Abstract
Significance: Hyperbaric oxygen therapy (HBOT) is an important advanced therapy in the treatment of problem wounds, including diabetic foot ulcers and late effect radiation injury. HBOT remains among the safest therapies used today. Nonetheless, there are side effects associated with HBOT. It is important for providers to be able to identify, understand, and quantify these side effects for prevention, management, and informed consent.
Recent Advances: The past two decades have seen significant advancements in our understanding of the underlying mechanisms of HBOT. This has led to a better understanding of the underlying reason for clinical benefit. It has also led to a better understanding of its side effects. Moreover, more recent literature allows for better quantification of these side effects. This review will highlight these side effects.
Critical Issues: Wound healing in the case of problem nonhealing wounds requires the use of various advanced treatment modalities, including HBOT. HBOT has been shown to significantly improve healing rates in certain problem wounds, including advanced diabetic foot ulcers and late effect radiation injury. It is provided in a variety of clinical settings by providers with varying levels of expertise. It is important for those providing this therapy to understand the potential side effects.
Future Directions: Research in HBOT has led to significant advancements in the area of wound healing. At the same time, there remains a variety of treatment protocols used at different institutions. It is important to quantify risk and benefit at different treatment pressures and times to better standardize treatment and improve patient care.
Keywords: : hyperbaric, oxygen, side, effects

Marvin Heyboer III, MD, FACEP, FUHM, FACCWS
Scope and Significance
Hyperbaric oxygen therapy (HBOT) has been identified as a useful advanced adjunctive therapy in the promotion of healing in certain problem wounds in addition to its application to a variety of other medical conditions. As with all medical treatments, HBOT has known potential side effects as a result of treatment. A side effect here is considered a known potential secondary and usually adverse effect. This review will attempt to describe and quantify these side effects. This should lead to better consideration of risk and benefit in discussions with the patient when considering HBOT.
Translational Relevance
HBOT works through both primary and secondary effects. Primary effects involve both increased pressure and hyperoxia. Secondary effects as a result of a controlled oxidative stress include antimicrobial effects, blunting of ischemia–reperfusion injury, and wound healing. Wound healing is the result of both local and systemic effects. Local effects include a steepened oxygen gradient, macrophage recruitment, and release of multiple growth factors. Systemic effects result in progenitor stem cell mobilization and release from bone marrow in addition to improved homing to the site of injury by these cells.1–4 The results of both local and systemic effects include neovasculogenesis and collagen formation, which promote wound healing.1–4 These same mechanisms that result in HBOT beneficial effects can also cause the known side effects in some patients.
Clinical Relevance
The use of HBOT has grown significantly in the past 2 decades. Its application has a variety of recognized indications as outlined by the Undersea and Hyperbaric Medicine Society,5 although a majority of patients are receiving treatment for late effect radiation injury and problem wounds (primarily advanced diabetic foot ulcers). Treatment is provided in a variety of clinical settings by providers and staff with differing levels of expertise. Having an understanding of the potential side effects of HBOT is critical to providing safe medical care with complete patient informed consent.
Background and Overview
HBOT is the treatment of patients with 100% oxygen at higher than atmospheric pressure.1 This is provided in either a monoplace (single person) chamber typically compressed with oxygen or a multiplace chamber (multiple persons) compressed with air where oxygen is delivered by either a hood or mask. The benefits of treatment are the result of both primary and secondary effects. Primary effects are the result of increased pressure and hyperoxia. Indeed, PaO2 can increase from less than 200 mmHg at 1 atmospheres absolute (ATA) room air to more than 2,000 mmHg at 3 ATA. This also translates into significant increases in tissue oxygen partial pressure.6 Meanwhile, secondary effects are the result of a controlled oxidative stress. HBOT produces reactive oxygen species (ROS) and reactive nitrogen species, which function as signaling molecules in multiple pathways, including those involved in wound healing.1 The result is an array of secondary effects that include improved leukocyte function, amelioration of ischemia–reperfusion injury, and neovascularization as a result of increased local growth factors and release of autologous progenitor stem cells.7
It is these very same primary and secondary effects that can cause the side effects associated with HBOT. These include various forms of barotrauma, central nervous system (CNS) and pulmonary oxygen toxicity, and ocular side effects. There are additionally issues of claustrophobia. It is important to understand and quantify these side effects. This assists with creating protocols to minimize risk in addition to better weighing risk and benefit of treatment for the patient. It is important to note that HBOT remains among the safest therapies used today.8 The following is an exhaustive list of potential side effects, some of which are more common (middle ear barotraumas [MEB], claustrophobia) and others that are theoretical risks unlikely to occur clinically with appropriate screening precautions (pulmonary barotrauma [PBT]).
Discussion
Effects of pressure
HBOT by definition means treatment with 100% oxygen at higher than atmospheric pressure where increased pressures depend on treatment guidelines and indications. Hence, the side effects of HBOT are based on the physiologic response to this high pressure–high oxygen environment and the psychological response that patients experience from the closed confines of the treatment chamber—monoplace or multiplace. Boyle's Law states that the volume of a gas at a fixed temperature is inversely proportional to the ambient pressure. Lowering the ambient pressure causes increased gas volume; the converse is also true (Fig. 1). These effects of pressure are experienced within physiologic and pathologic air cavities, including the middle ear, paranasal sinuses, pathologic dental spaces, and emphysematous bulla.
Figure 1.
Gas volume change with pressure.
Middle ear barotrauma
MEB is one of the most common side effects of HBOT. Patients may report difficulty with ear equalization, a feeling of pressure, ear pain, and discomfort during compression, which is the initial phase of HBOT.9
If not attended to, this can lead to edema in the middle ear, retraction, and in rare cases, rupture of the tympanic membrane with conductive hearing deficit. In rare cases, MEB can be transmitted to the inner ear, with risk of rupture of the round or oval window membranes and impairment of inner ear function, causing vertigo and sensorineural hearing loss.10 MEB is most commonly classified using the modified TEED score (Table 1). More recently, the O'Neill grading system was proposed as a newer and more practical grading system.11
Table 1.
Modified TEED score
| Grade | Findings on Otoscopy |
|---|---|
| 0 | Normal examination |
| 1 | TM injection or retraction |
| 2 | Slightly hemorrhagic TM |
| 3 | Grossly hemorrhagic TM |
| 4 | Hemotympanum |
| 5 | TM perforation |
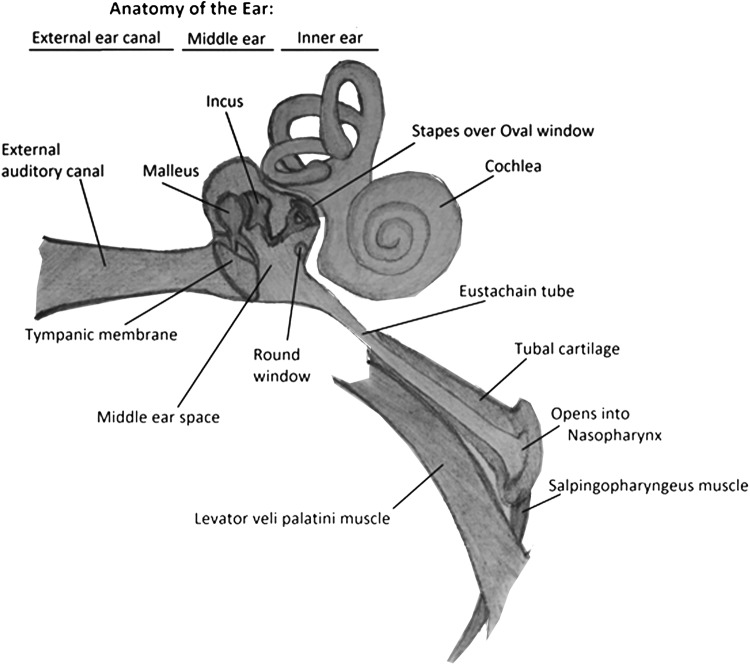
For effective air equalization in the middle ear to occur, the Eustachian tube (ET), which connects the middle ear to the nasopharynx, positioned superior, posterior, and lateral to the nasopharynx, needs to be open and functioning (Fig. 2).12 The ET is collapsed at rest and needs to be actively opened by the patient using valsalva maneuvers, swallowing, chewing, or by attempting to create positive pressure by blowing air against the pinched nares, thereby opening the collapsed ET.10 During compression, the relative negative middle ear pressure (compared with the dive chamber pressure) causes collapse and closure of the ET.13 Inability to open the ET prevents equalization of the middle ear pressure with the higher outside pressure. This results in gas volume contraction in the middle ear, which initially causes pain. Subsequent inward retraction of the tympanic membrane and adjoining ossicles, followed by middle ear mucosal swelling, capillary dilation, and transudate leakage, causes fluid extravasation into the middle ear space, with blood vessel rupture resulting in hemotympanum and possible tympanic membrane perforation.10,13
Figure 2.
Anatomy of the ear (D.S.).
Alternatively, if air equalization on either side of the tympanic membrane (TM) does not occur during decompression, positive pressure increase in the middle ear may also lead to similar middle ear trauma, although less likely since the positive pressure helps to open the ET.
Causes of ET dysfunction may be inherent craniofacial features such as palatal muscle anomalies or a result of infectious or allergic reactions. These include upper respiratory infections, environmental allergies, or enlarged adenoids, to name a few.14
The reported incidence of MEB in patients undergoing HBOT varies significantly from ∼2% to 84% in nonintubated and upward of 94% in intubated patients, making MEB one of the most common side effects of HBOT.8,15 The wide range of incidence is due to varying criteria used to define MEB, variations in patient population, and variation in patient instruction of how to equalize pressure in the middle ear.8 A recent publication of a large number of patient treatments demonstrated an overall incidence of 43%. The vast majority of cases were minor—84% were TEED 1 (TM injection/retraction) or TEED 2 (TM slight hemorrhage) with no episodes of TM rupture.8
Rate of compression does play a role in risk of MEB. A previous study demonstrated that a high rate of compression (4.1 psi/min) increased risk of MEB.16 On the other hand, a more recent study suggested that a very slow rate of compression (1 psi/min) also increases risk of MEB. It found that 2 psi/min was the best compression rate for minimizing MEB.8 There is an increased risk of MEB during initial treatments and no increased risk associated with a longer treatment course.8,17 Other risk factors indentified include intubation, active upper respiratory infection, diabetes (presumptively due to neuropathy), and a history of head and neck malignancy.8,15,18 Intuitively, there is higher risk of MEB in intubated patients with mixed evidence in the literature supporting this.8,19,20
MEB can be avoided and its incidence reduced. Adequate patient education, training, and assistance through active coaching during compression can help mitigate MEB cases. In instances where patients are unable to successfully equalize air pressure across the middle ear, needle myringotomy may be performed for emergent patients or tympanostomy tubes placed for the duration of extended treatments.10 For patients with inherent ET dysfunction with known allergic or inflammatory etiology, the use of decongestants and antihistamines can reduce obstruction and facilitate successful pressure equalization.
Most cases of MEB resolve in the absence of repetitive trauma.8 Long-term sequelae from MEB during HBOT are rare. The sequelae reported include sensorineural hearing loss, ossicular disruption, and perilymphatic fistula. Management requires referral to otolaryngology for possible surgical interventions such as tympanoplasty or surgical repair of the round or oval window.10
Most injuries, including tympanic membrane rupture, serous otitis, and tympanic membrane edema, heal spontaneously and medications such as antibiotics, decongestants, and steroids are not indicated. Although MEB is the most common of HBOT side effects, it is also most easily and effectively ameliorated with patient coaching, topical medications, and (in less common circumstances) relatively benign surgical intervention.
Sinus/paranasal barotrauma
Sinus and paranasal barotrauma is the second most common manifestation of barotrauma after MEB often occurring in the setting of upper respiratory infections or allergic rhinitis.15
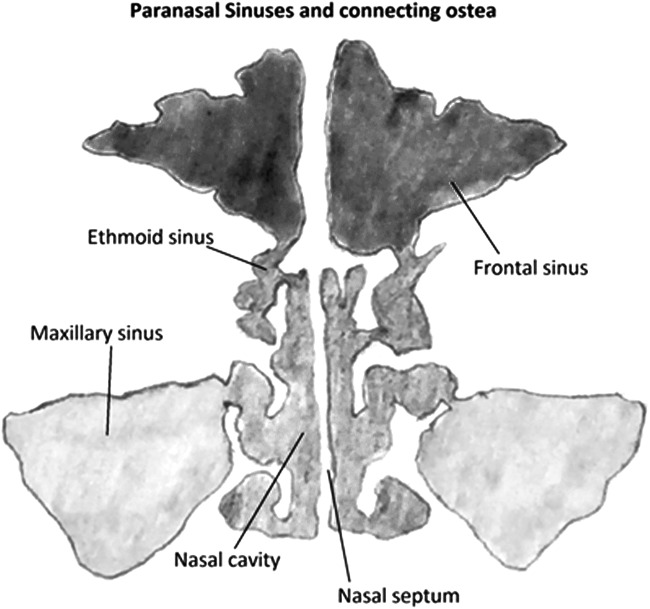
Barotrauma of the paranasal sinuses is characterized by pressure sensation, most commonly felt over the frontal sinuses, resulting in barosinusitis. The ostea draining the sinuses into the nasal cavity are small in diameter compared with the spaces they drain. With HBOT, changes in pressure occur during the compression phase inside sinus cavities, similar to those described in MEB above. A negative pressure gradient causes inflammation of the mucosal surfaces of sinuses and osteal openings, thereby occluding these sinuses. This compression of the sinus space leads to congestion and edema, which is accompanied by facial pain and relieved as the pressure is eliminated (Fig. 3).21 The resulting edema can create a closed air space within the sinuses, resulting in paradoxical pain during chamber decompression as the air volume expands, but is unable to escape.
Figure 3.
Anatomy of sinuses (D.S.).
Barosinusitis can lead to epistaxis, although such cases are rare, with incidence reported at 1 case per 10,000 treatments.15 The pressure changes that occur during HBOT are similar to pressure changes that occur during underwater recreational diving up to 60 feet in sea water (2.8 ATA). A case report for optic neuropathy was reported from possible ethmoidal and sphenoidal barotrauma after recreational diving, with resolution of symptoms.22
There is a propensity for barosinusitis to occur in the setting of upper airway inflammation due to underlying upper respiratory infections, allergic rhinitis, or mucociliary dysfunction. Indeed, acute upper respiratory infection is a relative contraindication to elective HBOT. When HBOT is provided, symptoms should be controlled with a regimen of decongestant nasal spray, antihistamines, and/or steroid nasal spray just before compression.15
Dental barotrauma
Dental barotrauma (barodontalgia/odontocrexis), which is commonly called tooth squeeze, is pain in a tooth caused by a change in atmospheric pressure. This phenomenon was first observed in air crews during World War II and subsequently reported in divers.23
Dental pain can occur during either compression or decompression. It is classically experienced as a decrease in pressure during decompression causes expansion of air bubbles trapped under a root filling or against dentin activating nociceptors. It can also be referred to pain with stimulation of nociceptors in the maxillary sinuses. If expanding trapped gas results in dental stress, it may cause tooth fracture, a process called odontecrexis (tooth explosion in Greek).24 Possible etiologies include dental infections, sinusitis, differences in the expansion behavior of dental enamel and pulp, and pressure-induced movement of fluids from exposed dentine to the pulp.23
Barodontalgia can occur as a result of any change in pressure, including HBOT. However, the majority of studies evaluating this side effect come from aviation and dive medicine. It has been reported during flying at altitudes higher than 600 m and during diving at depths of more than 10 m, with incidence at 0.26–2.8% in aircraft personnel, air passengers, and divers.23 Barodontalgia has been reported by 9.2–21.6% of American and Australian civilian divers.25
Preventive measures should include a dental examination by the hyperbaric medicine physician before HBOT and treatment of carious lesions and defective restorations when known before HBOT.23
Pulmonary barotrauma
As previously mentioned, Boyle's Law states that the volume of a gas at a fixed temperature is inversely proportional to the ambient pressure. Similar volume changes can be seen in the lungs of patients undergoing HBOT if a closed system is created. Normally, there is no risk of PBT in patients with normal lungs and an open glottis. The potential exists for PBT from lung overinflation for patients at risk for air trapping during decompression (asthma or chronic obstructive pulmonary disease [COPD] with active bronchospasm, mucous plugging, and bullous lung disease) and those with a prolonged closed glottis.
Should the lung parenchyma be disrupted from overinflation during decompression, the potential manifestations are pneumomediastinum, subcutaneous emphysema, intrapulmonary hemorrhage, simple pneumothorax (PTX), and tension PTX. Pneumomediastinum and intrapulmonary hemorrhage are generally self-limited and require only conservative management with supplemental oxygen to achieve resolution. PTX, however, is a potentially life-threatening phenomenon especially given the increased risk of exacerbating a simple PTX into a tension PTX during decompression in a hyperbaric chamber. Tension PTX can rapidly lead to cardiovascular collapse and death if not treated quickly with thoracostomy tube insertion. Cases of tension PTX during hyperbaric oxygen treatment are very rare, but have been reported.26,27
Arterial gas embolism (AGE) can result from PBT when alveolar air passes into the ruptured pulmonary vessels. The air bubbles then enter the left heart and embolize through systemic arteries leading, potentially, to vascular occlusion in the brain or heart.28 The most severe clinical manifestations of AGE include apnea, unconsciousness, and cardiac arrest. Other symptoms include loss of consciousness, confusion, aphasia, dysarthria, vertigo, visual disturbance, unilateral sensory and motor changes, and seizure. Cases of PBT resulting in AGE have been reported in the medical literature.29 Treatment of choice for AGE from any etiology is HBOT.5,6
The lung is an open air space system. As such, PBT is not expected with HBOT in the absence of pulmonary disease. As such, all potential candidates for HBOT must be thoroughly screened for pulmonary disease, which may increase the risk of PBT. Screening begins with a thorough history and physical examination. Historical features, including COPD, asthma, bronchiectasis, cancer, prior spontaneous PTX, or prior chest surgery, should prompt further investigation inclusive of, at minimum, a chest x-ray. Indeed, an unvented PTX is an absolute contraindication to HBOT due to the potential of creating a tension PTX during the decompression phase of treatment. This requires treatment to create an open system before any hyperbaric treatment. Other pulmonary historical features may not preclude a potential patient from HBOT, but treatment protocols may need adjustment such as a slower decompression rate to suit the needs of a particular patient. This may be the case in a patient with significant blebs who is decompressed at a slow 1 psi/min rate. Additionally, a careful evaluation of risk versus benefit should be undertaken in circumstances where significant pulmonary disease is present to mitigate any potential untoward outcome during HBOT.
CNS oxygen toxicity
As we have stated, HBOT is the treatment of patients with 100% oxygen at higher than atmospheric pressures. HBOT can result in arterial oxygen tensions of greater than 2,000 mmHg and tissue levels of 200–400 mmHg and higher.6 HBOT has many of its therapeutic benefits through controlled oxidative stress. Antioxidant defenses are usually adequate during the hyperoxic exposure created by a typical clinical hyperbaric oxygen treatment.1 Nonetheless, CNS oxygen toxicity does occur. The recognized presentation of CNS oxygen toxicity during clinical hyperbaric oxygen treatment is an oxygen toxicity seizure. The link between hyperbaric oxygen and seizure was first recognized by Paul Bert in 1878.30,31 Dr. Christian J. Lambertsen described it in this way: ‘The convulsion is usually preceded but not always by the occurrence of localized muscular twitching… Eventually an abrupt spread of excitation occurs and the rigid “tonic” phase of the convulsions begins. Vigorous clonic contractions of the muscle groups of the head and neck, trunk and limbs then occur becoming progressively less violent over 1 minute.’32,33
The exact underlying pathophysiology is not understood. It appears to be the result of direct oxygen toxicity. The increased ROS and free radical intermediates interact with the neuronal cell plasma membrane.34–36 This causes lipid peroxidation at the plasma membrane, resulting in a change in brain electrical activity.35 Nitric oxide (NO) has been implicated as a mediator for CNS oxygen toxicity through formation of peroxynitrite (ONOO−). In addition, there is vasodilation secondary to NO, which counteracts the cerebral vasoconstriction normally seen secondary to hyperoxia.37 Gross retention of carbon dioxide (CO2) in brain tissue and intense vasoconstriction have been shown to be unlikely causes.34
Prodromal symptoms have been reported, although they appear in <50% of cases. These include twitching, staring gaze, auditory hallucinations, visual changes, nausea, vertigo, anxiety, and irritability.38,39 This is rapidly followed by tonic–clonic seizure activity. This is reversible with no residual neurological damage and resolution with a decrease in the inspired partial pressure of oxygen (PO2), resulting in a reduced cerebral PO2.32 The occurrence of generalized convulsions requires sufficient oxygen pressure and duration of exposure. When this is sufficient, the required length of exposure varies inversely with the PO2 breathed.34 No pathologic changes have been found to be associated with an isolated oxygen-mediated seizure.36
An oxygen toxicity seizure is relatively rare at typical clinical treatment pressures (2 ATA–3 ATA). It is difficult to predict on an individual basis. It was traditionally reported at ∼1 in 10,000 treatments.40 However, more recent evidence over the past 15 years puts the incidence at ∼1 in 2,000–3,000 treatments.7,38,41,42 The reason for the increased incidence over the past 15–20 years appears to be related to patient selection (sicker patients with more comorbid illness) and changes in hyperbaric oxygen treatment protocols.42 Risk factors identified include higher treatment pressure, CO2 retention, brain tumor/brain soft tissue radionecrosis, hypoglycemia, hyperthyroid, and carbon monoxide poisoning.7,38,43–46 No link to increased risk has been identified with monoplace versus multiplace chamber, neurologic versus non-neurologic treatment indication, or past medical history of stroke, diabetes, alcoholism, or epilepsy.7,38,47 Table 2 shows the incidence reported in published studies. Table 3 lists risk factors to having an oxygen toxicity seizure that have been identified.
Table 2.
Incidence of oxygen toxicity seizure
| Study | Incidence | Percent | Tx Pressure | Indications |
|---|---|---|---|---|
| Hart 198789 | 1 in 12,253 | 0.008 | 2–3 ATA | All |
| 0.8% per 10,000 | ||||
| Davis40 | 1 in 10,552 | 0.01 | 2.4 ATA | All |
| 0.95 per 10,000 | ||||
| Welslau and Almeling44 | 1 in 6,704 | 0.05 | 2.4–2.8 ATA | All |
| 1.5 per 10,000 | ||||
| Plafki et al.41 | 1 in 2,844 | 0.035 | 2.4–2.5 ATA | All |
| 3.5 per 10,000 | ||||
| Hampson and Atik42 | 1 in 3,388 | 0.03 | 2–2.8 ATA | All |
| 3 per 10,000 | ||||
| Yildiz et al.43 | 1 in 40,339 | 0.0025 | 2–2.8 ATA | All |
| 0.25 per 10,000 | ||||
| Banham38 | 1 in 1,651 | 0.06 | 1.9–4 ATA | All |
| 6 per 10,000 | ||||
| Heyboer et al.7 | 1 in 2,121 | 0.05 | 2–2.8 ATA | All |
| 5 per 10,000 | ||||
| Hadanny et al.47 | 1 in 8,945 | 0.011 | 1.5–2.8 ATA | All |
| 1.1 per 10,000 |
ATA, atmospheres absolute.
Table 3.
Risk factors for oxygen toxicity seizure
| Risk Factors | Study |
|---|---|
| Increased treatment pressure (2 vs. 2.4/2.5 vs. 2.8 ATA) | Heyboer et al.7 |
| Other CNS (brain tumor/STRN brain) | |
| Air break | |
| Increased treatment pressure (2 vs. 2.4 ATA) | Banham38 |
| Carbon monoxide poisoning | |
| Increased treatment pressure (1.5 vs. 2 vs. 2.4 vs. 2.8 ATA) | Hadanny et al.47 |
| Carbon monoxide poisoning | Hampson et al.45 |
| Hypoglycemia, hyperthyroid | Welslau and Almeling44 |
| Hood over mask (multiplace chamber) | Yildiz et al.43 |
CNS, central nervous system.
Preventive measures include the use of air breaks at given intervals during hyperbaric oxygen breathing. This allows for interval breaks in overt exposure to oxygen free radicals and resulting seizure. While the use of air breaks to decrease the incidence of CNS oxygen toxicity has not been directly demonstrated, there is a large amount of published data on the cause of oxygen toxicity related directly to a combination of the level of PO2 exposure and time. As such, these air breaks limit the interval time exposure and are expected to decrease the risk of oxygen toxicity.34 Indeed, the U.S. Navy has used air breaks successfully for many years. Yildiz found that the use of masks was protective over hoods due to less risk of CO2 retention when undergoing treatment in a multiplace chamber.43 Finally, screening for risk factors and optimizing antiseizure medications in known epileptics before hyperbaric oxygen treatment are thought to be protective.
There are no long-term sequelae as a result of an oxygen toxicity seizure. No pathophysiologic changes have been found to be associated with an isolated oxygen-mediated seizure.36 Indeed, patients who have had an oxygen toxicity seizure may still go on to complete their recommended course of treatment. While their risk of subsequent oxygen toxicity seizure is increased, it is still less than 10%.7,38,44,47 Adjustments can be made to subsequent treatments, including lower treatment pressure and additional air breaks. While oxygen toxicity seizure is one of the more feared side effects of HBOT, its incidence remains low with no evidence of long-term sequelae as a result of an episode.
Pulmonary oxygen toxicity
Continuous exposure of the lungs to elevated levels of oxygen, either at atmospheric or hyperbaric pressure, leads to progressively severe toxic effects as the duration of exposure, FiO2, or pO2 increases. Pathological manifestations of pulmonary oxygen toxicity are differentiated into two overlapping phases: the acute exudative phase and the subacute proliferative phase. Pulmonary changes in the acute exudative phase include interstitial and alveolar edema, intra-alveolar hemorrhage, fibrinous exudate, hyaline membrane swelling, and destruction of capillary endothelial cells and type I alveolar epithelial cells. Interstitial fibrosis, fibroblastic proliferation, and hyperplasia of type II alveolar epithelial cells characterize the subacute proliferative phase (Table 4).48
Table 4.
Pathologic manifestations of pulmonary oxygen toxicity
| Acute Exudative Phase | Subacute Proliferative Phase |
|---|---|
| Interstitial edema | Interstitial fibrosis |
| Alveolar edema | Fibroblastic proliferation |
| Intra-alveolar hemorrhage | Type II alveolar epithelial cell hyperplasia |
| Fibrinous exudate | |
| Capillary endothelium destruction | |
| Type I alveolar epithelial cell destruction |
Pulmonary mechanical function is negatively affected by these pathological changes (Table 5). Changes in pulmonary function include decreased lung compliance, decrements in inspiratory and expiratory lung volumes and rates, and decreased CO2 diffusing capacity. Progressive reduction in vital capacity is seen in pulmonary oxygen toxicity. Indeed, decreased vital capacity remains a consistent and sensitive manifestation of pulmonary oxygen toxicity. The rate of pulmonary oxygen toxicity development correlates with pO2 and the duration of exposure.49 Symptoms of toxicity typically begin after ∼12–16 h at 1.0 ATA, 8–14 h at 1.5 ATA, and 3–6 h at 2.0 ATA. Symptoms occur earlier at 2.5 and 3.0 ATA, but are milder since exposure time is limited by neurologic oxygen toxicity.48–51
Table 5.
Pulmonary function changes in oxygen toxicity
| Decreased lung compliance |
| Decreased inspiratory lung volume and rate |
| Decreased expiratory lung volume and rate |
| Decreased carbon monoxide diffusing capacity |
| Decreased vital capacity |
Symptoms of pulmonary oxygen toxicity are insidious in onset and present as mild substernal chest discomfort accentuated by inspiration. As oxygen exposure continues, this progresses to widespread pleuritic chest pain, cough, chest tightness, and dyspnea.49,51 The severity of symptoms diminishes quickly within the first few hours postexposure and the sensation of pulmonary irritation completely disappears over the next 1–3 days.51
Pulmonary oxygen toxicity is not expected from routine daily HBOT. The possibility of development does exist with prolonged exposure most typically related to long treatment tables such as US Navy Treatment Table 6 used for decompression illness, but even these cases would be mild and self-limiting. Pulmonary oxygen toxicity can be avoided if oxygen is provided in the proper dose. Humans exposed to hyperoxia at 0.55 ATA for 7 days showed no manifestations of pulmonary toxicity.52 Likewise, exposure to 0.3 ATA for 30 days produced no toxicity.53 Most current applications of HBOT do not cause pulmonary symptoms or clinically significant pulmonary functional deficits.54
Ocular side effects
Increased partial pressures of oxygen can potentially pose harm to multiple body tissues, including the eye. Under normoxic conditions, oxygen metabolism produces superoxide radicals and other toxic reactive species. Removal of these harmful substances is mediated by superoxide dismutase and other cellular defense mechanisms. Under hyperoxic conditions, these defense mechanisms may become overwhelmed due to the increased free radical production leading to oxygen toxicity and subsequent ocular side effects.55
In addition to the inspired PO2 and exposure duration, many other variables can play a role in the development of ocular manifestations of oxygen toxicity. These include the age of the exposed individual whether advanced age (i.e., cataract promotion) or young age (i.e., retrolental fibroplasia), the method use for oxygen delivery, and the presence of undiagnosed comorbid conditions that may affect the patient's susceptibility to oxygen toxicity.56,57
Hyperoxic myopia
Myopia can have direct toxic effects of oxygen on the crystalline lens and is one of the most common side effects.57,58 An acute myopic shift may be due to osmotic changes in the lens of the eye, systemic medications (i.e., diuretics), miotic eye medications, and ciliary spasm. Under repeated exposures to hyperoxia, hyperoxic myopia is also included in the list of differential diagnoses.59
Progressive myopic changes are a known side effect of repetitive treatments with HBOT.59 The rate of this change has been reported in the literature to be ∼0.25 diopter per week and progressive throughout the course of ongoing treatment.60 Myopia has been reported in 25–100% of patients undergoing HBOT after several weeks at pressures of 2.0 ATA and greater.61 The exact mechanisms for myopic change are not fully known. Proposed hypotheses have included a reduction in backscattered light and lens optical density with hyperbaric oxygen through oxidative damage to the crystalline structure of the lens proteins and high partial pressures of oxygen in direct contact with the eye, resulting in oxygen toxicity due to local hyperoxia.62–64
A thorough ocular examination by the diving and hyperbaric physician can provide an objective assessment of visual status and ocular function before the patient beginning HBOT. If the patient is likely to undergo a prolonged course of treatment, one may consider a more thorough examination by an optometrist or ophthalmologist that includes documentation of corrected and uncorrected visual acuity, refraction, color vision, status of the crystalline lens, and fundus examination. By accurately documenting ocular function, the benefit of HBOT can be weighed against any adverse effects on visual acuity and thus be used to guide therapeutic decisions.
Clinical experience suggests that such a change in a patient's vision may affect their overall quality of life. Their driving habits may be affected, routine tasks may become more challenging, and patient safety becomes a concern, placing those with mobility issues at an increased risk of falls and subsequent injury. One may consider optometry evaluation for temporary visual correction during HBOT when the degree of myopia becomes a safety concern. Utilizing 5-min periods of air breathing every 20–25 min can reduce the risk of oxygen toxicity.57 In addition, the use of mask over hood in a multiplace chamber setting would theoretically decrease the risk due to a lower topical PO2. It is important to note that this myopic shift is reversible after discontinuation of HBOT usually returning to baseline within 3–6 weeks, but as long as 6–12 months.59
Cataracts
HBOT leads to an increase in pO2 and concentration of ROS in blood and tissues, including the lens stroma of the cornea, where it plays a role in cataractogenesis.57 The relationship between senile nuclear cataract formation and myopia is well known and reported and both can be considered to represent two levels of severity of lenticular oxygen toxicity. The development of cataracts in humans has been reported only after prolonged exposure to hyperbaric oxygen, usually 150 treatments or more.65 In the United States, de novo cataract genesis is rare as the maximum number of treatments rarely exceeds 75.66
Cataracts remain the leading cause of visual impairment and blindness worldwide and usually present after age 65.67 Risk factors include advanced age, female gender, smoking, diabetes, ethanol use, and corticosteroids. Incidence increases over the age of 65. Three types of cataracts have been identified, which are cortical, subcapsular, and nuclear, which is the most common.68 They are identified by a yellow-brown discoloration and hazy appearance of the lens in conjunction with the visual changes experienced by the patient.
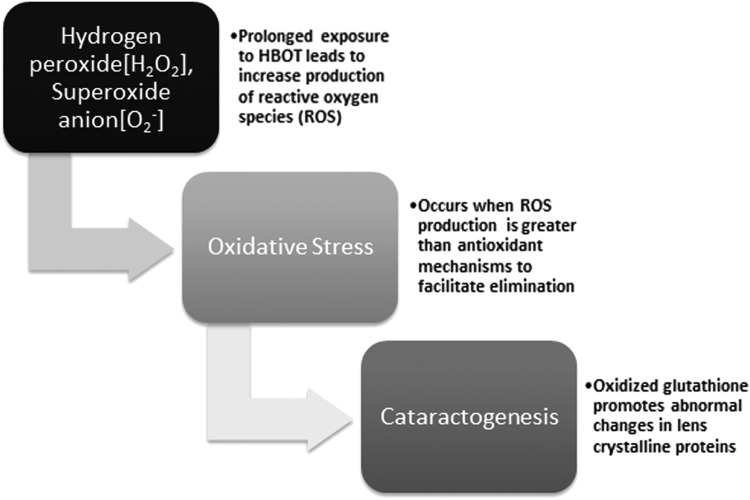
The exact mechanism of cataractogenesis is not completely understood. As reported, long-term exposure to HBOT resulting in oxidative damage to the lens proteins plays a critical role.69 Oxidative stress occurs when the production of ROS overwhelms the normal capacity of antioxidant defense mechanisms to facilitate their elimination. Glutathione is an important antioxidant that functions to prevent damage to cellular structures caused by ROS such as free radicals, peroxides, and lipid peroxides (Fig. 4). Glutathione, in its reduced form, plays a critical role in the lens stroma by maintaining the transparency of lens crystalline proteins.70,71 When oxidized, this can promote abnormal protein cross-linking, increased production of insoluble proteins, and abnormal colorization resulting in nuclear light scatterings—an early finding in cataract formation.69
Figure 4.
Oxygen free radical effect on ocular lens.
To date, there has only been one report of nuclear cataract development in a human receiving less than 50 HBOT treatments. This occurred in a 49-year-old woman who had received 48 treatments over a period of 11 weeks.69 This appears to be an exception to the general observation that cataract formation occurs only in treatment series greatly exceeding 20–60 treatments. Undiagnosed pre-existing conditions may play a role in such circumstances. Increased pO2 in the precorneal gas space has been reported to play a more significant role than inspired PO2 in determining the physiologic effects of the gas mix on the cornea.72 Thus, the use of a oronasal mask in lieu of a hood as an oxygen delivery device in a multiplace chamber should reduce the amount of oxygen to the eye, keeping the precorneal gas closer to normal oxygen fraction. This was shown to result in a 50% reduction in myopic shift and overall lenticular oxygen toxicity.63 Patients therefore should be offered the choice between delivery systems when available and clinically appropriate.
Retrolental fibroplasia following hyperoxic exposure
Retrolental fibroplasia also known as retinopathy of prematurity is a potentially blinding condition affecting the retina of premature infants caused by exposure to hyperoxia. It is vasoproliferative retinopathy that occurs in preterm babies with immature retinal vasculature.73,74 In the 1950s, it was associated with the use of high amounts of oxygen in the premature infant.75
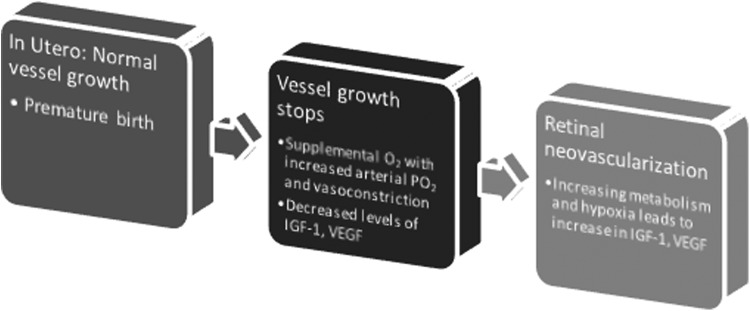
The two factors associated with pathophysiologic development are an incompletely vascularized retina and increased arterial pO2 with relative retinal hyperoxia. Both of these contribute to vasoconstriction of the developing retinal vessels and a decrease in growth factors, most notably insulin-like growth factor (IGF-1) and vascular endothelial growth factor (VEGF). Consequently, there is arrest of retinal neovascularization and capillary obliteration, leading to a decrease in perfusion and subsequent retinal ischemia and hypoxia. Normal response to these changes is an upregulation of various growth and angiogenic factors, including IGF-1 and VEGF (Fig. 5). If this response is exaggerated, abnormal and disorganized angiogenesis may ensue leading to inflammation, proliferative retinopathy, significant fibrosis, and ultimately irreversible retinal detachment and permanent blindness.76
Figure 5.
Retrolental fibroplasia.
The incidence in premature infants in Western countries has been reported to vary between 35% and 60%.74 Despite current neonatal care management maintaining moderate oxygenation with arterial pO2 in the range of 60–80 mmHg in attempts to curb the incidence, retinopathy of prematurity remains prevalent among small premature infants.77,78 About 4.5% of surviving babies weighing less than 1 kg are legally blind with a larger percentage exhibiting significant visual impairment. Neurocognitive and developmental abnormalities may also be seen leading to profound disability, including the inability to provide self-care, incontinence, motor disabilities, and altered social–personal skills.76
The discussion of HBOT in the setting of retrolental fibroplasia should include two factors: hyperoxia and neovascularization through angiogenesis. Oxygen saturations of 88–95% will maintain an arterial pO2 above 45 mmHg and usually less than 75–80 mmHg. Targeting oxygen saturation between 88% and 93% has been shown to result in a significant reduction in retinopathy of prematurity.77 Arterial pO2 is 90 to 110 mmHg at sea level. HBOT has been shown to increase the arterial pO2 to well above 2,000 mmHg.57 Such high partial pressures (and oxygen saturations) could potentially increase the risk of development. As discussed previously, an abnormal angiogenic process occurs in the retina in response to decreased perfusion and hypoxia. HBOT has been shown to increase angiogenesis through upregulation of VEGF production. These two benefits of HBOT not only play a central role in other conditions (i.e., wound healing) but have also been the focus of discussion and the cornerstone of research for therapeutic options in the setting of retrolental fibroplasias.
Retrolental fibroplasia remains an unfortunate growing global problem and a devastating complication of premature infants in both industrialized and developing nations. When severe, visual outcomes are unfavorable even with treatment and it still remains the most common cause of blindness.76 Prevention lies with a focus on research investigating preventative treatments that can modulate angiogenesis, for example, and sound clinical practice regarding oxygen supplementation and delivery in the premature infant.
Claustrophobia
Claustrophobia is the fear of being enclosed in small spaces with no escape and may be triggered by different stimuli in the daily environment such as elevators, tunnels, small windowless rooms, basements, or even tight clothing. Symptoms may include sweating, palpitations, hyperventilation, light-headedness, choking, chest tightness, increased blood pressure, trembling, anxiety, headache, confusion, or even disorientation. Claustrophobia is thought to be caused by classical conditioning, a result of past (usually childhood) experience or a learned behavior from parents or peers. Other theories attribute the size of the amygdala to a person experiencing claustrophobia, with persons suffering from panic disorders having smaller amygdala.79
Psychological effects of claustrophobia are experienced by some patients when placed in the tube-like cylindrical monoplace chambers or under hoods and face masks in multiplace chambers. Between 15% and 37% of people worldwide are affected by claustrophobia, with 5–7% affected by severe claustrophobia.80 It appears to be present in about 2% of the general patient population and may cause some degree of confinement anxiety, even in a multiplace chamber. Incidence of confinement anxiety in monoplace chambers is reported at 8 events per 10,000 treatments.15
Mild confinement anxiety is easily controlled with sedation before treatments such that individuals may continue to receive daily HBOT.15 More severe cases may need referral to a psychiatrist or a psychologist, and cognitive-behavioral therapy, relaxation exercises, and/or long-term drug therapy might be necessary.79 Preventive measures with adequate patient history, patient education, reassurance, and coaching are the most effective means of anticipating episodes of claustrophobia and treating them effectively before HBOT.
Other side effects of HBOT
Blood pressure effects
HBOT causes an increase in both systolic and diastolic blood pressure (DBP). This holds true for both hypertensive and nonhypertensive patients.81,82 Overall, the effect on blood pressure is mild. One study reported an overall increase in systolic blood pressure (SBP) of 6% and DBP of 12%.81 Another study reported increases in SBP of 7 mmHg, DBP of 4 mmHg, and MAP of 5 mmHg.82 No patients experienced signs of hypertensive urgency or emergency in these studies.81,82 The effect diminished with each additional treatment (protective).82 Calcium channel blockers and beta-blockers exacerbated HBOT effect on blood pressure.81,82 Proposed mechanisms include increased systemic vascular resistance through alpha-receptor-induced vasoconstriction. Endothelin-1 is elevated during HBOT and endothelin-1-induced vasoconstriction may be involved.82–84
Pulmonary edema
There is a theoretical risk of pulmonary edema in patients with compromised left ventricular function who are undergoing HBOT. There are limited published data, although the risk is low based off the data available. Two studies reported their incidence at 1 in 1,000 (0.1%) and 1 in 4,500 (0.02%).83,84 While the etiology is not fully known, it appears to be related to hyperbaric oxygen, inducing increased systemic vascular resistance and decreased cardiac output.83,85 A recent study evaluating the effects of hyperbaric oxygen at 243 kPa found a reduction in cardiac output due only to a decrease in heart rate with no impact on other cardiac function.86 Other potential mechanisms of hyperbaric-induced pulmonary edema include increased pulmonary capillary wedge pressure, myocardial damage, or consumption of endothelial-derived NO by oxygen free radicals, or cardiac output imbalance between the right and left heart.84 Noncardiac acute pulmonary edema is not expected in patients with normal cardiac function treated with HBOT. Nonetheless, it may occur in those with compromised baseline cardiac function. Patients with a history of congestive heart failure should have a baseline echocardiogram before HBOT and caution should be taken in patients with a low ejection fraction (EF) (i.e., <35–40%). Patients should not be fluid overloaded. In addition, consideration should be given to treatment in a multiplace chamber in these patients where they can sit upright and a tender is immediately available.84
Hypoglycemia in diabetics
HBOT stimulates residual insulin secretion in diabetics and increases glucose utilization in the brain.81 This combination, along with other potential mechanisms, can theoretically lead to hypoglycemia in diabetic patients undergoing HBOT. As a result, hyperbaric medicine facilities set minimum pretreatment serum glucose levels to undergo treatment. These minimum levels are as high as 150 mg/dL to as low as 100 mg/dL.
Overall published data suggest an overall mean decrease in the serum glucose levels for diabetics undergoing HBOT, but with a large range and a high percentage of patients who actually had an increase in their serum glucose. These include a relatively low number of clinically relevant post-HBOT hypoglycemia episodes with no poor outcomes reported. Finally, there are no clear risk factors for development of hypoglycemia, although lack of control appears to be a consistent theme.81,87,88 One study had post-treatment serum glucose levels higher in 54% of treatments and lower in 46% of treatments. They had only 1.5% <70 mg/dL, only 0.19% symptomatic, and no extreme hypoglycemia requiring assistance with treatment.87 Another study had a median change in serum glucose of −25 mg/dL, but with a range of +240 mg/dL to −374 mg/dL. Only 1.2% of treatments had a post-treatment serum glucose <90 mg/dL and no one was symptomatic.88 Another had an average drop of 22%, but only 0.3% of cases where patients had symptomatic hypoglycemia.81
In light of these findings, current protocols may require too high a minimum serum glucose level before treatment, although further evidence would be helpful. This is important since HBOT is an adjunctive therapy to standard care, which includes tight glycemic control for these patients. An absolute pretreatment minimum level of 100 mg/dL appears to be sufficient.87 In addition, the decision to pretreat with glucose and/or abort HBOT should include consideration of a patient's documented level of control and serum glucose trend on day of treatment.
Summary
HBOT is an important advanced adjunctive therapy in the treatment of certain problem wounds, primarily those as a result of late effect radiation injury and diabetes. HBOT remains among the safest therapies used today. The side effects that have been described are self-limiting and often can be avoided with adequate screening. One of the most common side effects related to pressure changes is MEB. While it is commonly encountered, it is typically mild and self-limiting, and it can be prevented by ongoing teaching of middle ear clearing techniques and appropriate compression rates. PBT is unlikely and can be avoided with appropriate pretreatment screening. Oxygen toxicity is rare and most commonly encountered as a CNS oxygen toxicity seizure. This resolves with withdrawal of oxygen and does not have any permanent implications. In addition, further HBOT is typically tolerated. Adjustment of the treatment protocol to decrease maximum pressure and provide additional air breaks may be undertaken, but is not required. Pulmonary oxygen toxicity is not seen with typical elective treatments provided for problem wounds. Ocular side effects should be monitored. Hyperoxic myopia, which is one of the most common side effects, is considered reversible. Providers should monitor the degree of change during treatment to assure safety with driving and instruct patients to avoid a new permanent prescription until at least 8 weeks after treatment is completed. Claustrophobia may be managed with coaching and anxiolytic medications. Intolerance of a monoplace chamber may warrant referral to the closest multiplace chamber facility. Hypoglycemia during HBOT is a legitimate concern, but clinically relevant hypoglycemia is not common. Minimum pretreatment thresholds should not be too laboriously high and overall individual patient glycemic control and day of treatment trend should be of greater importance than the absolute number. Finally, HBOT effects on BP are not typically of clinical relevance except in the case of patients with a low EF or severe aortic stenosis. Care should be taken in consideration of treatment in these patients to avoid acute pulmonary edema.
Take-Home Messages.
• HBOT remains among the safest therapies used today.
• HBOT is the treatment of patients with 100% oxygen at higher than atmospheric pressure. It is both the primary and secondary effects that result in its beneficial effects and side effects.
• One of the most common side effects identified in the peer-reviewed literature is MEB. It is typically mild and self-limited. Patient instruction on middle ear clearing, daily monitoring with otoscopic examination, and appropriate compression rates are important to its prevention.
• Oxygen toxicity seizure is one of the most feared side effects of HBOT. It is important to remember that this is an uncommon and self-limiting side effect. It is resolved with withdrawal of 100% oxygen and has no long-term implications. Continued HBOT is permissible and may be done with adjustment to maximum pressure and addition of air breaks. Higher occurrence rates have been linked to higher treatment pressures.
Abbreviations and Acronyms
- AGE
arterial gas embolism
- ATA
atmospheres absolute
- CNS
central nervous system
- CO2
carbon dioxide
- COPD
chronic obstructive pulmonary disease
- DBP
diastolic blood pressure
- EF
ejection fraction
- ET
Eustachian tube
- HBOT
hyperbaric oxygen therapy
- IGF-1
insulin-like growth factor
- MEB
middle ear barotrauma
- NO
nitric oxide
- ONOO-
peroxynitrite
- PaO2
partial pressure of oxygen in arterial blood
- PBT
pulmonary barotrauma
- pO2
partial pressure of oxygen
- PTX
pneumothorax
- ROS
reactive oxygen species
- SBP
systolic blood pressure
- TM
tympanic membrane
- VEGF
vascular endothelial growth factor
Acknowledgements and Funding Sources
There were no funding sources related to the work presented. The authors thank the Upstate University Hospital staff, including Kimberly Rouselle and Sarah Seargent, for their support and assistance in various studies, including quality studies that have been carried out. The authors also thank Susan Wojcik, PhD, and William Grant, EDD, from the Department of Emergency Medicine, SUNY Upstate Medical University, for their assistance in study design and statistical analysis.
Author Disclosures and Ghostwriting
The authors do not have any commercial conflicts of interest. The article was written exclusively by the authors.
About the Authors
Marvin Heyboer III, MD, FACEP, FUHM, FACCWS, is an Associate Professor of Emergency Medicine at SUNY Upstate Medical University. He is board certified in Undersea and Hyperbaric Medicine and Emergency Medicine. He is the Medical Director of Upstate University Hospital Hyperbaric Medicine and Wound Care Center and the Director of the Fellowship in Undersea and Hyperbaric Medicine. He is actively involved in research and has published multiple articles in the areas of hyperbaric medicine and wound care. William Santiago, MD, is an Assistant Professor of Emergency Medicine at SUNY Upstate Medical University. He is board certified in Undersea and Hyperbaric Medicine and Emergency Medicine. He is a Hyperbaric Medicine and Wound Care physician and an Emergency Medicine physician at Upstate University Hospital. Norman McCulloch, MD, MBA, is board certified in Internal Medicine. He is fellowship trained in Undersea and Hyperbaric Medicine and board eligible in Undersea and Hyperbaric Medicine with the American Board of Preventive Medicine. He is also an NOAA Dive Medical Officer. Deepali Sharma, MD, is a Fellow in Undersea and Hyperbaric Medicine at SUNY Upstate Medical University. She is residency trained in Emergency Medicine. She is a practicing Emergency Medicine physician at Upstate University Hospital.
References
- 1.Thom SR. Hyperbaric oxygen–its mechanism and efficacy. Plast Reconstr Surg 2011;127(S1):131S–141S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thom SR, Bhopale VM, Velazquez OC, Goldstein LJ, Thom LH, Buerk DG. Stem cell mobilization by hyperbaric oxygen. Am J Physiol Heart Circ Physiol 2006;290:1378–1386 [DOI] [PubMed] [Google Scholar]
- 3.Goldstein LJ, Gallagher KA, Bauer SM, et al. Endothelial progenitor cell release into circulation is triggered by hyperoxia-induced increases in bone marrow nitric oxide. Stem Cells 2006;24:2309–2318 [DOI] [PubMed] [Google Scholar]
- 4.Liu ZJ, Velazquez OC. Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing. Antioxid Redox Signal 2008;10:1869–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weaver LK. Hyperbaric Oxygen Therapy Indications, 13th ed. North Palm Beach, FL: Best Publishing Company, 2014 [Google Scholar]
- 6.Thom SR. Hyperbaric oxygen therapy. J. Intensive Care Med 1989;4:58–74 [Google Scholar]
- 7.Heyboer III M, Jennings S, Grant WD, Ojevwe C, Byrne J, Wojcik SM. Seizure incidence by treatment pressure in patients undergoing hyperbaric oxygen therapy. UHM 2014;41:380–385 [PubMed] [Google Scholar]
- 8.Heyboer III M, Wojcik S, Grant WD, Chambers P, Jennings S, Adcock P. Middle ear barotrauma in hyperbaric oxygen therapy. UHM 2014;41:359–363 [PubMed] [Google Scholar]
- 9.Jain KK. Textbook of Hyperbaric Medicine, 5th ed. Cambridge, MA: Hogrefe Publishing, 1999 [Google Scholar]
- 10.Shupak A, Gilbey P. Effects of pressure. In: Neuman TS, Thom SR. Physiology and Medicine of Hyperbaric Oxygen Therapy. Philadelphia, PA: Saunders Elsevier, 2008:513–526 [Google Scholar]
- 11.O'Neill OJ, Weitzner ED. The O'Neill grading system for evaluation of the tympanic membrane: a practical approach for clinical hyperbaric patients. UHM 2015;42:265–271 [PubMed] [Google Scholar]
- 12.Bluestone CD. Eustachian Tube Structure, Function, Role in Otitis Media. Hamilton, ON: BC Decker, Inc., 2005 [Google Scholar]
- 13.Beuerlein M, Nelson RN, Wellin DB. Inner and middle ear hyperbaric oxygen-induced barotrauma. Laryngoscope 1997;107:1350–1356 [DOI] [PubMed] [Google Scholar]
- 14.Bluestone CD. Studies in otitis media: Children's Hospital of Pittsburgh-University of Pittsburgh progress report—2004. Laryngoscope 2004;114(S105):1–26 [DOI] [PubMed] [Google Scholar]
- 15.Camporesi EM. Side effects of hyperbaric oxygen therapy. UHM 2014;41:253–257 [PubMed] [Google Scholar]
- 16.Vahidova D, Sen P, Papesch M, Zein-Snachez M, Mueller P. Does the slow compression technique of hyperbaric oxygen therapy decrease the incidence of middle-ear barotraumas? J Laryngol Otol 2006;120:446–449 [DOI] [PubMed] [Google Scholar]
- 17.Fitzpatrick D, Franck B, Mason K, Shannon S. Risk factors for symptomatic otic and sinus barotrauma in a multiplace hyperbaric chamber. Undersea Hyperb Med 2010;37:203–208 [PubMed] [Google Scholar]
- 18.Clements KS, Vrabee JT, Mader JT. Complications of tympanostomy tubes inserted for facilitation of hyperbaric oxygen therapy. Arch Otolaryngol Head Neck Surg 1998;124:278. [DOI] [PubMed] [Google Scholar]
- 19.Presswood G, Zamboni WA, Stephenson LL, Santos PM. Effect of artificial airway on ear complications from hyperbaric oxygen. Laryngoscope 1994;104:1383–1384 [DOI] [PubMed] [Google Scholar]
- 20.Bessereau J, Tabah A, Genotelle N, Francais A, Coulange M, Annane D. Middle-ear barotraumas after hyperbaric oxygen therapy. UHM 2010;37:203–208 [PubMed] [Google Scholar]
- 21.Skevas T, Baumann I, Bruckner T, Clifton N, Plinkert PK, Klingmann C. Medical and surgical treatment in divers with chronic rhinosinusitis and paranasal sinus barotrauma. Eur Arch Otorhinolaryngol 2012;269:853–860 [DOI] [PubMed] [Google Scholar]
- 22.Gunn DJ, O'Hagan S. Unilateral optic neuropathy from possible sphenoidal sinus barotrauma after recreational scuba diving: a case report. UHM 2013;40:81–86 [PubMed] [Google Scholar]
- 23.Stoetzer M, Kuehlhorn C, Ruecker M, Ziebolz D, Gellrich NC, von See C. Pathophysiology of barodontalgia: a case report and review of the literature. Case Rep Dent 2012;2012:453415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robichaud R, McNally ME. Barodontalgia as a differential diagnosis: symptoms and findings. J Can Dent Assoc 2005;71:39–42 [PubMed] [Google Scholar]
- 25.Zadik Y, Drucker S. Diving dentistry: a review of the dental implications of scuba diving. Australian Dent J 2011;56:265–271 [DOI] [PubMed] [Google Scholar]
- 26.Cakmak T, Battal B, Kara K, et al. A case of tension pneumothorax during hyperbaric oxygen therapy in an earthquake survivor with crush injury complicated by ARDS (adult respiratory distress syndrome). Undersea Hyperb Med 2015;42:9–13 [PubMed] [Google Scholar]
- 27.Unsworth IP. Pulmonary barotrauma in a hyperbaric chamber. Anesthesia 1973;28:675–678 [DOI] [PubMed] [Google Scholar]
- 28.Wolf HK, Moon RE, Mitchell PR, Burger PC. Barotrauma and air embolism in hyperbaric oxygen therapy. Am J Forensic Med Pathol 1990;11:149–153 [DOI] [PubMed] [Google Scholar]
- 29.Rivalland G, Mitchell SJ, Van Schalkwyk JM. Pulmonary barotrauma and cerebral gas embolism during hyperbaric oxygen therapy. Aviat Space Environ Med 2010;81:888–890 [DOI] [PubMed] [Google Scholar]
- 30.Kellogg RH. La pression barometrique: paul bert's hypoxia theory and its critics. Respir Physiol 1978;34:1–28 [DOI] [PubMed] [Google Scholar]
- 31.Bert P. La Pression Barométrique. Paris: Masson et Cie, 1878 [Google Scholar]
- 32.Lambertsen CJ. Effects of oxygen at high partial pressure. In: Fenn WO, Rahn H, eds. Handbook of Physiology. Bethesda, MD: Am Physiol Soc, 1965:1027–1046 [Google Scholar]
- 33.Bitterman N. CNS oxygen toxicity. UHM 2004;31:63–72 [PubMed] [Google Scholar]
- 34.Lambertsen CJ, Kough RH, Cooper DY, Emmel GL, Loeschcke HH, Schmidt CF. Oxygen toxicity. Effects in man of oxygen inhalation at 1 and 3.5 atmospheres upon blood gas transport, cerebral circulation and cerebral metabolism. Jr Applied Phys 1953;5:471–486 [DOI] [PubMed] [Google Scholar]
- 35.Torbati D, Church DF, Keller JM, Pryor W. Free radical generation in the brain precedes hyperbaric oxygn-induced convulsions. Free Radic Biol Med 1992;13:101–106 [DOI] [PubMed] [Google Scholar]
- 36.Clark JM. Oxygen toxicity. In: Neuman TS, Thom SR, eds. Physiology and Medicine of Hyperbaric Oxygen Therapy. Philadelphia, PA: Saunders Elsevier, 2008:527–564 [Google Scholar]
- 37.Chavko M, Auker CR, McCarron RM. Relationship between protein nitration and oxidation and development of hyperoxic seizures. Nitric Oxide 2003;9:18–23 [DOI] [PubMed] [Google Scholar]
- 38.Banham NDG. Oxygen toxicity seizures: 20 years experience from a single hyperbaric unit. Diving Hyperb Med 2011;41:202–210 [PubMed] [Google Scholar]
- 39.Donald KW. Oxygen poisoning in man. BMJ 1947;1:712–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis JC. Hyperbaric oxygen therapy. J Intensive Care Med 1989;4:55–57 [Google Scholar]
- 41.Plafki C, Peters P, Almeling M, Welslau W, Busch R. Complications and side effects of hyperbaric oxygen therapy. Aviat Space Environ Med 2000;71:119–124 [PubMed] [Google Scholar]
- 42.Hampson N, Atik D. Central nervous system oxygen toxicity during routine hyperbaric oxygen therapy. UHM 2003;30:147–153 [PubMed] [Google Scholar]
- 43.Yildiz S, Aktas S, Cimsit M, Ay H, Togrol E. Seizure incidence in 80,000 patient treatments with hyperbaric oxygen. Aviat Space Environ Med 2004;75:992–994 [PubMed] [Google Scholar]
- 44.Welslau W, Almeling M. Toxicity of hyperbaric oxygen (HBO)–incidence of major CNS-intoxications. Strahlenther Onkol 1996;172(S2):10–12 [PubMed] [Google Scholar]
- 45.Hampson NB, Simonson SG, Kramer CC, Piantadosi CA. Central nervous system oxygen toxicity during hyperbaric treatment of patients with carbon monoxide poisoning. UHM 1996;23:215–219 [PubMed] [Google Scholar]
- 46.Sanders RW, Katz KD, Suyama J, et al. Seizure during hyperbaric oxygen therapy for carbon monoxide toxicity: a case series and five-year experience. J Emerg Med 2012;42:e69–e72 [DOI] [PubMed] [Google Scholar]
- 47.Hadanny A, Meir O, Bechor Y, Fishlev G, Bergan J, Efrati S. Seizures during hyperbaric oxygen therapy: retrospective analysis of 62,614 treatment sessions. UHM 2016;43:21–28 [PubMed] [Google Scholar]
- 48.Clark JM, Lambertsen CJ. Pulmonary oxygen toxicity: a review. Pharmacol Rev 1971;23:37–133 [PubMed] [Google Scholar]
- 49.Clark JM, Lambertsen CJ, Gelfand R, et al. Effects of prolonged oxygen exposure at 1.5, 2.0; or 2.5 ATA on pulmonary function in men (Predictive Studies V). J Appl Physiol 1999;86:243–259 [DOI] [PubMed] [Google Scholar]
- 50.Comroe JH, Dripps RD, Dumke PR, Deming M. The effect of inhalation of high concentrations of oxygen for twenty-four hours on normal men at sea level and at a simulated altitude of 18,000 feet. JAMA 1945;128:710–717 [Google Scholar]
- 51.Clark JM, Lambertsen CJ. Rate of development of pulmonary O2 toxicity in man during O2 breathing at 2.0 atm abs. J Appl Physiol 1971;30:739–752 [DOI] [PubMed] [Google Scholar]
- 52.Michel EL, Langevin RW, Gell CF. Effect of continuous human exposure to oxygen tension of 418 mmHg for 168 hours. Aerosp Med 1960;31:138–144 [Google Scholar]
- 53.Herlocher JE, Quigley DG, Behar VS, et al. Physiologic response to increased oxygen partial pressure. I. Clinical observations. Aerosp Med 1964;35:613–618 [PubMed] [Google Scholar]
- 54.Thorsen E, Aanderud L, Aasen TB. Effects of a standard hyperbaric oxygen treatment protocol on pulmonary function. Eur Respir J 1998;12:1442–1445 [DOI] [PubMed] [Google Scholar]
- 55.Butler FK. Diving and Hyperbaric ophthalmology. Surv Ophthalmol 1995;39:347–396 [DOI] [PubMed] [Google Scholar]
- 56.Nichols CW, Lambertsen CJ. Effects of high oxygen pressures on the eye. N Engl J Med 1969;281:25–30 [DOI] [PubMed] [Google Scholar]
- 57.McMonnies CW. Hyperbaric oxygen therapy and the possibility of ocular complications or contradictions. Clin Exp Optom 2015;98:122–125 [DOI] [PubMed] [Google Scholar]
- 58.Tibbles PM, Edelsberg JS. Hyperbaric-oxygen therapy. N Engl J Med 1996;334:1642–1648 [DOI] [PubMed] [Google Scholar]
- 59.Butler FK, Hagan C. Ocular complications in hyperbaric oxygen therapy. In: Neuman TS, Thom SR, eds. Physiology and Medicine of Hyperbaric Oxygen Therapy. Philadelphia, PA: Saunders Elsevier, 2008:565–572 [Google Scholar]
- 60.Anderson B, Farmer JC. Hyperoxic myopia. Trans Am Ophthalmol Soc 1978;76:116–124 [PMC free article] [PubMed] [Google Scholar]
- 61.Churchill S, Deru K, Wilson G, Cable R, Bell JE, Weaver LK. Rates of visual acuity change in patients receiving hyperbaric oxygen in monoplace and multiplace chambers. UHM 2016:43:217–223 [PubMed] [Google Scholar]
- 62.Evanger K, Pierscionek BK, Vaagbo G, Thorsen E, Haugen OH. Myopic shift during hyperbaric oxygenation attributed to lens index changes. Optom Vis Sci 2015;92:1076–1084 [DOI] [PubMed] [Google Scholar]
- 63.Evanger K, Haugen OH, Irgens A, Aanderud L, Thorsen E. Ocular refractive changes in patients receiving hyperbaric oxygen administered by oronasal mask or hood. Acta Ophthalmol Scand 2004;82:449–453 [DOI] [PubMed] [Google Scholar]
- 64.Giblin FJ, Padgaonkar VA, Leverenz VR, et al. Nuclear light scattering, disulfide formation and membrane damage in lenses of older guinea pigs treated with hyperbaric oxygen. Exp Eye Res 1995;60:219–235 [DOI] [PubMed] [Google Scholar]
- 65.Palmquist BM, Philipson B, Barr PO. Nuclear cataract and myopia during hyperbaric oxygen therapy. B J Ophthalmol 1984;68:113–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kindwall EP, Whelan HT. Hyperbaric Medicine Practice, 3rd ed. Flagstaff, AZ: Best Publishing Company, 2008 [Google Scholar]
- 67.Solomon R, Donnenfeld ED. Recent advances and future frontiers in treating age-related cataracts. JAMA 2003;290:248–251 [DOI] [PubMed] [Google Scholar]
- 68.Allen D, Vasavada A. Cataract and surgery for cataract. BMJ 2006;333:128–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gesell LB, Trott A. De Novo cataract development following a standard course of Hyperbaric Oxygen Therapy. UHM 2007;34:389–392 [PubMed] [Google Scholar]
- 70.Weikel KA, Garber C, Baburins A, Taylor A. Nutritional modulation of cataract. Nutr Rev 2014;72:30–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giblin FJ. Glutathione: a vital lens antioxidant. J Ocul Phamacol Ther 2000;16:121–135 [DOI] [PubMed] [Google Scholar]
- 72.Butler FK. The eye in the wilderness. In: Auerbach P, eds. Wilderness Medicine, 5th ed. St Louis: Mosby, 2007:604–624 [Google Scholar]
- 73.Ashton N, Ward B, Serpell G. Role of oxygen in the genesis of retrolental fibroplasia: a preliminary report. Br J Ophthalmol 1953;37:513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Teoh SL, Boo NY, Ong LC, Nyein MK, Lye MS, Au MK. Duration of oxygen therapy and exchange transfusion as risk factors associated with retinopathy of prematurity in very low birth weight infants. Eye 1995;9:733–737 [DOI] [PubMed] [Google Scholar]
- 75.Patz A. The role of oxygen in retrolental fibroplasia. Tr Am Ophth Soc 1968;66:940–985 [PMC free article] [PubMed] [Google Scholar]
- 76.Sola A, Chow L, Rogido M. Retinopathy of prematurity and oxygen therapy: a changing relationship. An Pediatr (Barc) 2005;62:48–61 [DOI] [PubMed] [Google Scholar]
- 77.Chow LC, Wright KW, Sola A. Can changes in clinical practice decrease the incidence of severe retinopathy of prematurity in very low birth weight infants? Pediatrics 2003;111:339–345 [DOI] [PubMed] [Google Scholar]
- 78.Weinberger B, Laskin DL, Heck DE, Laskin JD. Oxygen toxicity in premature infants. Toxicol Appl Pharmacol 2002;181:60–67 [DOI] [PubMed] [Google Scholar]
- 79.Paddock M. Claustrophobia: causes, symptoms and treatments. June 25, 2015. www.medicalnewstoday.com/articles/37062 (last accessed November7, 2016)
- 80.Davey GC. Phobias: A Handbook of Theory, Research and Treatment, 1st ed. West Sussex, England: John Wiley & Sons Ltd, 1997 [Google Scholar]
- 81.Al-Waili NS, Butler GJ, Beale J, et al. Influences of hyperbaric oxygen on blood pressure, heart rate and blood glucose levels in patients with diabetes mellitus and hypertension. Arch Med Res 2006;37:991–997 [DOI] [PubMed] [Google Scholar]
- 82.Heyboer M, III, Smith G, Santiago W, Wojcik SM. Effect of hyperbaric oxygen therapy on blood pressure. UHM;.Accepted September 27, 2016 [DOI] [PubMed] [Google Scholar]
- 83.Abel FL, Mcnamee JE, Cone DL, Clarke D, Tao J. Effects of hyperbaric oxygen on ventricular performance, pulmonary blood volume, and systemic and pulmonary vascular resistance. UHM 2000;27:67–73 [PubMed] [Google Scholar]
- 84.Weaver LK, Churchill S. Pulmonary edema associated with hyperbaric oxygen therapy. Chest 2001;120:1407–1409 [DOI] [PubMed] [Google Scholar]
- 85.Whalen RE, Saltzman HA, Holloway DH Jr, et al. Cardiovascular and blood gas response to hyperbaric oxygenation. Am J Cardiol 1965;15:638–646 [DOI] [PubMed] [Google Scholar]
- 86.Gawthrope IC, Playford DA, King B, Brown K, Wilson C, McKeown B. The cardiac effects of hyperbaric oxygen at 243 kPa using inchamber echocardiography. Diving Hyperb Med 2014;44:141–145 [PubMed] [Google Scholar]
- 87.Stevens SL, Narr AJ, Claus PL, et al. The incidence of hypoglycemia during HBO therapy: a retrospective review. UHM 2015;42:191–196 [PubMed] [Google Scholar]
- 88.Swaby J, Heyboer M, Wojcik S, Grant W. Effects of hyperbaric oxygen therapy on diabetic serum glucose levels: an extended study. Undersea Hyperb Med J 2015;42:190 [Google Scholar]
- 89.Hart GB, Strauss MB. Central nervous system oxygen toxicity in a clinical setting. In: Bove AA, Bachrach AJ, Greenbaum LJ, eds. Undersea and Hyperbaric Physiology IX. Proceedings of the Ninth International Symposicum on Underwater and Hyperbaric Physiology Bethesda, MD: Undersea and Hyperbaric Medical Society, 1987:695–699 [Google Scholar]