Summary
MRSA and P. aeruginosa are two of the most prevalent bacteria isolated from CF sputum from patients in the United States (29). In addition, several gram-negative bacteria, which rapidly become resistant to multiple antibiotics, have been described over the last several years. These bacteria are often difficult to treat and have garnered much attention from clinicians, investigators, and pharmaceutical companies with respect to the development of drugs for the treatment of acute exacerbations and chronic suppression. Antibiotics have been the cornerstone of CF care for decades (11). However, the frequent use of antibiotics likely alters the host’s microbiota with yet poorly defined consequences. Because of this selective pressure and with the advent of new laboratory isolation techniques, many previously unrecognized microorganisms are being identified from CF lung secretions. The pathogenic significance of many of these microorganisms is still unknown. This information is important to the CF clinician and the patient with CF, as we need to understand which organisms to treat, whereas treatment of other organisms may actually be detrimental by enabling pathogenic bacteria to expand. As antimicrobials will likely remain a cornerstone of CF therapy far into the future, research into CF lung microbiology must continue until that time when the disease is cured and its associated airway infection is eradicated.
Acknowledgments
Acknowledgment
The authors thank Jay Hilliard for his help with editing and refining the figures and tables.
Footnotes
Supported by the National Institutes of Health grant P30-DK27651 and the U.S. Cystic Fibrosis Foundation.
Author Contributions: J.F.C. organized the manuscript, wrote the introduction and summary, and edited the manuscript. E.C.D. was the lead author for the section on MRSA and organized the references. J.J.L. was the lead author for the section on the Lung Microbiome in CF and created Figure 1. V.J.W. was the lead author for the section on Gram-Negative Bacteria and created Tables 1 and 2. J.S.E. was the lead author for the section on Treating Multiple Infections and created Tables 3 and 4. T.R.A., S.H.C., and S.C.R. reviewed, edited, and revised the manuscript. F.A.R. wrote the abstract, co-wrote the introduction and summary, and served as the deciding editor of the manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Chmiel JF, Berger M, Konstan MW. The role of inflammation in the pathophysiology of CF lung disease. Clin Rev Allergy Immunol. 2002;23:5–27. doi: 10.1385/CRIAI:23:1:005. [DOI] [PubMed] [Google Scholar]
- 2.Szaff M, Høiby N, Flensborg EW. Frequent antibiotic therapy improves survival of cystic fibrosis patients with chronic Pseudomonas aeruginosa infection. Acta Paediatr Scand. 1983;72:651–657. doi: 10.1111/j.1651-2227.1983.tb09789.x. [DOI] [PubMed] [Google Scholar]
- 3.Chmiel JF, Davis PB. State of the art: why do the lungs of patients with cystic fibrosis become infected and why can’t they clear the infection? Respir Res. 2003;4:8–21. doi: 10.1186/1465-9921-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tunney MM, Field TR, Moriarty TF, Patrick S, Doering G, Muhlebach MS, Wolfgang MC, Boucher R, Gilpin DF, McDowell A, et al. Detection of anaerobic bacteria in high numbers in sputum from patients with cystic fibrosis. Am J Respir Crit Care Med. 2008;177:995–1001. doi: 10.1164/rccm.200708-1151OC. [DOI] [PubMed] [Google Scholar]
- 5.Tunney MM, Klem ER, Fodor AA, Gilpin DF, Moriarty TF, McGrath SJ, Muhlebach MS, Boucher RC, Cardwell C, Doering G, et al. Use of culture and molecular analysis to determine the effect of antibiotic treatment on microbial community diversity and abundance during exacerbation in patients with cystic fibrosis. Thorax. 2011;66:579–584. doi: 10.1136/thx.2010.137281. [DOI] [PubMed] [Google Scholar]
- 6.LiPuma J. The new microbiology of cystic fibrosis: it takes a community. Thorax. 2012;67:851–852. doi: 10.1136/thoraxjnl-2012-202018. [DOI] [PubMed] [Google Scholar]
- 7.Waters V, Ratjen F. Standard versus biofilm antimicrobial susceptibility testing to guide antibiotic therapy in cystic fibrosis. Cochrane Database Syst Rev. 2012;11:CD009528. doi: 10.1002/14651858.CD009528.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Sanders DB, Bittner RC, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med. 2010;182:627–632. doi: 10.1164/rccm.200909-1421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Döring G, Conway SP, Heijerman HG, Hodson ME, Høiby N, Smyth A, Touw DJ. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: a European consensus. Eur Respir J. 2000;16:749–767. doi: 10.1034/j.1399-3003.2000.16d30.x. [DOI] [PubMed] [Google Scholar]
- 10.Döring G, Flume P, Heijerman H, Elborn JS Consensus Study Group. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros. 2012;11:461–479. doi: 10.1016/j.jcf.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Flume PA, Mogayzel PJ, Jr, Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ, Marshall BC Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180:802–808. doi: 10.1164/rccm.200812-1845PP. [DOI] [PubMed] [Google Scholar]
- 12.Flume PA, O’Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ, Jr, Willey-Courand DB, Bujan J, Finder J, Lester M, Quittell L, et al. Cystic Fibrosis Foundation, Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957–969. doi: 10.1164/rccm.200705-664OC. [DOI] [PubMed] [Google Scholar]
- 13.Mogayzel PJ, Jr, Naureckas ET, Robinson KA, Mueller G, Hadjiliadis D, Hoag JB, Lubsch L, Hazle L, Sabadosa K, Marshall B Pulmonary Clinical Practice Guidelines Committee. Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2013;187:680–689. doi: 10.1164/rccm.201207-1160oe. [DOI] [PubMed] [Google Scholar]
- 14.Cox MJ, Allgaier M, Taylor B, Baek MS, Huang YJ, Daly RA, Karaoz U, Andersen GL, Brown R, Fujimura KE, et al. Airway microbiota and pathogen abundance in age-stratified cystic fibrosis patients. PLoS ONE. 2010;5:e11044. doi: 10.1371/journal.pone.0011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fodor AA, Klem ER, Gilpin DF, Elborn JS, Boucher RC, Tunney MM, Wolfgang MC. The adult cystic fibrosis airway microbiota is stable over time and infection type, and highly resilient to antibiotic treatment of exacerbations. PLoS ONE. 2012;7:e45001. doi: 10.1371/journal.pone.0045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guss AM, Roeselers G, Newton IL, Young CR, Klepac-Ceraj V, Lory S, Cavanaugh CM. Phylogenetic and metabolic diversity of bacteria associated with cystic fibrosis. ISME J. 2011;5:20–29. doi: 10.1038/ismej.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris JK, De Groote MA, Sagel SD, Zemanick ET, Kapsner R, Penvari C, Kaess H, Deterding RR, Accurso FJ, Pace NR. Molecular identification of bacteria in bronchoalveolar lavage fluid from children with cystic fibrosis. Proc Natl Acad Sci USA. 2007;104:20529–20533. doi: 10.1073/pnas.0709804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klepac-Ceraj V, Lemon KP, Martin TR, Allgaier M, Kembel SW, Knapp AA, Lory S, Brodie EL, Lynch SV, Bohannan BJ, et al. Relationship between cystic fibrosis respiratory tract bacterial communities and age, genotype, antibiotics and Pseudomonas aeruginosa. Environ Microbiol. 2010;12:1293–1303. doi: 10.1111/j.1462-2920.2010.02173.x. [DOI] [PubMed] [Google Scholar]
- 19.Rogers GB, Carroll MP, Serisier DJ, Hockey PM, Jones G, Bruce KD. Characterization of bacterial community diversity in cystic fibrosis lung infections by use of 16s ribosomal DNA terminal restriction fragment length polymorphism profiling. J Clin Microbiol. 2004;42:5176–5183. doi: 10.1128/JCM.42.11.5176-5183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sibley CD, Parkins MD, Rabin HR, Duan K, Norgaard JC, Surette MG. A polymicrobial perspective of pulmonary infections exposes an enigmatic pathogen in cystic fibrosis patients. Proc Natl Acad Sci USA. 2008;105:15070–15075. doi: 10.1073/pnas.0804326105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stressmann FA, Rogers GB, van der Gast CJ, Marsh P, Vermeer LS, Carroll MP, Hoffman L, Daniels TW, Patel N, Forbes B, et al. Long-term cultivation-independent microbial diversity analysis demonstrates that bacterial communities infecting the adult cystic fibrosis lung show stability and resilience. Thorax. 2012;67:867–873. doi: 10.1136/thoraxjnl-2011-200932. [DOI] [PubMed] [Google Scholar]
- 22.van der Gast CJ, Walker AW, Stressmann FA, Rogers GB, Scott P, Daniels TW, Carroll MP, Parkhill J, Bruce KD. Partitioning core and satellite taxa from within cystic fibrosis lung bacterial communities. ISME J. 2011;5:780–791. doi: 10.1038/ismej.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao J, Schloss PD, Kalikin LM, Carmody LA, Foster BK, Petrosino JF, Cavalcoli JD, VanDevanter DR, Murray S, Li JZ, et al. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci USA. 2012;109:5809–5814. doi: 10.1073/pnas.1120577109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filkins LM, Hampton TH, Gifford AH, Gross MJ, Hogan DA, Sogin ML, Morrison HG, Paster BJ, O’Toole GA. Prevalence of streptococci and increased polymicrobial diversity associated with cystic fibrosis patient stability. J Bacteriol. 2012;194:4709–4717. doi: 10.1128/JB.00566-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers GB, Carroll MP, Serisier DJ, Hockey PM, Jones G, Kehagia V, Connett GJ, Bruce KD. Use of 16S rRNA gene profiling by terminal restriction fragment length polymorphism analysis to compare bacterial communities in sputum and mouthwash samples from patients with cystic fibrosis. J Clin Microbiol. 2006;44:2601–2604. doi: 10.1128/JCM.02282-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madan JC, Koestler DC, Stanton BA, Davidson L, Moulton LA, Housman ML, Moore JH, Guill MF, Morrison HG, Sogin ML, et al. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposures. MBio. 2012;3:e00251–12. doi: 10.1128/mBio.00251-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goddard AF, Staudinger BJ, Dowd SE, Joshi-Datar A, Wolcott RD, Aitken ML, Fligner CL, Singh PK. Direct sampling of cystic fibrosis lungs indicates that DNA-based analyses of upper-airway specimens can misrepresent lung microbiota. Proc Natl Acad Sci USA. 2012;109:13769–13774. doi: 10.1073/pnas.1107435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carmody LA, Zhao J, Schloss PD, Petrosino JF, Murray S, Young VB, Li JZ, LiPuma JJ. Changes in cystic fibrosis airway microbiota at pulmonary exacerbation. Ann Am Thorac Soc. 2013;10:179–187. doi: 10.1513/AnnalsATS.201211-107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cystic Fibrosis Foundation. Patient registry 2012 annual data report. Bethesda, MD; 2013
- 30.Goss CH, Muhlebach MS. Review: Staphylococcus aureus and MRSA in cystic fibrosis. J Cyst Fibros. 2011;10:298–306. doi: 10.1016/j.jcf.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Dasenbrook EC, Checkley W, Merlo CA, Konstan MW, Lechtzin N, Boyle MP. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA. 2010;303:2386–2392. doi: 10.1001/jama.2010.791. [DOI] [PubMed] [Google Scholar]
- 32.Dasenbrook EC, Merlo CA, Diener-West M, Lechtzin N, Boyle MP. Persistent methicillin-resistant Staphylococcus aureus and rate of FEV1 decline in cystic fibrosis. Am J Respir Crit Care Med. 2008;178:814–821. doi: 10.1164/rccm.200802-327OC. [DOI] [PubMed] [Google Scholar]
- 33.Lo DK, Hurley MN, Muhlebach MS, Smyth AR. Interventions for the eradication of methicillin-resistant Staphylococcus aureus (MRSA) in people with cystic fibrosis. Cochrane Database Syst Rev. 2013;2:CD009650. doi: 10.1002/14651858.CD009650.pub2. [DOI] [PubMed] [Google Scholar]
- 34.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 35.Walkey AJ, O’Donnell MR, Wiener RS. Linezolid vs glycopeptide antibiotics for the treatment of suspected methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a meta-analysis of randomized controlled trials. Chest. 2011;139:1148–1155. doi: 10.1378/chest.10-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zobell JT, Epps K, Young DC, Hatch M, Olson J, Ampofo K, Chin M, Marshall B, Dasenbrook EC. Utilization of antibiotics for methicillin-resistant Staphylococcus aureus infection in cystic fibrosis patients. Pediatr Pulmonol. 2013;48:311. doi: 10.1002/ppul.23132. [DOI] [PubMed] [Google Scholar]
- 37.Santos RP, Prestidge CB, Brown ME, Urbancyzk B, Murphey DK, Salvatore CM, Jafri HS, McCracken GH, Jr, Ahmad N, Sanchez PJ, et al. Pharmacokinetics and pharmacodynamics of linezolid in children with cystic fibrosis. Pediatr Pulmonol. 2009;44:148–154. doi: 10.1002/ppul.20966. [DOI] [PubMed] [Google Scholar]
- 38.Keel RA, Schaeftlein A, Kloft C, Pope JS, Knauft RF, Muhlebach M, Nicolau DP, Kuti JL. Pharmacokinetics of intravenous and oral linezolid in adults with cystic fibrosis. Antimicrob Agents Chemother. 2011;55:3393–3398. doi: 10.1128/AAC.01797-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.U.S. Food and Drug Administration. FDA Drug Safety Communication: Serious CNS reactions possible when linezolid (Zyvox) is given to patients taking certain psychiatric medications. 2011 Jul 26 [accessed 2013 Nov 15]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm265305.htm
- 40.Endimiani A, Blackford M, Dasenbrook EC, Reed MD, Bajaksouszian S, Hujer AM, Rudin SD, Hujer KM, Perreten V, Rice LB, et al. Emergence of linezolid-resistant Staphylococcus aureus after prolonged treatment of cystic fibrosis patients in Cleveland, Ohio. Antimicrob Agents Chemother. 2011;55:1684–1692. doi: 10.1128/AAC.01308-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doe SJ, McSorley A, Isalska B, Kearns AM, Bright-Thomas R, Brennan AL, Webb AK, Jones AM. Patient segregation and aggressive antibiotic eradication therapy can control methicillin-resistant Staphylococcus aureus at large cystic fibrosis centres. J Cyst Fibros. 2010;9:104–109. doi: 10.1016/j.jcf.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Rifampin [package insert]. Cincinnati, OH: Pantheon Pharmaceuticals Inc; 2004
- 43.Di Sant’Agnese PE, Andersen DH. Celiac syndrome: chemotherapy in infections of the respiratory tract associated with cystic fibrosis of the pancreas: observations with penicillin and drugs of the sulfonamide group, with special reference to penicillin aerosol. Am J Dis Child. 1946;72:17–61. [PubMed] [Google Scholar]
- 44.Trapnell BC, McColley SA, Kissner DG, Rolfe MW, Rosen JM, McKevitt M, Moorehead L, Montgomery AB, Geller DE Phase 2 FTI Study Group. Fosfomycin/tobramycin for inhalation in patients with cystic fibrosis with pseudomonas airway infection. Am J Respir Crit Care Med. 2012;185:171–178. doi: 10.1164/rccm.201105-0924OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Generali J, Cada D. Off-label drug uses: vancomycin: aerolsolizaton. Hosp Pharm. 2004;39:638–647. [Google Scholar]
- 46.Jennings MJ, Boyle MP, Bucur C, Konstan MW, Dasenbrook EC. Pharmacokinetics and safety of inhaled vancomycin in patients with cystic fibrosis. Pediatr Pulmonol. 2012;47:320. [Google Scholar]
- 47.Savara, IncEfficacy and safety study of AeroVanc for the treatment of persistent MRSA lung infection in cystic fibrosis patients. Clinicaltrials.Gov. NLM identifier: NCT01746095. Bethesda, MD: National Library of Medicine (US). 2012[accessed 2014 Jan 20]. Available from: http://clinicaltrials.Gov/ct2/show/nct01746095
- 48.Boyle MP.Persistent methicillin resistant Staphylococcus aureus eradication protocol (PMEP). Clinicaltrials.Gov. NLM identifier: NCT01594827. Bethesda, MD: National Library of Medicine (US). 2012[accessed 2014 Jan 20]. Available from: http://clinicaltrials.Gov/ct2/show/nct01594827
- 49.Muhlebach M.Early methicillin-resistant Staphylococcus aureus (MRSA) therapy in cystic fibrosis (CF) (STAR-Too). Clinicaltrials.Gov. NLM identifier: NCT01349192. Bethesda, MD: National Library of Medicine (US). 2011 [accessed 2014 Jan 20]. Available from: http://clinicaltrials.Gov/ct2/show/nct01349192
- 50.Al-Aloul M, Crawley J, Winstanley C, Hart CA, Ledson MJ, Walshaw MJ. Increased morbidity associated with chronic infection by an epidemic Pseudomonas aeruginosa strain in CF patients. Thorax. 2004;59:334–336. doi: 10.1136/thx.2003.014258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alexander BD, Petzold EW, Reller LB, Palmer SM, Davis RD, Woods CW, Lipuma JJ. Survival after lung transplantation of cystic fibrosis patients infected with Burkholderia cepacia complex. Am J Transplant. 2008;8:1025–1030. doi: 10.1111/j.1600-6143.2008.02186.x. [DOI] [PubMed] [Google Scholar]
- 52.Corey M, Farewell V. Determinants of mortality from cystic fibrosis in Canada, 1970-1989. Am J Epidemiol. 1996;143:1007–1017. doi: 10.1093/oxfordjournals.aje.a008664. [DOI] [PubMed] [Google Scholar]
- 53.Tablan OC, Chorba TL, Schidlow DV, White JW, Hardy KA, Gilligan PH, Morgan WM, Carson LA, Martone WJ, Jason JM, et al. Pseudomonas cepacia colonization in patients with cystic fibrosis: risk factors and clinical outcome. J Pediatr. 1985;107:382–387. doi: 10.1016/s0022-3476(85)80511-4. [DOI] [PubMed] [Google Scholar]
- 54.Waters V, Atenafu EG, Lu A, Yau Y, Tullis E, Ratjen F. Chronic Stenotrophomonas maltophilia infection and mortality or lung transplantation in cystic fibrosis patients. J Cyst Fibros. 2013;12:482–486. doi: 10.1016/j.jcf.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 55.Waters V, Yau Y, Prasad S, Lu A, Atenafu E, Crandall I, Tom S, Tullis E, Ratjen F. Stenotrophomonas maltophilia in cystic fibrosis: serologic response and effect on lung disease. Am J Respir Crit Care Med. 2011;183:635–640. doi: 10.1164/rccm.201009-1392OC. [DOI] [PubMed] [Google Scholar]
- 56.Waters V. New treatments for emerging cystic fibrosis pathogens other than Pseudomonas. Curr Pharm Des. 2012;18:696–725. doi: 10.2174/138161212799315939. [DOI] [PubMed] [Google Scholar]
- 57.Clinical Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Wayne, PA: CLSI; 2012 [Google Scholar]
- 58.Geller DE, Flume PA, Griffith DC, Morgan E, White D, Loutit JS, Dudley MN. Pharmacokinetics and safety of MP-376 (levofloxacin inhalation solution) in cystic fibrosis subjects. Antimicrob Agents Chemother. 2011;55:2636–2640. doi: 10.1128/AAC.01744-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Geller DE, Konstan MW, Smith J, Noonberg SB, Conrad C. Novel tobramycin inhalation powder in cystic fibrosis subjects: pharmacokinetics and safety. Pediatr Pulmonol. 2007;42:307–313. doi: 10.1002/ppul.20594. [DOI] [PubMed] [Google Scholar]
- 60.McCoy KS, Quittner AL, Oermann CM, Gibson RL, Retsch-Bogart GZ, Montgomery AB. Inhaled aztreonam lysine for chronic airway Pseudomonas aeruginosa in cystic fibrosis. Am J Respir Crit Care Med. 2008;178:921–928. doi: 10.1164/rccm.200712-1804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moriarty TF, McElnay JC, Elborn JS, Tunney MM. Sputum antibiotic concentrations: implications for treatment of cystic fibrosis lung infection. Pediatr Pulmonol. 2007;42:1008–1017. doi: 10.1002/ppul.20671. [DOI] [PubMed] [Google Scholar]
- 62.Ratjen F, Rietschel E, Kasel D, Schwiertz R, Starke K, Beier H, van Koningsbruggen S, Grasemann H. Pharmacokinetics of inhaled colistin in patients with cystic fibrosis. J Antimicrob Chemother. 2006;57:306–311. doi: 10.1093/jac/dki461. [DOI] [PubMed] [Google Scholar]
- 63.Reed MD, Stern RC, O’Riordan MA, Blumer JL. The pharmacokinetics of colistin in patients with cystic fibrosis. J Clin Pharmacol. 2001;41:645–654. doi: 10.1177/00912700122010537. [DOI] [PubMed] [Google Scholar]
- 64.Clancy JP, Dupont L, Konstan MW, Billings J, Fustik S, Goss CH, Lymp J, Minic P, Quittner AL, Rubenstein RC, et al. Arikace Study Group. Phase II studies of nebulised Arikace in CF patients with Pseudomonas aeruginosa infection. Thorax. 2013;68:818–825. doi: 10.1136/thoraxjnl-2012-202230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Canis F, Husson MO, Turck D, Vic P, Launay V, Ategbo S, Vincent A, Courcol RJ. Pharmacokinetics and bronchial diffusion of single daily dose amikacin in cystic fibrosis patients. J Antimicrob Chemother. 1997;39:431–433. doi: 10.1093/jac/39.3.431. [DOI] [PubMed] [Google Scholar]
- 66.LiPuma JJ. Microbiological and immunologic considerations with aerosolized drug delivery. Chest. 2001;120:118S–123S. doi: 10.1378/chest.120.3_suppl.118s. [DOI] [PubMed] [Google Scholar]
- 67.Konstan MW, Flume PA, Kappler M, Chiron R, Higgins M, Brockhaus F, Zhang J, Angyalosi G, He E, Geller DE. Safety, efficacy and convenience of tobramycin inhalation powder in cystic fibrosis patients: The EAGER trial. J Cyst Fibros. 2011;10:54–61. doi: 10.1016/j.jcf.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ratjen A, Yau Y, Wettlaufer J, Matukas L, Ratjen F, Zlosnik J, Speert DP, Tullis E, Waters V. Tobramycin susceptibilities of Burkholderia cepacia complex isolates from pediatric and adult cystic fibrosis patients. Pediatr Pulmonol. 2013:31837. [Google Scholar]
- 69.Assael BM, Pressler T, Bilton D, Fayon M, Fischer R, Chiron R, Larosa M, Knoop C, McElvaney N, Lewis SA, et al. For the AZLI Active Comparator Study Group Inhaled aztreonam lysine vs. inhaled tobramycin in cystic fibrosis: a comparative efficacy trial J Cyst Fibros(In press) [DOI] [PubMed] [Google Scholar]
- 70.Tullis DE, Burns JL, Retsch-Bogart GZ, Bresnik M, Henig NR, Lewis SA, Lipuma JJ. Inhaled aztreonam for chronic Burkholderia infection in cystic fibrosis: a placebo-controlled trial. J Cyst Fibros. 2014;13:296–305. doi: 10.1016/j.jcf.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 71.Hassett DJ, Cuppoletti J, Trapnell B, Lymar SV, Rowe JJ, Yoon SS, Hilliard GM, Parvatiyar K, Kamani MC, Wozniak DJ, et al. Anaerobic metabolism and quorum sensing by Pseudomonas aeruginosa biofilms in chronically infected cystic fibrosis airways: rethinking antibiotic treatment strategies and drug targets. Adv Drug Deliv Rev. 2002;54:1425–1443. doi: 10.1016/s0169-409x(02)00152-7. [DOI] [PubMed] [Google Scholar]
- 72.Yu Q, Griffin EF, Moreau-Marquis S, Schwartzman JD, Stanton BA, O’Toole GA. In vitro evaluation of tobramycin and aztreonam versus Pseudomonas aeruginosa biofilms on cystic fibrosis-derived human airway epithelial cells. J Antimicrob Chemother. 2012;67:2673–2681. doi: 10.1093/jac/dks296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Waters V, Ratjen F, Tullis E, Corey M, Matukas L, Leahy R, Yau Y. Randomized double blind controlled trial of the use of a biofilm antimicrobial susceptibility assay to guide antibiotic therapy in chronic Pseudomonas aeruginosa infected cystic fibrosis patients. Pediatr Pulmonol. 2010:330. [Google Scholar]
- 74.Geller DE, Flume PA, Staab D, Fischer R, Loutit JS, Conrad DJ Mpex 204 Study Group. Levofloxacin inhalation solution (MP-376) in patients with cystic fibrosis with Pseudomonas aeruginosa. Am J Respir Crit Care Med. 2011;183:1510–1516. doi: 10.1164/rccm.201008-1293OC. [DOI] [PubMed] [Google Scholar]
- 75.Wu K, Yau YC, Matukas L, Waters V. Biofilm compared to conventional antimicrobial susceptibility of Stenotrophomonas maltophilia Isolates from cystic fibrosis patients. Antimicrob Agents Chemother. 2013;57:1546–1548. doi: 10.1128/AAC.02215-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsivkovskii R, Sabet M, Tarazi Z, Griffith DC, Lomovskaya O, Dudley MN. Levofloxacin reduces inflammatory cytokine levels in human bronchial epithelia cells: implications for aerosol MP-376 (levofloxacin solution for inhalation) treatment of chronic pulmonary infections. FEMS Immunol Med Microbiol. 2011;61:141–146. doi: 10.1111/j.1574-695X.2010.00755.x. [DOI] [PubMed] [Google Scholar]
- 77.McCaughey G, Gilpin D, Elborn J, Tunney MM. The future of antimicrobial therapy in the era of antibiotic resistance in cystic fibrosis pulmonary infection. Expert Rev Respir Med. 2013;7:385–396. doi: 10.1586/17476348.2013.814411. [DOI] [PubMed] [Google Scholar]
- 78.Pattison SH, Rogers GB, Crockard M, Elborn JS, Tunney MM. Molecular detection of CF lung pathogens: current status and future potential. J Cyst Fibros. 2013;12:194–205. doi: 10.1016/j.jcf.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Foweraker JE, Govan JR. Antibiotic susceptibility testing in early and chronic respiratory infections with Pseudomonas aeruginosa. J Cyst Fibros. 2013;12:302. doi: 10.1016/j.jcf.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 80.Gillham MI, Sundaram S, Laughton CR, Haworth CS, Bilton D, Foweraker JE. Variable antibiotic susceptibility in populations of Pseudomonas aeruginosa infecting patients with bronchiectasis. J Antimicrob Chemother. 2009;63:728–732. doi: 10.1093/jac/dkp007. [DOI] [PubMed] [Google Scholar]
- 81.Hurley MN, Ariff AH, Bertenshaw C, Bhatt J, Smyth AR. Results of antibiotic susceptibility testing do not influence clinical outcome in children with cystic fibrosis. J Cyst Fibros. 2012;11:288–292. doi: 10.1016/j.jcf.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parkins MD, Rendall JC, Elborn JS. Incidence and risk factors for pulmonary exacerbation treatment failures in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa. Chest. 2012;141:485–493. doi: 10.1378/chest.11-0917. [DOI] [PubMed] [Google Scholar]