Abstract
The individual innate immune components, interleukin-6 and complement component C3, play a role in the development of acute seizures in the Theiler's murine encephalomyelitis virus-induced seizure model. We examined the mRNA expression of various other complement components, cytokines, chemokines, and major histocompatibility complex antigens both within brain and in isolated ramified microglial and infiltrating macrophage/activated microglial cell populations over a time course covering the first 3 days postinfection. We found that complement component C3 showed the greatest increase in expression in brain of all of the complement components assayed and its level of expression was higher in infiltrating macrophages/activated microglia than in ramified microglial cells.
Keywords: : Theiler's murine encephalomyelitis virus, seizures, viral encephalitis, picornavirus, innate immune response, complement
Introduction
Microglia (myeloid-derived cells) play a vital role in central nervous system (CNS) development, maintenance, and neuroinflammatory responses to invading pathogens [reviewed in Ref. (32, 36, 38)]. Insults occurring in the brain parenchyma activate and induce the migration of microglia to the damaged tissue (38, 45, 47). Monocytes, a myeloid-derived cell type found in the periphery, have been found to infiltrate the CNS upon an insult to the CNS (3, 33). These myeloid-derived cell types have been observed to upregulate expression of interleukin (IL)-6 and complement proteins at various time points following insult (4, 9, 36, 38, 47). IL-6, a pro-inflammatory cytokine, is known to be produced in the brain early after viral infection by both resident cells (microglia, astrocytes, and neurons) of the CNS and by cells infiltrating from the periphery (macrophages) (23, 31, 35). In terms of complement, others found, through the use of genetic approaches, that altering complement expression, specifically the expression of complement components C1q and C3, can affect CNS development and lead to neuronal hyperexcitability and epileptiform activity (7, 39). In addition, the levels of various complement components are known to be increased in the CNS, localized to microglia/macrophages, following viral infection [(10, 11), reviewed in Ref. (28)].
The Theiler's murine encephalomyelitis virus (TMEV)-induced model of seizures is a model in which TMEV infection of C57BL/6J mice induces acute behavioral seizures [reviewed in Ref. (25)]. Neuronal cell death was noted within the hippocampus of TMEV-infected C57BL/6J mice during the peak of seizure activity (27, 40). Howe et al. demonstrated that infiltrating inflammatory monocytes are the critical mediators of hippocampal injury during acute TMEV infection of the brain (5, 18, 19). Neuronal loss through both direct killing and excitotoxicity would disrupt the hippocampal circuitry and result in seizures (17). Previously, we found that IL-6-producing macrophages (which differentiate from monocytes) infiltrating into the brains of TMEV-infected mice were important for the development of acute seizures starting at day 3 postinfection (p.i.) in these mice (9, 21, 26). IL-6 can provoke neuronal hyperexcitability/excitotoxicity through excess activation of glutamate receptors, such as the N-methyl-d-aspartate (NMDA) subtype of glutamate receptors, thus resulting in the development of acute symptomatic seizures (37).
In addition to IL-6, the role of complement component C3 in the development of acute seizures was examined in this model (28). In the CNS, neurons, microglia, astrocytes, and oligodendrocytes constitutively produce complement proteins (13, 14, 16, 46). The importance of the complement system within the CNS in the development of seizures in the TMEV-induced seizure model was demonstrated through the use of mice deficient in C3 and through the depletion of C3 in the periphery (28). However, complement component C3 is but a single member of a complex network or cascade. The complement system consists of more than 40 proteins and is highly regulated by the expression of complement receptors and complement inhibitors (1, 36). Complement can be activated through three different pathways, the classical, lectin, and alternative pathways, all of which use complement component C3 at some point in their cascade (1, 36). The contribution of complement component C3 to the development of seizures may be through its effect on the IL-6 pathway (48).
Determining what cell types and factors are involved in seizure development could provide some insight into how a viral infection of the brain can lead to long-term physiological changes in the CNS, thereby potentially leading to the development of recurrent and spontaneous seizures, even after the virus is cleared. Our earlier studies focused on determining what immune components (IL-6 and C3) were involved in the development of acute seizures in the TMEV-induced seizure model. To date, early kinetic studies assaying for both IL-6 and complement in response to viral infection with TMEV have not been thoroughly explored. Therefore, in this study, we examined the mRNA expression of various complement components, cytokines, chemokines, and major histocompatibility complex (MHC) class I and II antigens both within brain and in isolated ramified microglial (R1) and infiltrating macrophage/activated microglial (R2) cell populations before the onset of seizures (the first 3 days p.i.). We found that in brain complement component C3 showed the greatest increase in expression of all of the complement components assayed. The level of expression of complement component C3 was found to be higher in the infiltrating macrophage/activated microglial (R2) cell population compared to the ramified microglial (R1) cell population. In addition, we examined the expression of various complement components in the serum over a time course covering the first 3 days p.i. through the use of enzyme-linked immunosorbent assay (ELISA); however, no changes were found in the complement components in the serum, suggesting that this process is specific to the CNS.
Materials and Methods
Animal experiments
All animal experiments were conducted in accordance with the guidelines prepared by the Committee on Care and Use of Laboratory Animals, Institute of Laboratory Animals Resources, National Research Council. C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Five- to 6-week-old male C57BL/6J mice were infected intracerebrally (i.c.) with 3 × 105 plaque forming units (pfu) of the Daniels (DA) strain of TMEV. Mice were euthanized with isoflurane at 6, 12, 18, 24, 48, and 72 h p.i. Mice were perfused with phosphate-buffered saline (PBS), and serum was collected and stored frozen. For examination of brains (three mouse brains per time point), the brains were harvested and stored frozen. Brains from PBS-injected (20 μL volume) mock infected control mice (n = 3) were harvested and frozen at the 72 h time point. Brains from naive mice (not infected, n = 3) were harvested and frozen as a normal control. For examination of ramified microglial (R1) and infiltrating macrophage/activated microglial (R2) cell populations, brains from 20 mice per group (naive, 6, 12, 18, 24, 48, and 72 h time points) were harvested at the indicated times, followed by cell isolation and cell sorting.
Cell isolation and sorting
Briefly, brains were finely chopped and incubated with Trypsin LE (Invitrogen, San Diego, CA) for 30 min at 37°C. The tissues were then mashed through cell dissociation sieves (Chemglass Life Sciences, Vineland, NJ) in the presence of Hibernate A (Invitrogen) and filtered through 100 and 40 μm filters. The pooled cells were centrifuged at 400 g for 5 min and resuspended in 7 mL Hibernate A. The cell suspension was mixed with 3 mL Stock Isotonic Percoll (90% percoll made with Hibernate A) and layered onto 70% percoll made with PBS. The samples were centrifuged at 430 g for 30 min with low acceleration and no brakes. The cells were collected from the interface and stained with anti-CD45–v500 and anti-CD11b–APC (eBioscience, San Diego, CA) anti-mouse antibodies for 30 min at 4°C. Cell sorting of the CD45hi CD11b+ (R2) and CD45lo/int CD11b+ (R1) cell populations and quantification were performed at the University of Utah core facility on a FACSAria-II SORP (BD Bioscience, San Jose, CA) (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/vim). The isolated R1 and R2 cell populations were mixed with 0.5 mL TRIzol reagent (Invitrogen) and stored frozen at −80°C.
RNA isolation
RNA was isolated from frozen brains by homogenizing individual brains in TRIzol reagent for ≥1 min, performing a chloroform extraction, and then further purifying the RNA from the aqueous layer by means of the RNeasy Midi Kit (Qiagen, Chatsworth, CA). A DNase digestion was performed on the column during the RNA purification, as per the manufacturer's recommendations. The RNA was quantified and stored at −80°C until use.
RNA was isolated from frozen R1 and R2 cell populations by thawing, mixing by pipetting, and incubating at room temperature for 5 min. Chloroform was then added, and the method outlined above for isolation of RNA from frozen brains was followed with the modification that the RNeasy Mini Kit (Qiagen) was used. The RNA was quantified and stored at −80°C until use.
PCR arrays
First-strand cDNA was synthesized from 0.5 μg RNA per sample using the RT2 First Strand Kit (Qiagen), according to the manufacturer's recommendations. Quantitative real-time polymerase chain reaction (PCR) was performed to assay for the expression of individual complement component, cytokine, and chemokine mRNAs, using the RT2 SYBR Green Master Mix (Qiagen) and both a Modified RT2 Profiler PCR array: the Mouse Inflammatory Response and Autoimmunity PCR Array (Qiagen), to which were added the genes for complement receptors C5ar1, Cr2, Cr3 (CD11b/CD18), and C3ar1 (brain only), and a 32 × 3 Custom RT2 Profiler PCR array (Qiagen) (brain and R1/R2 cell populations), developed based on the results from the initial Modified RT2 Profiler PCR array. The PCR arrays were assayed on a LC480 LightCycler (Roche, Indianapolis, IN) 96-well block, using the following cycling parameters: denaturation at 95°C for 10 min, followed by 45 cycles of 95°C for 15 sec, and 60°C for 1 min, as per the manufacturer's instructions.
PCR array data analysis
The Crossing point (Cp) cycle values were calculated for each well using the Second Derivative Maximum analysis method within the LC480 software. The Cp data were then exported to an Excel spreadsheet and subsequently uploaded to the RT2 Profiler PCR Array Data Analysis (version 3.5) web-based analysis suite (www.sabiosciences.com/pcrarraydataanalysis.php). The 24 individual arrays per experiment on brain (three mice per time point times six time points plus mock and naive) were grouped according to time point, mock or naive, and the naive mouse group was used as the Control group for the data analysis. This naive mouse group was also used as the Control group for the data analysis of the four R1 and R2 cell population individual arrays (24 and 72 h time points). Each group was assessed for PCR reproducibility, reverse transcription efficiency, and the presence of genomic DNA contamination using the quality control function of the analysis suite. The Hsp90ab1 gene was used as the housekeeping gene for data normalization by arithmetic means. The relative expression levels for each gene, given as both fold-change and fold-regulation (which presents fold-change results in a biologically meaningful way), were then calculated by the software through the ΔΔCt method.
ELISA
Serum, which was collected from the perfused mice and had been stored frozen, was assayed for the expression of individual complement components (C1q, C2, C3, C4, C4b, C5, and C9), using commercially available ELISA Kits (BlueGene, Shanghai, China), according to the manufacturer's recommendations.
Results and Discussion
Modified mouse inflammatory response and autoimmunity PCR array: Brain
The Mouse Inflammatory Response and Autoimmunity PCR Array had only the complement genes C3, C3ar1, and C4b present in the array. The basic array was modified through the addition of genes for complement receptors C5ar1, Cr2, Cr3 (CD11b/CD18), and C3ar1 (duplicate control). All of the time points, mock and naive groups (eight groups with three mice per group), passed the PCR reproducibility, reverse transcription efficiency, and presence of genomic DNA contamination quality control assessments (data not shown). Comparison of the mock infected (PBS) group to the control naive group demonstrated only seven genes upregulated and one gene downregulated more than twofold from control (data not shown). Examination of the C3ar1 duplicate gene present on the array also demonstrated high PCR reproducibility (data not shown). Comparison of the six time point groups to the control naive group for the 92 individual genes on the modified array demonstrated increased expression (more than twofold from control) over all time points for many genes (33), notably including C3, C3ar1, C4b, and C5ar1 (Table 1). Decreased expression (more than twofold from control) was demonstrated for very few genes (4) within the brain (Table 1). The expression of 27 genes, to include Cr2, remained unchanged over all time points (Table 1). The expression of the remaining genes (28), varied greatly as to whether the expression was increased over a small range of time points, was increased over all but one time point, or was sporadically increased (increased at some time points but not others without an apparent pattern) (more than twofold from control) (Table 1). The expression of Cr3 (CD11b/CD18) was increased over a small range of time points, 18–72 h time points, in the time course study (Table 1). IL1a was unique in that this gene showed sporadic upregulation at 6, 12, 48, and 72 h, but was downregulated at 18 h (Table 1).
Table 1.
Modified Mouse Inflammatory Response and Autoimmunity Polymerase Chain Reaction Array: Brain
| Upregulation all time points | Upregulation early (6–18 h) | Upregulation all except 6 h | Sporadic upregulation | No change in regulation |
|---|---|---|---|---|
| B2m | Ccl11 | Tlr1 | Ccl22 (6 and 18 h) | Actb |
| C3 | Cxcl5 | Ccr4 (24 h) | Bcl6 | |
| C3ar1 | Cd14 (6, 12, and 72 h) | Ccl1 | ||
| C4b | Crp (12 h) | Ccl17 | ||
| C5ar1 | Cxcl3 (12 h) | Ccl24 | ||
| Ccl12 | Up all except 12 h | Cxcr4 (72 h) | Ccl25 | |
| Ccl2 | Tlr7 | Fasl (72 h) | Cd40lg | |
| Ccl3 | Fos (72 h) | Cr2 | ||
| Ccl4 | Il10 (18, 24, and 72 h) | Cxcr1 | ||
| Ccl5 | Il1a (6, 12, 48, and 72 h) | Gapdh | ||
| Ccl7 | Il1r1 (6 h) | Gusb | ||
| Ccl8 | Up mid (12–24 h) | Il22 (24 h) | Hsp90ab1 | |
| Ccr1 | Ccl19 | Up all except 24 h | Il6ra (72 h) | Il10rb |
| Ccr3 | Ccr2 | Ltb (72 h) | Il17a | |
| Ccr7 | Tlr4 (12, 24, and 72 h) | Il18 | ||
| Cd40 | Tlr6 (24 and 72 h) | Il1rap | ||
| Cebpb | Il23a | |||
| Cxcl1 | Il23r | |||
| Cxcl10 | Il9 | |||
| Cxcl11 | Up all except 48 h | Kng1 | ||
| Cxcl2 | Csf1 | Lta | ||
| Cxcl9 | Ptgs2 | Nfkb1 | ||
| Ifng | Up late (18–72 h) | Downregulation | Nr3c1 | |
| Il1b | Cr3 | Il1a (18 h) | Ripk2 | |
| Il1rn | Il5 | Ly96 (48 h) | Tirap | |
| Il6 | Itgb2 | Nos2 (72 h) | Tlr5 | |
| Il7 | Up all except 72 h | Tnfsf14 (6 h) | Tollip | |
| Myd88 | Ccl20 | |||
| Sele | Cxcr2 | |||
| Tlr2 | ||||
| Tlr3 | ||||
| Tlr9 | ||||
| Tnf |
32 × 3 Custom RT2 Profiler PCR array: brain
From the initial screen of 92 individual genes, a subset of genes that were up- or downregulated plus 8 additional genes for complement components (C1qa, C2, C5, and C9), cytokines (Tgfb1), chemokines (Cx3cr1), and class II antigens (H2-Ab1 and H2-Ea-ps) not present on the initial array were used to construct a 32 × 3 Custom RT2 PCR array. The sample cDNAs (three mice per group) were then assayed in triplicate. Again all of the time points, mock and naive groups (eight groups with three mice per group assayed in triplicate), passed the PCR reproducibility, reverse transcription efficiency, and presence of genomic DNA contamination quality control assessments (data not shown). Comparison of the mock infected (PBS) group to the control naive group demonstrated that no genes were up- or downregulated more than twofold from control (data not shown). This result differs from the initial 92 gene screen, most likely due to the samples being assayed in triplicate. Comparison of the time point groups to the control naive group for the 29 individual genes on the 32 × 3 array demonstrated increased expression of many genes (more than twofold from control), either over all time points (14), over a small range of time points (3), or sporadically (4), but no decreased expression of any genes (more than twofold from control) within the brain (Table 2). This result differs from the initial 92 gene screen and demonstrates that the 92 gene screen, where each gene is assayed only once per sample, can result in false downregulation results. The expression of two of the genes (Ly96 and Nos2) for which expression was shown to be downregulated on the 92 gene screen was found to be unchanged over all time points on the 32 × 3 array, and the expression of two of the genes (Il1a and Tnfsf14) for which expression was shown to be downregulated on the 92 gene screen was found to be upregulated at all time points on the 32 × 3 array (compare Tables 1 and 2). The remaining genes (8) showed no change in expression over the entire time course (Table 2). In terms of complement, certain complement components were upregulated (more than twofold from control) through the 72 h time point, C3, C3ar1, C4b, and C2, following virus infection, while two complement components remained unaffected, C5 and C9 (Table 2). Still other complement components were upregulated (more than twofold from control) starting at either 18 h [Cr3 (CD11b/CD18)] or 24 h (C1qa) following infection and remained upregulated through the 72 h time point (Table 2). The cytokine Tgfb1 showed increased expression only at 72 h (Table 2). The expression of the chemokine Cx3cr1 remained unchanged over the course of the study (Table 2). This chemokine receptor is expressed exclusively by microglia in the brain (15, 20). Previous studies using mice deficient in CX3CR1 demonstrated a role for CX3CR1 in CNS development and synaptic pruning, and loss of this chemokine receptor led to immature synaptic function and aberrant neuronal excitability (29). In addition, mice deficient in CX3CR1 demonstrated a reduction in seizure susceptibility to pentylenetetrazole-induced seizures (29). Therefore, analysis of this chemokine in relation to a possible role in virus-induced seizures in the current study was warranted. The expression of the class II antigen H2-Ab1 was increased (more than twofold from control) over a small range of time points, 18–72 h time points, while the expression of the pseudogene H2-Ea-ps remained unchanged over the time course (Table 2).
Table 2.
32 × 3 Custom RT2 Profiler Polymerase Chain Reaction Array: Brain
| Upregulation all time points | Upregulation late (18–72 h) | Sporadic upregulation | No change in regulation |
|---|---|---|---|
| B2m | Cr3 | Cd14 (6, 12, 48, and 72 h) | C5 |
| C2 | H2-Ab1 | Fos (72 h) | C9 |
| C3 | Ptgs2 (6, 12, 18, and 72 h) | Cx3cr1 | |
| C3ar1 | Tgfb1 (72 h) | H2-Ea-ps | |
| C4b | Hsp90ab1 | ||
| Ccl12 | Il1rap | ||
| Ccl2 | Ly96 | ||
| Ccl5 | Up later (24–72 h) | Nos2 | |
| Cxcl10 | C1qa | ||
| Il1a | |||
| Il1rn | |||
| Il6 | |||
| Tlr3 | |||
| Tnfsf14 |
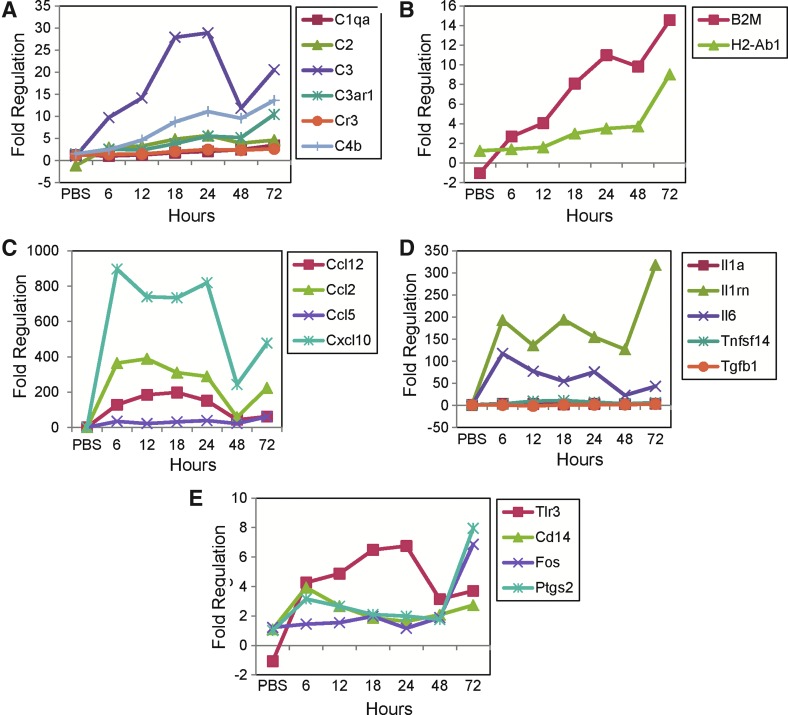
Of those genes that showed upregulation (more than twofold from the control naive group) in their expression in the brain, the level of expression varied greatly from gene to gene. Of all of the complement components that showed increased expression, complement component C3 showed the greatest increase (Fig. 1A). Both B2m and H2-Ab1, MHC class I and II antigens, respectively, had increased expression (Fig. 1B). Of the chemokines analyzed, Cxcl10 had the greatest increase in expression (Fig. 1C). Of the cytokines analyzed, Il1rn had the greatest increase in expression (Fig. 1D). Of the other interesting genes analyzed, Tlr3 was increased over the 6 to 24 h time points, while Ptgs2 and Fos increased at the 72 h time point (Fig. 1E). TLR3 is a pattern recognition receptor that recognizes double-stranded RNA, a common intermediate during viral replication [reviewed in Ref. (30)]; it mediates a protective response against picornaviruses, of which TMEV is a member (41); and TLR3 protein has been shown to be expressed by astrocytes, oligodendrocytes, and neurons, but not microglia [reviewed in Ref. (24)]. Recognition of double-stranded RNA by TLR3 has been demonstrated in bone marrow-derived macrophages (2). Therefore, although TLR3 can be expressed by macrophages, cells other than microglia and macrophages are expressing TLR3 in the CNS as its expression was negligible in both the R1 (Fig. 4E) and R2 (Fig. 5E) cell populations.
FIG. 1.
Brain gene expression as determined using the 32 × 3 Custom RT2 Profiler PCR array. Gene expression, for the mock infected group (PBS) and the time point groups, is given as fold regulation, compared to the control naive group, for complement components (A), MHC class I and II antigens (B), chemokines (C), cytokines (D), and other genes (E) with expression increased more than twofold from control. Note the changes in scale for the y-axis from graph to graph. MHC, major histocompatibility complex; PBS, phosphate-buffered saline; PCR, polymerase chain reaction.
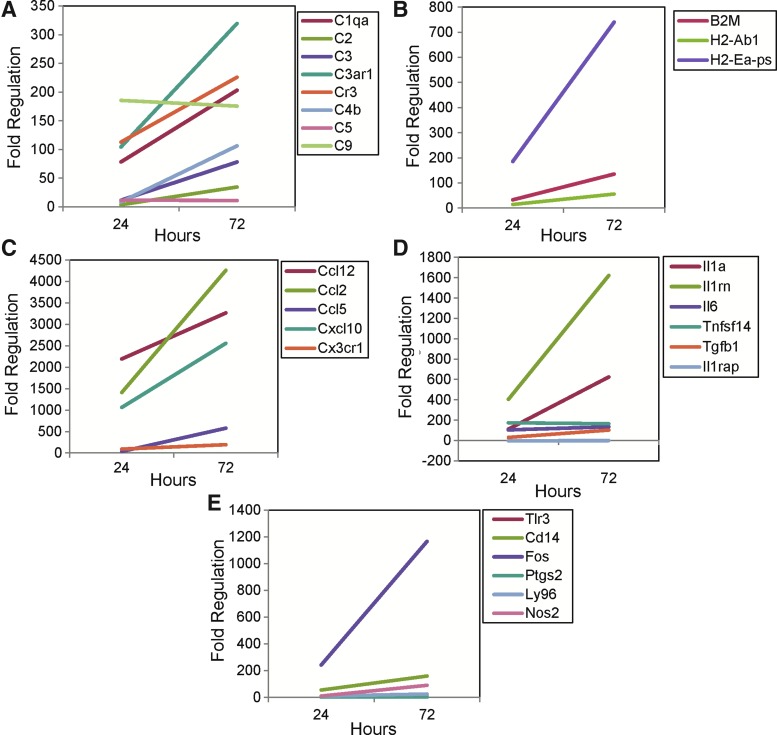
FIG. 4.
Ramified microglia (R1) gene expression as determined using the 32 × 3 Custom RT2 Profiler PCR array. Gene expression, for the 24 and 72 h time point groups, is given as fold regulation, compared to the control naive group, for complement components (A), MHC class I and II antigens (B), chemokines (C), cytokines (D), and other genes (E) with expression increased more than twofold from control. Note the changes in scale for the y-axis from graph to graph.
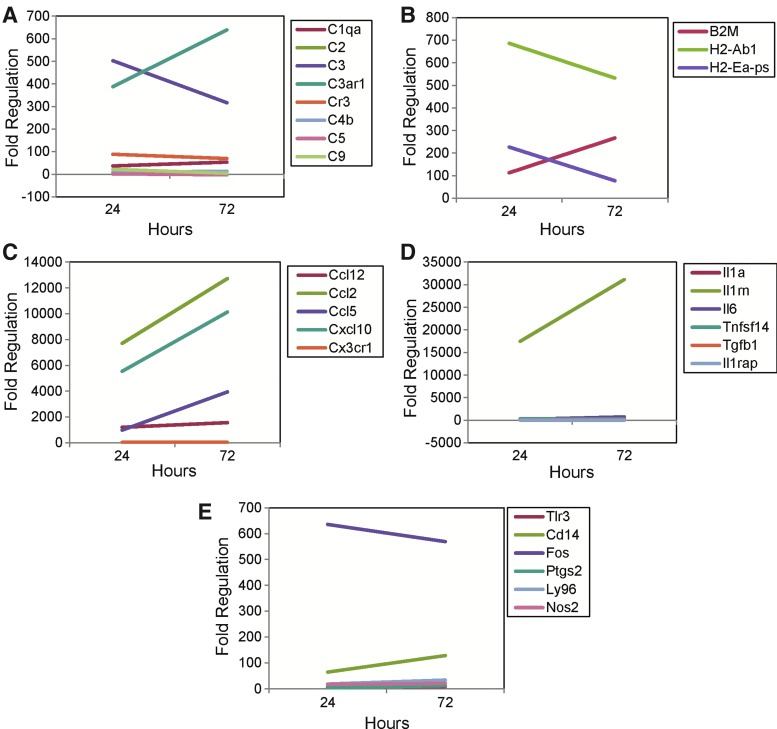
FIG. 5.
Infiltrating macrophage/activated microglial (R2) gene expression as determined using the 32 × 3 Custom RT2 Profiler PCR array. Gene expression, for the 24 and 72 h time point groups, is given as fold regulation, compared to the control naive group, for complement components (A), MHC class I and II antigens (B), chemokines (C), cytokines (D), and other genes (E) with expression increased more than twofold from control. Note the changes in scale for the y-axis from graph to graph.
32 × 3 Custom RT2 Profiler PCR array: ramified microglia (R1) versus infiltrating macrophages/activated microglia (R2)
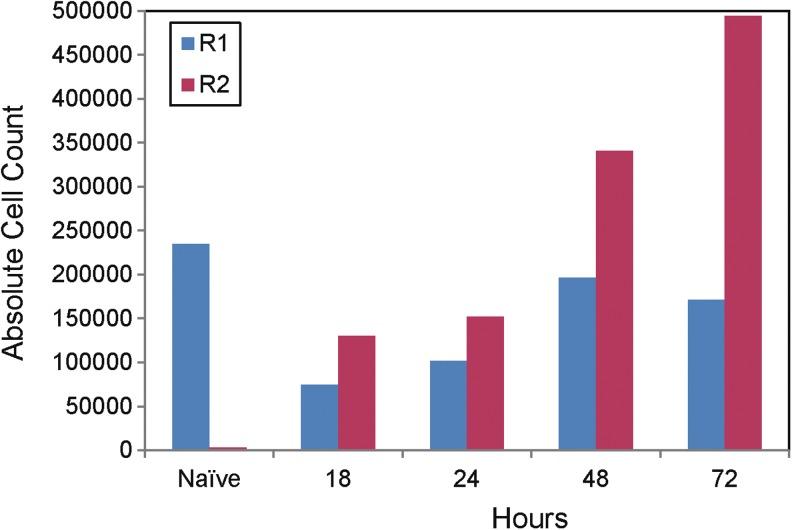
During sorting of cells isolated from naive mice and mice from the 18, 24, 48, and 72 h time points (20 mice per group), ramified microglial (R1) and infiltrating macrophage/activated microglial (R2) cell population counts were obtained (Fig. 2). As seen for naive mice (Fig. 2), and for the 6 and 12 h time points (20 mice per group; data not shown), the R2 cell population was negligible (as expected); therefore we were unable to perform the RNA isolation and PCR array procedures for these time points. RNA isolation and PCR array analysis were performed on the 24 and 72 h time points (below).
FIG. 2.
Cell counts for ramified microglial (R1) and infiltrating macrophage/activated microglial (R2) cell populations. Absolute R1 and R2 cell population counts obtained during sorting of cells isolated from naive mice and mice from the 18, 24, 48, and 72 h time points (20 mice per group) are shown.
Isolated RNAs from infiltrating macrophage/activated microglia (R2) and ramified microglia (R1) were used to generate cDNAs for use with the same 32 × 3 Custom RT2 Profiler PCR array used for brain. In this way we have begun to assign cell types to the various complement components and cytokine and chemokine mRNAs, which are up- or downregulated in the brain. The 24 and 72 h time points, assayed in triplicate, passed the PCR reproducibility, reverse transcription efficiency, and presence of genomic DNA contamination quality control assessments (data not shown). In contrast to the results from the brain, nearly all of the genes examined were upregulated more than twofold from control in both the R1 and R2 cell populations. In the R1 cell population, only IL1rap was not upregulated more than twofold from control; it was downregulated more than twofold from control at the 24 h time point and showed no change from control at the 72 h time point. In the R2 cell population, IL1rap was downregulated more than twofold from control at both the 24 and 72 h time points. Ptgs2, also known as Cox-2, was highly expressed at the 72 h time point in the brain (Fig. 1E), but its expression was negligible in both the R1 (Fig. 4E) and R2 (Fig. 5E) cell populations. PTGS2 is an inducible enzyme that functions in prostaglandin biosynthesis and is tightly coupled with glutamate-mediated neuronal excitotoxic death (6). Expression of PTGS2 in oligodendrocytes in brains, at 2 weeks p.i., and in spinal cords, at 3, 4, 5, and 9 weeks p.i., has been demonstrated following TMEV infection, i.c. with 2 × 105 pfu of the DA strain, of SJL/J mice (6). Therefore, cells other than microglia and macrophages, such as neurons and oligodendrocytes, are expressing PTGS2 in the CNS. In the R2 cell population C5 showed no change from control at the 24 h time point, but was downregulated more than twofold from control at the 72 h time point.
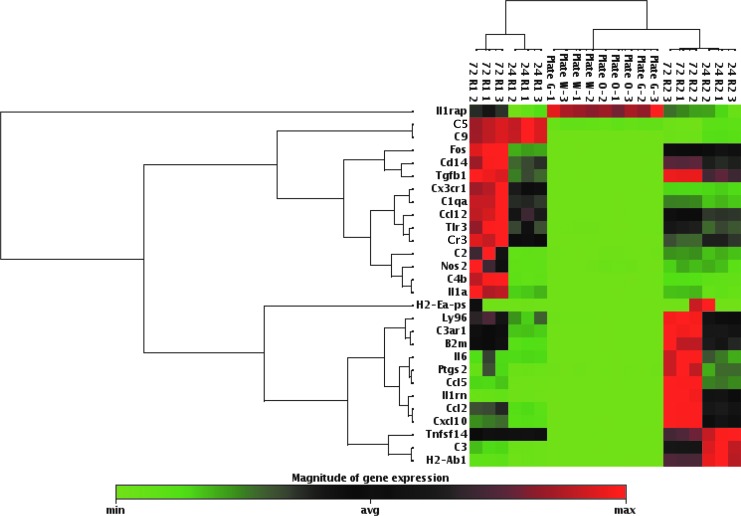
As was seen with brain, the level of expression of the different genes examined in the R1 and R2 cell populations varied greatly from gene to gene. Cluster analysis within the data analysis suite was used to generate a nonsupervised hierarchical clustering of the entire dataset. The resultant cluster analysis is displayed as a heat map with dendrograms indicating coregulated genes across the individual samples assayed in triplicate (Fig. 3). The heat map and upper dendrogram group the R1 cell population and the R2 cell population separately by gene expression (Fig. 3). The heat map and left-hand dendrogram group individual genes together by expression, such as the C5 and C9 complement components (Fig. 3).
FIG. 3.
Cluster analysis for the ramified microglial (R1) and infiltrating macrophage/activated microglial (R2) cell populations. Clustering of the entire dataset is displayed as a heat map with dendrograms indicating coregulated genes across individual samples assayed in triplicate. Plate G, Plate O, and Plate W represent the three individual samples obtained from the control naive mice assayed in triplicate (1–3).
The levels of expression of individual complement components (Fig. 4A), MHC class I and II antigens (Fig. 4B), chemokines (Fig. 4C), cytokines (Fig. 4D), and other interesting genes (Fig. 4E) in the R1 cell population assayed at the 24 and 72 h time points were compared. Similarly, the levels of expression of individual complement components (Fig. 5A), MHC class I and II antigens (Fig. 5B), chemokines (Fig. 5C), cytokines (Fig. 5D), and other genes (Fig. 5E) in the R2 cell population assayed at the 24 and 72 h time points were compared. The complement component C3ar1 showed high levels of expression in both the R1 (Fig. 4A) and R2 (Fig. 5A) cell populations and this expression increased from 24 to 72 h in both cell populations. In contrast complement component C3 showed the greatest increase in expression of all of the complement components assayed (Fig. 1A) and the level of expression was higher in the R2 cell population (Fig. 5A) than in the R1 cell population (Fig. 4A); the expression decreased from 24 to 72 h in the R2 cell population, whereas the expression increased from 24 to 72 h in the R1 cell population. The complement receptor Cr3 (CD11b/CD18), of which the CD11b subunit binds to the major complement opsonic C3 fragment iC3b (43), was also expressed in the brain following viral infection (Tables 1 and 2) and expression increased from 24 to 72 h in the ramified microglial (R1) cell population (Fig. 4A) while remaining constant from 24 to 72 h in the infiltrating macrophage/activated microglial (R2) cell population (Fig. 5A). The CD11b surface marker, in conjunction with CD45, was used to isolate the ramified microglial and infiltrating macrophage/activated microglial cell populations through cell sorting. Due to the intertwining of the many components of the complement system, it is difficult to specify one single dominant complement activation pathway over the others. However, the alternative activation pathway may be favored by infiltrating macrophages/activated microglia (R2) which highly express C3 (Fig. 5A), while the classical activation pathway may be favored by ramified microglia (R1) which highly express C1qa (Fig. 4A). The lack of increase in expression of C5 and C9 (Table 2) may possibly be explained by their late involvement in the complement cascade, although these components would be expected to be present within hours and not days (44).
As for the MHC class I and II antigens, the expression of both B2m and H2-Ab1, respectively, was higher in the R2 cell population (Fig. 5B) than in the R1 cell population (Fig. 4B), and whereas the expression of H2-Ab1 increased from 24 to 72 h in the R1 cell population, its expression decreased from 24 to 72 h in the R2 cell population.
Of the chemokines analyzed, the R2 cell population had high, and increasing, levels of expression of Ccl2 and Cxcl10 (Fig. 5C), while the R1 cell population had high, and increasing, levels of expression of these two chemokines plus Ccl12 (Fig. 4C). The chemokine ligand Cxcl10, which was highly expressed in the brain (Fig. 1C) as well, binds to the CXCR3 chemokine receptor (not on our arrays) and is chemotactic for CXCR3+ monocytes and T lymphocytes (42). Cxcl10 is also known as interferon-γ inducible protein-10 kDa (IP-10), and expression of this chemokine in the CNS has been demonstrated following TMEV infection, i.c. with 2 × 105 pfu of the DA strain, of SJL/J mice (42).
Of the cytokines analyzed, IL1rn had high and increasing levels of expression in both the R1 (Fig. 4D) and the R2 (Fig. 5D) cell populations; however, its level of expression was much higher in the R2 cell population (compare the y-axes of Figs. 4D and 5D). The cytokine inhibitor Il1rn was highly expressed in the brain (Fig. 1D) as well. IL1RN is a receptor antagonist that inhibits the activities of IL-1α and IL-1β by binding to the IL-1 receptor-1 and preventing its association with its coreceptor IL1RAP (12). Previously, we found that mice deficient in either IL-1 receptor-1 or myeloid differentiation primary response gene 88 (MyD88), through which IL-1 signals, developed seizures at frequencies comparable to wild-type mice (21). We also demonstrated that IL-1α and IL-1β mRNA expression was not greatly increased at day 6 p.i. in infected mice experiencing seizures compared to infected mice not experiencing seizures (21). Based on both the presence of seizures in mice deficient in IL-1 receptor 1 or MyD88 and the examination of the mRNA expression of IL-1α and IL-1β, it appears that IL-1 does not play a major role in the development of seizures in the TMEV-induced seizure model. The increased expression of Il1rn that we see in this study readily explains and supports the lack of involvement of IL-1 signaling in the development of seizures that was seen previously using mice deficient in IL-1 receptor 1 or MyD88.
Of the other interesting genes analyzed, the Fos gene had high levels of expression, with the expression increasing from 24 to 72 h in the R1 cell population (Fig. 4E) and decreasing in the R2 cell population (Fig. 5E). FOS is a nuclear phosphoprotein which when bound to JUN forms the inducible transcription factor complex AP-1 (8). FOS is one member of a multimeric complex involved with regulating transforming growth factor (TGF)-β-mediated signaling (49). Previously, TGF-β expression was found to correlate with seizure activity in the hippocampus in the TMEV-induced model of seizures (27). In addition, FOS is induced by seizures and as such has been used as a marker of repetitive neuronal activation in audiogenic seizures and other seizure types (22). Finally, expression of Fos (both mRNA and protein) was found to be induced early following TMEV infection (BeAn strain) both in vitro in pure primary cultured astrocytes isolated from SJL/J mice and in vivo in SJL/J mouse brains (i.c. with 3 × 106 pfu) (34).
ELISA
Serum was assayed for the expression of individual complement components (C1q, C2, C3, C4, C4b, C5, and C9). Sera collected from mice at the 18, 24, and 72 h time points were pooled and assayed. We do not see any changes in the complement components assayed in the serum for the time points assayed (data not shown).
Conclusions
In the TMEV-induced model of seizures, the host's innate immune response to TMEV infection contributes to the development of seizures (25). Using PCR arrays we have examined the expression of various innate immune response genes within the CNS early following infection: from 6 h to 3 days p.i., with 3 days p.i. being the earliest time point at which seizures are observed. We have started to assign cell types to the various complement components and cytokine and chemokine mRNAs that are up- or downregulated in the brain. Upon examination of the brain, many components of the complement system were found to be expressed following viral infection (Fig. 1A), where previously we had only implicated C3 (28). We confirmed the importance of C3 in the development of seizures and saw higher expression of it in the infiltrating macrophage/activated microglial (R2) cell population compared to the ramified microglial (R1) cell population. Cxcl10 and Il1rn were both expressed in both infiltrating macrophages/activated microglia and ramified microglia. Tlr3 and Ptgs2, which were highly expressed in the brain, were not expressed to any great extent in either infiltrating macrophages/activated microglia or ramified microglia. Finally, the expression of Fos varied between the infiltrating macrophage/activated microglial and ramified microglial cell populations. Each unique component's contribution to the development of seizures in the TMEV-induced seizure model remains to be elucidated. In addition, how the upregulation of these immunologic factors contributes to the development of recurrent and spontaneous seizures in this model is currently being investigated.
Supplementary Material
Acknowledgments
The authors thank F. Lynn Sonderegger, PhD, and Ana Beatriz DePaula-Silva, PhD, for helpful discussions, Jordan T. Sim, BA, and Mitchell A. Wilson for excellent technical assistance, and Daniel J. Harper for the outstanding preparation of the article. This work was supported by NCRR of the NIH 1S10RR026802-01 (Flow Cytometry Core Facility), NIH T32AI055434 (M.F.C.), and CURE (R.S.F.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Alexander JJ, Anderson AJ, Barnum SR, et al. . The complement cascade: Yin-Yang in neuroinflammation—neuro-protection and -degeneration. J Neurochem 2008;107:1169–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexopoulou L, Holt AC, Medzhitov R, et al. . Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001;413:732–738 [DOI] [PubMed] [Google Scholar]
- 3.Ashhurst TM, van Vreden C, Niewold P, et al. . The plasticity of inflammatory monocyte responses to the inflamed central nervous system. Cell Immunol 2014;291:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnum SR. Complement biosynthesis in the central nervous system. Crit Rev Oral Biol Med 1995;6:132–146 [DOI] [PubMed] [Google Scholar]
- 5.Buenz EJ, Sauer BM, Lafrance-Corey RG, et al. . Apoptosis of hippocampal pyramidal neurons is virus independent in a mouse model of acute neurovirulent picornavirus infection. Am J Pathol 2009;175:668–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlson NG, Hill KE, Tsunoda I, et al. . The pathologic role for COX-2 in apoptotic oligodendrocytes in virus induced demyelinating disease: implications for multiple sclerosis. J Neuroimmunol 2006;174:21–31 [DOI] [PubMed] [Google Scholar]
- 7.Chu Y, Jin X, Parada I, et al. . Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc Natl Acad Sci U S A 2010;107:7975–7980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curran T, Franza BR., Jr. Fos and Jun: the AP-1 connection. Cell 1988;55:395–397 [DOI] [PubMed] [Google Scholar]
- 9.Cusick MF, Libbey JE, Patel DC, et al. . Infiltrating macrophages are key to the development of seizures following virus infection. J Virol 2013;87:1849–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Depboylu C, Schafer MK, Schwaeble WJ, et al. . Increase of C1q biosynthesis in brain microglia and macrophages during lentivirus infection in the rhesus macaque is sensitive to antiretroviral treatment with 6-chloro-2',3'-dideoxyguanosine. Neurobiol Dis 2005;20:12–26 [DOI] [PubMed] [Google Scholar]
- 11.Dietzschold B, Schwaeble W, Schafer MK, et al. . Expression of C1q, a subcomponent of the rat complement system, is dramatically enhanced in brains of rats with either Borna disease or experimental allergic encephalomyelitis. J Neurol Sci 1995;130:11–16 [DOI] [PubMed] [Google Scholar]
- 12.Gabay C, Lamacchia C, and Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol 2010;6:232–241 [DOI] [PubMed] [Google Scholar]
- 13.Gasque P, Dean YD, McGreal EP, et al. . Complement components of the innate immune system in health and disease in the CNS. Immunopharmacology 2000;49:171–186 [DOI] [PubMed] [Google Scholar]
- 14.Gasque P, Fontaine M, and Morgan BP. Complement expression in human brain. Biosynthesis of terminal pathway components and regulators in human glial cells and cell lines. J Immunol 1995;154:4726–4733 [PubMed] [Google Scholar]
- 15.Harrison JK, Jiang Y, Chen S, et al. . Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A 1998;95:10896–10901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosokawa M, Klegeris A, Maguire J, et al. . Expression of complement messenger RNAs and proteins by human oligodendroglial cells. Glia 2003;42:417–423 [DOI] [PubMed] [Google Scholar]
- 17.Howe CL, LaFrance-Corey RG, Mirchia K, et al. . Neuroprotection mediated by inhibition of calpain during acute viral encephalitis. Sci Rep 2016;6:28699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howe CL, Lafrance-Corey RG, Sundsbak RS, et al. . Hippocampal protection in mice with an attenuated inflammatory monocyte response to acute CNS picornavirus infection. Sci Rep 2012;2:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howe CL, Lafrance-Corey RG, Sundsbak RS, et al. . Inflammatory monocytes damage the hippocampus during acute picornavirus infection of the brain. J Neuroinflammation 2012;9:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung S, Aliberti J, Graemmel P, et al. . Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 2000;20:4106–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirkman NJ, Libbey JE, Wilcox KS, et al. . Innate but not adaptive immune responses contribute to behavioral seizures following viral infection. Epilepsia 2010;51:454–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein BD, Fu YH, Ptacek LJ, et al. . c-Fos immunohistochemical mapping of the audiogenic seizure network and tonotopic neuronal hyperexcitability in the inferior colliculus of the Frings mouse. Epilepsy Res 2004;62:13–25 [DOI] [PubMed] [Google Scholar]
- 23.Konsman JP, Drukarch B, and Van Dam AM. (Peri)vascular production and action of pro-inflammatory cytokines in brain pathology. Clin Sci (Lond) 2007;112:1–25 [DOI] [PubMed] [Google Scholar]
- 24.Lee H, Lee S, Cho IH, et al. . Toll-like receptors: sensor molecules for detecting damage to the nervous system. Curr Protein Pept Sci 2013;14:33–42 [DOI] [PubMed] [Google Scholar]
- 25.Libbey JE, and Fujinami RS. Neurotropic viral infections leading to epilepsy: focus on Theiler's murine encephalomyelitis virus. Future Virol 2011;6:1339–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Libbey JE, Kennett NJ, Wilcox KS, et al. . Interleukin-6, produced by resident cells of the central nervous system and infiltrating cells, contributes to the development of seizures following viral infection. J Virol 2011;85:6913–6922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Libbey JE, Kirkman NJ, Smith MC, et al. . Seizures following picornavirus infection. Epilepsia 2008;49:1066–1074 [DOI] [PubMed] [Google Scholar]
- 28.Libbey JE, Kirkman NJ, Wilcox KS, et al. . Role for complement in the development of seizures following acute viral infection. J Virol 2010;84:6452–6460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paolicelli RC, Bolasco G, Pagani F, et al. . Synaptic pruning by microglia is necessary for normal brain development. Science 2011;333:1456–1458 [DOI] [PubMed] [Google Scholar]
- 30.Perales-Linares R, and Navas-Martin S. Toll-like receptor 3 in viral pathogenesis: friend or foe? Immunology 2013;140:153–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raivich G, Jones LL, Kloss CU, et al. . Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration. J Neurosci 1998;18:5804–5816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ransohoff RM, and Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature 2010;468:253–262 [DOI] [PubMed] [Google Scholar]
- 33.Rezai-Zadeh K, Gate D, and Town T. CNS infiltration of peripheral immune cells: D-Day for neurodegenerative disease? J Neuroimmune Pharmacol 2009;4:462–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubio N, and Martin-Clemente B. Theiler's murine encephalomyelitis virus infection induces early expression of c-fos in astrocytes. Virology 1999;258:21–29 [DOI] [PubMed] [Google Scholar]
- 35.Rubio N, and Sierra A. Interleukin-6 production by brain tissue and cultured astrocytes infected with Theiler's murine encephalomyelitis virus. Glia 1993;9:41–47 [DOI] [PubMed] [Google Scholar]
- 36.Shastri A, Bonifati DM, and Kishore U. Innate immunity and neuroinflammation. Mediators Inflammation 2013;2013:342931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singhi P. Infectious causes of seizures and epilepsy in the developing world. Dev Med Child Neurol 2011;53:600–609 [DOI] [PubMed] [Google Scholar]
- 38.Smith JA, Das A, Ray SK, et al. . Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull 2012;87:10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevens B, Allen NJ, Vazquez LE, et al. . The classical complement cascade mediates CNS synapse elimination. Cell 2007;131:1164–1178 [DOI] [PubMed] [Google Scholar]
- 40.Stewart KA, Wilcox KS, Fujinami RS, et al. . Theiler's virus infection chronically alters seizure susceptibility. Epilepsia 2010;51:1418–1428 [DOI] [PubMed] [Google Scholar]
- 41.Tatematsu M, Nishikawa F, Seya T, et al. . Toll-like receptor 3 recognizes incomplete stem structures in single-stranded viral RNA. Nature Commun 2013;4:1833. [DOI] [PubMed] [Google Scholar]
- 42.Tsunoda I, Lane TE, Blackett J, et al. . Distinct roles for IP-10/CXCL10 in three animal models, Theiler's virus infection, EAE, and MHV infection, for multiple sclerosis: implication of differing roles for IP-10. Mult Scler 2004;10:26–34 [DOI] [PubMed] [Google Scholar]
- 43.Ueda T, Rieu P, Brayer J, et al. . Identification of the complement iC3b binding site in the beta 2 integrin CR3 (CD11b/CD18). Proc Natl Acad Sci U S A 1994;91:10680–10684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vakeva A, Morgan BP, Tikkanen I, et al. . Time course of complement activation and inhibitor expression after ischemic injury of rat myocardium. Am J Pathol 1994;144:1357–1368 [PMC free article] [PubMed] [Google Scholar]
- 45.Yenari MA, Kauppinen TM, and Swanson RA. Microglial activation in stroke: therapeutic targets. Neurotherapeutics 2010;7:378–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu JX, Bradt BM, and Cooper NR. Constitutive expression of proinflammatory complement components by subsets of neurons in the central nervous system. J Neuroimmunol 2002;123:91–101 [DOI] [PubMed] [Google Scholar]
- 47.Zabel MK, and Kirsch WM. From development to dysfunction: microglia and the complement cascade in CNS homeostasis. Ageing Res Rev 2013;12:749–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Kimura Y, Fang C, et al. . Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood 2007;110:228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Feng XH, and Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription. Nature 1998;394:909–913 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.