Abstract
Significance: Pancreatic tumors express high level of nitric oxide synthases (NOSs) in particular inducible (iNOS/NOS2) and endothelial (eNOS/NOS3) forms. However, the role of nitric oxide (NO•) in the development and progression of pancreatic cancer is not clearly defined. Delineating the NO•-induced signaling in pancreatic cancer and its potential contribution in disease aggressiveness may provide therapeutic targets to improve survival in this lethal malignancy.
Recent Advances: An increased expression of NOS2/iNOS in tumors is associated with poorer survival in early stage resected patients with pancreatic ductal adenocarcinoma (PDAC). Furthermore, genetic deletion of NOS2 enhanced survival in mice with autochthonous PDAC. Additionally, targeting NOS3/eNOS reduced the abundance of precursor lesions in mice, which trended toward improved survival.
Critical Issues: The extremely poor prognosis in pancreatic cancer is due to the late diagnosis and lack of effective therapy in advanced disease. One of the most critical issues is to decipher the underlying mechanism of disease aggressiveness and therapeutic resistance for identifying potential therapeutic target and effective treatment. Given the evidence of a strong association between inflammation and pancreatic cancer and clinical evidence, which suggests an association between NOS2 and disease aggressiveness, it is critical to define the role of NO• signaling in this lethal malignancy.
Future Directions: Recent preclinical and clinical evidences indicate a potential therapeutic significance of targeting NO• signaling in pancreatic cancer. With the emergence of new preclinical models, including the patient-derived organoids, further preclinical evaluation using clinically tested NOS inhibitors is needed for designing future clinical investigation. Antioxid. Redox Signal. 26, 1000–1008.
Keywords: : nitric oxide, pancreatic cancer, microRNA, nitric oxide synthase, Nostrin, pancreatic ductal adenocarcinoma (PDAC)
Introduction
Pancreatic cancer is one of the most lethal malignancies and is ranked as fourth leading cause of death due to cancer with an estimated 53,070 new pancreatic cancer cases and 41, 780 deaths due to this malignancy in 2016 alone (68). A mere 8% of the patients with advanced stage of the disease survive more than 5 years after diagnosis. Alarmingly, a consistent rise in incidence and death in pancreatic cancer is estimated to make it the second leading cause of cancer-related deaths by 2030 (61). The lack of a reliable biomarker and asymptomatic nature of the early stages of the disease prevent early detection and therefore a majority of the pancreatic cancer patients are diagnosed at an advanced stage and are refractory to the available treatments. A small number of patients, detected at an early stage, undergo surgical resection with some curable potential; however, a large percentage of resected patients show recurrence within 2 years.
Among different forms of pancreatic cancer, the most common is pancreatic ductal adenocarcinoma (PDAC) and is commonly referred as pancreatic cancer. Several risk factors, including smoking, alcohol use, inflammation, family history, diabetes, obesity, and race, have been associated with pancreatic cancer (4, 10, 14, 17, 20–22, 26, 40, 45, 78). Investigations of tumor biology have identified several genetic alterations, with the activating mutations in KRAS and inactivation of the CDKN2A gene (loss of p16 protein) as the most common, occurring in more than 90% of PDACs. In addition, TP53 alterations in about 50–75% and the loss of DPC4 (deleted in pancreatic cancer) are recorded in about 50% of all PDAC cases (33, 34). Furthermore, PDAC is characterized by a highly reactive, dense, and poorly vascularized stroma, called desmoplasia. Molecular analysis of desmoplastic stroma revealed its highly complex architecture comprising fibroblasts, pancreatic stellate cells, endothelial cells, and immune and inflammatory cells intermingled with a dense extracellular matrix containing collagen, laminin, and fibronectin [reviewed in ref. (27)]. The role of desmoplastic stroma in PDAC is highly complex and in addition to providing growth advantage and maintenance of tumor cells, it is implicated in restricting the access to chemotherapeutic drugs (56, 59). In contrast, however, recent studies have shown that stroma, in fact, act as a barrier restraining the pancreatic cancer growth and metastasis rather than supporting it (58, 63). Therefore, further studies are needed to delineate the role of desmoplastic stroma in pancreatic cancer that would allow its reprogramming in tumors with a distinct molecular makeup to achieve precise therapeutic intervention.
Epidemiological and molecular evidence corroborate a role of inflammation in pancreatic tumorigenesis and therapeutic resistance in pancreatic cancer (21, 89). One such evidence is the observation that the risk of developing pancreatic cancer increases severalfold in patients with hereditary and sporadic pancreatitis (43, 86). An increasing level of inflammation is accompanied with the progression of precancerous lesions to advanced disease in pancreatic cancer (11, 15). A strong inflammatory microenvironment generates an enhanced level of protumorigenic cytokines, chemokines, and reactive species and leads to the activation of oncogenic signaling pathways contributing to tumorigenesis (23, 30, 65, 76). Additionally, tumor cells also produce many of the inflammatory mediators, including nitric oxide (NO•) and macrophage migration inhibitory factor (MIF). An increased MIF in tumors is associated with poorer survival in patients with PDAC (88). Nevertheless, a clear understanding of the role of inflammatory mediators in the development, progression, and therapeutic resistance of pancreatic cancer is still lacking. An in-depth knowledge of the contribution of inflammatory mediators in pancreatic cancer progression may identify unique therapeutic vulnerability for the management of this lethal malignancy.
One of the inflammatory mediators that is implicated in the development and progression of many cancer types, including that of pancreas, is NO• (72, 73) [reviewed in refs. (30, 84)]. NO• is a free radical and is involved in a number of critical physiological processes, including vasodilation, neurotransmission, immune regulation, inflammation, and host defense (5, 8, 12, 13, 19, 28, 35, 46, 49, 50). NO• is produced by a family of nitric oxide synthase (NOS) enzymes, which includes neuronal NOS (NOS1/nNOS), inducible NOS (NOS2/iNOS), and endothelial NOS (NOS3/eNOS). Whereas, NOS1 and NOS3 are the constitutive isoforms and produce a small amount of NO• at picomolar to nanomolar range, NOS2 is an inducible isoform and can produce a higher and sustained level of NO• in micromolar range in response to inflammatory stimuli. Therefore, NOS2 is primarily responsible for an enhanced level of NO• production (72). The role of NO• in tumorigenesis is highly complex and both pro- and antineoplastic functions have been reported, which largely depends on the amount of NO•, cell types, cellular microenvironment, its interaction with other reactive species, and the presence of metals, and has been extensively reviewed elsewhere (30, 73, 75). For example, genetic deletion of NOS2 in p53-deficient mice can either suppress or enhance lymphomagenesis depending on the inflammatory microenvironment (29, 31). Furthermore, NOS2 deficiency decreased lung tumor growth and oncogenic KRAS-mediated inflammatory response and increased survival in a genetic mouse model of lung cancer with conditional activation of mutant KRAS (54). This short review is focused on the evidence of a role of NO in the development and progression of pancreatic cancer and the potential of NO•-mediated signaling to be targeted or modulated for therapeutic intervention.
NO• and Pancreatic Cancer: A Survey of Evidence
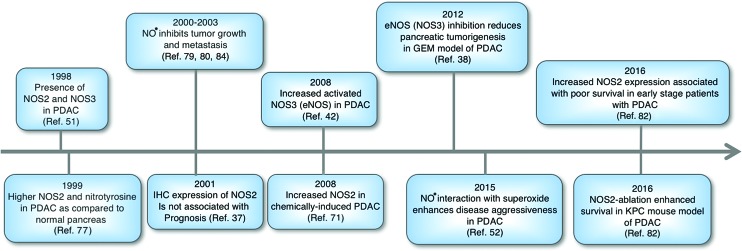
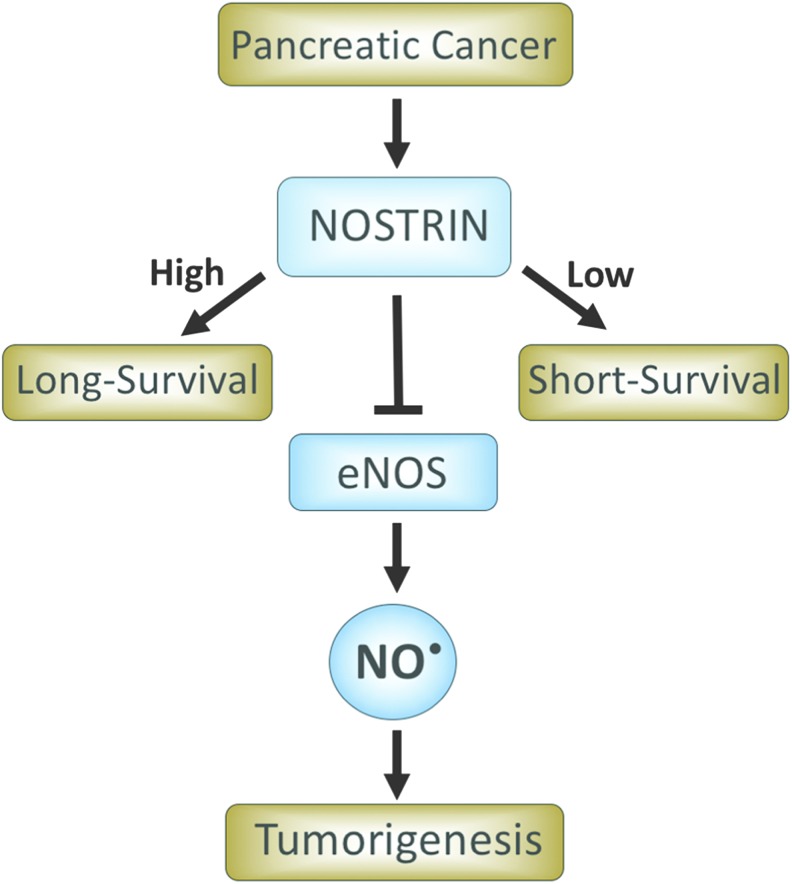
It is important to first discuss the relevance of a role of NO• in the pathogenesis of pancreatic cancer based on clinical observations. There have been several studies showing the high expression of NOS2 and NOS3 in tumors from patients with pancreatic cancer. In one of the earliest studies, examination of 12 PDACs showed a higher expression of NOS2 and nitrotyrosine, which is formed due to the interaction of NO• with superoxide anion and formation of a reactive nitrogen species, peroxynitrite, compared with normal pancreas (77). Furthermore, immunohistochemical analysis of tumors from 72 PDAC cases found the presence of NOS2 in about 66% of these cases (37). Similarly other studies have also reported the presence of NOS2 either at the mRNA or protein level in a significant number of PDACs analyzed in respective studies (16, 36, 51). Interestingly, in one of these studies (51), 60% of the tumors showed NOS2 mRNA expression, but they failed to show any NOS2 protein expression. Most recently, about 71% of the tumors from PDAC patients were found to express a high level of nitrotyrosine compared with its undetectable level in adjacent normal pancreatic ducts (52). Recently, in a largest study so far (N = 107) to examine the role of NO• in human pancreatic cancer, a higher NOS2 gene expression was associated with poorer survival in early stage resected patients with PDAC (82). Furthermore, the study also showed that in a genetically engineered autochthonous mouse model of pancreatic cancer, which faithfully mirrors the development and progression of human PDAC, genetic ablation of NOS2 significantly enhanced survival and reduced tumor severity. These findings showed that NOS2 is a candidate predictor of prognosis in PDAC patients and provided proof of principle that NOS2 may be targeted for improving disease outcome in this lethal malignancy. In an earlier study, however, the presence or absence of NOS2 expression, as determined by immunohistochemistry, did not associate with overall survival (37). In addition to NOS2, the presence of NOS3 or eNOS has also been reported in PDAC and is implicated in neovascularization (51). Moreover, an increased expression of activated eNOS was found in PDAC compared with nontumor control (42). Consistent with these findings, eNOS positivity was noted in about half of the PDACs (38). Additionally, genetic ablation and pharmacological inhibition of eNOS reduced tumorigenicity in a genetically engineered mutant KRAS mouse model of PDAC (38). Endothelial nitric oxide synthase traffic inducer (NOSTRIN) translocates and sequesters eNOS in a vesicular structure, which inhibits NO• production (53, 92). Recently, we have shown that a lower expression level of NOSTRIN in tumors is associated with worst survival in early stage resected patients with PDAC (83). NOSTRIN reduced the activation of eNOS and NO• production, inhibited migration and invasion, and enhanced sensitivity of human pancreatic cancer cell lines to chemotherapeutic drugs (83). These findings suggested a potential regulatory role of NO•-mediated signaling in the development and/or progression of pancreatic cancer (Figs. 1–3).
FIG. 1.
Schematic representation of a brief history suggesting a role of NO• in the development and progression of pancreatic cancer. NO•, nitric oxide. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
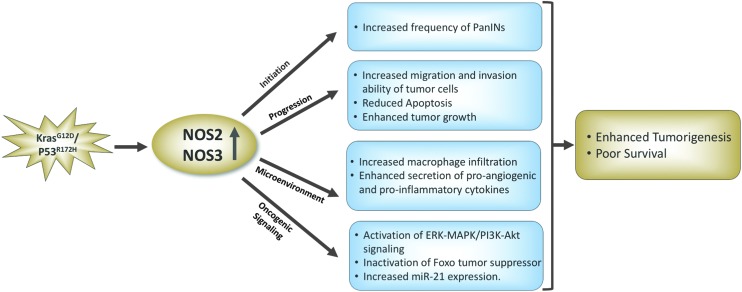
FIG. 2.
Protumorigenic role of NOS2 and NOS3 based on the studies involving genetically engineered mouse model of pancreatic cancer with pancreas-specific mutation in the Kras and P53 genes. eNOS/NOS3, endothelial nitric oxide synthase; iNOS/NOS2, inducible nitric oxide synthase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 3.
NOSTRIN is a predictor of prognosis in early stage resected patients with PDAC. Patients with a higher NOSTRIN expression in tumors survive longer compared with patients with a lower expression of NOSTRIN. NOSTRIN inhibits NOS3 (eNOS) and reduces NO production. NOSTRIN, endothelial nitric oxide synthase traffic inducer; PDAC, pancreatic ductal adenocarcinoma. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Biological Significance of NO in Pancreatic Cancer
NO• regulates a number of signaling pathways and critical cellular functions, for example, apoptosis, cell cycle arrest, and senescence, and may have both pro- and antitumorigenic functions, which are found to be largely context dependent. A concentration-dependent mapping of the biological response of NO• showed that a distinct level of NO• results in the post-translation modification and activation/stabilization of a specific protein implicated in cancer (74). For example, a lower concentration of NO• ranging from 10 to 30 nM leads to the phosphorylation of ERK, whereas 400 nM level of NO• is required for phosphorylation and stabilization of p53. An extensive mechanistic and functional investigation using pancreatic cancer cell lines expressing various levels of NOS2 and mouse xenograft model showed that NOS2 and NO• production are vastly heterogeneous and that the physiological level of NO• inhibits tumor growth and metastasis (67, 79–81, 85). However, depletion of interferon-γ abrogated the NO•-mediated antitumor effect and resulted in aggressive tumor growth and metastasis of the highly metastatic pancreatic cancer cell line, Panc02-H7 (80). Therefore, interferon-γ expression was found to be essential for NOS2/NO•-mediated antitumor effects in this model. The antitumorigenic effect of NO• in these studies is attributed to NO•-mediated apoptosis (39, 87). In contrast, however, NO• has been found to be responsible for enhanced invasion of pancreatic cancer cells following exposure to carbon ion radiation (18). This NO•-mediated increase in invasiveness of pancreatic cancer cells involved the activation of PI3K-AKT and RhoA pathways. Furthermore, primary tumor cells isolated from NOS2-deficient mouse model of pancreatic cancer, LSL-KrasG12D/+/LSL-Trp53R172H/Pdx-1-Cre (KPC mice), showed reduced proliferation index and invasive ability compared with the primary tumor cells from KPC mice with wild-type NOS2 (82). Mechanistic study revealed that NO• enhances phosphorylation/inactivation of forkhead box transcription factor O (FOXO3) through the ERK signaling pathway. In a recent study, the interaction of NO• with superoxide and formation of peroxynitrite with subsequent increase in nitration, as determined by 3-nitrotyrosine immunostaining, was found to enhance disease aggressiveness and poor survival in PDAC patients (52). The increase in superoxide and its interaction with NO• in PDAC resulted due to the loss of extracellular superoxide dismutase (SOD3). Furthermore, treatment of NOS inhibitor also inhibited pancreatic tumor growth in xenograft models. An increased expression of NOS2 was found in a chemical carcinogen-induced animal model of pancreatic cancer (71). The treatment with NOS2 inhibitor in this model reduced the multiplicity of precancerous lesions and incidence of PDAC, supporting a role of NO• in growth and progression of PDAC.
Several in vivo studies have examined the effect of NO-donating nonsteroidal anti-inflammatory drug (NSAID) on pancreatic carcinogenesis. Treatment of NO•-donating Aspirin (NO-ASA) drastically reduced the multiplicity and incidence of chemically induced pancreatic cancer in hamsters (57). Similarly, treatment of a genetically engineered mouse model of pancreatic cancer with NO-ASA resulted in reduced number of PanIN3 lesion incidence of PDAC (62). Mechanistic investigation showed a decrease in NOS2 expression in NO-ASA-treated mice in addition to proliferating cell nuclear antigen, cyclooxygenase-2, cyclin D1, and Bcl2 and increase in p21, p38, and p53 (62). However, the role of NO• in these studies is not clear.
One of the characteristics of pancreatic cancer is its resistance to chemotherapeutic drugs, resulting in poor disease outcome. A number of factors have been attributed to the refractory nature of PDAC to therapy with major emphasis on massive desmoplastic hypovascular stroma and enhanced interstitial pressure, resulting in constriction of vessels (56, 59, 60). In the context of tumor–stromal interaction and resistance to chemotherapy, coculturing of chemosensitive pancreatic cancer cells with fibroblasts rendered them less sensitive to etoposide-induced killing. This chemoresistance was mediated by NO•, released by fibroblasts, and subsequent NO•-mediated upregulation of interleukin (IL)-1β in cancer cells, which could be abolished by the treatment with NOS2 inhibitor (47). Further mechanistic investigation revealed that a cellular adhesion molecule, L1 cell adhesion molecule, upregulated NO• secretion, and IL-1β and NO• resulted in the inhibition of caspase activity and apoptosis (48, 66). These findings are consistent with a highly complex role of NO• in pancreatic cancer, as has been earlier described, for its contribution to tumorigenesis in general.
NO• and MicroRNA in Pancreatic Cancer
Altered expression of microRNAs (miRNAs) is implicated in the development, progression, and aggressiveness of pancreatic cancer. Earlier studies, examining miRNA expression, have reported their differential expression in pancreatic cancer compared with nontumorous pancreas (1, 6, 41, 64). A lower expression of miR-200 family is associated with pancreatic cancer aggressiveness, and re-expression of miR-200c leads to reduced tumor cell aggressiveness (70). Likewise, miR-146a suppressed the invasive ability of pancreatic cancer cells (41). In contrast, a reduced miR-21 expression level is associated with better outcome following adjuvant treatment in resectable pancreatic cancer patients (32). Examining 95 miRNAs, which are functionally related to cancer biology, cell development, and apoptosis, in pancreatic cancer tissue and cell lines showed different profiling patterns among individual cases and cell lines, suggesting individual molecular makeup in pancreatic cancer (91). miRNA analysis in fine needle aspirates from PDAC patients showed a unique signature compared with control subjects (2). Aligned with these findings, several miRNAs are identified as potential target for therapy in pancreatic cancer. Modulation of miR-200 and miR-21 expression enhanced gemcitabine sensitivity of pancreatic cancer cells (1). Recently, a system biology approach to identify and exploit epigenetic regulation of miRNA has been described as a potential therapeutic strategy in pancreatic cancer (3). Several cancer-related miRNAs are regulated by inflammatory and immune mediators (28, 65). Altered expression of oncogenic miRNA-21 has been associated with increased NOS2 level in chronic inflammatory cancer-prone diseases (69). Moreover, NOS2-deficient tumors in a mutant KRAS lung cancer mouse model showed a lower expression of miR-21 compared with tumors from NOS2 wild-type mice (55). Consistent with this finding, pancreatic tumors from NOS2-deficient KPC mice showed a decrease in mir-21expression compared with tumors in KPC mice with the intact NOS2 gene (82). However, the mechanism of a potential regulation of mir-21 by NO• is not clear. Examination of miRNA expression profile following Corynebacterium parvum-induced inflammation in mice revealed NO•-dependent modulation of miR-29b/c (44). Interestingly, a paradoxical role of NO• is described in the regulation of miR-155. An exogenous NO• exposure at higher level increased miR-155 expression, whereas the endogenous basal level of NO• inhibits it (90). In addition to the NO•-mediated regulation of miRNAs, there are several miRNAs that are recently described as the regulator of NOS2 [reviewed in ref. (24)]. miR-939 and miR-26a bind to the 3′UTR on the NOS2 gene and block its translation (25). Despite convincing evidence of an interaction between NO• and miRNA, and their individual contribution in pancreatic cancer, the interactive role of NO• and miRNA in pancreatic cancer warrants further investigation.
NO• and Pancreatic Cancer Therapy
Accumulating evidence of a biological role of NO•-mediated signaling in pancreatic cancer progression and therapeutic resistance underscores its therapeutic potential to be explored in preclinical and clinical trials. As discussed in this review, some preclinical studies using NOS inhibitors in chemically induced or genetically engineered animal models of pancreatic cancer are highly encouraging. Similarly, several studies have used NO•-donating NSAIDs in vitro and in vivo, describing its tumor inhibitory effects on the growth and progression of pancreatic cancer. However, the role of NO• in these studies is not clearly understood. Interestingly, the use of NOS inhibitor, N-nitro-l-arginine, either alone or in combination with the VEGF-R2 inhibitor suppressed tumor vascularization and growth of orthotopic tumors (9). However, the highly hypovascular nature of human PDAC and the fact that an increase in drug delivery following the enhanced vascularization results in increase in survival observed in an autochthonous mouse model of pancreatic cancer argue against the benefit of targeting NO•-induced tumor vascularization. Moreover, the recent understanding of the complexity of tumor–stromal interaction in pancreatic cancer (58, 63) warrants further investigation into the possibility of NO•-mediated stromal reprogramming that can be altered to achieve therapeutic advantage. To further assess the potential therapeutic significance of NOS inhibitors, patient-derived tumor xenografts and recently developed human PDAC organoid model (7) could be highly useful.
Summary
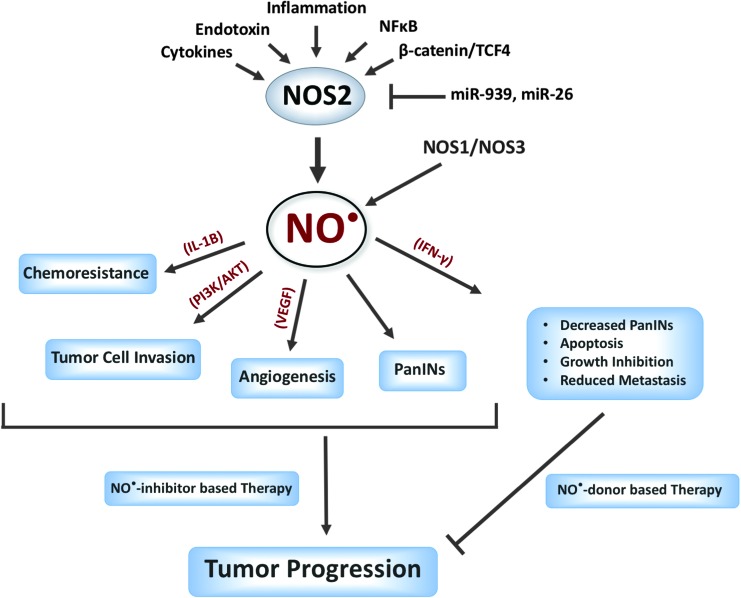
Although both pro- and antitumorigenic functions of NO• have been described (Fig. 4), the evidence of a role of NO•-mediated signaling in the development and progression of pancreatic cancer is becoming a bit more convincing. Association of a higher expression of NOS2 with poorer survival in a large cohort of patients with multiple validations in independent cohorts suggests a role of NO• in pancreatic cancer progression and disease aggressiveness. Furthermore, NOS2 ablation and increased survival of mice with lethal PDAC provide proof of principal that targeting NOS2 may have potential therapeutic significance in this lethal malignancy. Additionally, genetic ablation and pharmacological inhibition of eNOS/NOS3 reduced pancreatic lesions and showed a trend of increasing survival in a mutant Kras mouse model. However, future preclinical and clinical trials using NOS2/NOS3 inhibitors are warranted to further assess its therapeutic potential in patients with PDAC.
FIG. 4.
The principal source of a high and sustained level of NO• is the NOS2. However, NO• produced by NOS2 and NOS3 is implicated in pancreatic cancer progression. NO• signaling is reported to have both pro- and antitumorigenic functions. Further mechanistic, functional, preclinical, and clinical studies are warranted to guide the potential therapeutic benefits of NO•-based or NO•-targeted treatment strategies. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Abbreviations Used
- eNOS/NOS3
endothelial nitric oxide synthase
- IL
interleukin
- iNOS/NOS2
inducible nitric oxide synthase
- KPC
LSL-KrasG12D/+/LSL-Trp53R172H/Pdx-1-Cre
- MIF
migration inhibitory factor
- miRNA
microRNA
- nNOS/NOS1
neuronal nitric oxide synthase
- NO
nitric oxide
- NO-ASA
nitric oxide donating aspirin
- NOS
nitric oxide synthase
- NOSTRIN
endothelial nitric oxide synthase traffic inducer
- NSAID
nonsteroidal anti-inflammatory drug
- PDAC
pancreatic ductal adenocarcinoma
Acknowledgment
This work was supported by the Intramural Research Program of the Center for Cancer Research, NCI, NIH.
References
- 1.Ali S, Ahmad A, Banerjee S, Padhye S, Dominiak K, Schaffert JM, Wang Z, Philip PA, and Sarkar FH. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res 70: 3606–3617, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Ali S, Saleh H, Sethi S, Sarkar FH, and Philip PA. MicroRNA profiling of diagnostic needle aspirates from patients with pancreatic cancer. Br J Cancer 107: 1354–1360, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azmi AS, Beck FW, Bao B, Mohammad RM, and Sarkar FH. Aberrant epigenetic grooming of miRNAs in pancreatic cancer: a systems biology perspective. Epigenomics 3: 747–759, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao B, Wang Z, Li Y, Kong D, Ali S, Banerjee S, Ahmad A, and Sarkar FH. The complexities of obesity and diabetes with the development and progression of pancreatic cancer. Biochim Biophys Acta 1815: 135–146, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billiar TR, Hoffman RA, Curran RD, Langrehr JM, and Simmons RL. A role for inducible nitric oxide biosynthesis in the liver in inflammation and in the allogeneic immune response. J Lab Clin Med 120: 192–197, 1992 [PubMed] [Google Scholar]
- 6.Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP, Liu CG, Bhatt D, Taccioli C, and Croce CM. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA 297: 1901–1908, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS, Gracanin A, Oni T, Yu KH, van Boxtel R, Huch M, Rivera KD, Wilson JP, Feigin ME, Ohlund D, Handly-Santana A, Ardito-Abraham CM, Ludwig M, Elyada E, Alagesan B, Biffi G, Yordanov GN, Delcuze B, Creighton B, Wright K, Park Y, Morsink FH, Molenaar IQ, Borel Rinkes IH, Cuppen E, Hao Y, Jin Y, Nijman IJ, Iacobuzio-Donahue C, Leach SD, Pappin DJ, Hammell M, Klimstra DS, Basturk O, Hruban RH, Offerhaus GJ, Vries RG, Clevers H, and Tuveson DA. Organoid models of human and mouse ductal pancreatic cancer. Cell 160: 324–338, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bredt DS. and Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem 63: 175–195, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Camp ER, Yang A, Liu W, Fan F, Somcio R, Hicklin DJ, and Ellis LM. Roles of nitric oxide synthase inhibition and vascular endothelial growth factor receptor-2 inhibition on vascular morphology and function in an in vivo model of pancreatic cancer. Clin Cancer Res 12: 2628–2633, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Chang KJ, Parasher G, Christie C, Largent J, and Anton-Culver H. Risk of pancreatic adenocarcinoma: disparity between African Americans and other race/ethnic groups. Cancer 103: 349–357, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Chu GC, Kimmelman AC, Hezel AF, and DePinho RA. Stromal biology of pancreatic cancer. J Cell Biochem 101: 887–907, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Coleman JW. Nitric oxide in immunity and inflammation. Int Immunopharmacol 1: 1397–1406, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Dedon PC. and Tannenbaum SR. Reactive nitrogen species in the chemical biology of inflammation. Arch Biochem Biophys 423: 12–22, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Everhart J. and Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA 273: 1605–1609, 1995 [PubMed] [Google Scholar]
- 15.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, and Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res 18: 4266–4276, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franco L, Doria D, Bertazzoni E, Benini A, and Bassi C. Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in pancreatic cancer. Prostaglandins Other Lipid Mediat 73: 51–58, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Fuchs CS, Colditz GA, Stampfer MJ, Giovannucci EL, Hunter DJ, Rimm EB, Willett WC, and Speizer FE. A prospective study of cigarette smoking and the risk of pancreatic cancer. Arch Intern Med 156: 2255–2260, 1996 [PubMed] [Google Scholar]
- 18.Fujita M, Imadome K, Endo S, Shoji Y, Yamada S, and Imai T. Nitric oxide increases the invasion of pancreatic cancer cells via activation of the PI3K-AKT and RhoA pathways after carbon ion irradiation. FEBS Lett 588: 3240–3250, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Furchgott RF. and Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J 3: 2007–2018, 1989 [PubMed] [Google Scholar]
- 20.Gapstur SM, Gann PH, Lowe W, Liu K, Colangelo L, and Dyer A. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA 283: 2552–2558, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Garcea G, Dennison AR, Steward WP, and Berry DP. Role of inflammation in pancreatic carcinogenesis and the implications for future therapy. Pancreatology 5: 514–529, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Greer JB. and Whitcomb DC. Inflammation and pancreatic cancer: an evidence-based review. Curr Opin Pharmacol 9: 411–418, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Gukovsky I, Li N, Todoric J, Gukovskaya A, and Karin M. Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology 144: 1199–1209 e4, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo Z. and Geller DA. microRNA and human inducible nitric oxide synthase. Vitam Horm 96: 19–27, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Guo Z, Shao L, Zheng L, Du Q, Li P, John B, and Geller DA. miRNA-939 regulates human inducible nitric oxide synthase posttranscriptional gene expression in human hepatocytes. Proc Natl Acad Sci U S A 109: 5826–5831, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, and Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev 20: 1218–1249, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Hidalgo M. Pancreatic cancer. N Engl J Med 362: 1605–1617, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Hussain SP. and Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer 121: 2373–2380, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Hussain SP, He P, Subleski J, Hofseth LJ, Trivers GE, Mechanic L, Hofseth AB, Bernard M, Schwank J, Nguyen G, Mathe E, Djurickovic D, Haines D, Weiss J, Back T, Gruys E, Laubach VE, Wiltrout RH, and Harris CC. Nitric oxide is a key component in inflammation-accelerated tumorigenesis. Cancer Res 68: 7130–7136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hussain SP, Hofseth LJ, and Harris CC. Radical causes of cancer. Nat Rev Cancer 3: 276–285, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Hussain SP, Trivers GE, Hofseth LJ, He P, Shaikh I, Mechanic LE, Doja S, Jiang W, Subleski J, Shorts L, Haines D, Laubach VE, Wiltrout RH, Djurickovic D, and Harris CC. Nitric oxide, a mediator of inflammation, suppresses tumorigenesis. Cancer Res 64: 6849–6853, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Hwang JH, Voortman J, Giovannetti E, Steinberg SM, Leon LG, Kim YT, Funel N, Park JK, Kim MA, Kang GH, Kim SW, Del Chiaro M, Peters GJ, and Giaccone G. Identification of microRNA-21 as a biomarker for chemoresistance and clinical outcome following adjuvant therapy in resectable pancreatic cancer. PLoS One 5: e10630, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iacobuzio-Donahue CA. Genetic evolution of pancreatic cancer: lessons learnt from the pancreatic cancer genome sequencing project. Gut 61: 1085–1094, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iacobuzio-Donahue CA, Velculescu VE, Wolfgang CL, and Hruban RH. Genetic basis of pancreas cancer development and progression: insights from whole-exome and whole-genome sequencing. Clin Cancer Res 18: 4257–4265, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ignarro LJ. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol 30: 535–560, 1990 [DOI] [PubMed] [Google Scholar]
- 36.Kasper HU, Wolf H, Drebber U, Wolf HK, and Kern MA. Expression of inducible nitric oxide synthase and cyclooxygenase-2 in pancreatic adenocarcinoma: correlation with microvessel density. World J Gastroenterol 10: 1918–1922, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong G, Kim EK, Kim WS, Lee YW, Lee JK, Paik SW, Rhee JC, Choi KW, and Lee KT. Inducible nitric oxide synthase (iNOS) immunoreactivity and its relationship to cell proliferation, apoptosis, angiogenesis, clinicopathologic characteristics, and patient survival in pancreatic cancer. Int J Pancreatol 29: 133–140, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Lampson BL, Kendall SD, Ancrile BB, Morrison MM, Shealy MJ, Barrientos KS, Crowe MS, Kashatus DF, White RR, Gurley SB, Cardona DM, and Counter CM. Targeting eNOS in pancreatic cancer. Cancer Res 72: 4472–4482, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lancaster JR, Jr., and Xie K. Tumors face NO problems? Cancer Res 66: 6459–6462, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Landi S. Genetic predisposition and environmental risk factors to pancreatic cancer: a review of the literature. Mutat Res 681: 299–307, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Vandenboom TG, 2nd, Wang Z, Kong D, Ali S, Philip PA, and Sarkar FH. miR-146a suppresses invasion of pancreatic cancer cells. Cancer Res 70: 1486–1495, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim KH, Ancrile BB, Kashatus DF, and Counter CM. Tumour maintenance is mediated by eNOS. Nature 452: 646–649, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, Dimagno EP, Andren-Sandberg A, and Domellof L. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med 328: 1433–1437, 1993 [DOI] [PubMed] [Google Scholar]
- 44.Mathe E, Nguyen GH, Funamizu N, He P, Moake M, Croce CM, and Hussain SP. Inflammation regulates microRNA expression in cooperation with p53 and nitric oxide. Int J Cancer 131: 760–765, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, and Fuchs CS. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA 286: 921–929, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Moncada S, Palmer RM, and Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43: 109–142, 1991 [PubMed] [Google Scholar]
- 47.Muerkoster S, Wegehenkel K, Arlt A, Witt M, Sipos B, Kruse ML, Sebens T, Kloppel G, Kalthoff H, Folsch UR, and Schafer H. Tumor stroma interactions induce chemoresistance in pancreatic ductal carcinoma cells involving increased secretion and paracrine effects of nitric oxide and interleukin-1beta. Cancer Res 64: 1331–1337, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Muerkoster SS, Lust J, Arlt A, Hasler R, Witt M, Sebens T, Schreiber S, Folsch UR, and Schafer H. Acquired chemoresistance in pancreatic carcinoma cells: induced secretion of IL-1beta and NO lead to inactivation of caspases. Oncogene 25: 3973–3981, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Murad F. The nitric oxide-cyclic GMP signal transduction system for intracellular and intercellular communication. Recent Prog Horm Res 49: 239–248, 1994 [DOI] [PubMed] [Google Scholar]
- 50.Nathan C. and Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell 78: 915–918, 1994 [DOI] [PubMed] [Google Scholar]
- 51.Nussler AK, Gansauge S, Gansauge F, Fischer U, Butzer U, Kremsner PG, and Beger HG. Overexpression of endothelium-derived nitric oxide synthase isoform 3 in the vasculature of human pancreatic tumor biopsies. Langenbecks Arch Surg 383: 474–480, 1998 [DOI] [PubMed] [Google Scholar]
- 52.O'Leary BR, Fath MA, Bellizzi AM, Hrabe JE, Button AM, Allen BG, Case AJ, Altekruse S, Wagner BA, Buettner GR, Lynch CF, Hernandez BY, Cozen W, Beardsley RA, Keene J, Henry MD, Domann FE, Spitz DR, and Mezhir JJ. Loss of SOD3 (EcSOD) expression promotes an aggressive phenotype in human pancreatic ductal adenocarcinoma. Clin Cancer Res 21: 1741–1751, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oess S, Icking A, Fulton D, Govers R, and Muller-Esterl W. Subcellular targeting and trafficking of nitric oxide synthases. Biochem J 396: 401–409, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okayama H, Kohno T, Ishii Y, Shimada Y, Shiraishi K, Iwakawa R, Furuta K, Tsuta K, Shibata T, Yamamoto S, Watanabe S, Sakamoto H, Kumamoto K, Takenoshita S, Gotoh N, Mizuno H, Sarai A, Kawano S, Yamaguchi R, Miyano S, and Yokota J. Identification of genes upregulated in ALK-positive and EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Res 72: 100–111, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Okayama H, Saito M, Oue N, Weiss JM, Stauffer J, Takenoshita S, Wiltrout RH, Hussain SP, and Harris CC. NOS2 enhances KRAS-induced lung carcinogenesis, inflammation and microRNA-21 expression. Int J Cancer 132: 9–18, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, and Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324: 1457–1461, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ouyang N, Williams JL, Tsioulias GJ, Gao J, Iatropoulos MJ, Kopelovich L, Kashfi K, and Rigas B. Nitric oxide-donating aspirin prevents pancreatic cancer in a hamster tumor model. Cancer Res 66: 4503–4511, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, De Jesus-Acosta A, Sharma P, Heidari P, Mahmood U, Chin L, Moses HL, Weaver VM, Maitra A, Allison JP, LeBleu VS, and Kalluri R. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25: 719–734, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, and Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21: 418–429, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Provenzano PP. and Hingorani SR. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br J Cancer 108: 1–8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, and Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 74: 2913–2921, 2014 [DOI] [PubMed] [Google Scholar]
- 62.Rao CV, Mohammed A, Janakiram NB, Li Q, Ritchie RL, Lightfoot S, Vibhudutta A, and Steele VE. Inhibition of pancreatic intraepithelial neoplasia progression to carcinoma by nitric oxide-releasing aspirin in p48(Cre/+)-LSL-Kras(G12D/+) mice. Neoplasia 14: 778–787, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW, Westphalen CB, Kitajewski J, Fernandez-Barrena MG, Fernandez-Zapico ME, Iacobuzio-Donahue C, Olive KP, and Stanger BZ. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25: 735–747, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roldo C, Missiaglia E, Hagan JP, Falconi M, Capelli P, Bersani S, Calin GA, Volinia S, Liu CG, Scarpa A, and Croce CM. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol 24: 4677–4684, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Schetter AJ, Heegaard NH, and Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis 31: 37–49, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sebens Muerkoster S, Werbing V, Sipos B, Debus MA, Witt M, Grossmann M, Leisner D, Kotteritzsch J, Kappes H, Kloppel G, Altevogt P, Folsch UR, and Schafer H. Drug-induced expression of the cellular adhesion molecule L1CAM confers anti-apoptotic protection and chemoresistance in pancreatic ductal adenocarcinoma cells. Oncogene 26: 2759–2768, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Shi Q, Xiong Q, Wang B, Le X, Khan NA, and Xie K. Influence of nitric oxide synthase II gene disruption on tumor growth and metastasis. Cancer Res 60: 2579–2583, 2000 [PubMed] [Google Scholar]
- 68.Siegel RL, Miller KD, and Jemal A. Cancer statistics, 2016. CA Cancer J Clin 66: 7–30, 2016 [DOI] [PubMed] [Google Scholar]
- 69.Sohn JJ, Schetter AJ, Yfantis HG, Ridnour LA, Horikawa I, Khan MA, Robles AI, Hussain SP, Goto A, Bowman ED, Hofseth LJ, Bartkova J, Bartek J, Wogan GN, Wink DA, and Harris CC. Macrophages, nitric oxide and microRNAs are associated with DNA damage response pathway and senescence in inflammatory bowel disease. PLoS One 7: e44156, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soubani O, Ali AS, Logna F, Ali S, Philip PA, and Sarkar FH. Re-expression of miR-200 by novel approaches regulates the expression of PTEN and MT1-MMP in pancreatic cancer. Carcinogenesis 33: 1563–1571, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takahashi M, Kitahashi T, Ishigamori R, Mutoh M, Komiya M, Sato H, Kamanaka Y, Naka M, Maruyama T, Sugimura T, and Wakabayashi K. Increased expression of inducible nitric oxide synthase (iNOS) in N-nitrosobis(2-oxopropyl)amine-induced hamster pancreatic carcinogenesis and prevention of cancer development by ONO-1714, an iNOS inhibitor. Carcinogenesis 29: 1608–1613, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Tamir S, deRojas-Walker T, Gal A, Weller AH, Li X, Fox JG, Wogan GN, and Tannenbaum SR. Nitric oxide production in relation to spontaneous B-cell lymphoma and myositis in SJL mice. Cancer Res 55: 4391–4397, 1995 [PubMed] [Google Scholar]
- 73.Tamir S. and Tannenbaum SR. The role of nitric oxide (NO.) in the carcinogenic process. Biochim Biophys Acta 1288: F31–F36, 1996 [DOI] [PubMed] [Google Scholar]
- 74.Thomas DD, Ridnour LA, Espey MG, Donzelli S, Ambs S, Hussain SP, Harris CC, DeGraff W, Roberts DD, Mitchell JB, and Wink DA. Superoxide fluxes limit nitric oxide-induced signaling. J Biol Chem 281: 25984–25993, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C, Paolocci N, Ambs S, Colton CA, Harris CC, Roberts DD, and Wink DA. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med 45: 18–31, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tlsty TD. and Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol 1: 119–150, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Vickers SM, MacMillan-Crow LA, Green M, Ellis C, and Thompson JA. Association of increased immunostaining for inducible nitric oxide synthase and nitrotyrosine with fibroblast growth factor transformation in pancreatic cancer. Arch Surg 134: 245–251, 1999 [DOI] [PubMed] [Google Scholar]
- 78.Vincent A, Herman J, Schulick R, Hruban RH, and Goggins M. Pancreatic cancer. Lancet 378: 607–620, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang B, Xiong Q, Shi Q, Le X, Abbruzzese JL, and Xie K. Intact nitric oxide synthase II gene is required for interferon-beta-mediated suppression of growth and metastasis of pancreatic adenocarcinoma. Cancer Res 61: 71–75, 2001 [PubMed] [Google Scholar]
- 80.Wang B, Xiong Q, Shi Q, Le X, and Xie K. Genetic disruption of host interferon-gamma drastically enhances the metastasis of pancreatic adenocarcinoma through impaired expression of inducible nitric oxide synthase. Oncogene 20: 6930–6937, 2001 [DOI] [PubMed] [Google Scholar]
- 81.Wang B, Xiong Q, Shi Q, Tan D, Le X, and Xie K. Genetic disruption of host nitric oxide synthase II gene impairs melanoma-induced angiogenesis and suppresses pleural effusion. Int J Cancer 91: 607–611, 2001 [PubMed] [Google Scholar]
- 82.Wang J, He P, Gaida MM, Yang S, Schetter A, Gaedcke J, Ghadimi BM, Ried T, Yfantis HG, Lee DH, Weiss JM, Stauffer J, Hanna N, Alexander HR, and Hussain SP. Inducible nitric oxide synthase enhances disease aggressiveness in pancreatic cancer. Oncotarget [Epub ahead of print]; DOI: 10.18632/oncotarget.10323, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang J, Yang S, He P, Schetter A, Gaedcke J, Ghadimi BM, Ried T, Yfantis HG, Lee DH, Gaida MM, Hanna N, Alexander HR, and Hussain SP. Endothelial Nitric oxide synthase traffic inducer (NOSTRIN) is a negative regulator of disease aggressiveness in pancreatic cancer. Clin Cancer Res 2016. [Epub ahead of print]; DOI: 10.1158/1078-0432.CCR-16-0511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang L. and Xie K. Nitric oxide and pancreatic cancer pathogenesis, prevention, and treatment. Curr Pharm Des 16: 421–427, 2010 [DOI] [PubMed] [Google Scholar]
- 85.Wei D, Richardson EL, Zhu K, Wang L, Le X, He Y, Huang S, and Xie K. Direct demonstration of negative regulation of tumor growth and metastasis by host-inducible nitric oxide synthase. Cancer Res 63: 3855–3859, 2003 [PubMed] [Google Scholar]
- 86.Whitcomb DC, Applebaum S, and Martin SP. Hereditary pancreatitis and pancreatic carcinoma. Ann N Y Acad Sci 880: 201–209, 1999 [DOI] [PubMed] [Google Scholar]
- 87.Xie K. and Huang S. Contribution of nitric oxide-mediated apoptosis to cancer metastasis inefficiency. Free Radic Biol Med 34: 969–986, 2003 [DOI] [PubMed] [Google Scholar]
- 88.Yang S, He P, Wang J, Schetter A, Tang W, Funamizu N, Yanaga K, Uwagawa T, Satoskar AR, Gaedcke J, Bernhardt M, Ghadimi BM, Gaida MM, Bergmann F, Werner J, Ried T, Hanna N, Alexander HR, and Hussain SP. A novel MIF signaling pathway drives the malignant character of pancreatic cancer by targeting NR3C2. Cancer Res 76: 3838–3850, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ying H, Elpek KG, Vinjamoori A, Zimmerman SM, Chu GC, Yan H, Fletcher-Sananikone E, Zhang H, Liu Y, Wang W, Ren X, Zheng H, Kimmelman AC, Paik JH, Lim C, Perry SR, Jiang S, Malinn B, Protopopov A, Colla S, Xiao Y, Hezel AF, Bardeesy N, Turley SJ, Wang YA, Chin L, Thayer SP, and DePinho RA. PTEN is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-kappaB-cytokine network. Cancer Discov 1: 158–169, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yuhas Y, Berent E, and Ashkenazi S. Effect of nitric oxide on microRNA-155 expression in human hepatic epithelial cells. Inflamm Res 63: 591–596, 2014 [DOI] [PubMed] [Google Scholar]
- 91.Zhang Y, Li M, Wang H, Fisher WE, Lin PH, Yao Q, and Chen C. Profiling of 95 microRNAs in pancreatic cancer cell lines and surgical specimens by real-time PCR analysis. World J Surg 33: 698–709, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zimmermann K, Opitz N, Dedio J, Renne C, Muller-Esterl W, and Oess S. NOSTRIN: a protein modulating nitric oxide release and subcellular distribution of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A 99: 17167–17172, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]