In October 2016, the American Joint Committee on Cancer (AJCC; www.cancerstaging.org) published the eighth edition of the AJCC/TNM cancer staging system, which will replace the seventh edition that has been in use by clinicians, cancer registries, and researchers since 2009 (1). Unlike the American Thyroid Association (ATA) risk stratification system that is designed to predict disease recurrence, the AJCC/TNM system is optimized to predict survival in patients with cancer (2,3). While clinicians are encouraged to use the scientific content of the eighth edition staging manual to enhance patient care, the actual implementation date for the eighth edition cancer staging system is planned to be January 1, 2018, in order to allow the cancer care community to make the infrastructure changes needed for data collection and implementation. All newly diagnosed cases through December 31, 2017, will continue to be staged by tumor registries according to the seventh edition staging system (https://cancerstaging.org/About/news/Pages/Implementation-of-AJCC-8th-Edition-Cancer-Staging-System.aspx). This commentary examines how the eighth edition differs from the seventh edition in the staging of differentiated and anaplastic thyroid cancers. Examination of the changes in the staging for medullary thyroid cancer will be presented in a follow-up commentary in the near future.
Using an evidenced-based medicine approach to literature review and grading, a multidisciplinary expert committee identified several specific areas in the seventh edition staging system that needed to be modified in order to optimize initial staging. While it is beyond the scope of this commentary to explore fully the reasons underpinning the changes in the eighth edition, the details and rationale for each of these changes with corresponding literature review is presented in the text of the eighth edition staging system for those interested in the details (1). As will be seen in the discussion below, the net effect of most of the changes in the eighth edition will be to downstage a significant number of patients into lower stages that more accurately reflect their low risk of dying from thyroid cancer. More individualized and accurate assessments of the risk of dying from thyroid cancer and the risk of disease recurrence should have a significant impact on both initial therapeutic decision making (e.g., extent of thyroid surgery, need for radioactive iodine ablation/therapy, and/or need for thyrotropin suppressive therapy) and on follow-up management strategies.
Description of the AJCC/TNM Eighth Edition Staging System
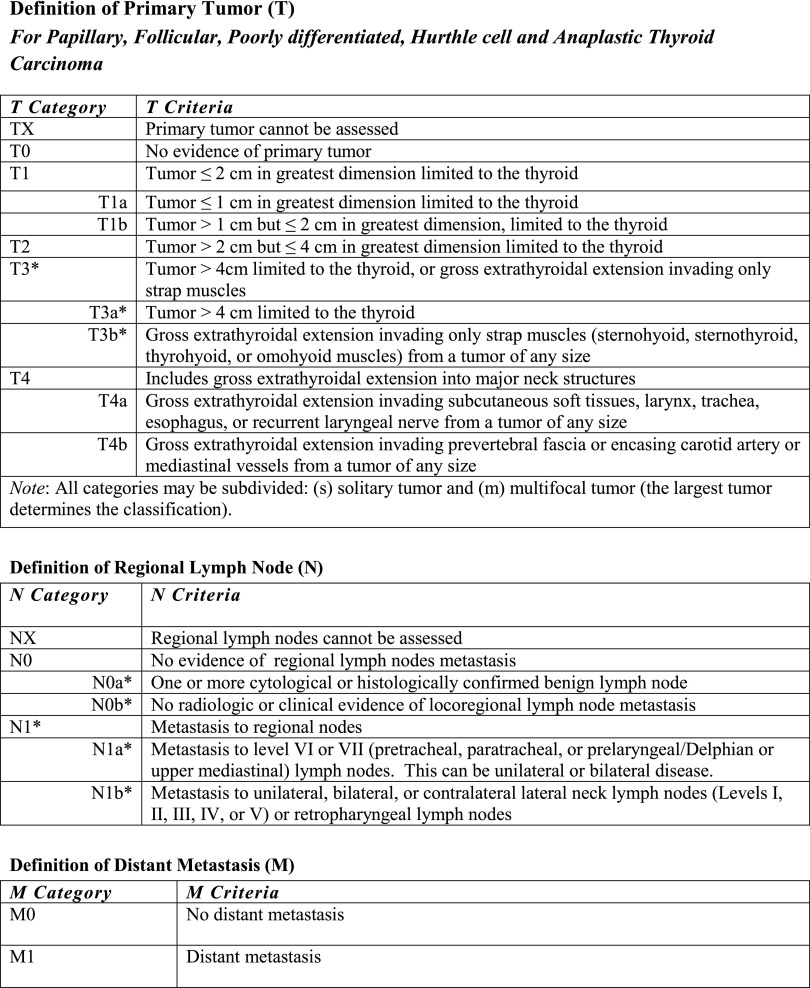
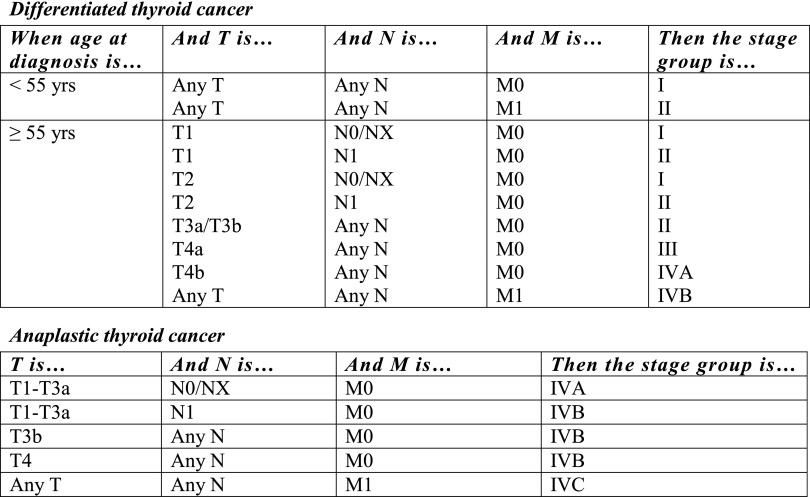
The eighth edition T, N, and M definitions are presented in Figure 1, with the corresponding stages (I, II, III, and IV) presented in Figure 2 (1). Table 1 summarizes the major changes to the AJCC/TNM staging of differentiated and anaplastic thyroid cancers in the eighth edition. While still retaining the basic anatomic pathology T-N-M staging approach, the eighth edition downstages a significant number of patients by (i) raising the age cutoff from 45 to 55 years of age at diagnosis and (ii) removing regional lymph node metastases and microscopic extrathyroidal extension from the definition of T3 disease. The eighth edition also re-emphasizes the critical importance of gross extrathyroidal extension as an unfavorable prognostic factor while minimizing the significance of minor extension through the thyroid capsule, which is identified only on histological examination. The eighth edition makes it clear that gross extrathyroidal extension is a clinical finding based on radiologic and/or clinical evidence of macroscopic tumor extending outside the thyroid gland. Consistent with previous editions, all data that are accumulated preoperatively, intraoperatively, and during the first four months of follow-up after thyroid surgery should be used to define the initial N and M status. The increase in the age cutoff from 45 to 55 years of age at diagnosis downstaged a significant number of patients into stage I without significantly altering the mortality associated with the various stages (4). However, it is recognized that mortality increases progressively with advancing age, beginning at about 35 years of age. Thus, any single cut point for age is likely to perform less well than models that consider age as a continuous variable (such as the MACIS system or nomograms) (5–8).
FIG. 1.
Eighth edition definitions for primary tumor (T), lymph node status (N), and distant metastasis (M). Changes from the seventh edition are marked with an asterisk (see text for descriptions).
FIG. 2.
Eighth edition American Joint Committee on Cancer prognostic stage groups for differentiated thyroid cancer (top panel) and anaplastic thyroid cancer (bottom panel).
Table 1.
Major Changes to the AJCC/TNM Staging of Differentiated and Anaplastic Thyroid Cancers in the Eighth Edition
| Differentiated | 1. | The age cutoff used for staging was increased from 45 to 55 years of age at diagnosis. |
| 2. | Minor extrathyroidal extension detected only on histological examination was removed from the definition of T3 disease and therefore has no impact on either T category or overall stage. | |
| 3. | N1 disease no longer upstages a patient to stage III. If <55 years of age at diagnosis, N1 disease is stage I. If ≥55 years of age, N1 disease is stage II. | |
| 4. | T3a is a new category for tumors >4 cm confined to the thyroid gland. | |
| 5. | T3b is a new category for tumors of any size demonstrating gross extrathyroidal extension into strap muscles (sternohyoid, sternothyroid, thyrohyoid, or omohyoid muscles). | |
| 6. | Level VII lymph nodes, previously classified as lateral neck lymph nodes (N1b), were reclassified as central neck lymph nodes (N1a) to be more anatomically consistent and because level VII presented significant coding difficulties for tumor registrars, clinicians, and researchers. | |
| 7. | In differentiated thyroid cancer, the presence of distant metastases in older patients is classified as stage IVB disease rather than stage IVC disease. Distant metastasis in anaplastic thyroid cancer continues to be classified as stage IVC disease. | |
| Anaplastic | 1. | Unlike previous editions where all anaplastic thyroid cancers were classified as T4 disease, anaplastic cancers will now use the same T definitions as differentiated thyroid cancer. |
| 2. | Intrathyroidal disease is stage IVA, gross extrathyroidal extension or cervical lymph node metastases is stage IVB, and distant metastases are stage IVC. |
AJCC, American Joint Committee on Cancer; TNM, tumor-node-metastasis.
Likewise, by removing lymph node metastases and minor extrathyroidal extension from the definition of T3 disease, a significant number of patients (45–54 years old, N1, M0) will be downstaged to stage I, and older patients will be downstaged to either stage I (≥55 years old, minor extrathyroidal extension, N0, M0) or stage II (≥55 years old, N1, M0). It does appear that the presence of clinically significant lymph node metastases is associated with poorer outcomes in adults of all ages, but the impact on survival in younger patients (<55 years), even though statistically significant, is relatively minor (9) (hence classified as stage I), while the impact on survival in older patients is more clinically significant (hence classification as stage II disease). It is important to note that pathologic confirmation of lymph node status is not required, and patients can be classified as having N0 disease, as long as there is no evidence of lymph node metastasis on routine preoperative and intraoperative evaluations (clinical examination, imaging, and intraoperative findings). As can be seen in Figure 1, the AJCC subclassifies N0 disease as either cytologically/histologically confirmed (N0a) or the absence of radiologic or clinical evidence of disease (N0b). However, in differentiated and anaplastic thyroid cancer, the subtype of N0 disease, location (N1a or N1b), or presence/absence of extranodal extension does not influence AJCC staging.
In addition to the critical factors necessary to stage patients (T, N, M) appropriately, the eighth edition also provides a list of additional clinical factors that would be considered to aid in risk stratification for routine clinical care. These include presence/absence of microscopic extrathyroidal extension, the location of the involved lymph nodes (N1a vs. N1b), the number of involved lymph nodes, the number of lymph nodes sampled, the size of the largest involved lymph node, the size of the largest metastatic focus within a lymph node, the presence/absence of extranodal extension, the presence/absence of vascular invasion, the postoperative serum thyroglobulin, the completeness of surgical resection (R stage), and the specific histological subtypes. Currently, these additional clinical factors are useful in assessing the risk of recurrence and early response to therapy. It is likely that some of these additional clinical features may be incorporated into future editions of the AJCC/TNM staging systems to refine and optimize initial risk stratification further. Even though molecular characterization of tumors has the potential to refine risk estimates, none of the current molecular markers were considered to have sufficient independent prognostic significance to merit inclusion in the eighth edition staging definitions.
Comparison of the AJCC/TNM Seventh and Eighth Edition Staging Systems
A comparison of the seventh and eighth edition staging system definitions and anticipated 10-year disease-specific survival rates are presented in the eighth edition text (1) and are summarized in Table 2. The 10-year disease-specific survival rates presented in Table 2 represent the best estimates based on the published literature (see eighth edition text for detailed description and specific references) (1) but will require further studies involving long-term follow-up in large multicenter data sets for validation and refinement.
Table 2.
Comparison of the AJCC Seventh and Eighth Edition Staging System
| Stage | 7th edition description | 7th edition 10-year DSS | 8th edition description | 8th edition expected 10-year DSS | |
|---|---|---|---|---|---|
| Younger patients | I | <45 years old All patients without distant metastases, regardless of tumor size, lymph node status, or extrathyroidal extension |
97–100% | <55 years old All patients without distant metastases, regardless of tumor size, lymph node status, or extrathyroidal extension |
98–100% |
| II | <45 years old Distant metastases |
95–99% | <55 years old Distant metastases |
85–95% | |
| Older patients | I | ≥45 years old ≤2 cm tumor Confined to the thyroid |
97–100% | ≥55 years old ≤4 cm tumor Confined to the thyroid |
98–100% |
| II | ≥45 years old 2–4 cm tumor Confined to the thyroid |
97–100% | ≥55 years old Tumors >4 cm, or tumors of any size with central or lateral neck lymph nodes, or gross extrathyroidal extension into strap muscles |
85–95% | |
| III | ≥45 years old >4 cm tumor, or minimal extrathyroidal extension, or central neck lymph node metastasis |
88–95% | ≥55 years old Tumors of any size with gross extrathyroidal extension into subcutaneous tissue, larynx, trachea, esophagus, recurrent laryngeal nerve |
60–70% | |
| IV | ≥45 years old Gross extrathyroidal extension, or lateral neck lymph node metastasis, or distant metastasis |
50–75% | ≥55 years old Tumors of any size, or lymph node status with gross extrathyroidal extension into prevertebral fascia, encasing major vessels, or distant metastasis |
<50% |
DSS, disease-specific survival.
For younger patients, the only differences in the definitions of stage I and stage II disease relate to the age cutoff (45 years old in the seventh edition vs. 55 years old in the eighth edition). A recent international multi-institutional validation study of 9484 patients (median follow-up of five years) demonstrated that an increase in the age cutoff from 45 to 55 years of age at diagnosis downstaged 12% of patients and was associated with a 10-year disease-specific survival of 98% in the downstaged group (4). However, the very small number of patients that transitioned from seventh edition stage IV to eighth edition stage II (aged 45–54 years with M1 disease, 29/9484 patients, 0.3% of the entire cohort) demonstrated a 10-year disease-specific survival of 68%, indicating that the increase in age cutoff will move a few higher-risk patients into the eighth edition younger stage II group (4). Nonetheless, since the majority of younger patients with M1 disease will do well, it is anticipated that this small number of higher-risk patients who are moved into the eighth edition stage II disease will have only a small impact on the long-term disease-specific survival for this stage group.
In older patients, there are significant differences in the staging definitions between the seventh and eighth editions. In the eighth edition, all patients with differentiated thyroid cancer ≤4 cm that is confined to the thyroid will be stage I, while the seventh edition had previously classified smaller tumors (≤2 cm) as stage I and larger tumors (2–4 cm) as stage II. Since the disease-specific survival did not differ by tumor size for these intrathyroidal lesions, it was appropriate to combine these tumors into a single stage group (eighth edition stage I).
In the eighth edition, older patients (>55 years old) with metastatic spread to either central or lateral neck lymph nodes or gross extrathyroidal extension involving only the overlying strap muscles will be classified as stage II disease. Since lymph node metastases and gross extrathyroidal extension in these older patients are important prognostic factors, stage II disease is expected to have a 10-year disease-specific survival that is worse than stage I disease (see Table 2).
Stage III in the seventh edition included patients at relatively low risk of dying from thyroid cancer (primarily patients with central neck lymph node metastases and or microscopic extrathyroidal extension), while stage III in the eighth edition is composed of high-risk patients demonstrating gross extrathyroidal extension into major structures in the neck without distant metastases at diagnosis. The eighth edition stage III patients should have outcomes slightly worse than the seventh edition stage IVa disease (T4a disease or N1b disease without distant metastasis) in which 10-year disease-specific survival approximated 75% (10). Therefore, the eighth edition stage III is expected to have a significantly poorer disease-specific survival than the seventh edition stage III category (see Table 2).
Similarly, stage IV in the seventh edition included all patients with gross extrathyroidal extension or distant metastases at diagnosis, but also included all patients with lateral neck lymph node involvement, which, as mentioned above, is not associated with a high risk of early death from thyroid cancer. Conversely, stage IV in the eighth edition excludes patients with just lateral neck lymph node metastases and includes only the patients at highest risk of dying from thyroid cancer (≥55 years old with extensive gross extrathyroidal extension defined as T4b disease or distant metastases at diagnosis). As a result, the eighth edition classifies fewer patients as having stage IV disease, but conveys a much poorer prognosis for this category than would have been predicted using the seventh edition definition that included patients without distant metastases with T4b or N1b disease.
With regard to anaplastic cancer, the major change involves the definition of the T category. In the past, all anaplastic thyroid cancer was classified as T4 disease, with intrathyroidal disease classified as T4a and tumors with gross extrathyroidal extension classified as T4b disease. For uniformity, the anaplastic thyroid cancer T category in the eighth edition will follow the same definitions as those used for differentiated thyroid cancers (see Fig. 1). However, the stage groups remain effectively the same, with intrathyroidal disease classified as stage IVA, while the presence of lymph node metastases or gross extrathyroidal extension mandates stage IVB and distant metastases are classified as stage IVC disease.
Practical Application of the AJCC/TNM Eighth Edition Staging System in Clinical Practice
From a practical implementation standpoint, it is easier to rearrange the eighth edition staging table so that the proper stage can be easily identified based on the most important clinical factors (age, distant metastases, and the presence or absence of gross extrathyroidal extension), as presented in Table 3.
Table 3.
A Clinically Based Approach to Staging in Differentiated Thyroid Cancer Using the Eighth Edition AJCC/TNM Update
| Distant metastasis | Gross ETE present? | Structures involved with gross ETE | T category | N category | Stage | |
|---|---|---|---|---|---|---|
| <55 years | No | Yes or no | Any or none | Any | Any | I |
| Yes | Yes or no | Any or none | Any | Any | II | |
| ≥55 years | No | No | None | ≤4 cm (T1–2) | N0/Nx | I |
| N1a/N1b | II | |||||
| >4 cm (T3a) | N0/Nx/N1a/N1b | II | ||||
| Yes | Only strap muscle (T3b) | Any | Any | II | ||
| Subcutaneous, larynx, trachea, esophagus, recurrent laryngeal nerve (T4a) | Any | Any | III | |||
| Prevertebral fascia, encasing major vessels (T4b) | Any | Any | IVA | |||
| Yes | Yes or no | Any or none | Any | Any | IVB |
The expected 10-year DSS estimates presented in this table are approximate figures based on a review of previously published retrospective studies evaluating the AJCC staging system and/or the prognostic importance of critical clinical features (age, gross extrathyroidal extension, cervical lymph node metastases, and distant metastases).
• In patients <55 years old, all patients are stage I (regardless of tumor size, lymph node status, histological subtype, or the presence/absence of extrathyroidal extension), unless they have distant metastases, in which case they are stage II.
• In patients ≥55 years of age, the presence of distant metastases mandates classification as stage IVB, while older patients without distant metastases are further characterized based on the presence/absence of gross extrathyroidal extension, tumor size, and lymph node status.
• Older patients with tumors ≤4 cm (T1–2) are stage I if confined to the thyroid (N0/Nx) or stage II if lymph node metastases are present (N1a or N1b).
• Older patients with tumors >4 cm confined to the thyroid (T3a) are classified as stage II, regardless of the lymph node status.
• Older patients demonstrating gross extrathyroidal extension are classified as stage II if only the strap muscles are grossly invaded (T3b); stage III if there is gross invasion of the subcutaneous tissue, larynx, trachea, esophagus, or recurrent laryngeal nerve (T4a); and stage IVA if there is gross invasion of the prevertebral fascia or tumor encasing major vessels (T4b).
Conclusions
In summary, the net effect of the changes in the eighth edition staging system for differentiated thyroid cancer will be to classify the vast majority of thyroid cancer patients appropriately as being at low risk for dying from thyroid cancer (stage I or stage II disease). However, it is important to remember that the risk of death from thyroid cancer does not parallel the risk of recurrence in many patients. This is particularly true in younger (<55 years old) patients with stage I disease, as this cohort will include the full spectrum of recurrence risk, ranging from patients at very low risk of recurrence to patients at high risk of recurrence. Therefore, clinical management should be guided both by an assessment of the risk of dying from thyroid cancer and the risk of recurrence. We endorse the management approach described in the recent ATA guidelines in which initial risk estimates (for both risk of recurrence and risk of dying from thyroid cancer) are formulated based on all the information available at diagnosis and are then modified over time as new data becomes available (2). This dynamic risk-assessment approach will further refine the initial risk estimates and identify patients who are doing worse (or better) than would have been predicted by their initial staging. While additional studies are needed to provide further validation of this updated staging system, the eighth edition is a significant step forward in initial risk stratification for patients with differentiated thyroid cancer.
Acknowledgments
This work was funded in part by the NIH/NCI Cancer Center Support Grant P30 CA008748 (Craig Thompson, PI).
Author Disclosure Statement
The authors have nothing to disclose.
References
- 1.Tuttle M, Morris LF, Haugen B, Shah J, Sosa JA, Rohren E, Subramaniam RM, Hunt JL, Perrier ND. 2017. Thyroid-differentiated and anaplastic carcinoma. In: Amin MB, Edge SB, Greene F, Byrd D, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley J, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR, (eds) AJCC Cancer Staging Manual. Eighth edition. Springer International Publishing, New York, New York [Google Scholar]
- 2.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2016. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Momesso DP, Tuttle RM. 2014. Update on differentiated thyroid cancer staging. Endocrinol Metab Clin North Am 43:401–421 [DOI] [PubMed] [Google Scholar]
- 4.Nixon IJ, Wang LY, Migliacci JC, Eskander A, Campbell MJ, Aniss A, Morris L, Vaisman F, Corbo R, Momesso D, Vaisman M, Carvalho A, Learoyd D, Leslie WD, Nason RW, Kuk D, Wreesmann V, Morris L, Palmer FL, Ganly I, Patel SG, Singh B, Tuttle RM, Shaha AR, Gonen M, Pathak KA, Shen WT, Sywak M, Kowalski L, Freeman J, Perrier N, Shah JP. 2016. An international multi-institutional validation of age 55 years as a cutoff for risk stratification in the AJCC/UICC staging system for well-differentiated thyroid cancer. Thyroid 26:373–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganly I, Nixon IJ, Wang LY, Palmer FL, Migliacci JC, Aniss A, Sywak M, Eskander AE, Freeman JL, Campbell MJ, Shen WT, Vaisman F, Momesso D, Corbo R, Vaisman M, Shaha A, Tuttle RM, Shah JP, Patel SG. 2015. Survival from differentiated thyroid cancer: what has age got to do with it? Thyroid 25:1106–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. 1993. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 114:1050–1057; discussion 1057–1058 [PubMed] [Google Scholar]
- 7.Pathak KA, Mazurat A, Lambert P, Klonisch T, Nason RW. 2013. Prognostic nomograms to predict oncological outcome of thyroid cancers. J Clin Endocrinol Metab 98:4768–4775 [DOI] [PubMed] [Google Scholar]
- 8.Adam MA, Thomas S, Hyslop T, Scheri RP, Roman SA, Sosa JA. 2016. Exploring the relationship between patient age and cancer-specific survival in papillary thyroid cancer: rethinking current staging systems. J Clin Oncol 34:4415–4420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adam MA, Pura J, Goffredo P, Dinan MA, Reed SD, Scheri RP, Hyslop T, Roman SA, Sosa JA. 2015. Presence and number of lymph node metastases are associated with compromised survival for patients younger than age 45 years with papillary thyroid cancer. J Clin Oncol 33:2370–2375 [DOI] [PubMed] [Google Scholar]
- 10.Verburg FA, Mader U, Tanase K, Thies ED, Diessl S, Buck AK, Luster M, Reiners C. 2013. Life expectancy is reduced in differentiated thyroid cancer patients >/ = 45 years old with extensive local tumor invasion, lateral lymph node, or distant metastases at diagnosis and normal in all other DTC patients. J Clin Endocrinol Metab 98:172–180 [DOI] [PubMed] [Google Scholar]