Abstract
The 57th annual Thomas L. Petty Aspen Lung Conference, entitled “Rebuilding the Injured Lung,” was held from June 4 to 7, 2014 at the Gant Conference Center in Aspen, Colorado. Investigators from a wide range of disciplines and perspectives convened to discuss the biology of lung injury, how the lung repairs itself, how and why repair fails, and how the repair process can be enhanced. Among the challenges identified in the course of the conference was how to develop more predictive experimental models that capture the multidimensional complexity of lung injury and repair in a tractable manner. From such approaches that successfully fuse the biological and physical sciences, the group envisioned that new therapies for acute and chronic lung injury would emerge. The discussion of experimental therapeutics ranged from pharmaceuticals and cells that interdict fibrosis and enhance repair to a de novo lung derived from stem cells repopulating a decellularized matrix.
Keywords: biomechanics, injury, repair, regeneration, fibrosis
I would like to thank the conference organizers (Chair, David Riches; Co-chairs Ellen Burnham, Gregory Downey, Marc Moss, and Eric Schmidt) and benefactors for the honor of serving as summarizer. As I begin this task, I am guided by the authoritative and inspirational book written by Tom Petty, entitled History of the Aspen Lung Conference (1). In it, Tom chronicled what can be considered the modern history of pulmonary medicine. It is an anthology of paradigms established—and paradigms shifted—by an interdisciplinary assembly of clinicians and scientists dedicated to improving the lives of patients with lung disease. This year’s conference is no exception to that tradition. It includes contributions from scientists across the spectrum of modern biomedical science, striving to understand the intricacies of acute and chronic lung injury, how the lung repairs itself, and how we might assist the process of lung repair. This scientific scope, encompassing novel and exciting data from across the physical and biological sciences, makes creating a meaningful synthesis a serious challenge. In accepting this challenge, I would like to begin with a quote from a former chair of my Department of Medicine at the University of Minnesota, Dick Ebert, who served as the summarizer for the inaugural Aspen Emphysema Conference (as it was known then in 1957, until its renaming as the Aspen Lung Conference in 1974). Dick wrote, “I think it will be a little difficult to summarize this conference. One of its strengths is that so many divergent views were presented.”
With Dick’s prescient comments in mind, I offer this summary. It is based on the knowledge provided by the legion of pulmonary scientists represented in the preceding 56 conferences (I should note parenthetically that my first Aspen Lung Conference was the 23rd, held in 1980, entitled “The Environment and the Lung,” summarized by Margaret Becklake) and is offered with the humility that comes from more than 3 decades of experience in the field of lung injury, fibrosis, and repair. Here, I will do my best to honor this heritage and accurately represent the conference content, albeit with some editorial license.
I begin by highlighting some important themes that emerged during the conference, reflecting the contribution of investigators across the wide range of disciplines represented (Figure 1).
Figure 1.
Rebuilding the injured lung: central themes. The importance of biomechanics and the collaboration between extracellular matrix (ECM) and cell in shaping tissue and organ function received considerable attention, as more crossover scientists (i.e., hybrid physical and biological scientists) bring computational and engineering approaches to understanding form, function, and pathobiology. Fibrosis is hard to stop and reverse because it is driven by a robust network of positive feedback and feed-forward loops that are just beginning to come into focus. The comment about homeostasis being the preferred state emerged in several talks and posters as a strong counter to what many perceived as widespread therapeutic nihilism about arresting or even reversing fibrosis or about promoting lung regeneration. A corollary is that understanding the natural mechanisms involved when injury is effectively repaired and controlled may be one path forward to new therapeutic approaches for acute and chronic lung injury. This includes the immune system and stem and progenitor cells. These natural mechanisms and their response to our interventions are strongly influenced by each individual’s genetic makeup, and we are just beginning to learn how to incorporate OMICs into our clinical trial designs.
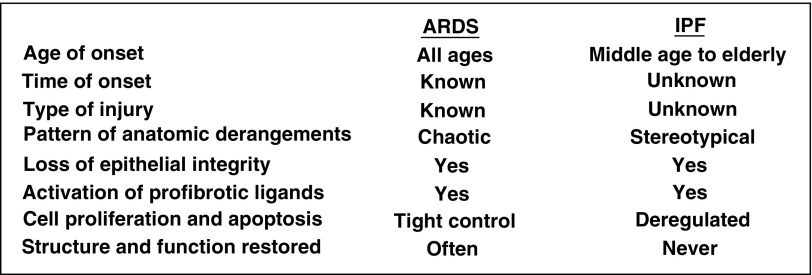
As these themes resonated throughout the conference, several presentations unveiled gaps in our knowledge about lung biology that we need to address if we are to discover promising new therapeutic targets to speed lung repair and reverse fibrosis. As examples, here I will highlight two. The first is whether chronic lung injury as observed in idiopathic pulmonary fibrosis (IPF) or chronic obstructive pulmonary disease (COPD) is accurately conceptualized as a series of discrete low-intensity acute injuries that accumulate over time or whether the nature of the injury and response are fundamentally different than in acute injury. The second is our incomplete understanding of the mechanisms determining which injury-induced anatomic derangements are reversible and which are not. In conceptualizing these knowledge gaps, it is instructive to compare features of prototype acute and chronic lung disorders associated with epithelial injury and expansion of stromal cells and the extracellular matrix (ECM), the acute respiratory distress syndrome (ARDS), and IPF (Figure 2).
Figure 2.
Comparison of a prototypical acute lung disorder, acute respiratory distress syndrome (ARDS), with a chronic disorder, idiopathic pulmonary fibrosis (IPF). These conditions highlight two major unresolved questions in the field of lung injury. (1) Are acute and chronic injury qualitatively different or do they just differ in degree and kinetics? (2) Because even catastrophic insults such as ARDS can heal, whereas IPF apparently never does (at least not once it is diagnosed as such), it remains to be determined which injury-induced anatomic derangements are reversible and which are not.
In the course of this summary, I attempt to emphasize data and enabling technologies presented at this conference that advance our understanding of the key biological properties distinguishing acute from chronic injuries and reversible from irreversible anatomic changes to the lung.
Mechanisms of Acute Lung Injury
Mechanical Ventilation: Too Much or Too Little of a Good Thing
Marco Ranieri convened the conference with a discussion of the relationships among physical and biological events in the development and perpetuation of lung injury by positive pressure mechanical ventilation. Marco emphasized the role played by lung inhomogeneity in amplifying injury due to regional overdistension or atelectrauma (i.e., the repetitive cycles of airway opening and closing that occur with regional underventilation). In addition to direct mechanical injury, regional over- or underdistention causes alveolar epithelial and endothelial injury with resultant activation of inflammatory cascades that further amplify the injury. Even in cases of the most severe acute lung injury, ventilatory strategies that minimize overdistension and alveolar collapse can be accompanied by complete resolution of the injury. Don Gaver provided primary data that supported these concepts in a talk focused on combining computational modeling, microparticle-based velocity measurements, and biomimetic airway constructs to the problem of atelectrauma. His group found that interfacial flows and injury were directly related and that pulsatile flow can ameliorate the injury by enhancing surfactant distribution and function.
Injury and Repair: Multifaceted Processes with Precise Choreography
Peter Henson provided the group with a thoughtful review of the cellular context for acute lung injury, grounded in the fundamentals of anatomy and the innate immune response. The response to lung injury is precisely choreographed in time and space, leading to resolution of inflammation and fibrosis with restoration of structure and function. Perturbations of cell–cell interactions or to components of the inflammatory cascade that add, subtract, accelerate, or delay any component can have disastrous consequences. In the two talks that followed Peter’s presentation, the speakers focused on different phases of resolution after acute lung injury. In the first, Sekhar Reddy studied the role of the transcription factor NRF2 using NRF2 knockout mice exposed to hyperoxic stress. The Reddy group found that NRF2 was necessary for macrophage-mediated neutrophil efferocytosis and timely resolution of neutrophilic inflammation and that the mechanism involved decreased macrophage expression of the scavenger receptors required for efferocytosis. In the second, Elizabeth Redente examined resolution of fibrosis after bleomycin-induced acute lung injury in mice and found that exogenous tumor necrosis factor (TNF)-α decreases fibrosis. Companion in vitro studies revealed that fibroblasts isolated from resolving lungs were more susceptible to apoptosis and that TNF-α shifts the phenotype of fibrogenic lung myofibroblasts toward more apoptosis-prone lipofibroblasts.
Mechanisms of Progressive Lung Injury and Fibrosis
Initiation and Perpetuation of Fibrosis: Linking Molecular Mechanisms with Experimental Therapeutics
Rachel Chambers provided a comprehensive overview of IPF pathogenesis with a strong focus on how mechanistic insights have informed current and planned clinical trials. Rachel provided one example in which the high-affinity thrombin receptor, proteinase activator-1 (PAR-1), mediates crosstalk between the coagulation system and transforming growth factor (TGF)-β signaling. When activated, PAR-1 signaling in epithelial cells and fibroblasts leads to integrin-mediated TGF-β activation. This knowledge forms the rationale for considering trials of PAR-1 antagonists in IPF. A second example relates to the striking similarity of IPF fibroblasts to cancer. Although the pathological phenotype is not mediated by known somatic mutations as is the case in cancer, IPF fibroblasts are in many ways a cancer phenocopy likely due to epigenetic alterations. They are invasive, hyperproliferative, and apoptosis resistant, manifesting a deficiency of the tumor suppressor PTEN and activation of the PI3K/Akt/mTOR signaling cascade. These striking similarities motivated the successful clinical trial of nintedanib (2), a multifunctional kinase inhibitor developed as an anticancer agent, along with other ongoing trials of anticancer agents. As in cancer, where it is known that only some of the cells in a tumor have progenitor properties and are tumorigenic, a recent study in IPF indicates that there are fibrogenic mesenchymal progenitor cells among the fibroblasts populating the fibrotic lung (3). This finding has important therapeutic implications.
Dean Sheppard advanced the theme of progressive fibrosis with an authoritative discussion of a critical point of vulnerability in the TGF-β profibrotic pathway involving αv-integrins that has applicability across many forms of organ fibrosis. The αv-integrin family plays a central role in the untethering of inactive TGF-β from latency-associated protein. When expressed with a partner β-integrin on epithelial cells or myofibroblasts, αv-integrin liberates active TGF-β, which can initiate and amplify a profibrotic signaling cascade. Once initiated, an embedded positive feedback loop is activated: TGF-β drives myofibroblast collagen synthesis, which stiffens the surrounding ECM, which in turn amplifies myofibroblast contractile forces, resulting in more activation of latent TGF-β. These data highlight the rationale to target αv-integrin–mediated activation of TGF-β in fibrotic disorders, an approach that is now being advanced toward clinical application.
The accompanying talks in this session highlighted molecular mechanisms that could be harnessed to enhance lung repair after acute injury. Xiaoli Zhao presented data implicating TRIM72 in the repair of epithelial cell plasma membrane damage, using TRIM72 wild-type, knockout, and conditionally overexpressing mice. The theme of restoring epithelial integrity was further advanced by the next presentations. In the first, Rachel Zemans showed that the transcription factor HIF1α accelerated recovery of barrier function in a mouse model of keratinocyte-derived chemokine-induced lung injury. Companion in vitro studies implicated transcriptional activation of CXCR4 and its ligand SDF1. In the second, Xinping Yue used a conditional knockout mouse to identify a role for Sulf2 in the ability of type II epithelial cells to repair bleomycin-induced lung injury. Benjamin Singer explored a novel approach to enhancing lung repair. Using an LPS mouse model, his group showed that the DNA methyltransferase inhibitor 5-aza-2′-deoxycytidine decreased mortality and established that Tregs were the target cells mediating the effect.
Mesenchyme, Matrix, and Mechanotransduction
In the ECM Jungle: The Hippopotamus Rules
Dan Tschumperlin detailed key molecular mechanisms by which mesenchymal cells sense and transduce the topography, chemistry, and mechanical properties of the ECM into functional outputs. Hippo, the master regulator of organ size during development, served to illustrate several of the important principles (4). When activated downstream of G-protein–coupled receptors, the Hippo kinase cascade restrains cell growth and proliferation by inactivating a positive transcriptional regulatory network mediated by YAP and TAZ. Focusing on integrins and their interaction with synthetic ECM of differing stiffness, Dan outlined an alternative means to regulate YAP and TAZ in which ECM stiffness per se governs the subcellular localization of these critical transcriptional regulators through an integrin-initiated signaling cascade involving focal adhesion kinase and Rock/Rho. When fibroblasts reside on stiff matrices, YAP and TAZ are localized to the nucleus, and fibroblasts manifest enhanced contractility and proliferation in vitro and fibrogenicity in vivo in a YAP/TAZ-dependent manner. The clear implication of Dan’s presentation was that rebuilding the injured lung may require reprogramming cells to deposit a physiological ECM.
Picking up on the theme of cell reprogramming, Hesham Basma addressed the degree to which epigenetic changes shape fibroblast gene expression and phenotype using lung fibroblasts from patients with COPD and control subjects as the experimental system. After reprogramming control and COPD lung fibroblasts into iPSCs and permitting redifferentiation into fibroblasts, gene expression and functional differences observed in the primary cells were nearly completely ablated. This strongly implicates epigenetic changes in the COPD fibroblast phenotype. Lynn Schnapp followed with a talk focused on the role of perivascular mesenchymal cell α8-integrin in lung injury. Using the mouse model of bleomycin lung injury and a Cre-Lox system to delete perivascular mesenchymal cell α8-integrin, Lynn reported worse barrier function and a trend toward increased mortality in the α8-null state. However, in surviving mice, there was significant attenuation of hydroxyproline accumulation when α8 was deleted. This result highlights the critical role of integrin-mediated mesenchymal cell–ECM interactions in dictating lung injury outcome.
The Matrix Is Indeed the Message
Eric White presented a compelling argument for using decellularized lung ECM as a platform for studying human lung cell biology in health and disease. It is challenging to capture the mechanical microheterogeneity of the lung parenchyma with current synthetic matrix models of uniform stiffness. Over a distance of a few hundred micrometers, the elastic modulus ranges across one order of magnitude in normal lung and across three orders of magnitude in fibrotic lung. The value of decellularized ECM is that it preserves the topography, chemical composition, and mechanical properties of the intact lung. As a result, many disease-relevant properties of primary human lung fibroblasts can be studied using this system. For example, myofibroblast differentiation as reflected by α-smooth muscle actin expression is promoted by decellularized IPF lung ECM but not normal lung ECM. A general principle that emerged is the powerful influence of the ECM on cell function, location, and phenotype. A corollary of this is that we should exercise caution when extrapolating the salutary effects of cellular therapies, such as mesenchymal stem cells in acute injury settings, to a setting in which the administered cells will reside in a profoundly altered ECM.
The two accompanying talks examined how cells sense their matrix environment. In a study to identify a fibroblast mechanosensor that mediates fibrosis, Mitch Olman focused on TRPV4, a calcium ion channel known to be responsive to stretch. He reported that TRPV4 is required for myofibroblast differentiation in response to TGF-β, matrix stiffness, or fibrotic tissue and that TRPV4 deficiency in mice attenuates the fibrotic response to bleomycin. These data identify TRPV4 as a core fibroblast mechanosensor. The antifibrotic microRNA miR-29 is down-regulated by decellularized IPF lung ECM, but the sensing mechanism is undefined. Jeremy Herrera reported that stiff synthetic matrices actually increase fibroblast miR-29 with a concomitant decrease in its target collagen I, exactly the opposite of what occurs on IPF ECM. Instead, Jeremy found that α2-integrin was required for the inhibition of miR-29 and the increase in collagen I on IPF ECM. These results showed that stiff synthetic matrices initiate a negative feedback loop in which increased miR-29 shuts down collagen I production, whereas IPF ECM initiates a pathological positive feedback loop in which decreased miR-29 fails to terminate collagen I production.
Tissue Scaffolds and Regeneration
IPF and Chronic Lung Allograft Rejection: The Case for Developing a Parts List
In an effort to identify core fibrotic pathways, Oliver Eickelberg reviewed the state of the art in progressive airway and parenchymal fibrosis. Both processes include epithelial stress, in chronic lung allograft rejection (CLAD) from immune injury and in IPF from several putative sources, eventually leading to epithelial cell dysfunction and death. In CLAD, well-defined innate and adaptive immune responses are important drivers of fibrosis, whereas in IPF the role of immune mechanisms is less well defined. In both situations, fibrogenic mesenchymal progenitor cells are implicated in the expansion of the fibroblast population and generation of myofibroblasts. Biomarkers for fibrosis are a work in progress. There is support for the MUC5B genotype and MMP7 blood levels in IPF but no validated biomarkers in CLAD. Based on the current state of knowledge, Oliver made a strong case for a rigorously obtained parts list of fibrosis including quantitative data about the transcriptome, proteome, and matrisome.
To complement this review of fibrogenesis, the accompanying talks focused on reversibility of fibrosis. Based on the expanded network of lymphatics observed in fibrotic lungs, Abigail Lara used the bleomycin mouse model of fibrosis to show that vascular endothelial cell growth factor receptor blocking antibodies antagonize lymphangiogenesis and attenuate fibrosis. Yu-chun Lin sought to reverse fibrosis by simultaneously targeting myofibroblasts and the collagen cross-linking enzyme lysyl oxidase. Using a mouse tracheal transplant model, Yu-chun showed that combined treatment with Relaxin and β-aminopropionitrile diminished airway fibrosis and promoted airway reepithelialization.
Lung on a Chip
In an introduction to next-generation tissue culture, Don Huh presented a powerful new microsystem that combines multiple cell types, vascular conduits, physiological surfaces, mechanical forces, advanced optical imaging, and microfluidics to model a living, breathing lung with a bona fide alveolar–capillary interface. Examples of situations that could be modeled included bacterial infections, nanotoxicology, and pulmonary edema in response to IL-2. Don suggested that this system could be used as an alternative to—and to prioritize and streamline—preclinical and clinical studies for biomedical, pharmaceutical, and environmental applications. The accompanying talks provided examples of specific molecular mechanisms that could be examined in such a system. Studying the effect of alcohol on lung barrier function, Michael Koval showed that the tight junction protein claudin 5 was increased by alcohol and that this led to disruption of tight junctions by preventing the association of claudin 18 with ZO-1. Rama Mallampalli, examining the mechanism of mitochondrial injury in Staphylococcus aureus–induced acute lung injury in vitro, showed that S. aureus activates ubiquitin-mediated degradation of the enzyme generating the mitochondrial-specific lipid cardiolipin. Companion in vivo studies showed that mice lacking a key component of the degradation machinery were protected from S. aureus–induced lung injury.
Rebuilding the Lung
The Matrix Is the Message—Or Is It? Part 2
Harold Ott provided an authoritative recounting of the current state of the field of engineering a bioartificial lung. He summarized proof of principle experiments establishing that a decellularized lung could serve as a scaffold for progenitor cells to form a lung that could be transplanted into a recipient and carry out gas exchange. Harold also carefully reviewed the limitations of current empiric approaches, which are a long distance from yielding a structurally normal lung. Much needs to be learned about the appropriate progenitor cell types and their niches to move the field forward. A particularly large gap in knowledge relates to whether progenitor cells can use a mature adult lung matrix to repopulate the lung in a manner that restores normal structure and function. Understanding the crosstalk between matrix and progenitor cell during self-renewal, asymmetric cell division, and differentiation will be critical if the goal of off-the-shelf lungs is to be realized. Examining pathways relevant to lung organogenesis, Melanie Konigshoff presented data using human emphysema lung slices showing that induction of Wnt/b-catenin signaling led to increased expression of alveolar epithelial proteins such as surfactant protein C. Stijn De Langhe followed with an examination of the relationships among integrin-linked kinase (ILK), matrix rigidity, and Hippo pathway maintenance of airway stem cell quiescence. Matrix-controlled airway epithelial cell ILK resides at the apex of a pathway that maintains a hippo “on” state to preserve homeostasis. Loss of ILK silences the Hippo pathway, which transcriptionally activates epithelial cell release of Wnt7b leading to smooth muscle cell release of Fgf10, which in turn stimulates airway stem cell proliferation.
It Is Both the Niche and the Knowledge
Darrel Kotton advanced the theme of missing data in the lung regeneration field and made a compelling argument for systematic, carefully conducted studies using well-characterized stem and progenitor cell populations. Lessons from developmental biology teach us that organogenesis—and by inference regeneration—is mediated by stem cells residing in highly structured niches with precisely balanced cell populations distributed along a differentiation hierarchy, interacting with a dynamically changing extracellular milieu containing morphogens, cytokines, and matrix macromolecules (5). In development, if any steps are delayed or out of order, the system fails. Darrel made a strong case for more basic science knowledge before giving the general public the mistaken impression that stem cell science is almost there. Sarah Gilpin presented evidence that expansion of human iPSC-derived endothelial and epithelial precursors could be achieved on decellularized lung scaffolds in biomimetic culture and that further expansion and differentiation ensued after orthotopic transplantation. Luis Ortiz focused on the salutary effects of mesenchymal stem cells (MSCs). Using the bleomycin mouse model of fibrogenic lung injury, which is accompanied by right ventricular dysfunction, Luis showed that administration of MSCs or a component of their secretome, the hyaluronan-binding protein TSG-6, significantly ameliorated the cardiac pathology.
Strategic Approaches to Improving therapy
Can We Harness the Healing Power of MSCs in the Acutely Injured Lung?
Michael Matthay provided an overview of MSC biology and detailed the compelling rationale and preliminary studies that motivated the first in humans phase 1 and 2 clinical trials. Foundational data included basic cell and molecular biology of putative mechanisms including paracrine release of keratinocyte growth factor and mitochondrial transfer to the lung epithelium; preclinical studies in mouse and sheep models; and, finally, information from phase 1/2 clinical trials. A lively discussion ensued about important differences between the progressive fibrosis seen in IPF and the reversible, wound-like expansion of myofibroblasts seen in ARDS. Although all points of disagreement were not resolved, there was consensus that MSC therapy in humans with acute organ injury is a highly promising experimental direction to pursue for the treatment of many disorders for which supportive care is currently the only option. As a counterpoint, caution is needed when extrapolating these findings to IPF or other forms of progressive fibrosis.
Continuing the theme of experimental therapeutics for lung injury and fibrosis, Lorraine Ware and Richard Gomer presented the results of early-phase clinical trials. In a phase 2 study, Lorraine examined acetaminophen for severe sepsis, the most common cause of ARDS, a trial motivated by prior work showing cell-free hemoglobin in the airspaces of patients with ARDS and the ability of acetaminophen to mitigate its ability to mediate oxidant injury. In all patients enrolled and in the subgroup with ARDS, acetaminophen attenuated oxidant injury and improved kidney function. Richard showed preclinical data in rodent models of lung injury that serum amyloid P attenuates neutrophil influx, promotes M2 macrophage differentiation, and attenuates fibrosis. In a phase 1b trial in pulmonary fibrosis, serum amyloid P improved lung function. These promising gateway studies are being moved forward to larger, late-phase trials.
Designing a Good Clinical Trial Is a Serious Challenge… and Be Mindful of Genetics
Gordon Reubenfeld discussed the basics of clinical trial design and interpretation of the resultant data using ARDS as the paradigm. He began with the current standard, the randomized (blinded, when feasible) trial design, and reviewed the importance of choosing the right sample size and optimizing the treatment effect to noise ratio by tuning the intervention, standardizing ancillary care, and centralizing adjudication. He reminded the group that a trial can be negative because the intervention does not work, but can also be negative because the sample or effect size was too small or the noise was too large. Beyond this, Reuben discussed some alternative study designs that might be suitable for trials in the critical care unit. Particularly attractive to patients and clinicians are adaptive trial designs that allow modifications to the design and analysis plan based on results as they accrue. Of course, adaptive designs have their statistical price but are particularly efficient when the primary and secondary outcomes can be ascertained quickly, which is often the case in critical care clinical trials.
One emerging area of importance in trial design is prior knowledge about a subject’s genetic make-up, enabling far more precise risk stratification and pharmacogenomic inferences to guide eligibility, treatment assignment, and treatment dose/schedule. Earlier in the conference, Judie Howrylak foreshadowed this concept, presenting a transcriptional profile that distinguishes pediatric and adult patients with sepsis who go on to develop ARDS from those who do not. Mark Wurfel presented a genomewide association study for ARDS risk based on data (from the identification of SNPs Predisposing to Altered ALI Risk Consortium) and identified an intronic variant of FARP1 as a novel ARDS risk locus. These data have important implications for trials designed to interdict ARDS in high-risk patients. Based on the hypothesis that ARDS is a heterogeneous disease cluster, Carolyn Calfee applied latent class modeling to analyze patients enrolled in two independent randomized trials. Carolyn reported that two classes emerged in both trials, with one class having higher levels of inflammatory biomarkers, supportive care needs, prevalence of sepsis as the antecedent condition, and mortality. In one trial, the treatment effect differed significantly by class. It is becoming clear that a trial design team that does not incorporate genetic/genomic/pharmacogenomics data will do so at their peril.
Concluding Remarks
In many ways, this conference unveiled the fault line between how we conceptualized lung disease prior to—and how we conceptualize lung disease after—the widespread availability of advanced OMICs tools and biomimetic ex vivo tissue culture systems to study lung biology. As “classical” molecular, cellular, and developmental biology approaches converge with next-generation technologies, it is apparent that we are well positioned to advance toward our goal of reversing fibrosis and rebuilding the injured lung. In doing so, the important biological differences between the sequelae of acute and chronic lung injury will need to be elucidated and accounted for, as will any differences in the types and properties of the cell populations and the ECM found in the two settings. As we approach this goal, in addition to the obvious scientific challenges to be met is the challenge of improving our ability to communicate scientific findings to policy makers and the public with clarity, optimism, and objectivity.
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Petty TL History of the aspen lung conference. 2005. Aspen, CO: Snowdrift Pulmonary. [Google Scholar]
- 2.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 3.Xia H, Bodempudi V, Benyumov A, Hergert P, Tank D, Herrera J, Braziunas J, Larsson O, Parker M, Rossi D, et al. Identification of a cell-of-origin for fibroblasts comprising the fibrotic reticulum in idiopathic pulmonary fibrosis. Am J Pathol. 2014;184:1369–1383. doi: 10.1016/j.ajpath.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov. 2014;13:63–79. doi: 10.1038/nrd4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clevers H, Loh KM, Nusse R. Stem cell signaling: an integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]