Abstract
Continuous Glucose Monitoring (CGM) has been demonstrated to be clinically valuable, reducing risks of hypoglycemia and hyperglycemia, glycemic variability (GV), and improving patient quality of life for a wide range of patient populations and clinical indications. Use of CGM can help reduce HbA1c and mean glucose. One CGM device, with accuracy (%MARD) of approximately 10%, has recently been approved for self-adjustment of insulin dosages (nonadjuvant use) and approved for reimbursement for therapeutic use in the United States. CGM had previously been used off-label for that purpose. CGM has been demonstrated to be clinically useful in both type 1 and type 2 diabetes for patients receiving a wide variety of treatment regimens. CGM is beneficial for people using either multiple daily injections (MDI) or continuous subcutaneous insulin infusion (CSII). CGM is used both in retrospective (professional, masked) and real-time (personal, unmasked) modes: both approaches can be beneficial. When CGM is used to suspend insulin infusion when hypoglycemia is detected until glucose returns to a safe level (low-glucose suspend), there are benefits beyond sensor-augmented pump (SAP), with greater reduction in the risk of hypoglycemia. Predictive low-glucose suspend provides greater benefits in this regard. Closed-loop control with insulin provides further improvement in quality of glycemic control. A hybrid closed-loop system has recently been approved by the U.S. FDA. Closed-loop control using both insulin and glucagon can reduce risk of hypoglycemia even more. CGM facilitates rigorous evaluation of new forms of therapy, characterizing pharmacodynamics, assessing frequency and severity of hypo- and hyperglycemia, and characterizing several aspects of GV.
Keywords: : Continuous glucose monitoring (CGM), Flash glucose monitoring, Multiple daily injections (MDI), Continuous subcutaneous insulin infusion (CSII), Sensor-augmented pump (SAP), Automated insulin delivery (AID), Closed-loop control (CLC), Hypoglycemia, Hyperglycemia, Time in range (TIR), Ambulatory glucose profile (AGP), Glycemic variability (GV), Artificial pancreas (AP), Type 1 diabetes (T1DM), Type 2 diabetes (T2DM).
Introduction
We shall review recent developments regarding continuous glucose monitoring (CGM) with focus on demonstrable clinical outcomes. Part I discusses several aspects of use of CGM and related techniques for open-loop control of glucose: type 1 diabetes, special populations (hypoglycemia unawareness, pregnancy, hospitalized patients), use of CGM combined with multiple daily injections (MDI) versus continuous subcutaneous insulin infusion (CSII), flash glucose monitoring in type 1 and type 2 diabetes, and use of CGM and flash glucose monitoring in type 2 diabetes. Part II discusses recent progress using several approaches to closed-loop control: threshold suspend, predictive threshold suspend, hyperglycemia–hypoglycemia minimizer, hybrid closed loop, and full closed loop using insulin alone or insulin and glucagon.
Part I: Open-Loop Control
Clinical application of modern-era continuous glucose sensing began in 2000.1–4 CGM was widely heralded as a great advance. However, early CGM sensors had limited accuracy, limited duration of use, and limited usability. After 10 years of clinical experience, an evaluation by the Cochrane collaboration,5 based on 22 randomized controlled trials (through 2011, including the JDRF-CGM studies6,7), was extremely cautious5:
“There is limited evidence [emphasis added] for the effectiveness of real-time CGM use in children, adults and patients with poorly controlled diabetes. The largest improvements in glycemic control were seen for sensor-augmented insulin pump therapy in patients with poorly controlled diabetes who had not used an insulin pump before. The risk of severe hypoglycemia or ketoacidosis was not significantly increased [sic.] for CGM users, but as these events occurred infrequent these results have to be interpreted cautiously. There are indications that higher compliance of wearing the CGM device improves glycosylated hemoglobin HbA1c level (HbA1c) to a larger extent.”5
An independent review by Liebl et al.8 focusing on eight major studies (also including the JDRF-CGM studies) reached somewhat stronger, but still guarded, conclusions:
“Randomized controlled studies have provided evidence that hemoglobin HbA1c (HbA1c) results can be improved in patients with type 1 diabetes with elevated baselineHbA1c when using CGM frequently enough and that the frequency and duration of hypoglycemic events can be reduced in patients with satisfactory baselineHbA1c.”[emphasis added]8
Price and coworkers have pointed out a number of pitfalls in meta-analyses.9 With the benefit of time and the large number of studies that have followed, we can now be much more assertive regarding the clinical benefits of CGM. The JDRF cooperative studies6,7 reported in 2008 and 2010 represented a breakthrough in terms of size, rigor, use of three different CGM systems, multiple patient populations (children, adolescents, adults), and evaluation of factors such as the extent of usage. The JDRF study made several critically important observations based on a large number of subjects and 6-month follow-up:
(1) In people with elevated HbA1c at baseline, introduction of CGM usually resulted in significant and sometimes substantive reductions in HbA1c.
(2) The magnitude of reduction in HbA1c is dependent on the baseline HbA1c. If the HbA1c is close to the desired or target HbA1c level, then further decline in HbA1c was difficult to achieve. Improvements in HbA1c were directly related to the level of usage of CGM.
(3) Improvement in HbA1c was demonstrated for subjects in all age groups. Improvement was smaller for adolescents compared with children (ages 8–14 years) or adults (age >25 years). The relationships with age were primarily attributable to the rate of utilization of CGM in the various groups.
(4) For individuals experiencing a high risk of hypoglycemia, real-time CGM usually resulted in significant clinically meaningful reductions in risk of hypoglycemia by 33% to 50%.)6,7
In addition to quantifying the changes in mean glucose, and risks of hypo- and hyperglycemia, CGM enables the patient and physician to visualize the typical patterns of glucose throughout the day, including changes following meals, exercise, medications, and in response to changes in treatment regimen. One can also evaluate glycemic variability (GV) within and between days or between days of the week. One can analyze the average or typical glucose patterns by time of day for any specified day or for various periods of time for one subject and for groups of subjects. For example, Forlenza et al. characterized the average patterns of glucose by time of day for six groups of subjects in the JDRF studies (adults, adolescents, and children either with HbA1c levels above or within target levels) (cf. Fig. 2 of Forlenza et al.)10
El-Laboudi et al.11 have recently reported a new more detailed analysis of the data from the JDRF studies.6,7 They studied more than two dozen criteria, including measures of overall quality of glycemic control, hypoglycemia, hyperglycemia, and GV [Table 5 of El-Laboudi et al.11 and Supplementary Table S1 to the present article (available online at www.liebertpub.com/dia)]. Use of CGM resulted in a dramatic and significant reduction in both HbA1c and mean glucose.7,9,11 There were highly significant improvements in five measures of hypoglycemia, (LBGI, %GRADEhypoglycemia, and percentage of time glucose was ≤2.8, ≤3.3, or ≤3.9 mM (≤50, ≤60, or ≤70 mg/dL)).7,9,11 There were significant reductions in three measures of hyperglycemia (HBGI, %GRADEhyperglycemia, and the percentage of time with glucose >7.8 mM [> 140 mg/dL]).7,9,11 Six of eight measures of GV were markedly reduced after 26 weeks of use of CGM (P < 0.001), and GV was progressively reduced as the number of days of CGM used per week increased. Some of the changes (HbA1c, HBGI, LBGI, and several measures of GV) were significant, not only at the usual P-values (e.g., P < 0.05, 0.01, or 0.001) but also with extraordinary P-values ranging from P ∼ 10−4 to P ∼ 10−6 (Table 5 of El-Laboudi et al.11 and Supplementary Table S1 to this article). Reduction in GV is important because GV is highly correlated with the risk of hypoglycemia: for any specified average blood glucose (and the corresponding HbA1c), the percentage of glucose values below the threshold defining hypoglycemia will decrease as GV is reduced, as shown theoretically and empirically.12,13
Based on the findings accumulated through 2013, The German Diabetes Association developed recommendations for use of CGM in T1DM.8 The Endocrine Society,14,15 NICE,16 ISPAD,17 the American Association of Clinical Endocrinologists/American College of Endocrinology,18 and the American Diabetes Association19 have published recommendations regarding clinical indications for use of CGM.
Numerous studies have confirmed the major findings of the JDRF-CGM studies for a wide range of patient populations and indications, using a wide variety of CGM sensors.15,17,18 We shall now discuss several aspects of CGM related to clinical outcomes, which have appeared subsequent to the truly pioneering JDRF studies.6,7
Improved accuracy of CGM
There has been steady improvement in the accuracy of glucose sensors.20–27 The best sensors now have an accuracy of approximately ±10% MARD. This has led to greater acceptance by patients and physicians and has enabled users of CGM to reduce the number of measurements of capillary blood glucose (CBG). Based on modeling studies, Kovatchev et al.28 demonstrated that a 10% MARD should be sufficient to permit self-adjustment of insulin dosage without the need for a confirmatory CBG. Thus, CGM is ready for nonadjuvant use—no longer just an adjuvant to self-monitoring of blood glucose (SMBG).
Reduced need for calibration
The improvement in accuracy of CGM sensors has been accompanied by a reduced need for frequent calibration29—or any calibration30—by the user. Indeed, flash glucose monitoring (discussed further below) uses factory calibration, which does not require calibration by the patient.30
Approval for nonadjuvant use
The CE (Conformité Européene) Mark and FDA approved CGM for nonadjuvant use, i.e., adjustment of insulin dosage without confirmatory CBG measurement.31–33 The initial approval applied to only one specific model, the Dexcom G5 sensor. Hopefully, this will serve as an important precedent that will also facilitate the approval of other CGM sensors that offer similar levels of accuracy. Many CGM users had already discovered that CGM provided sufficient information, accuracy, and reliability to enable them to make insulin dosage adjustments based on CGM alone.33,34
Effectiveness of CGM in conjunction with MDI as well as with CSII
Most of the early studies with CGM were on people with type 1 diabetes who were also using an insulin pump (CSII). There have been questions as to whether similar benefits would be seen in people using basal–bolus therapy with multiple daily injections (MDI). The statement from ISPAD17 and AACE/ACE18 hinted that there might be differences in the extent of improvement in mean glucose and risk of hypoglycemia for users of MDI and CSII.
There is now considerable evidence that the improvement in quality of glycemic control is essentially equivalent in users of MDI and CSII.35–42 The effectiveness of CGM for people with T1 DM using MDI as well as CSII was clearly demonstrated in recent randomized clinical trials, which used flash glucose monitoring or flash glucose monitoring variation of CGM.40–42 Changes in mean glucose were identical for users of MDI and CGM.40–42
CGM studies in special populations
Patients at high risk for hypoglycemia
One of the most important applications of CGM is for the management of patients with frequent severe hypoglycemia, often associated with hypoglycemia unawareness. A recent clinical trial evaluated the effectiveness of CGM for this patient population. Van Beers et al.37 used a crossover randomized study and demonstrated a dramatic increase in % of time in the target range that was identical for users of MDI or CSII (cf Figs. 2 and 4 of van Beers et al.37) with concomitant marked reduction in frequency of hypoglycemia, as ascertained using three different thresholds (≤3.9, ≤3.5 and ≤2.8 mM or ≤70, ≤ 63, or ≤50 mg/dL) (Fig. 3 of van Beers et al.37). Despite a twofold or greater reduction in the % of glucose values falling below 2.8 mM, there was surprisingly little or no change in the small, but definite, fraction (∼5% of all hypoglycemic episodes) that was designated as severe hypoglycemia, as defined by coma, seizure, or hospital admission. (cf. Fig. 3 of van Beers et al.37).
If one postulates that the major effect of CGM in terms of reducing the frequency of hypoglycemia is due to a reduction in GV, then one could predict theoretically that the odds ratio for reduction of hypoglycemia will increase progressively as the threshold defining hypoglycemia is lowered, that is, greater effectiveness using a threshold of 40 mg/dL compared with a threshold of 70 mg/dL. This effect has been seen in most studies involving CGM. Thus, it was unexpected that the frequency of severe hypoglycemia was not reduced by use of CGM.37 This suggests that severe hypoglycemia manifested by coma, seizure, or hospital admission may be governed by factors in addition to the duration of blood glucose levels below the usual thresholds.
Pregnancy
There have been numerous studies of CGM in pregnancy.43–48 Use of CGM during pregnancy has been reported to improve quality of glycemic control, but does not reduce the risk of macrosomia.46,47 Additional randomized trials have been initiated.48
Hospitalized patients
Usage of CGM in the hospitalized patient and in the ICU remains a work in progress.49,50 There has been considerable interest in the use of CGM in the hospitalized patient to assist with glycemic control in patients continuing the therapies they used as an outpatient, for control of insulin infusions, and for use in the intensive care unit. The continuing improvement in the accuracy, robustness, and usability of CGM sensors offers considerable promise for an increasing role for the hospitalized patient. Thabit et al. have recently reported significant improvement in glycemic control in a randomized parallel-arm study of 40 inpatients with type 2 diabetes when using closed-loop control without premeal boluses: percentage of time in the target range was improved from 38.1% in the control group to 59.8%, a change of 21.8% [95% CI 10.4–33.1]; P = 0.0004.51 CGM by itself cannot be expected to be effective if the patient, nursing staff, and physician are unable to respond to the data generated; in contrast, incorporation of CGM into a conservative closed-loop system may be more feasible in the hospital.51
Flash glucose monitoring
Flash glucose monitoring is sometimes regarded as a separate entity from CGM. Alternatively, flash glucose monitoring can be regarded as a special case or subset of CGM. Rather than updating a display of glucose continuously at 5-min intervals, glucose values are reported only when the user scans the sensor by passing a reader or a cell phone close to the sensor. Flash glucose monitoring forfeits the abilities to display a continuous real-time graph of glucose versus time, rate of change of glucose, alarms, remote monitoring, and suitability for use in closed-loop systems. Flash glucose monitoring can still generate retrospective graphical displays of glucose versus date and time of day. Current flash glucose monitoring systems have good accuracy, factory calibration, a 2-week sensor lifetime, small size, light weight, excellent usability, good user acceptance, low cost, and an improved method for sensor insertion.
The IMPACT study evaluated flash glucose monitoring in people with type 1 diabetes42; the REPLACE study evaluated flash glucose monitoring in people with type 2 diabetes.52 Participants using flash glucose monitoring stopped using CBG almost entirely. People with T1DM obtained flash glucose monitoring glucose sensor measurements about 15 times per day initially, with further slow decline in frequency of scanning thereafter. Using flash glucose monitoring in type 1 diabetes for 6 months, the average time spent in hypoglycemia was reduced by 38%, dropping by 1.30 h/day from 3.38 h/day at baseline to 2.03 h/day at 6 months compared with negligible changes in the control group. There were minimal changes in HbA1c in either group. However, there was marked improvement in quality of glycemic control as reflected in BGRI, LBGI, and time in target range. There was a reduction in the number, duration, and magnitude (area below a specified threshold or AUC) for hypoglycemia.42 There was also a reduction in the number, duration, and area under the curve for hyperglycemia defined by a threshold of >13.3 mM (>240 mg/dL) (Supplementary Table S1 Bolinder et al.42) and a reduction in GV measured as SD, %CV, CONGA1, CONGA2, CONGA4, and CONGA6 (all P < 0.0001), and in MAGE (P < 0.001) (cf. Supplementary Table S2 of Bolinder et al.42).
Flash glucose monitoring users showed improvement in subjective factors such as satisfaction with treatment measured by the Diabetes Treatment Satisfaction Questionnaire (DTSQ) (P < 0.001) (cf. Supplementary Fig. S5 of Bolinder et al.42) and participants were aware of reduced hypoglycemia. There were no significant changes in the Diabetes Distress Survey, nor in the Fear of Hypoglycemia Survey (cf. Supplementary Fig. S5 of Bolinder et al.42).
Use of flash glucose monitoring in people with type 2 diabetes showed similar results52 with reductions in the risk of hypoglycemia of 55%, 68%, and 75% using thresholds of ≤3.9, ≤ 3.1, and ≤2.5 mM (≤70, ≤55, and ≤45 mg/dL), respectively (P < 0.001 for all three thresholds). Only minimal changes were observed in mean glucose and HbA1c. Flash glucose monitoring participants reduced usage of CBG by 90% from a baseline of 3.8 CBG readings per day. Sensor scanning frequency averaged 8.3/day (median 6.9/day). Treatment satisfaction by the DTSQ and Diabetes Quality of Life (DQoL) measure improved52 (P < 0.001 and P < 0.05, respectively).
Applicability of CGM and flash glucose monitoring to type 2 diabetes
CGM and flash glucose monitoring are applicable to management of people with type 2 diabetes.52,53 People with type 2 diabetes who require basal–bolus insulin therapy may be regarded as nearly equivalent to people with type 1 diabetes. People with frequent severe hypoglycemia with hypoglycemia unawareness are also candidates for CGM, just as their counterparts with type 1 diabetes. CGM may be used in continuous real-time mode (personal mode) or for short periods in a masked mode, sometimes called professional mode. Vigersky and Shrivastav recently reviewed the status of both the personal and professional modes.53
Mazze et al.54 and Hill et al.55 characterized CGM findings in normal subjects. These studies provide the necessary reference data for evaluation of CGM in people with obesity, prediabetes, and type 2 diabetes. Vigersky and others have also conducted studies of CGM in people with prediabetes and with morbid obesity.56,57 They reported an increase in GV in people with obesity. GV increases progressively as people develop impaired fasting glucose, impaired glucose tolerance, and finally overt type 2 diabetes.58–61
After development of type 2 diabetes, with progressive loss of beta cell function, there is usually periodic advancement in the intensity of therapy, from mono-, to dual-, to triple-therapy, and finally to injectables (insulin, GLP-1RA agents, or both). Accordingly, the form of therapy can be used as a surrogate for the duration and severity of diabetes. Kohnert et al. compared the extent of GV in patients with type 2 diabetes receiving different classes of therapy and with patients with type 1 diabetes.62 Augstein et al.63 also examined quality of glycemic control (heavily influenced by GV) in relation to form of therapy. These studies showed a progressive increase in GV with duration and severity of T2DM.
Professional CGM versus personal CGM
When professional (masked, short-term) CGM was introduced, it was suggested that periodic brief 3–6-day periods of observation might provide physicians with sufficient information to adjust therapy and advise the patient regarding medications, diet, and lifestyle. The rationale for the short term, 3–6 days, may have included several pragmatic considerations such as the approved duration of use for CGM sensors at that point in time, cost, potential reimbursement for CGM as a diagnostic test, convenience for the patient (short term, no alarms), and workflow issues for the healthcare provider. Three to 6 days of data can sometimes be sufficient to identify major problems that were not readily apparent from HbA1c or from a small number of SMBG measurements, for example, hyper- or hypoglycemia, inconsistencies between mean glucose by CGM, mean glucose by SMBG and HbA1c, large GV, excessive postprandial excursions, and nocturnal patterns and variability. Use of masked CGM was intended to ensure that the data would be representative of actual experience without changes that patients might introduce if they had received instant feedback regarding their glucose levels.
A few studies have claimed benefits from short-term (3–5 days) professional CGM.64–66 However, other studies failed to demonstrate a clinical benefit for short-term professional CGM.67
How long a series of CGM data is required before one can characterize glucose patterns and statistics? Four studies have addressed this question.
Mazze indicated that one needs about 15–30 days of CGM to obtain a stable pattern for the ambulatory glucose profile54,68. Dunn and Crouther also reported that 14 days provide a good snapshot.69 Xing et al.70 recommended the use of at least 12–15 days of data to ensure that results would be correlated with results based on a 3-month study to characterize the overall level of glycemic control, mean glucose, coefficient of variation of glucose (%CV), and percentages of glucose values within the hypoglycemic, hyperglycemic, and target ranges.
Neylon et al.71 also concluded that one needs at least 12 days of CGM data to obtain reliable, consistent, and stable estimates of GV using SD and %CV. Parameters such as MAGE and CONGAn require more data and their estimation can be seriously impaired in the face of missing data.
Clinicians and researchers should be extremely cautious when interpreting CGM data based on less than 14 days of CGM data. Two weeks is the minimum amount of time required to begin to detect reproducible changes in patterns related to the day of the week. Thus, it is no surprise that 3- or 6-day professional CGM has not been demonstrated to be very useful clinically.
Impact of CGM on clinical trials of new therapeutic agents
One of the most important applications of CGM is for purposes of clinical research to evaluate and compare different forms of treatment, both for type 1 and type 2 diabetes. Ever since the DCCT study, clinical research was focused almost entirely on HbA1c values, fasting blood glucose, and perhaps a glucose profile obtained using SMBG seven or eight times per day for 1, 2, or 3 days. These studies required a large number of subjects to obtain sufficiently reliable data. It was recognized that the 8-point SMBG profile would miss many hypo- and hyperglycemic episodes, especially at night. Today it is feasible to utilize CGM to compare treatment regimens with other interventions, other active agents, or placebo. This has been applied to rapid-acting72,73 and long-acting74 insulins, SGLT2 inhibitors,75 GLP-1 RAs,72,76,77 and other classes78 of medications. CGM is critical when we wish to compare GV for different forms of therapy, evaluate nocturnal patterns, frequency, severity, and duration of hypo- and hyperglycemia, and postprandial patterns. CGM is essential for many pharmacodynamic studies. One can expect that CGM will play a progressively larger role in clinical trials. The barriers to use of CGM to date might have included uncertainty whether regulatory bodies would accept CGM data. It would be helpful if CE and FDA would make an unambiguous declaration that they will accept CGM data as part of the formal evaluation of new forms of therapy. The improving accuracy, ease of use, duration of use, user acceptance, reduced costs of CGM systems, and the success of studies reported to date72–78 should spur increasing use of CGM in clinical trials.
Part II: Closed-Loop Control
We will now consider the recent developments regarding the use of CGM as one of the pillars of closed-loop control systems for automatic delivery of insulin and glucagon.
We acknowledge the importance of the “#we are not waiting community” that developed Nightscout for remote sharing and monitoring of glucose levels obtained using real-time CGM79,80 and then turned to developing an open access artificial pancreas system (OpenAPS).81,82
These open-source, do-it-yourself (DIY) endeavors provided proof of concept that undoubtedly spurred the development of artificial pancreas systems by academia and industry, energized regulatory agencies and the general public, and raised awareness of the urgent need for these systems. Many of the current ventures by the commercial sector, government, academia, and diabetes organizations had already been well underway at the time the open-source community announced their work. Open-source systems only serve one person at a time and have a very high barrier to entry: one must construct the system oneself. Open-source systems are not readily generalizable to a large and diverse community and there are no financial resources, incentives, and infrastructure to test, disseminate, and support these systems—which should be regarded as experimental prototypes. The systems developed by industry and academia have been rigorously and painstakingly developed in terms of safety, robustness, reliability, clinical trials, documentation, development of resources for user training and support, and scalability. Products should be designed to survive in the face of rapidly changing technologies and user expectations. Some active innovators in the OpenAPS community subsequently moved to industry, whereupon they quickly discovered that they would need 100–200 million dollars to begin to reach commercial viability.
We note the work of a few of the pioneers in the long quest for an artificial pancreas.83–87 We consider five levels in the development of an artificial pancreas:
(1) Threshold low-glucose suspend of insulin infusion
(2) Predictive low-glucose suspend of insulin infusion
(3) The hyperglycemia/hypoglycemia minimizer (control to range)
(4) Hybrid closed-loop systems with user adjustment of premeal boluses
(5) Full closed-loop systems (insulin only)
(6) Dual-hormone closed-loop systems utilizing insulin and glucagon
Recent research utilizing several of these approaches will be discussed in more detail later throughout this special Supplement issue of Diabetes Technology and Therapeutics.
Low-glucose suspend of insulin infusion
Rather than giving an alarm when hypoglycemia is detected (which may or may not be heard) and relying on the patient or a family member to take the necessary action, real-time CGM can be used to suspend insulin delivery from an insulin pump. This simple and straightforward algorithm was shown to be very effective in reducing the risk of nocturnal hypoglycemia88–91: there was a 38% reduction in the AUC below 70 mg/dL for subjects using the threshold suspend. There was approximately a 25% reduction in time with glucose in the range 51–70 mg/dL and more than a 50% reduction in time with glucose ≤50 mg/dL. One should generally expect a greater relative reduction in time spent in the more severe levels of hypoglycemia (≤50 mg/dL) than with less stringent criteria (e.g., glucose ≤70). There were no meaningful changes in HbA1c and no increase in hyperglycemia.88–91
Predictive low-glucose suspend of insulin infusion
Rather than waiting until the observed glucose has fallen below a specified threshold glucose level, one can predict when such an event is likely to happen within a short period of time. There are several algorithms for making that prediction.92 Use of a predictive algorithm improves the performance compared with low-glucose suspend: the number and duration of nocturnal hypoglycemic events were both reduced, with the greatest reduction for events with glucose <60 mg/dL and lasting >180 min for children (ages 4–10) and adolescents (ages 11–14).93,94
Calhoun et al.94 reported that a system utilizing overnight predictive glucose suspend achieved a twofold reduction in risk of hypoglycemia. This effect was so robust that it was unaffected by 14 factors describing the patient and conditions at bedtime: (1) age; (2) gender; (3) diabetes duration; (4) baseline HbA1c at onset of study; (5) daily %basal insulin; (6) total daily dose of insulin; (7) bedtime blood glucose; (8) presence or absence of a bedtime snack; (9) calculated amount of insulin on board at bedtime; (10) CGM rate of change at bedtime; (11) day of the week; (12) time of day when the low-glucose suspend system was activated; (13) exercise intensity; and (14) the number of hypoglycemic CGM values ≤60 mg/dL between noon and 8 pm on the day preceding the overnight study.
Hypoglycemia/hyperglycemia minimizer: control to range
Following the use of a low-glucose suspend algorithm, one of the next logical steps was to introduce a similar kind of control algorithm to help prevent excursions on the hyperglycemia side of the target range, thereby creating a control-to-range algorithm. In addition to a low-glucose suspend to address actual or predicted hypoglycemia, one can increase insulin infusion rates if the glucose level goes above (or is expected to go above) a specified threshold. This has been called a hypoglycemia/hyperglycemia minimizer.95 As expected, this approach can provide improved control and increase the percentage of time in the target range. The authors also examined the effects of aggressiveness of the algorithm for control of hypoglycemia.96
Hybrid closed-loop control
Early studies with closed-loop control systems consistently demonstrated excellent control for the overnight period, but experienced greater difficulty in achieving good control during the day. One of the major problems is estimating the dose of the premeal insulin boluses. Accordingly, a number of groups utilized a hybrid closed-loop system, where each person controls her own premeal boluses, possibly using the same logic or same bolus calculator she had been using previously with an open-loop insulin pump. Then, the closed loop would handle the glucose control between meals. The premeal boluses taken under the control of the user were reduced by 30% to 50% relative to the calculated amount based on the supposition that this could give rise to fewer hypoglycemia events, and the closed loop could make up for any underestimate for the insulin bolus. Numerous algorithms have been used for the hybrid closed loop. Clinical studies have ranged from overnight to 1 year, from a clinical research center to outpatient studies in a restricted and protected environment with close monitoring, to free living conditions, diabetes camps, and skiing expeditions.97–113 These studies have shown a consistent increase in the percentage of time spent in the target range (%time in range or %TIR), a modest reduction in the percentage of time in the hyperglycemic range, and reduction in the hypoglycemic range for adults and adolescents. Improvement during the nocturnal period is consistently superior to that observed for the full 24-h period.95–113 A recent study112,113 provided the basis for approval of the first hybrid closed-loop system by the FDA.114,115
Thabit and Hovorka116 summarized 12 studies of the artificial pancreas involving 339 children, adolescents, and adults in transitional and home settings using a wide variety of experimental designs (e.g., overnight to 12 weeks, nocturnal only, or 24 h/day) and using a variety of comparators (insulin pump, sensor-augmented pump [SAP], or threshold suspend). Eight studies utilized single-hormone (insulin) closed-loop control; three involved bihormonal control. In nine cases, the percentage of glucose values within the target range (%TIR) (with various definitions) was the primary or coprimary response parameter. %TIR increased in eight of the nine cases from an average of 53.1% ± 13.7% (SD) to 69.5% ± 6.8%. The average change in %TIR was 12.9% ± 9.3% (SEM ±3.1%) (P < 0.01).
The % of glucose values in the hypoglycemic range was the primary or coprimary response variable in six studies. The mean % hypoglycemia was reduced from 6.8% ± 2.4% (SD) to 4.9% ± 2.7%. The relative reduction of % hypoglycemia using closed-loop control was 34% ± 20% (±8.0% SEM), (P < 0.01).
Two studies used other primary endpoints. In one study, the risk of severe hypoglycemia was reduced threefold; in another, the LBGI was reduced by 43%. FPG and 24-h mean glucose were the primary endpoints in two studies, with reductions of 1.4 and 1.7 mM (25 and 31 mg/dL), respectively.
A late-breaking abstract at the 2016 ADA scientific meetings,112 presented a pivotal study leading to FDA approval of the first closed-loop system.112–114 Time in range (%TIR) improved from a median of 67.8% to 73.4% and to 76.4% for the overnight period. Mean %TIR increased from 66.7% ± 12.2% to 72.2% ± 8.8% overall and from 66.8% ± 14% to 75.3 ± 9.8 (nocturnal). Results of this study were presented in greater detail by Garg et al.113 Results are consistent with those from 12 studies discussed above.116
The studies cited here97–117 used a wide range of technologies for glucose sensor, controller, algorithms, and insulin infusion devices. All achieved excellent safety, efficacy, and user acceptance.
While insulin can be used to reduce glucose, there is no good way for a system using only insulin to increase glucose rapidly except to ask the subject to consume carbohydrates. Management of premeal insulin is challenging. In the hybrid system, the user takes his/her own insulin bolus before the meal based on carbohydrate content and other factors. The bolus may be calculated conservatively since the closed-loop system could be expected to provide additional insulin if needed. Several studies are underway to automatically and reliably detect the onset of meals and administer insulin at an appropriate rate.
Use of closed-loop systems often results in a two- to threefold reduction in rates of hypoglycemia compared with CGM and insulin pump (SAP) depending on the patient population, study design, and criteria utilized. Closed-loop control sometimes results in reduction of the total insulin dose per day, which might be regarded as more efficient, effective, and physiological use of insulin. Effects on HbA1c have been variable and dependent on the baseline value, and on target levels for mean glucose.
Dual-hormone (insulin and glucagon) closed-loop systems
The next level in the evolution of the closed-loop system is a dual-hormone system providing the ability to infuse either or both insulin and glucagon. Several groups have investigated this option and several systems have been brought to fruition and extensively tested clinically both for inpatients and for long-term ambulatory use.118–125 The dual-hormone control systems are able to reduce the risk of hypoglycemia by approximately a factor of three relative to the risk when using SAP. The dual-loop algorithm as used by Russell, Damiano, El-Khatib, and coworkers requires minimal specification of parameters for each individual patient—only the patient's weight is required.123,124 There is an urgent need for an FDA-approved stable and soluble glucagon preparation. The increased complexity of dual-hormone control systems might be expected to lead to increased costs. It remains to be seen which subgroups of patients would benefit from a dual-hormone system in a cost-effective manner.
Discussion
Clinical benefits and application of CGM
CGM technology has improved dramatically over the past 5 years. The accuracy, reliability, and robustness of CGM permits use for adjustment of insulin dosage for basal insulin and premeal and correction boluses, without confirmation by a blood glucose meter. There is no question that relative to SMBG, use of CGM can dramatically improve the quality of glycemic control both in type 1 and type 2 diabetes. The benefit is especially pronounced in high-risk patients with frequent or severe hypoglycemia, often associated with hypoglycemia unawareness. CGM is also finding increasing use in the hospitalized patient for monitoring and for control of insulin infusions. CGM can be used effectively with either MDI or with CSII.35–42
Short-term masked CGM can be used to detect problems, evaluate quality of glycemic control, describe patterns of glucose, assess risks of hypoglycemia and hyperglycemia by time of day and day of the week, and evaluate GV. This can be helpful to the physician and patient.
Diabetes management options
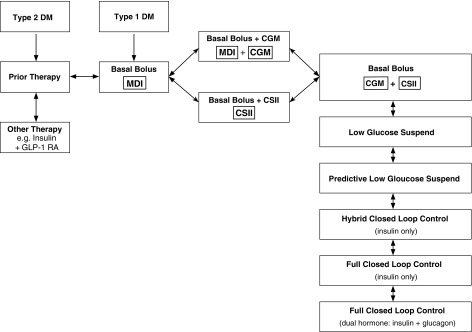
For many patients using basal–bolus insulin with MDIs, the benefits of CGM and the benefits of CSII are roughly equivalent while combination of CSII and CGM can provide additional benefits. Accordingly, the management of a patient using basal–bolus insulin therapy, who has not achieved their individualized target level for mean glucose with an acceptable risk of hypoglycemia, can be schematized as shown in Figure 1.
FIG. 1.
Flowchart of current and potential future options for management of patients receiving basal–bolus therapy. The first clinical decision is whether to add CGM, to add CSII, or introduce both CGM and CSII. CGM, continuous glucose monitoring; CSII, continuous subcutaneous insulin infusion.
Hypoglycemia
In addition to the enormous cost (2–4 billion US dollars per year) for emergency room visits and hospitalization,126–129 there is another cost to hypoglycemia. Brod130–132 and coworkers have reported a series of studies showing the enormous economic, psychological, and loss-of-work-productivity costs of nonsevere hypoglycemia. This factor must also be considered when evaluating the cost-effectiveness of approaches to mitigate hypoglycemia. The cost-effectiveness of CGM and closed-loop systems is considered in more detail elsewhere.133–135
CGM also serves as a tool that can assist evaluation of new and improved methods for medical therapy of diabetes, for earlier detection of prediabetes, and earlier diagnosis of diabetes.
Other indications for CGM
CGM (real-time, personal, and masked, professional) and the closely related technique, FGM, are now well established as important tools for the management of patients with diabetes—both type 1 and type 2. CGM is also clinically useful when dealing with other conditions, for example, diabetes associated with cystic fibrosis, excessive glycemic excursions seen following bariatric surgery, evaluation of hypoglycemia related to insulinoma, Hirata's disease (anti-insulin autoantibodies), and rare conditions attributable to autoantibodies to the insulin receptor.
Clinical management strategies
Additional clinical studies and real-world follow-up will be necessary to develop strategies and guidelines for the allocation of advanced efficacious, but potentially costly resources, with the multiple new options that are just becoming available now (Fig. 1).
Educational materials and assisted interpretation
There is need for educational materials for HCPs and patients regarding retrospective interpretation and use of CGM data and for selecting patients and monitoring performance of closed-loop systems. Most physicians have not been trained in the interpretation of CGM data and use of those data for generating recommendations for revision of therapy, diet, or lifestyle. There is need for automated interpretation to alert users to specific patterns and make recommendations for changes in therapy and/or lifestyle. Finally, there will be need for guidelines for physicians, as to when to deploy these new systems for control of insulin administration.
Standardization of metrics for assessment of CGM and closed-loop control systems
There are now dozens of ways to express the changes in glycemic control, hypoglycemia, hyperglycemia, and GV following an intervention106 (e.g., Supplementary Table S1). This makes it difficult to compare and combine the results of multiple studies involving different patient populations, different sensors and control algorithms for closed-loop control, different settings and durations of studies, different definitions of hyperglycemia, target range, and hypoglycemia.106 It will be important to select a few criteria to be included routinely in the evaluation and characterization of CGM and closed-loop systems. Multiple ranges of blood glucose covering different degrees of severity of hypo- and hyperglycemia can be amalgamated using triads of metrics such as {HBGI, LBGI, BGRI} or {GRADEhypoglycemia, GRADEhyperglycemia, GRADE} or {Hypoglycemia Index, Hyperglycemia Index, IGC}. This can simplify the analysis and interpretation and reduce problem of conflicting results.
The ambulatory glucose profile (AGP) has proven to be extremely useful in summarizing the results of CGM and closed-loop studies. Several groups have superimposed control and intervention groups using different colors and displayed the percentiles (10th, 25th, 50th, 75th, and 90th) for each treatment group. The evolution of the AGP is described in the literature.54,68,136–141 The AGP has been employed in many of the studies cited here: it facilitates analysis of the percentages of time in the hyper-, target, and hypoglycemic ranges by time of day and the changes in these percentages attributed to various interventions. The AGP provides a qualitative picture, but does not provide a single metric. The IGC, BGRI, GRADE, Q-score, glucose pentagon/pentagram, ADRR, and %TIR enable us to reduce this dynamic picture to a single score. Since there are two aspects of poor control, one needs a minimum of two measures—one to reflect hypoglycemia, another to reflect hyperglycemia.141
Minimal duration of CGM data
If different days of the week show different glycemic patterns, it would be necessary to obtain least 2 (and preferably four) weeks of CGM recordings, followed by appropriate analyses. Two weeks appear to be the minimum time required for reliable, stable, and sensitive retrospective CGM.54,68–70
Implanted sensors
With proven clinical efficacy and safety of CGM and of closed-loop control, and the need of many patients to use CGM on a long-term continuous basis, there has been a resurgence of interest in long-term implantable glucose sensors.142 Several companies are developing prototypes of implantable sensors using a variety of techniques. We can expect continued rapid activity on that front, just as the percutaneous glucose sensors continue to improve.
Conclusions
CGM (including flash glucose monitoring) systems are safe and effective in both type 1 and type 2 diabetes and can improve quality of glycemic control, reduce risk of hypoglycemia, and permit selection of lower target levels for mean glucose and HbA1c. Recent CE and FDA approvals for use of CGM for adjustment of insulin dosages are a major step forward. The application of CGM for closed-loop control is advancing rapidly, with well-documented reduction in risk of hypoglycemia in many carefully controlled studies in multiple locations worldwide applied to multiple patient groups under diverse conditions with progressively more sophisticated sensors, algorithms, usability, and integration with the insulin pump. The FDA has approved one system for commercialization and one can expect many additional systems to follow.
Supplementary Material
Acknowledgments
The author wishes to thank Dr. El-Laboudi and Prof. Nick Oliver for permission to utilize the results shown in Supplementary material, Supplementary Table S1, which is based on Table 5 of El-Laboudi et al.,11 with additional results kindly provided by Dr. El-Laboudi (personal communication).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kaufman FR: Role of the continuous glucose monitoring system in pediatric patients. Diabetes Technol Ther 2000;2(Suppl.1):S49–S52 [DOI] [PubMed] [Google Scholar]
- 2.Chase HP, Kim LM, Owen SL, et al. : Continuous subcutaneous glucose monitoring in children with type 1 diabetes. Pediatrics 2001;107:222–226 [DOI] [PubMed] [Google Scholar]
- 3.Joseph H, Orville RS, David IR, et al. : Glucose monitoring system, US Patent US5497772A. www.google.com/patents/US5497772 (accessed January8, 2017)
- 4.Gilligan BJ, Shults MC, Rhodes RK, Updike SJ: Evaluation of a subcutaneous glucose sensor out to 3 months in a dog model. Diabetes Care 1994;17(8):882–887 [DOI] [PubMed] [Google Scholar]
- 5.Langendam M, Luijf YM, Hooft L, et al. : Continuous glucose monitoring systems for type 1 diabetes mellitus. Cochrane Database Syst Rev 2012;1:CD008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Tamborlane WV, Beck RW, et al. : Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 7.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group: Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care 2010;33:17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liebl A, Henrichs HR, Heinemann L, et al. : Continuous glucose monitoring: evidence and consensus statement for clinical use. J Diabetes Sci Technol 2013;7:500–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Price D, Graham C, Parkin CG, Peyser TA: Are systematic reviews and meta-analyses appropriate tools for assessing evolving medical device technologies? J Diabetes Sci Technol 2016;10:439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forlenza GP, Pyle LL, Maahs DM, Dunn TC: Ambulatory glucose profile analysis of the juvenile diabetes research foundation continuous glucose monitoring dataset - Applications to the pediatric diabetes population. Pediatr Diabetes 2016. [Epub ahead of print]; DOI: 10.1111/pedi.12474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Laboudi AH, Godsland IF, Johnston DG, Oliver NS: Measures of glycemic variability in type 1 diabetes and the effect of real-time continuous glucose monitoring. Diabetes Technol Ther 2016;18:806–812 [DOI] [PubMed] [Google Scholar]
- 12.Rodbard D: Hypo- and hyperglycemia in relation to the mean, standard deviation, coefficient of variation, and nature of the glucose distribution. Diabetes Technol Ther 2012;14:868–876 [DOI] [PubMed] [Google Scholar]
- 13.Dunn TC, Hayter GA, Doniger KJ, Wolpert HA: Development of the Likelihood of Low Glucose (LLG) algorithm for evaluating risk of hypoglycemia: a new approach for using continuous glucose data to guide therapeutic decision making. J Diabetes Sci Technol 2014;8:720–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klonoff DC, Buckingham B, Christiansen JS, et al. : Continuous glucose monitoring: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2011;96:2968–2979 [DOI] [PubMed] [Google Scholar]
- 15.Peters AL, Ahmann AJ, Battelino T, et al. : Diabetes technology - continuous subcutaneous insulin infusion therapy and continuous glucose monitoring in adults: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2016;101:3922–3937 [DOI] [PubMed] [Google Scholar]
- 16.National Institute for Health and Care Excellence (NICE): NICE Guideline (NG) 17, Type 1 diabetes in adults: diagnosis and management. August 2015. Last updated: July 2016. (cf. pages 25–26, sections 1.6.21–1.6.24). www.nice.org.uk/guidance/NG17 (accessed May3, 2017)
- 17.Rewers MJ, Pillay K, de Beaufort C, et al. : Assessment and monitoring of glycemic control in children and adolescents with diabetes. ISPAD Clinical Practice Consensus Guidelines 2014 Compendium. Pediatric Diabetes 2014;15(Suppl.20):102–114 [DOI] [PubMed] [Google Scholar]
- 18.Fonseca VA, Grunberger G, Anhalt H, et al. : Continuous Glucose Monitoring: A Consensus Conference of The American Association of Clinical Endocrinologists and American College of Endocrinology. Endocr Pract 2016;22:1008–1021 [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association: 2017 Standards of Medical Care in Diabetes. Standards of Medical Care in Diabetes—2017: Summary of Revisions. Diabetes Care 2017;40(Suppl.1):S4–S5 [DOI] [PubMed] [Google Scholar]
- 20.Damiano ER, El-Khatib FH, Zheng H, et al. : A comparative effectiveness analysis of three continuous glucose monitors. Diabetes Care 2013;36:251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailey TS, Ahmann A, Brazg R, et al. : Accuracy and acceptability of the 6-day Enlite continuous subcutaneous glucose sensor. Diabetes Technol Ther 2014;16:277–283 [DOI] [PubMed] [Google Scholar]
- 22.Matuleviciene V, Joseph JI, Andelin M, et al. : A clinical trial of the accuracy and treatment experience of the Dexcom G4 sensor (Dexcom G4 system) and Enlite sensor (guardian REAL-time system) tested simultaneously in ambulatory patients with type 1 diabetes. Diabetes Technol Ther 2014;16:759–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damiano ER, McKeon K, El-Khatib FH, et al. : A comparative effectiveness analysis of three continuous glucose monitors: the Navigator, G4 Platinum, and Enlite. J Diabetes Sci Technol 2014;8:699–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taleb N, Emami A, Suppere C, et al. : Comparison of two continuous glucose monitoring systems, Dexcom G4 Platinum and Medtronic Paradigm Veo Enlite System, at rest and during exercise. Diabetes Technol Ther 2016;18:561–567 [DOI] [PubMed] [Google Scholar]
- 25.Thabit H, Leelarathna L, Wilinska ME, et al. : Accuracy of Continuous Glucose Monitoring During Three Closed-Loop Home Studies Under Free-Living Conditions. Diabetes Technol Ther 2015;17:801–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laffel L: Improved accuracy of continuous glucose monitoring systems in pediatric patients with diabetes mellitus: results from two studies. Diabetes Technol Ther 2016;18(Suppl. 2):S223–S233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonora B, Maran A, Ciciliot S, et al. : Head-to-head comparison between flash and continuous glucose monitoring systems in outpatients with type 1 diabetes. J Endocrinol Invest 2016;39:1391–1399 [DOI] [PubMed] [Google Scholar]
- 28.Kovatchev BP, Patek SD, Ortiz EA, Breton MD: Theoretical and modeling studies demonstrating that a 10% MARD (Mean Absolute Relative Difference) is or should be sufficient to permit use of CGM as a basis for self-adjustment of insulin dosage without confirmatory capillary blood glucose. Assessing sensor accuracy for non-adjunct use of continuous glucose monitoring. Diabetes Technol Ther 2015;17:177–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acciaroli G, Vettoretti M, Facchinetti A, et al. : From Two to One Per Day Calibration of Dexcom G4 Platinum by a Time-Varying Day-Specific Bayesian Prior. Diabetes Technol Ther 2016;18:472–479 [DOI] [PubMed] [Google Scholar]
- 30.Bailey T, Bode BW, Christiansen MP, et al. : The Performance and Usability of a Factory-Calibrated Flash Glucose Monitoring System. Diabetes Technol Ther 2015;17:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.FDA News Release 12-20-2016: FDA expands indication for continuous glucose monitoring system, first to replace fingerstick testing for diabetes treatment decision. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm534056.htm (accessed May3, 2017)
- 32.Dexcom: Executive Summary for the Clinical Chemistry and Clinical Toxicology Devices Panel Meeting July 21, 2016. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/ClinicalChemistryandClinicalToxicologyDevicesPanel/UCM511811.pdf (accessed May3, 2017)
- 33.Edelman SV: Regulation Catches Up to Reality: Nonadjunctive Use of Continuous Glucose Monitoring Data. J Diabetes Sci Technol 2017;11:160–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pettus J, Edelman SV: Recommendations for Using Real-Time Continuous Glucose Monitoring (rtCGM) Data for Insulin Adjustments in Type 1 Diabetes. J Diabetes Sci Technol 2016;11:138–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foster NC, Miller KM, Tamborlane WV, et al. : T1D Exchange Clinic Network Continuous Glucose Monitoring in Patients With Type 1 Diabetes Using Insulin Injections. Diabetes Care 2016;39:e81–e82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller K, Foster N, Tamborlane W, et al. : Continuous glucose monitoring in T1D patients using injections of insulin: a report from the T1D Exchange clinic registry. Diabetes Technol Ther 2016;18:A–27. Abstract 069. [Google Scholar]
- 37.van Beers CA, DeVries JH, Kleijer SJ, et al. : Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol 2016;4:893–902 [DOI] [PubMed] [Google Scholar]
- 38.Lind M, Polonsky W, Hirsch IB, et al. : Design and Methods of a Randomized Trial of Continuous Glucose Monitoring in Persons With Type 1 Diabetes With Impaired Glycemic Control Treated With Multiple Daily Insulin Injections (GOLD Study). J Diabetes Sci Technol 2016;10:754–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Little SA, Leelarathna L, Walkinshaw E, et al. : Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care 2014;37:2114–2122 [DOI] [PubMed] [Google Scholar]
- 40.Beck RW, Riddlesworth T, Ruedy K, et al. : Effect of Continuous Glucose Monitoring on Glycemic Control in Adults With Type 1 Diabetes Using Insulin Injections: The DIAMOND Randomized Clinical Trial. JAMA 2017;317:371–378 [DOI] [PubMed] [Google Scholar]
- 41.Lind M, Polonsky W, Hirsch IB, et al. : Continuous Glucose Monitoring vs Conventional Therapy for Glycemic Control in Adults With Type 1 Diabetes Treated With Multiple Daily Insulin Injections: The GOLD Randomized Clinical Trial. JAMA 2017;317:379–387 [DOI] [PubMed] [Google Scholar]
- 42.Bolinder J, Antuna R, Geelhoed-Duijvestijn P, et al. : Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet 2016;388:2254–2263 [DOI] [PubMed] [Google Scholar]
- 43.Chen R, Yogev Y, Ben-Haroush A, et al. : Continuous glucose monitoring for the evaluation and improved control of gestational diabetes mellitus. J Matern Fetal Neonatal Med 2003;14:256–260 [DOI] [PubMed] [Google Scholar]
- 44.Su JB, Wang XQ, Chen JF, et al. : Glycemic variability in gestational diabetes mellitus and its association with β cell function. Endocrine 2013;43:370–375 [DOI] [PubMed] [Google Scholar]
- 45.Mazze R, Yogev Y, Langer O: Measuring glucose exposure and variability using continuous glucose monitoring in normal and abnormal glucose metabolism in pregnancy. J Matern Fetal Neonatal Med 2012;25:1171–1175 [DOI] [PubMed] [Google Scholar]
- 46.Secher AL, Ringholm L, Andersen HU, et al. : The effect of real-time continuous glucose monitoring in pregnant women with diabetes: a randomized controlled trial. Diabetes Care 2013;36:1877–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Law GR, Ellison GT, Secher AL, et al. : Analysis of Continuous Glucose Monitoring in Pregnant Women With Diabetes: Distinct Temporal Patterns of Glucose Associated With Large-for-Gestational-Age Infants. Diabetes Care 2015;38:1319–1325 [DOI] [PubMed] [Google Scholar]
- 48.Feig DS, Asztalos E, Corcoy R, et al. : CONCEPTT: Continuous Glucose Monitoring in Women with Type 1 Diabetes in Pregnancy Trial: a multi-center, multi-national, randomized controlled trial - Study protocol. BMC Pregnancy Childbirth 2016;16:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallia A, Umpierrez GE, Nasraway SA, et al. : Round Table Discussion on Inpatient Use of Continuous Glucose Monitoring at the International Hospital Diabetes Meeting. J Diabetes Sci Technol 2016;10:1174–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Preiser JC, Chase JG, Hovorka R, et al. : Glucose Control in the ICU: A Continuing Story. J Diabetes Sci Technol 2016;10:1372–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thabit H, Hartnell S, Allen JM, et al. : Closed-loop insulin delivery in inpatients with type 2 diabetes: a randomised, parallel-group trial. Lancet Diabetes Endocrinol 2017;5:117–124 [DOI] [PubMed] [Google Scholar]
- 52.Haak T, Hanaire H, Ajjan R, et al. : Flash Glucose-Sensing Technology as a Replacement for Blood Glucose Monitoring for the Management of Insulin-Treated Type 2 Diabetes: a Multicenter, Open-Label Randomized Controlled Trial. Diabetes Ther 2017;8:55–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vigersky R, Shrivastav M: Role of continuous glucose monitoring for type 2 in diabetes management and research. J Diabetes Complications 2017;31:280–287 [DOI] [PubMed] [Google Scholar]
- 54.Mazze RS, Strock E, Wesley D, et al. : Characterizing glucose exposure for individuals with normal glucose tolerance using continuous glucose monitoring and ambulatory glucose profile analysis. Diabetes Technol Ther 2008;10:149–159 [DOI] [PubMed] [Google Scholar]
- 55.Hill NR, Oliver NS, Choudhary P, et al. : Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther 2011;13:921–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salkind SJ, Huizenga R, Fonda SJ, et al. : Glycemic variability in nondiabetic morbidly obese persons: results of an observational study and review of the literature. J Diabetes Sci Technol 2014;8:1042–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma CM, Yin FZ, Wang R, Qin CM, Liu B, Lou DH, Lu Q: Glycemic variability in abdominally obese men with normal glucose tolerance as assessed by continuous glucose monitoring system. Obesity (Silver Spring). 2011. August;19(8):1616–1622. [DOI] [PubMed] [Google Scholar]
- 58.Madhu SV, Muduli SK, Avasthi R: Abnormal glycemic profiles by CGMS in obese first-degree relatives of type 2 diabetes mellitus patients. Diabetes Technol Ther 2013;15:461–465 [DOI] [PubMed] [Google Scholar]
- 59.Chon S, Lee YJ, Fraterrigo G, et al. : Evaluation of glycemic variability in well-controlled type 2 diabetes mellitus. Diabetes Technol Ther 2013;15:455–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zou CC, Liang L, Hong F, Zhao ZY: Glucose metabolism disorder in obese children assessed by continuous glucose monitoring system. World J Pediatr 2008;4:26–30 [DOI] [PubMed] [Google Scholar]
- 61.Rodbard D: Increased glycemic variability at the onset and during progression of type 2 diabetes-commentary Diabetes Technol Ther 2013;15:445–447. [DOI] [PubMed] [Google Scholar]
- 62.Kohnert K-D, Heinke P, Fritzsche G, et al. : Evaluation of the mean absolute glucose change as a measure of glycemic variability using continuous glucose monitoring data. Diabetes Technol Ther 2013;15:448–454 [DOI] [PubMed] [Google Scholar]
- 63.Augstein P, Heinke P, Vogt L, et al. : Q-Score: development of a new metric for continuous glucose monitoring that enables stratification of antihyperglycaemic therapies. BMC Endocr Disord 2015;15:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leinung M, Nardacci E, Patel N, et al. : Benefits of short-term professional continuous glucose monitoring in clinical practice. Diabetes Technol Ther 2013;15:744–747 [DOI] [PubMed] [Google Scholar]
- 65.Munshi MN, Segal AR, Suhl E, et al. : Frequent hypoglycemia among elderly patients with poor glycemic control. Arch Intern Med 2011;171:362–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gehlaut RR, Dogbey GY, Schwartz FL, et al. : Hypoglycemia in Type 2 Diabetes—More Common Than You Think: A Continuous Glucose Monitoring Study. J Diabetes Sci Technol 2015;9:999–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poolsup N, Suksomboon N, Kyaw AM: Systematic review and meta-analysis of the effectiveness of continuous glucose monitoring (CGM) on glucose control in diabetes. Diabetol Metab Syndr 2013;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mazze RS, Strock E, Borgman S, et al. : Evaluating the accuracy, reliability, and clinical applicability of continuous glucose monitoring (CGM): Is CGM ready for real time? Diabetes Technol Ther 2009;11:11–18 [DOI] [PubMed] [Google Scholar]
- 69.Dunn TC, Crouther N: Assessment of the variance of the Ambulatory Glucose Profile over 3 to 20 days of continuous glucose monitoring. 46th European Association for the Study of Diabetes Annual Meeting, Stockholm, 20–24 September 2010. <www.diabetesfrontier.us/ourresources/dunn_easd_2010_31aug10.pdf≥ (accessed January7, 2016)
- 70.Xing D, Kollman C, Beck RW, et al. : Optimal sampling intervals to assess long-term glycemic control using continuous glucose monitoring. Diabetes Technol Ther 2011;13:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neylon OM, Baghurst PA, Cameron FJ: The Minimum Duration of Sensor Data From Which Glycemic Variability Can Be Consistently Assessed. J Diabetes Sci Technol 2014;8:273–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jendle J, Testa MA, Martin S, et al. : Continuous glucose monitoring in patients with type 2 diabetes treated with glucagon-like peptide-1 receptor agonist dulaglutide in combination with prandial insulin lispro: an AWARD-4 substudy. Diabetes Obes Metab 2016;18:999–1005 [DOI] [PubMed] [Google Scholar]
- 73.Heise T, Stender-Petersen K, Hövelmann U, et al. : Pharmacokinetic and pharmacodynamic properties of faster-acting insulin aspart versus insulin aspart across a clinically relevant dose range in subjects with type 1 diabetes mellitus. Clin Pharmacokinet 2016. doi: 10.1007/s40262-016-0473-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bergenstal RM, Bailey TS, Rodbard D, et al. : Comparison of Insulin Glargine 300 U/mL and 100 U/mL in Adults with Type 1 Diabetes: Continuous Glucose Monitoring Profiles and Variability Using Morning or Evening Injections. Diabetes Care 2017;40:554–560 [DOI] [PubMed] [Google Scholar]
- 75.Rodbard HW, Peters AL, Slee A, et al. : The Effect of Canagliflozin, a Sodium Glucose Cotransporter 2 Inhibitor, on Glycemic End Points Assessed by Continuous Glucose Monitoring and Patient-Reported Outcomes Among People With Type 1 Diabetes. Diabetes Care 2017;40:171–180 [DOI] [PubMed] [Google Scholar]
- 76.FLAT-SUGAR Trial Investigators: Glucose Variability in a 26-Week Randomized Comparison of Mealtime Treatment with Rapid-Acting Insulin versus GLP-1 Agonist in Participants with Type 2 Diabetes at High Cardiovascular Risk. Diabetes Care 2016;39:973–981 [DOI] [PubMed] [Google Scholar]
- 77.Bergenstal RM, Strock E, Mazze R, et al. : Diurnal glucose exposure profiles of patients treated with lixisenatide before breakfast or the main meal of the day: an analysis using continuous glucose monitoring. Diabetes Metab Res Rev 2016. [Epub ahead of print]; DOI: 10.1002/dmrr.2879 [DOI] [PubMed] [Google Scholar]
- 78.Mazze RS, Strock ES, Monk AM, et al. : Diurnal glucose profiles using continuous glucose monitoring to identify the glucose-lowering characteristics of colesevelam HCl (Welchol). Endocr Pract 2013;19:275–283 [DOI] [PubMed] [Google Scholar]
- 79.Lee JM, Hirschfeld E, Wedding J: A Patient-Designed Do-It-Yourself Mobile Technology System for Diabetes: Promise and Challenges for a New Era in Medicine. JAMA 2016;315:1447–1448 [DOI] [PubMed] [Google Scholar]
- 80.The Nightscout Project. Nightscout. #WeAreNotWaiting. http://www.nightscout.info/ (accessed May3, 2017)
- 81.Lewis D, Leibrand S; #OpenAPS Community: Real-World Use of Open Source Artificial Pancreas Systems. J Diabetes Sci Technol 2016;10:1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.#WeAreNotWaiting; APS; DIY diabetes technology; OpenAPS; artificial pancreas; closed loop PMID: PMCID: DOI: 10.1177/1932296816665635 https://openaps.org (accessed May3, 2017) [DOI] [PMC free article] [PubMed]
- 83.Albisser AM, Leibel BS, Ewart TG, et al. : Clinical control of diabetes by the artificial pancreas. Diabetes 1974;23:397–404 [DOI] [PubMed] [Google Scholar]
- 84.Albisser AM, Leibel BS, Ewart TG, et al. : An artificial endocrine pancreas. Diabetes 1974;23:389–396 [DOI] [PubMed] [Google Scholar]
- 85.Shichiri M, Kawamori R, Hakui N, et al. : Closed-loop glycemic control with a wearable artificial endocrine pancreas. Variations in daily insulin requirements to glycemic response. Diabetes 1984;33:1200–1202 [DOI] [PubMed] [Google Scholar]
- 86.Kowalski AJ: Can we really close the loop and how soon? Accelerating the availability of an artificial pancreas: a roadmap to better diabetes outcomes. Diabetes Technol Ther 2009;11(Suppl.1):S113–S119 [DOI] [PubMed] [Google Scholar]
- 87.Steil GM, Rebrin K, Darwin C, et al. : Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes 2006;55:3344–3350 [DOI] [PubMed] [Google Scholar]
- 88.Bergenstal RM, Klonoff DC, Garg SK, et al. : Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013;369:224–232 [DOI] [PubMed] [Google Scholar]
- 89.Weiss R, Garg SK, Bode BW, et al. : Hypoglycemia Reduction and Changes in HemoglobinHbA1c in the ASPIRE In-Home Study. Diabetes Technol Ther 2015;17:542–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Agrawal P, Zhong A, Welsh JB, et al. : Retrospective analysis of the real-world use of the threshold suspend feature of sensor-augmented insulin pumps. Diabetes Technol Ther 2015;17:316–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Agrawal P, Welsh JB, Kannard B, et al. : Usage and effectiveness of the low glucose suspend feature of the Medtronic Paradigm Veo insulin pump. J Diabetes Sci Technol 2011;5:1137–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Facchinetti A, Sparacino G, Trifoglio E, Cobelli C: A new index to optimally design and compare continuous glucose monitoring glucose prediction algorithms. Diabetes Technol Ther 2011;13:111–119 [DOI] [PubMed] [Google Scholar]
- 93.Buckingham BA, Raghinaru D, Cameron F, et al. : Predictive low-glucose insulin suspension reduces duration of nocturnal hypoglycemia in children without increasing ketosis. Diabetes Care 2015;38:1197–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Calhoun PM, Buckingham BA, Maahs DM, et al. : Efficacy of an overnight predictive low-glucose suspend system in relation to hypoglycemia risk factors in youth and adults with type 1 diabetes. J Diabetes Sci Technol 2016;10:1216–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Finan DA, McCann TW, Jr, Mackowiak L, et al. : Closed-loop control performance of the Hypoglycemia-Hyperglycemia Minimizer (HHM) System in a feasibility study. J Diabetes Sci Technol 2014;8:35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Finan DA, Dassau E, Breton MD, et al. : Sensitivity of the Predictive Hypoglycemia Minimizer System to the Algorithm Aggressiveness Factor. J Diabetes Sci Technol 2015;10:104–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wolpert HA, Atakov-Castillo A, Smith SA, Steil GM: Dietary fat acutely increases glucose concentrations and insulin requirements in patients with type 1 diabetes: implications for carbohydrate-based bolus dose calculation and intensive diabetes management. Diabetes Care 2013;36:810–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Del Favero S, Place J, Kropff J, et al. : Multicenter outpatient dinner/overnight reduction of hypoglycemia and increased time of glucose in target with a wearable artificial pancreas using modular model predictive control in adults with type 1 diabetes. Diabetes Obes Metab 2015;17:468–476 [DOI] [PubMed] [Google Scholar]
- 99.Tauschmann M, Allen JM, Wilinska ME, et al. : Day-and-Night Hybrid Closed-Loop Insulin Delivery in Adolescents With Type 1 Diabetes: A Free-Living, Randomized Clinical Trial. Diabetes Care 2016;39:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tauschmann M, Allen JM, Wilinska ME, et al. : Home Use of Day-and-Night Hybrid Closed-Loop Insulin Delivery in Suboptimally Controlled Adolescents With Type 1 Diabetes: A 3-Week, Free-Living, Randomized Crossover Trial. Diabetes Care 2016;39:2019–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grosman B, Ilany J, Roy A, et al. : Hybrid Closed-Loop Insulin Delivery in Type 1 Diabetes During Supervised Outpatient Conditions. J Diabetes Sci Technol 2016;10:708–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ly TT, Weinzimer SA, Maahs DM, et al. : Automated hybrid closed-loop control with a proportional-integral-derivative based system in adolescents and adults with type 1 diabetes: individualizing settings for optimal performance. Pediatr Diabetes 2016. [Epub ahead of print]; DOI: 10.1111/pedi.12399 [DOI] [PubMed] [Google Scholar]
- 103.Thabit H, Hartnell S, Allen JM, et al. : Closed-loop insulin delivery in inpatients with type 2 diabetes: a randomised, parallel-group trial. Lancet Diabetes Endocrinol 2017;5:117–124 [DOI] [PubMed] [Google Scholar]
- 104.Stewart ZA, Wilinska ME, Hartnell S, et al. : Closed-Loop Insulin Delivery during Pregnancy in Women with Type 1 Diabetes. N Engl J Med 2016;375:644–654 [DOI] [PubMed] [Google Scholar]
- 105.Thabit H, Hovorka R: Coming of age: the artificial pancreas for type 1 diabetes. Diabetologia 2016;59:1795–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maahs DM, Buckingham BA, Castle JR, et al. : Outcome measures for artificial pancreas clinical trials: a consensus report. Diabetes Care 2016;39:1175–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kovatchev B, Cheng P, Anderson SM, et al. : Feasibility of Long-Term Closed-Loop Control: A Multicenter 6-Month Trial of 24/7 Automated Insulin Delivery. Diabetes Technol Ther 2017;19:18–24 [DOI] [PubMed] [Google Scholar]
- 108.Renard E, Farret A, Kropff J, et al. : Day-and-Night Closed-Loop Glucose Control in Patients With Type 1 Diabetes Under Free-Living Conditions: Results of a Single-Arm 1-Month Experience Compared With a Previously Reported Feasibility Study of Evening and Night at Home. Diabetes Care 2016;39:1151–1160 [DOI] [PubMed] [Google Scholar]
- 109.Sharifi A, De Bock MI, Jayawardene D, et al. : Glycemia, Treatment. Satisfaction, Cognition, and Sleep Quality in Adults and Adolescents with Type 1 Diabetes When Using a Closed-Loop System Overnight Versus Sensor-Augmented Pump with Low-Glucose Suspend Function: A Randomized Crossover Study. Diabetes Technol Ther 2016;18:772–783 [DOI] [PubMed] [Google Scholar]
- 110.Patel NS, Van Name MA, Cengiz E, et al. : Mitigating Reductions in Glucose During Exercise on Closed-Loop Insulin Delivery: The Ex-Snacks Study. Diabetes Technol Ther 2016;18:794–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nimri R, Bratina N, Kordonouri O, et al. : MD-Logic Overnight Type 1 Diabetes Control in Home Settings: Multicenter, Multinational, Single blind, Randomized Trial. Diabetes Obes Metab 2017;19:553–561 [DOI] [PubMed] [Google Scholar]
- 112.Bergenstal RM, Garg S, Weinzimer SA, et al. : Safety of a Hybrid Closed-Loop Insulin Delivery System in Patients With Type 1 Diabetes. JAMA 2016;316:1407–1408. (Also, Abstract LB99, American Diabetes Association meeting, 2016). http://app.core-apps.com/tristar_ada16/abstract/e44e3c20f4b9cf62bd1796c12c1cbb77 (accessed January8, 2016). https://ada.scientificposters.com/epsView.cfm?H%2BvYJa9uw%2Fhd865Iip7taZv%2BTNZ17E6JZR%2BpsmgVpvYUsfb%2FTDImzQ%3D%3D (accessed January 21, 2016) [DOI] [PubMed] [Google Scholar]
- 113.Garg SK, Weinzimer SA, Tamborlane WV, et al. : Glucose Outcomes with the In-Home Use of a Hybrid Closed-Loop Insulin Delivery System in Adolescents and Adults with Type 1 Diabetes. Diabetes Technol Ther 2017;19:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.JDRF Celebrates Historic Artificial Pancreas Success Bringing Life-changing Benefits to People with Type 1 Diabetes, FDA Approves Medtronic Hybrid Closed Loop System www2.jdrf.org/site/Donation2?18761.donation=form1df_id=18761s_src=jdrf.orgs_subsrc=PPCutm_source=Purutm_campaign=160928_APutm_medium=PPCutm_content=Ad2utm_term=ArtificialPancreasSystemgclid=CjwKEAiAkuLDBRCRguCgvITww0YSJAAHrpf-gmhcPPNRyBsET-KPFsRzU9N5EiRnowljzqo7EvXPdRoC4Vvw_wcB (accessed January8, 2016)
- 115.FDA approves first automated insulin delivery …<www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522974.htm> (accessed January8, 2016)
- 116.Thabit H, Hartnell S, Allen JM, et al. : Closed-loop insulin delivery in inpatients with type 2 diabetes: a randomised, parallel-group trial. Lancet Diabetes Endocrinol 2017;5:117–124 [DOI] [PubMed] [Google Scholar]
- 117.Bally L, Thabit H, Kojzar H, et al. : Day-and-night glycaemic control with closed-loop insulin delivery versus conventional insulin pump therapy in free-living adults with well controlled type 1 diabetes: an open-label, randomised, crossover study. Lancet Diabetes Endocrinol 2017;5:261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Castle JR, Engle JM, El Youssef J, et al. : Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care 2010;33:1282–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.El-Khatib FH, Jiang J, Damiano ER: Adaptive closed-loop control provides blood-glucose regulation using dual subcutaneous insulin and glucagon infusion in diabetic Swine. J Diabetes Sci Technol 2007;1:181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ward WK, Engle J, Duman HM, et al. : The benefit of subcutaneous glucagon during closed-loop glycemic control in rats with type 1 diabetes. IEEE Sensors J 2008;8:89–96 [Google Scholar]
- 121.El-Khatib FH, Russell SJ, Nathan DM, et al. : A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med 2010;2:27ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ward WK, Castle JR, El Youssef J: Safe glycemic management during closed-loop treatment of type 1 diabetes: the role of glucagon, use of multiple sensors, and compensation for stress hyperglycemia. J Diabetes Sci Technol 20111;5:1373–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Russell SJ, El-Khatib FH, Sinha M, et al. : Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014;371:313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.El-Khatib FH, Balliro C, Hillard MA, et al. : Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. Lancet 2017;389:369–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gingras V, Rabasa-Lhoret R, Messier V, et al. : Efficacy of dual-hormone artificial pancreas to alleviate the carbohydrate-counting burden of type 1 diabetes: A randomized crossover trial. Diabetes Metab 2016;42:47–54 [DOI] [PubMed] [Google Scholar]
- 126.Giorda CB, Rossi MC, Ozzello O, et al. : Healthcare resource use, direct and indirect costs of hypoglycemia in type 1 and type 2 diabetes, and nationwide projections: Results of the HYPOS-1 study. Nutr Metab Cardiovasc Dis 2017;27:209–216 [DOI] [PubMed] [Google Scholar]
- 127.Zhao Y, Shi Q, Wang Y, et al. : Economic burden of hypoglycemia: Utilization of emergency department and outpatient services in the United States (2005–2009). Med Econ 2016;19:852–857 [DOI] [PubMed] [Google Scholar]
- 128.Geller AI, Shehab N, Lovegrove MC, et al. : National estimates of insulin-related hypoglycemia and errors leading to emergency department visits and hospitalizations. JAMA Intern Med 2014;174:678–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Quilliam BJ, Simeone JC, Ozbay AB, Kogut SJ: The incidence and costs of hypoglycemia in type 2 diabetes. Am J Manag Care 2011;17:673–680 [PubMed] [Google Scholar]
- 130.Fulcher G, Singer J, Castañeda R, et al. : The psychosocial and financial impact of non-severe hypoglycemic events on people with diabetes: two international surveys. J Med Econ 2014;17:751–761 [DOI] [PubMed] [Google Scholar]
- 131.Brod M, Wolden M, Groleau D, Bushnell DM: Understanding the economic, daily functioning, and diabetes management burden of non-severe nocturnal hypoglycemic events in Canada: differences between type 1 and type 2. J Med Econ 2014;17:11–20 [DOI] [PubMed] [Google Scholar]
- 132.Brod M, Wolden M, Christensen T, Bushnell DM: Understanding the economic burden of nonsevere nocturnal hypoglycemic events: impact on work productivity, disease management, and resource utilization. Value Health 2013;16:1140–1149 [DOI] [PubMed] [Google Scholar]
- 133.Fonda SJ, Graham C, Munakata J, et al. : The Cost-Effectiveness of Real-Time Continuous Glucose Monitoring (RT-CGM) in Type 2 Diabetes. J Diabetes Sci Technol 2016;10:898–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Graham C: Continuous glucose monitoring and global reimbursement: an update. Diabetes Technol. Ther 2017;19(Suppl 3):S-60–S-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Parkin CG, Graham C, Smolskis J: Continuous glucose monitoring use in type 1 diabetes: longitudinal analysis demonstrates meaningful improvements in HbA1c and reductions in health care utilization. J Diabetes Sci Technol 2017;11(3): doi: 10.1177/19322968117693253 (accessed May3, 2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pernick NL, Rodbard D: Personal computer programs to assist with self-monitoring of blood glucose and self-adjustment of insulin dosage. Diabetes Care 1986;9:61–69 [DOI] [PubMed] [Google Scholar]
- 137.Mazze RS, Lucido D, Langer O, et al. : Ambulatory glucose profile: representation of verified self-monitored blood glucose data. Diabetes Care 1987;10:111–117 [DOI] [PubMed] [Google Scholar]
- 138.Rodbard D: Potential role of computers in clinical investigation and management of diabetes mellitus. Diabetes Care 1988;11(Suppl.1):54–61 [PubMed] [Google Scholar]
- 139.Bergenstal RM, Ahmann AJ, Bailey T, et al. : Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the Ambulatory Glucose Profile (AGP). Diabetes Technol Ther 2013;15:198–211 [DOI] [PubMed] [Google Scholar]
- 140.Rodbard D: Standardization versus customization of glucose reporting. Diabetes Technol Ther 2013;15:439–443 [DOI] [PubMed] [Google Scholar]
- 141.Rodbard D: Evaluating quality of glycemic control: graphical displays of hypo- and hyperglycemia, time in target range, and mean glucose. J Diabetes Sci Technol 2015;9:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kropff J, Choudhary P, Neupane S, et al. : Accuracy and Longevity of an Implantable Continuous Glucose Sensor in the PRECISE Study: A 180-Day, Prospective, Multicenter, Pivotal Trial. Diabetes Care 2017;40:63–68 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.