Abstract
Rationale: Approximately 20% of patients hospitalized for COPD exacerbations in the United States will be readmitted within 30 days. The Centers for Medicare and Medicaid Services has recently proposed to revise the Hospital Readmissions Reduction Program to financially penalize hospitals with high all-cause 30-day rehospitalization rates after a hospitalization for COPD exacerbation on or after October 1, 2014.
Objectives: To report the results of a systematic review of randomized clinical trials evaluating interventions to reduce the rehospitalizations after COPD exacerbations.
Methods: Multiple electronic databases were systematically searched to identify relevant studies published between January 1966 and June 2013. Titles, abstracts, and, subsequently, full-text articles were assessed for eligibility. Each study was appraised using predefined criteria.
Measurements and Main Results: Among 913 titles and abstracts screened, 5 studies (1,393 participants) met eligibility criteria. All studies had a primary outcome of rehospitalization at 6 or 12 months. No study examined 30-day rehospitalization as the primary outcome. Each study tested a different set of interventions. Two studies (one conducted in Canada and one conducted in Spain and Belgium) showed a decrease in all-cause rehospitalization over 12 months in the intervention group versus comparator group (mean number of hospitalizations per patient, 1.0 vs. 1.8; P = 0.01; percent hospitalized, 45 vs. 67%; P = 0.028; respectively). The only study conducted in the United States found a greater than twofold higher risk of mortality in the intervention group (17 vs. 7%, P = 0.003) but no significant difference in rehospitalizations. It was unclear which set of interventions was effective or harmful.
Conclusions: The evidence base is inadequate to recommend specific interventions to reduce rehospitalizations in this population and does not justify penalizing hospitals for high 30-day rehospitalization rates after COPD exacerbations.
Keywords: chronic obstructive pulmonary disease, rehospitalization, systematic review
Chronic obstructive pulmonary disease (COPD) affects approximately 24 million individuals and is the third leading cause of death in the United States (1). Each year, COPD leads to as many as 800,000 hospitalizations and nearly $50 billion in healthcare expenditures (1, 2). About 20% of patients hospitalized with COPD exacerbations are rehospitalized within 30 days, and healthcare expenditures for rehospitalizations in this population rank as the third highest among Medicare beneficiaries (3). It is therefore not surprising that provisions in the Affordable Care Act specify rehospitalizations after COPD exacerbations as a potential target for financial penalties by the Centers for Medicare and Medicaid Services (CMS) (4). In May 2013, CMS invited public comments on a proposal to add hospitalizations for COPD exacerbations to its Hospital Readmissions Reduction Program, which would in effect trigger financial penalties directed at hospitals if admissions for COPD exacerbations resulted in a higher-than-expected all-cause 30-day rehospitalization rate (i.e., even rehospitalizations not attributed to COPD) (5). The proposal would expand the CMS Hospital Readmissions Reduction Program, which is currently focused on reducing rehospitalizations after a hospital admission for heart failure, myocardial infarction, or pneumonia (6). Understandably, hospitals and provider groups are seeking evidence-based strategies to reduce rehospitalization rates for various patient populations, including for patients with COPD exacerbations. Although there are a number of interventions that have been shown to reduce rehospitalizations (7–10), none of these have been specifically tested in patients hospitalized for COPD exacerbations, and none include interventions to address the specific needs of this patient population (eg, oxygen titration and education, inhaler use teaching, noninvasive ventilatory support).
Previous systematic reviews in patients with COPD have studied self-management education (11, 12), integrated care (13–15), shared management between specialists and primary care physicians (16), and home care (17) in patients with stable COPD, or hospital at home for acutely ill patients with COPD exacerbations (18) or other conditions (19). Previous reviews on care transitions from hospital to home have focused on other patient populations (ie, not patients with COPD exacerbations) (20–22). However, no review to date has focused specifically on identifying interventions that reduce rehospitalizations in patients hospitalized for COPD exacerbations. We therefore conducted a systematic review of clinical trials to identify interventions that could reduce the risk of rehospitalization in patients initially hospitalized with COPD exacerbations. Preliminary findings were reported at the 2013 meeting of the American Thoracic Society International Conference (23).
Methods
Data Sources
With the assistance of a medical librarian, we conducted a systematic search of multiple electronic databases (PubMed, EMBASE, CINAHL, the Cochrane library) for studies published between January 1966 and June 2013. The PubMed search was constructed by using combinations of Medical Subject Heading (MeSH) search terms and keywords according to the following algorithm: (“COPD” [MeSH] AND “exacerbation”) AND (“self-management” OR “care facilitator” OR “coordinator” OR “case manage*” OR “facilitator” OR “patient navigator” OR “navigator” OR “integrated care”) OR (“COPD” [MeSH] AND (“patient discharge” [MeSH] OR “discharge planning” [MeSH] OR “care continuity, patient” [MeSH]) OR (“COPD” [MeSH] AND “hospital readmission” [MeSH])). The other databases were queried using similar terms (see Table E1 in the online supplement). We used Google Scholar and Web of Science for citation searches to identify additional articles. Reference lists in review articles were also examined to identify additional articles.
Study Selection
Inclusion criteria for full-text review and data abstraction were: (1) published in English, (2) randomized clinical trial design, (3) enrolled patients with COPD, (4) patients were hospitalized for COPD exacerbation within the previous 12 months, and (5) primary outcome was rehospitalization (all-cause or COPD-related) or composite endpoint that included rehospitalization. We excluded studies that focused exclusively on decreasing the hospital length of stay(e.g., early assisted discharge, hospital at home), because such interventions are not necessarily expected to also reduce the risk of rehospitalization. Studies suggest that multiple factors contribute to safe transitions from hospital to home (e.g., access/quality of ambulatory care, social support, no follow-up appointment, transportation problems, inadequate care coordination with ambulatory providers) (24). We therefore excluded studies limited to pharmacotherapy, procedures, or technology-based interventions, because these are not designed to address the multiple factors above. We also excluded studies that exclusively focused on pulmonary rehabilitation in the context of a recent hospitalization for COPD exacerbation, as this topic was addressed in a recently published systematic review (25). Two authors independently screened studies for eligibility criteria through title and abstract review (V.P. and M.A.M.). The full-text review of the preselected articles was performed independently by two authors (V.P., M.A.M., H.A.G., N.I.R., or J.A.K.) to identify the final set of eligible studies. Disagreements over study eligibility were resolved by third-party arbitration (N.I.R. or H.A.G.).
Data Abstraction and Risk of Bias Assessment
Two authors (V.P., M.A.M., M.J.J., N.I.R., H.A.G., B.P., or S.M.N.) independently abstracted data from each study’s published protocol (Table E2) and assessed risk of bias, per the Cochrane Effective Practice and Organization of Care (EPOC) Group’s risk of bias criteria (26). The EPOC risk of bias criteria includes (1) random sequence allocation, (2) concealed allocation, (3) masking of participants and personnel, (4) masking of outcome assessment, (5) incomplete outcome data, (6) selective reporting, and (7) other bias. The risk of bias for each of these domains was graded as high, low, or unclear. If necessary, disagreements were resolved through a third-party arbitrator (J.A.K.). Additionally, to minimize the risk of data abstraction errors, the corresponding authors of the reviewed studies were contacted for clarification of study procedures (27).
Data Synthesis and Analysis
Based on a previous study (22), we classified intervention components into three temporal categories: (1) predischarge interventions, (2) postdischarge interventions, or (3) bridging interventions (spanning the pre- and postdischarge periods). Because of the heterogeneity of interventions, measurements, and reporting of outcomes, we present a narrative synthesis (rather than a metaanalysis) of findings. Outcomes of interest were rehospitalizations (all-cause and COPD-related) and mortality (all-cause and COPD-related), as defined by the authors in the various studies.
Results
Study Selection
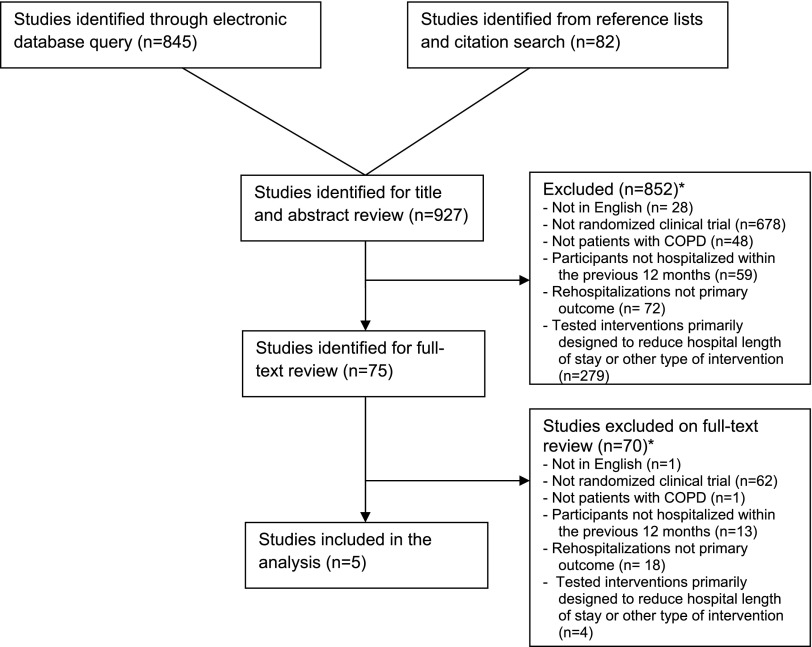
Our query of the electronic databases and review of reference lists and citation searches identified 913 studies (Figure 1). Based on review of titles and abstracts, 75 were selected for full-text review. Of those, five studies met all eligibility criteria and were selected for data abstraction and synthesis (Table 1).
Figure 1.
Summary of evidence search and selection. COPD = chronic obstructive pulmonary disease. *Reasons for exclusion exceed number of studies excluded due to studies being excluded for more than one reason.
Table 1.
Characteristics of clinical trials
| Author | Year | Country | No. Sites | No. Participants | Mean Age (yr) | % Women | FEV1 | Intervention Setting | Assessment Period (mo) | No. EPOC Criteria Satisfied (7 Possible) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bourbeau et al. (31) | 2003 | Canada | 7 | 191 | 69 | 45 | 0.99 L | 3 | 12 | 6 | |
| Kwok et al. (30) | 2005 | China | 2 | 157 | 74 | 29 | NR | 1, 2, 3 | 6 | 5 (of 6 evaluable domains) | |
| Casas et al. (29) | 2006 | Spain, Belgium | 2 | 155 | 71 | 17 | 42% pred | 1, 2, 3 | 12 | ||
| Bucknall et al. (32) | 2012 | UK | 6 | 464 | 69 | 63 | 41% pred | 3 | 12 | 6 | |
| Fan et al. (28) | 2012 | United States | 20 | 426 | 66 | 3 | 48% pred | 3 | 12 | 6 |
Definition of abbreviations: EPOC = Effective Practice and Organization of Care criteria; NR = not reported; % pred = percent of predicted spirometry value.
Summary: The five studies were performed in six countries, involving 2 to 20 sites per study with a number of participants ranging from 155 to 464 (total number of participants: 1,393). In three out of five studies the proportion of female participants was < 30%. Most studies focused on postdischarge interventions, with very few studies performing either predischarge or bridging interventions. The two largest studies were conducted in the UK (6 sites, 464 predominantly female participants) and the United States (20 sites, 426 predominantly male participants) and focused exclusively on postdischarge interventions with a duration of 12 months. Intervention setting: 1 = interventions performed in the predischarge period (implemented before hospital discharge). 2 = interventions performed as bridging interventions (same person conducted interventions in the pre- and postdischarge periods). 3 = interventions performed in the postdischarge period (implemented after hospital discharge).
Characteristics of Clinical Trials
The five studies enrolled a total of 1,393 participants (range, 155–464 per study) in six countries; only one of these studies was conducted in the United States (28). All studies included postdischarge interventions, but only two studies also included predischarge and bridging interventions (29, 30). The studies were designed to examine rehospitalizations at 6 months (one trial) (30) or 12 months (four trials) (28, 29, 31, 32), but not at 30 days. In the four studies that reported sufficient information to evaluate the risk of bias in all seven EPOC domains (Table E3), the risk of bias was considered low in all domains except masking of study participants and personnel to the intervention (28, 29, 31, 32).
Study Interventions
Interventions in each study are summarized in Tables 2 and 3, with more detailed descriptions in Tables E2, E4, and E5. More than 15 different strategies formed intervention bundles (i.e., multiple interventions implemented as part of a care strategy) across the various studies. The number of interventions in the bundle used in each study ranged from 9 to 11. All five studies provided patient education about use of respiratory inhalers, developed an action plan (instruction on steps to be taken in case of worsening symptoms), and provided participants a hotline (phone or pager number that patients could call as needed). The intervention bundle in four studies also included a different combination of education about COPD, general health counseling (e.g., living a healthy lifestyle), coordination with the patient’s primary provider, home visits, and a follow-up phone call. Less frequent interventions included smoking cessation counseling (three studies), social services referral (two studies), assessment of comorbidities (one study), discharge planning (one study), and pulmonary rehabilitation (one study). Medication reconciliation, scheduling follow-up appointments with the patient’s provider, provider continuity, patient-centered discharge instructions, or referral to a smoking cessation program were not components of the intervention bundle for any of the five studies. There was substantial heterogeneity between studies, such as the timing (eg, predischarge vs. postdischarge), frequency (e.g., number of home visits), and how each intervention was delivered (e.g., type and number of personnel conducting interventions). In two studies, the control group also received lower-intensity interventions; in the other studies, usual care was the comparator (28, 32). Two studies initiated interventions more than 28 days after hospital discharge (28, 31).
Table 2.
Interventions performed
| Interventions | Study, year |
|||||
|---|---|---|---|---|---|---|
| Bourbeau et al., 2003 (31) | Kwok et al., 2005 (30) | Casas et al., 2006 (29) | Bucknall et al., 2012 (32) | Fan et al., 2012 (28) | ||
| Discharge planning | 1 | |||||
| Disease education | 3 | 1 | 3 | 3* | ||
| Health counseling | 3 | 1 | 3 | 3 | ||
| Inhaler use teaching | 3 | 1 | 1 | 3* | 3 | |
| Development of action plan | 3 | 1 | 1 | 3 | 3 | |
| Medications given for action plan | 3 | 3 | 3 | |||
| Smoking cessation counseling | 3 | 3* | 3 | |||
| Assessment of comorbidities | 3 | |||||
| Referral to pulmonary rehabilitation | 3* | |||||
| Exercise program | 3 | 3 | 3 | |||
| Referral to social services | 3 | 3 | ||||
| Communication with patient’s PCP | 3 | 3 | 3 | 3* | ||
| Transition navigator | 2 | 2 | ||||
| Home visits | 3 | 3 | 3 | 3 | ||
| Follow-up telephone call | 3 | 3 | 3 | 3 | ||
| Patient hotline | 3 | 3 | 3 | 3 | 3* | |
Definition of abbreviations: PCP = primary care provider.
Intervention setting: 1 = Interventions performed in the predischarge period. 2 = Interventions performed as bridging interventions. 3 = Interventions performed in the postdischarge period.
Interventions that were also implemented in some form in the control group. See Table E2 in the online supplement for further details.
Table 3.
Home visit procedures
| Study, Year |
|||||
|---|---|---|---|---|---|
| Bourbeau et al., 2003 (31) | Kwok et al., 2005 (30) | Casas et al., 2006 (29) | Bucknall et al., 2012 (32) | Fan et al., 2012 (28) | |
| Staff performing home visit | RN, PT, RT | RN | RN, MD, SW | RN | None |
| Days from hospital discharge to first home visit | >28 | <7 | <3 | 29 (median) | |
| No. of home visits | 8 | 9 | 1 | >10 | |
| Months from first home visit to last home visit | 2 | 6 | n/a | 12 | |
Definition of abbreviations: MD = medical doctor; n/a = not applicable; PT = physical therapist; RN = registered nurse; RT = respiratory therapist; SW = social worker.
Outcomes (Rehospitalizations and Mortality)
Measurement and reporting of outcomes varied across studies. Rehospitalizations were assessed by reviewing hospital or national health records (two studies) (30, 32), patient interviews or questionnaires (one study) (31), or a combination of both (two studies) (28, 29). Mortality was assessed through review of medical records and interviews of family members in two studies (28, 29), review of national health records (32), or was not adequately described (30, 31). None of the studies reported 30-day rehospitalization rates, although one study involving 157 patients in China examined 28-day rehospitalization rates as a secondary outcome (30).
Two of the five studies demonstrated a significant decrease in all-cause rehospitalizations in the intervention group versus comparator group at 12 months (mean number of hospitalizations per patient, 1.0 vs. 1.8; P = 0.01; percent rehospitalized, 45 vs. 67%, P = 0.028) (29, 31), with one also reporting a significant decrease in COPD-related rehospitalizations at 12 months (32 vs. 50%, P = 0.01) (Table 4) (31). None of the studies demonstrated a significant reduction in all-cause mortality in the intervention group at 6 or 12 months, although one study (the only study conducted in the United States), was terminated prematurely by an independent Data Safety Monitoring Board due to excess risk of death in the intervention group (compared with usual care; 17 vs. 7%, P = 0.003) (28). An extensive evaluation by the authors of this study, a multicenter study conducted across 20 Veterans Affairs hospitals, failed to identify a reason that mortality was higher in the intervention group.
Table 4.
Rehospitalizations and mortality
| Intervention Components | Study, Year |
||||
|---|---|---|---|---|---|
| Bourbeau etal., 2003 (31) |
Kwok etal., 2005 (30) |
Casas etal., 2006 (29) |
Bucknall etal., 2012 (32) |
Fan etal., 2012 (28) |
|
| (n = 191) | (n = 157) | (n = 155) | (n = 464) | (n = 426) | |
| Assessment period, mo | 12 | 6 | 12 | 12 | 12 |
| Rehospitalizations, intervention vs. control | |||||
| All-cause | 1.0 vs. 1.8 (mean)* | 76 vs. 62 (at 28 d: 47 vs. 37) | 45 vs. 67* | 69 vs. 72 | 37 vs. 36 |
| COPD-related | 32 vs. 50* | 44 vs. 46 | 27 vs. 24 | ||
| Mortality, intervention vs. control | |||||
| All-cause | 5 vs. 9 | 4 vs. 8 | 18 vs. 16 | 13 vs. 9 | 17 vs. 7* |
| COPD-related | 10 vs. 7 | 7 vs. 3 | |||
Data are given as percent unless otherwise noted.
P < 0.05.
Discussion
To our knowledge, this report represents the first systematic review of literature to evaluate interventions to reduce rehospitalizations among patients hospitalized with COPD exacerbations. Results indicate that (1) there are no published clinical trials examining the effects of interventions to reduce 30-day rehospitalization rates in this population as the primary outcome, with studies largely designed to examine outcomes at 6 months or 12 months; (2) there is substantial heterogeneity in the design, measurement, and reporting of study results, precluding a formal metaanalysis; and (3) interventions tested to date in this population may result in benefit, harm, or have no discernible effect. Overall, it appears that there is inadequate evidence to recommend disease-specific interventions to reduce 30-day rehospitalization rates in patients initially admitted for COPD exacerbations.
Although 30-day rehospitalization rates among patients hospitalized for COPD exacerbations are of particular interest in the United States, given the potential financial penalties imposed by CMS, we did not identify any clinical trial designed specifically to address this outcome. Only one of the five studies we identified was conducted in the United States (28), and neither this study nor the other four were designed specifically to examine 30-day rehospitalization rates. Also, two studies initiated interventions 28 days or more after hospital discharge, limiting the usefulness of such studies to identify interventions to decrease 30-day rehospitalization rates (28, 31).
We were unable to combine the results from the different studies and carry out a metaanalysis due to several sources of heterogeneity. First, even among studies that implemented similar interventions (e.g., home visits), there was significant variability in both content (e.g., topics addressed during home visits) and context (e.g., number and timing of home visits, personnel conducting the home visits). Second, there was significant heterogeneity in the measurement (e.g., self-report, review of medical records) and reporting (e.g., percent rehospitalized, mean number of rehospitalizations per patient) of outcomes. Third, there was inconsistent reporting of patient characteristics such as socioeconomic status, level of social support, and comorbidities, all of which have been linked with rehospitalization rates (33–35). Establishing standards for measuring and reporting patient characteristics and outcomes will help readers determine whether study results are applicable to their clinical populations and can therefore be used to develop local quality-improvement programs. For example, although the average individual with COPD has six or more comorbid conditions and more than 95% have at least one condition that may complicate the treatment of COPD (36), only one study reported addressing participants’ comorbidities as part of their intervention (29). Even in this report, there was insufficient information about the evaluation or treatment of comorbidities (e.g., which comorbidities were assessed, how did this evaluation modify the postdischarge treatment plan) in the context of the interventions to reduce rehospitalizations.
Interventions had highly variable effects on rehospitalizations and mortality (from benefit to harm). Most of the studies focused on postdischarge interventions, with only two studies also including predischarge and bridging interventions (29, 30). It was unclear, however, which intervention or groups of interventions were most likely to cause benefit or harm. For example, three studies all used COPD education, health counseling, inhaler use teaching, action plans, smoking cessation counseling, and a patient hotline (28, 31, 32), yet observed vastly different outcomes. Although one study demonstrated a significant reduction in rehospitalizations (31), others showed either no significant effect or even an increase in mortality in the intervention group (28, 30). Differences in interventions or study populations likely contributed to heterogeneous findings.
In a previous systematic review (not focused on patients with COPD exacerbations), the authors concluded that that no specific intervention or intervention bundle was consistently associated with a reduction in 30-day rehospitalization rates (22). Only one of the five clinical trials in the current report was included in this previous systematic review (30). Our findings, however, are consistent with this previous report, and we were unable to identify intervention to reduce 30-day rehospitalizations in patients with COPD exacerbations; our findings also indicate that there do not appear to be interventions that consistently reduce rehospitalizations at subsequent time points (e.g., 6 or 12 months post discharge). Other randomized controlled trials that enrolled ambulatory patients or patients presenting to the emergency department with COPD exacerbations (but not hospitalized) also found inconsistent benefits of interventions designed to reduce hospitalizations (37–40). Although there appear to be a large number of studies examining strategies to reduce rehospitalizations, the lack of a standard approach to defining patient populations, study interventions and comparators, outcomes, timeframe for assessing outcomes, and settings in which the study was conducted are barriers to cross-study syntheses of evidence. The National Institute of Health recently convened a highly successful multidisciplinary conference on standardizing outcomes in clinical research for patients with asthma (41). A similar effort is needed to facilitate the conduct of systematic reviews of studies designed to reduce rehospitalizations.
This systematic review has potential limitations. We may have missed identifying some effective interventions because we restricted our review to randomized clinical trials and did not include evidence from other study designs; however, randomized clinical trials are generally considered as providing the strongest quality of evidence. Also, this review did not include studies that focused exclusively on pharmacological interventions, and we may have missed identifying pharmacological agents effective in reducing rehospitalizations. Another potential limitation involves availability of data. Results of this systematic review are largely based on descriptions of interventions and outcomes published in journal articles and supplemented with queries to study authors. The availability of detailed study protocols may have helped us understand the reasons for the heterogeneous effect of interventions across studies.
The findings of this report have two implications. First, it is not possible to make recommendations for COPD-specific interventions to reduce rehospitalizations after COPD exacerbations. In fact, our findings indicate that providers must be careful when implementing programs to reduce rehospitalizations in this population, given the twofold excess risk of death identified in one of the largest studies to date. Second, although nearly all patients with COPD have clinically relevant comorbidities, only one study in this report devoted attention to these conditions (29). We suspect that developing and implementing disease-specific strategies (e.g., separate strategies for patients with COPD and for patients with heart failure) to reduce rehospitalizations supplemented by interventions focusing on specific comorbid conditions will be burdensome and inefficient. A more general patient-centered approach that includes interventions that are likely to be effective for multiple populations (eg, coordination of care with ambulatory providers, addressing financial and social barriers to care) supplemented with disease-specific interventions as needed (e.g., inhaler use and supplemental oxygen teaching in patients with COPD exacerbations) may be more efficient for health systems and providers to implement but needs further testing (42). The Society of Hospital Medicine’s Project BOOST, for example, provides the opportunity to tailor the hospital-to-home transition process to the patient’s needs but would benefit from the addition of evidence-based modules that address the needs of patients with COPD exacerbations (10).
In conclusion, we found significant heterogeneity in the design and outcome of clinical trials to reduce rehospitalizations in patients with COPD exacerbations. Clinical trials identified in this review focused on reducing rehospitalizations at 6 and 12 months; no study focused on decreasing 30-day rehospitalizations as a primary endpoint. No specific intervention or bundle of interventions could be identified as effective in reducing the rate of rehospitalizations in this population. Moreover, the only clinical trial to date conducted in the United States documented a twofold excess risk of death in patients assigned to the intervention group and no significant effect on the risk of rehospitalization. The currently available evidence provides very limited guidance to practitioners and health systems seeking to reduce rehospitalizations in this population and does not justify penalizing hospitals for high 30-day rehospitalization rates after COPD exacerbations. Quality improvement initiatives should carefully monitor the effect of their intervention on mortality and rehospitalizations. An effort to promote the use of standardized descriptions of patient characteristics, interventions, and outcomes is needed.
Acknowledgments
Acknowledgment
The authors thank Ms. Lisa Massengale, M.L.I.S., for her help optimizing the literature search, and Drs. Christine Bucknall, Vincent Fan, and Josep Roca for providing us with additional information regarding their studies.
Footnotes
Supported by National Institutes of Health institutional training grant T32 2T32HL082547 (V.P.-C.) and by Patient-Centered Outcomes Research Institute contract IH-12–11–4365 (H.A.G., J.A.K.).
Author Contributions: All authors had access to the data and played a role in writing this manuscript. V.P.-C. contributed to the study conception, design, literature search, data analysis and interpretation, writing and revision of the manuscript. M.A.M. contributed to the literature search, data analysis and interpretation, revision of the manuscript. N.I.R. contributed to the literature search, data analysis and interpretation, revision of the manuscript. H.A.G. contributed to the literature search, data analysis and interpretation, writing and revision of the manuscript. S.M.N. contributed to the literature search, data analysis and interpretation, revision of the manuscript. M.J.J. contributed to the literature search, data analysis and interpretation, revision of the manuscript. B.P. contributed to the literature search, data analysis and interpretation, revision of the manuscript. N.B. contributed to the interpretation of the data and had substantial involvement in the revision of the manuscript. R.D. contributed to the interpretation of the data and had substantial involvement in the revision of the manuscript. P.O.G. contributed to the interpretation of the data and had substantial involvement in the revision of the manuscript. H.A.J. contributed to the interpretation of the data and had substantial involvement in the revision of the manuscript. R.K. contributed to the interpretation of the data and had substantial involvement in the revision of the manuscript. A.S.P. contributed to the interpretation of the data and had substantial involvement in the revision of the manuscript. B.R.P. contributed to the interpretation of the data and had substantial involvement in the revision of the manuscript. B.S. contributed to the interpretation of the data and had substantial involvement in the revision of the manuscript. B.M.T. contributed to the interpretation of the data and had substantial involvement in the revision of the manuscript. M.V.W. contributed to the interpretation of the data and had substantial involvement in the revision of the manuscript. J.L.S. contributed to the interpretation of the data and had substantial involvement in the revision of the manuscript. J.A.K. contributed to the study conception, design, literature search, data analysis and interpretation, writing and revision of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.National Heart, Lung, and Blood Institute. Morbidity and mortality: 2009. chart book on cardiovascular, lung, and blood diseases [2011; accessed 2013 July12]. Available from: http://www.nhlbi.nih.gov/about/factbook/chapter4.htm
- 2.Stein BD, Charbeneau JT, Lee TA, Schumock GT, Lindenauer PK, Bautista A, Lauderdale DS, Naureckas ET, Krishnan JA. Hospitalizations for acute exacerbations of chronic obstructive pulmonary disease: how you count matters. COPD. 2010;7:164–171. doi: 10.3109/15412555.2010.481696. [DOI] [PubMed] [Google Scholar]
- 3.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 4.US CongressHouse Committee on Ways and Means, Committee on Energy and Commerce, Committee on Education and Labor. Compilation of Patient Protection and Affordable Care Act: As amended through 1 November 2010, including Patient Protection and Affordable Care Act health-related portions of the Health Care and Education Reconciliation Act of 2010. Washington, DC: U.S. Government Printing Office; 2010. xxiii [Google Scholar]
- 5.Department of Health and Human ServicesCenters for Medicare and Medicaid Services. Medicare Program; Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long- Term Care Hospital Prospective Payment System and Proposed Fiscal Year 2014 Rates; Quality Reporting Requirements for Specific Providers; Hospital Conditions of Participation [Internet]. National Archives and Records Administration; 2013. May [accessed 2013 July 14]. 78 Federal Register 91. Available from: http://www.gpo.gov/fdsys/pkg/FR-2013-05-10/pdf/2013-10234.pdf [PubMed]
- 6.CMS.gov.Readmission reduction program [Internet]. Baltimore, MD: Centers for Medicare and Medicaid Services; [revised 2013 April 13; accessed 2013 July 14]. Available from: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html
- 7.Jack BW, Chetty VK, Anthony D, Greenwald JL, Sanchez GM, Johnson AE, Forsythe SR, O’Donnell JK, Paasche-Orlow MK, Manasseh C, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150:178–187. doi: 10.7326/0003-4819-150-3-200902030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822–1828. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- 9.Naylor M, Brooten D, Jones R, Lavizzo-Mourey R, Mezey M, Pauly M. Comprehensive discharge planning for the hospitalized elderly. A randomized clinical trial. Ann Intern Med. 1994;120:999–1006. doi: 10.7326/0003-4819-120-12-199406150-00005. [DOI] [PubMed] [Google Scholar]
- 10.Hansen LO, Greenwald JL, Budnitz T, Howell E, Halasyamani L, Maynard G, Vidyarthi A, Coleman EA, Williams MV. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8:421–427. doi: 10.1002/jhm.2054. [DOI] [PubMed] [Google Scholar]
- 11.Effing T, Monninkhof EM, van der Valk PD, van der Palen J, van Herwaarden CL, Partidge MR, Walters EH, Zielhuis GA. Self-management education for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2007;4:CD002990. doi: 10.1002/14651858.CD002990.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Walters JA, Turnock AC, Walters EH, Wood-Baker R. Action plans with limited patient education only for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010;5:CD005074. doi: 10.1002/14651858.CD005074.pub3. [DOI] [PubMed] [Google Scholar]
- 13.Adams SG, Smith PK, Allan PF, Anzueto A, Pugh JA, Cornell JE. Systematic review of the chronic care model in chronic obstructive pulmonary disease prevention and management. Arch Intern Med. 2007;167:551–561. doi: 10.1001/archinte.167.6.551. [DOI] [PubMed] [Google Scholar]
- 14.Lemmens KM, Nieboer AP, Huijsman R. A systematic review of integrated use of disease-management interventions in asthma and COPD. Respir Med. 2009;103:670–691. doi: 10.1016/j.rmed.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Taylor SJ, Candy B, Bryar RM, Ramsay J, Vrijhoef HJ, Esmond G, Wedzicha JA, Griffiths CJ. Effectiveness of innovations in nurse led chronic disease management for patients with chronic obstructive pulmonary disease: systematic review of evidence. BMJ. 2005;331:485. doi: 10.1136/bmj.38512.664167.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith SM, Allwright S, O’Dowd T. Effectiveness of shared care across the interface between primary and specialty care in chronic disease management. Cochrane Database Syst Rev. 2007;3:CD004910. doi: 10.1002/14651858.CD004910.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Smith B, Appleton S, Adams R, Southcott A, Ruffin R. Home care by outreach nursing for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2001;3:CD000994. doi: 10.1002/14651858.CD000994. [DOI] [PubMed] [Google Scholar]
- 18.Jeppesen E, Brurberg KG, Vist GE, Wedzicha JA, Wright JJ, Greenstone M, Walters JA.Hospital at home for acute exacerbations of chronic obstructive pulmonary disease Cochrane Database Syst Rev 20125CD003573 [DOI] [PubMed] [Google Scholar]
- 19.Shepperd S, Doll H, Angus RM, Clarke MJ, Iliffe S, Kalra L, Ricauda NA, Tibaldi V, Wilson AD. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180:175–182. doi: 10.1503/cmaj.081491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiu WK, Newcomer R.A systematic review of nurse-assisted case management to improve hospital discharge transition outcomes for the elderly Prof Case Manag 200712330–336.quiz 337–338 [DOI] [PubMed] [Google Scholar]
- 21.Preyde M, Macaulay C, Dingwall T. Discharge planning from hospital to home for elderly patients: a meta-analysis. J Evidence-Based Soc Work. 2009;6:198–216. doi: 10.1080/15433710802686898. [DOI] [PubMed] [Google Scholar]
- 22.Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528. doi: 10.7326/0003-4819-155-8-201110180-00008. [DOI] [PubMed] [Google Scholar]
- 23.Prieto-Centurion V, DiDomenico R, Godwin P, Grude-Bracken N, Gussin H, Jaffe HA, Joo MJ, Markos MA, Nyenhuis S, Pickard AS, et al. A systematic review of interventions to reduce readmissions following COPD exacerbations [abstract] Am J Respir Crit Care Med. 2013;187:A2340. [Google Scholar]
- 24.Kangovi S, Grande D. Hospital readmissions—not just a measure of quality. JAMA. 2011;306:1796–1797. doi: 10.1001/jama.2011.1562. [DOI] [PubMed] [Google Scholar]
- 25.Puhan MA, Gimeno-Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;10:CD005305. doi: 10.1002/14651858.CD005305.pub3. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Green S.Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [Internet]. The Cochrane Collaboration; 2011. March [accessed 2013 August 6]. Available from: www.cochrane-handbook.org
- 27.Gøtzsche PC, Hróbjartsson A, Maric K, Tendal B. Data extraction errors in meta-analyses that use standardized mean differences. JAMA. 2007;298:430–437. doi: 10.1001/jama.298.4.430. [DOI] [PubMed] [Google Scholar]
- 28.Fan VS, Gaziano JM, Lew R, Bourbeau J, Adams SG, Leatherman S, Thwin SS, Huang GD, Robbins R, Sriram PS, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann Intern Med. 2012;156:673–683. doi: 10.7326/0003-4819-156-10-201205150-00003. [DOI] [PubMed] [Google Scholar]
- 29.Casas A, Troosters T, Garcia-Aymerich J, Roca J, Hernández C, Alonso A, del Pozo F, de Toledo P, Antó JM, Rodríguez-Roisín R, et al. members of the CHRONIC Project. Integrated care prevents hospitalisations for exacerbations in COPD patients. Eur Respir J. 2006;28:123–130. doi: 10.1183/09031936.06.00063205. [DOI] [PubMed] [Google Scholar]
- 30.Kwok T, Lum CM, Chan HS, Ma HM, Lee D, Woo J. A randomized, controlled trial of an intensive community nurse-supported discharge program in preventing hospital readmissions of older patients with chronic lung disease. J Am Geriatr Soc. 2004;52:1240–1246. doi: 10.1111/j.1532-5415.2004.52351.x. [DOI] [PubMed] [Google Scholar]
- 31.Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupré A, Bégin R, Renzi P, Nault D, Borycki E, Schwartzman K, et al. Chronic Obstructive Pulmonary Disease axis of the Respiratory Network Fonds de la Recherche en Santé du Québec. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med. 2003;163:585–591. doi: 10.1001/archinte.163.5.585. [DOI] [PubMed] [Google Scholar]
- 32.Bucknall CE, Miller G, Lloyd SM, Cleland J, McCluskey S, Cotton M, Stevenson RD, Cotton P, McConnachie A. Glasgow supported self-management trial (GSuST) for patients with moderate to severe COPD: randomised controlled trial. BMJ. 2012;344:e1060. doi: 10.1136/bmj.e1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calvillo-King L, Arnold D, Eubank KJ, Lo M, Yunyongying P, Stieglitz H, Halm EA. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28:269–282. doi: 10.1007/s11606-012-2235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joynt KE, Orav EJ, Jha AK. Thirty-day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305:675–681. doi: 10.1001/jama.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ko FW, Tam W, Tung AH, Ngai J, Ng SS, Lai K, Au KF, Hui DS. A longitudinal study of serial BODE indices in predicting mortality and readmissions for COPD. Respir Med. 2011;105:266–273. doi: 10.1016/j.rmed.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 36.Schnell K, Weiss CO, Lee T, Krishnan JA, Leff B, Wolff JL, Boyd C. The prevalence of clinically-relevant comorbid conditions in patients with physician-diagnosed COPD: A cross-sectional study using data from NHANES 1999–2008. BMC Pulm Med. 2012;12:26. doi: 10.1186/1471-2466-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hermiz O, Comino E, Marks G, Daffurn K, Wilson S, Harris M. Randomised controlled trial of home based care of patients with chronic obstructive pulmonary disease. BMJ. 2002;325:938. doi: 10.1136/bmj.325.7370.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin IR, McNamara D, Sutherland FR, Tilyard MW, Taylor DR. Care plans for acutely deteriorating COPD: a randomized controlled trial. Chron Respir Dis. 2004;1:191–195. doi: 10.1191/1479972304cd047oa. [DOI] [PubMed] [Google Scholar]
- 39.Rice KL, Dewan N, Bloomfield HE, Grill J, Schult TM, Nelson DB, Kumari S, Thomas M, Geist LJ, Beaner C, et al. Disease management program for chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med. 2010;182:890–896. doi: 10.1164/rccm.200910-1579OC. [DOI] [PubMed] [Google Scholar]
- 40.Soler JJ, Martínez-García MA, Román P, Orero R, Terrazas S, Martínez-Pechuán A. [Effectiveness of a specific program for patients with chronic obstructive pulmonary disease and frequent exacerbations] Arch Bronconeumol. 2006;42:501–508. doi: 10.1016/s1579-2129(06)60576-4. [DOI] [PubMed] [Google Scholar]
- 41.Busse WW, Morgan WJ, Taggart V, Togias A. Asthma outcomes workshop: overview. J Allergy Clin Immunol. 2012;129:S1–S8. doi: 10.1016/j.jaci.2011.12.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams MV. A requirement to reduce readmissions: take care of the patient, not just the disease. JAMA. 2013;309:394–396. doi: 10.1001/jama.2012.233964. [DOI] [PubMed] [Google Scholar]