Abstract
Significance: With an aging population leading to an increase in diabetes and associated cutaneous wounds, there is a pressing clinical need to improve wound-healing therapies.
Recent Advances: Tissue engineering approaches for wound healing and skin regeneration have been developed over the past few decades. A review of current literature has identified common themes and strategies that are proving successful within the field: The delivery of cells, mainly mesenchymal stem cells, within scaffolds of the native matrix is one such strategy. We overview these approaches and give insights into mechanisms that aid wound healing in different clinical scenarios.
Critical Issues: We discuss the importance of the biomimetic niche, and how recapitulating elements of the native microenvironment of cells can help direct cell behavior and fate.
Future Directions: It is crucial that during the continued development of tissue engineering in wound repair, there is close collaboration between tissue engineers and clinicians to maintain the translational efficacy of this approach.
Keywords: : biomimetic, tissue engineering, wound healing

Umber Cheema, BSc (Hon), PhD
Scope and Significance
Cutaneous wound healing is a major burden for healthcare systems worldwide. Here, we review the key tissue engineering strategies in cutaneous wound healing, including scaffolds, growth factors, and cellular therapies, to create biological skin equivalents. We also address the current challenges and future implications to the ever-evolving scientific research and technology.
Translational Relevance
Normal wound healing is commonly described as four overlapping and coordinated stages: hemostasis, inflammation, proliferation, and remodeling. The role of endogenous stem cells is crucial to the process. Tissue-engineered solutions that combine stem cells, growth factors, and a supporting matrix are being used to create products for clinical wound care applications. There has been a recent research focus on systems of stem cell delivery to wound sites, which ensure cell viability and efficacy in promoting wound healing and the regeneration of its appendages.
Clinical Relevance
Management of wounds is a routine part of medical practice worldwide, and delays in healing represent a significant clinical and economic burden. From national data in the United Kingdom, the National Health Service manages 2.2 million patients, costing an estimated £5.3 billion. These numbers are ever increasing, especially with an aging population.1 They also have a higher mortality, prolonged hospital stays, poorer quality of life, and an increased rate of being in a long-term care facility when discharged.1–3
Background
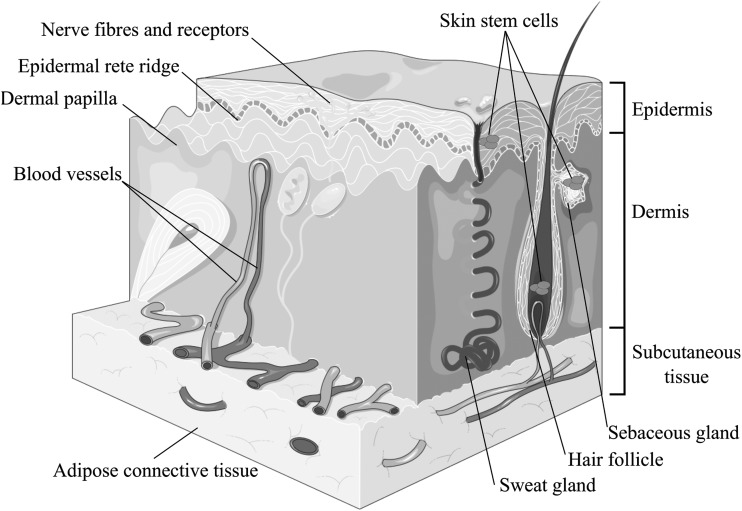
Skin is the largest organ in the body and it consists of the epidermis, dermis, subcutaneous tissue layers, as well as skin appendages such as hairs and glands, which expand from deep in the dermis to the superficial epidermal layers (Fig. 1). It is very vascular and highly innervated; is functionally responsible for maintenance of homeostasis of the living body by regulation of temperature, hydration, and vitamin D synthesis; as well as the all-important protective barrier against external chemicals and pathogens. Damage to any part of this organ from the development of a skin wound will inevitably compromise the functional properties mentioned earlier, exposing individuals to the risk of other health complications.
Figure 1.
Adult skin consists of epidermis, dermis, and appendages. Skin stem cells have been described in the hair follicles, sebaceous glands, and the interfollicular epidermis. Adapted from Servier Medical Art.
Normal wound healing is commonly described as four overlapping and precisely coordinated stages: hemostasis, inflammation, proliferation, and remodeling.4 During the first stage, when the epidermal barrier is violated, keratinocytes react to cell damage. Hemostasis is achieved by endothelial-activated vasoconstriction and the clotting cascade. Platelets degranulate alpha granules, leading to secretion of growth factors and pro-inflammatory cytokines.5 The inflammation phase also begins early with one of the predominant cell types at this stage being neutrophils acting to debride the wound. Monocytes constitute another important group of cells, which, regulated by transforming growth factor (TGF)-β, transform into macrophages. These further amplify the inflammatory response and the formation of granulation tissue as the proliferative phase is entered, lasting a maximum of 14 days.6,7 This phase encompasses the multiple processes of angiogenesis, epithelialization, and granulation tissue and collagen deposition. As part of the formation of granulation tissue and laying down of extracellular matrix (ECM), endothelial cell proliferation and angiogenesis have to occur. This is stimulated by vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF).2,8 Fibroblasts are the predominant cell type in the early stages. Some of this population transform into myofibroblasts, which are responsible for wound contraction. Fibroblasts secrete components of ECM, which form the foundations for the healing skin.9 The re-epithelialization process with epithelial cell proliferation and migration starts early after injury and continues into the remodeling phase, which can last from months to years. The initial increased fibroblast activity results in the laying down of type III collagen, which initially may account for 30% of the healing wound collagen. Gradually, this is replaced by type I collagen and by the second week, type I production is predominant again. Both type I and III collagen are produced during wound healing, but it is the ratio of their production that determines the proportion of collagen type. Net collagen accumulation peaks at around the third week after injury. Throughout the rest of the remodeling stage, collagen is produced at elevated rates without an overall net increase. This is due to collagen production being balanced by degradation.10,11
Chronic wounds have decreased levels of growth factors, display abnormal ECM function and poor blood supply; in addition, they show increased levels of the inflammatory interleukins and tumor necrosis factor (TNF), which prevents the start of the proliferative stage of healing and can hinder the remodeling process. An overview of the key contributing cells and factors involved in wound healing is presented in Table 1.
Table 1.
Key contributing cells and factors involved in the phases of wound healing
| Haemostasis | Inflammation | Proliferation | Remodelling |
|---|---|---|---|
| Typical timing | |||
| Hours | 4–5 days | Till 14 days | Lasts 12–18 months |
| Key contributing cells5,12 | |||
| Keratinocytes | Neutrophils | Macrophages | T-lymphocytes |
| Endothelial | Monocytes | Fibroblasts | Fibroblasts |
| Platelets | Macrophages | Myofibroblasts | Myofibroblasts |
| Endothelial cells | T-lymphocytes | ||
| Fibroblasts | |||
| Key contributing cytokines4,13 | |||
| IL-1 | EGF | EGF | TGF-β |
| TXA2 | PDGF | VEGF | PDGF |
| TGF-α | TGF-β | TGF-β | IGF |
| TGF-β | FGF | PDGF | |
| PDGF | IFN-α | FGF | |
| EGF | TNF-α | IL-6 | |
| VEGF | IL-1 | ||
| FGF | IL-8 | ||
| IL-10 | |||
FGF, fibroblast growth factor; IGF, insulin-like growth factor; IL-1, interleukin 1; PDGF, platelet-derived growth factor; TGF, transforming growth factor; TNF, tumor necrosis factor; TXA2, thromboxane; VEGF, vascular endothelial-derived growth factor.
Wounds in the clinical setting
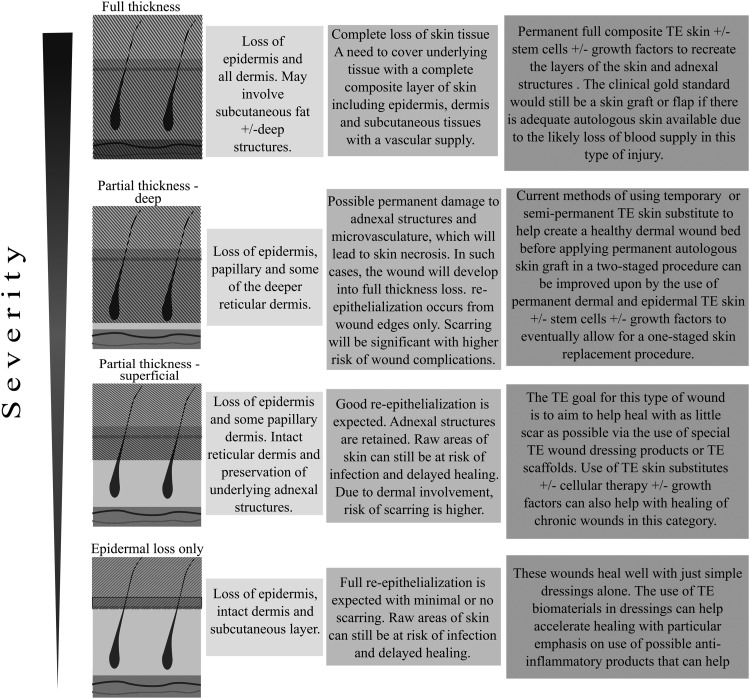
There have been significant advancements to the manufacturing of wound care products over the past few decades. To be able to maximize healing potential through the choice of management options, it is important to have an understanding of different wound types and the pathophysiology of wound environments. Figure 2 broadly defines the different types of wounds seen in a clinical setting.14 The most common types of chronic wounds being treated include leg ulcers of vasculopathic and diabetic origin, pressure ulcers, and surgical or traumatic wounds.1 It is well established that some patient characteristics predispose them to delayed wound healing. Local factors include oxygenation, infection, foreign bodies, or venous disease. Important systemic factors are age, stress, ischemic factors, obesity, immunosuppression, smoking, and nutrition.15 Certain co-morbidities have also been shown to be independent risk factors for developing open wounds or ulcers.
Figure 2.
A basic diagrammatic representation of different wound types, the treatment challenges, and possible tissue engineering solutions in relation to the varying wound severities.14 The hatched shading over diagrammatic skin layers represents tissue loss.
We have overviewed wound type by severity, and we have listed the specific challenges that each type of wound exhibits and the strategies used to overcome these (Fig. 2).14
There are several approaches to wound management in the clinical setting and to consider each one in detail would go beyond the scope of this article. As this article is the most concerned with current tissue-engineered strategies for wound healing, the use of skin substitutes will be discussed. Skin substitutes can be used either alone or as an adjunct to skin grafting for wound coverage, depending on which layers of the skin the product is designed to support. Horch et al. have described three types of skin substitutes that have been classified according to their relevant biological action in patients.16 These were historically developed from how surgeons treated wounds in clinical practice, and they are summarized in Table 2.
Table 2.
The classification of skin substitutes according to their biological actions
| Types of Skin Substitutes | Definition |
|---|---|
| Temporary | Materials that can be placed on a fresh wound (usually partial thickness) and left until healed |
| Semi-permanent | Materials that are left attached to the excised wound, and eventually added on with autologous skin grafts in a two-staged surgical procedure. |
| Permanent | Incorporating an epidermal analogue, dermal analogue, or both as a permanent skin replacement solution |
Adapted from Horch et al.16
The relevance of this table is seen in how the different commercially available skin products are aimed at different types of wound treatments (Fig. 2 and Table 3). This distinction is particularly helpful for researchers in this field to be able to tailor tissue-engineered products to the required patient groups.
Table 3.
Current available commercial tissue-engineered therapies for wound healing
| Commerical Products | Product Composition |
|---|---|
| Acellular products | |
| Biological matrix | |
| Promogram™ (Acelity L.P., Inc.) | Oxidized regenerated cellulose and collagen |
| Puraply® (Organogenesis, Inc.) | Porcine-derived type I collagen |
| MatriDerm® (MedSkin Solutions Dr. Suwelack AG) | Bovine collagen fibrils with elastin |
| Tisseel® (Baxter International, Inc.) | Fibrin |
| Beriplast P (CSL Behring) | Freeze-dried fibrinogen-factor XIII and thrombin |
| EVICEL® (Ethicon) | Fibrin |
| Hyaff® (ATGmed–AT Technologies GmbH) | Hyaluronic acid |
| Hycoat® (The Hymed Group) | Sodium hyaluronate |
| Synthetic/biosynthetic matrix | |
| Integra® (Integra Life Sciences Corp.) | Bilayer matrix bovine collagen and silicon |
| Hyalomatrix® (Anika Therapeutics) | Silicon membrane bound to hyaluronic acid |
| Biobrane® (Mylan and Smith & Nephew) | Silicon membrane bound to porcine collagen-coated nylon mesh |
| Suprathel® (Polymedics Innovation) | D.Lactide trimethylene carbonate and epsilon-capronolactone membrane |
| Terudermis (Olympus Terumo Biomaterial Corp.) | Bovine dermal cross-linked atelocollagen with or without silicone |
| Pelnac (Gunze Ltd., Medical Materials Centre) | Procine tendon-derived atelocollagen type I with or without silicone film |
| Biologically processed matrix | |
| OASIS Wound Matrix® (Cook Biotech, Inc.) | Acellular procine small intestinal submucosa |
| PriMatrix® (Integra Life Science Corp.) | Acellular fetal bovine dermis |
| MatriStem® (ACell, Inc.) | Acellular porcine urinary bladder |
| SurgiMend® (Integra Life Science Corp.) | Acellular fetal or neonatal bovine dermis |
| AlloDerm® (LifeCell Corp.) | Acellular human dermis |
| GraftJacket® (Wright Medical Technology, Inc.) | Acellular human dermis |
| DermaMatrix® (Musculoskeletal Transplant Foundation and Synthes CMF) | Acellular human dermis |
| EZ Derm® (Mölnlycke Healthcare, LLC) | Acellular silver-impregnated aldehyde cross-linked porcine dermis |
| Amnioexcel (Derma Sciences, Inc.) | Dehydrated amnion-derived tissue |
| Biovance® (Alliqua BioMedical, Inc.) | Dehydrated amnion-derived tissue |
| Grafix® (Osiris Therapeutics, Inc.) | Cryopreserved amnion-derived tissue |
| Epifix®/EpiBurn® (MiMedx Group, Inc.) | Dehydrated and sterilized human amnion/chorion tissue |
| Cellular products | |
| Epidermal products | |
| Epicel® (Genzyme Tissue Repair Corporation) | Cultured autologous keratinocytes |
| Keratinozyten Sheets (DIZG) | Cultured autologous keratinocytes |
| ReCell® (Avita Medical) | Autologous epidermal cells in liquid suspension |
| MySkin (Celltran Ltd.) | Cultured autologous keratinocytes on membrane |
| Laserskin®/Vivoderm® (Fidia Advanced Biopolymers) | Cultured autologous keratinocytes in laser-perforated hyaluronic acid |
| Dermal/epidermal-dermal (composite) products | |
| Theraskin (Soluble Systems LLC) | Human allogeneic split-skin graft, including keratinocytes and fibroblasts |
| Dermagraft® (Organogenesis, Inc.) | Neonatal foreskin-derived fibroblast seeded in polyglycolic acid or polyglactin-910 mesh |
| Apligraf® (Organogenesis, Inc.) | Bilayered human neonatal epidermal keratinocytes and neonatal foreskin-derived fibroblast seeded in bovine collagen matrix |
| StrataGraft® (Stratatech Corp.) | Bilayered human dermal fibroblast and human keratinocyte-derived fully stratified epidermis |
| Growth factor products | |
| Regranex® (Ortho-McNeil) | Human recombinant platelet-derived growth factor |
| Autologel® (Cytomedix, Inc.) | Platelet-rich plasma |
The challenge of generating tissue repair is, therefore, the ability to regenerate native tissue in a manner that allows for the restoration of function to the lost tissue in both acute and chronic wound settings. Tissue engineering can provide the necessary ingredients to replicate tissue via the use of three central components, scaffolds, cells, and growth factors, to develop three-dimensional (3D) structural units that aim at restoring the function to cutaneous tissue.17 Strategies mainly involve covering wounds with native matrix and/or polymer scaffold dressings, injection of cells directly to the wound site, or cell encapsulation within materials that can then be implanted.
In the next few sections, we review the key strategies in the use of tissue engineering in cutaneous wound healing, including scaffolds, cellular therapies, and growth factors, to create biological skin equivalents. We also aim at addressing the current challenges and future implications to the ever-evolving scientific research and technology.
Systematic Literature Review Methodology
To summarize the key research avenues that are currently being explored for addressing cutaneous wound healing, we have performed a systemic search of the available literature, with particular focus on the use of cells and scaffolds in animal wound-healing models. The final search term was chosen based on the raw number of hits and the proportion of experimental versus reviews or opinion papers. The search terms used were (Scaffold OR “Mesenchymal stem cell” OR Biomaterial OR “cell based therapy”) AND (“Diabetic wound” OR “skin wound”) AND (“ischemia” OR “hypoxia”). The search was performed in Google scholar with restrictions on date from 2006 to 2016, and on English language papers only. The first exclusion pass was based on only the title. Papers were excluded if they were found to meet any of the following criteria:
• Review papers.
• Abstract only.
• Unable to retrieve a full copy of the paper.
• Non-cutaneous wounding.
• Non-wound healing
• Non-English.
The initial search returned 3,901 papers. After the first pass, this was reduced to 238 papers. After the first pass, the remaining papers were reviewed for scientific quality and variables of interest.
A set of variables related to methodology and results were created, and any papers for which two or more of the chosen variables could not be extracted were excluded. After the second pass, 121 papers remained for comparative review.
The variables assessed in this article were chosen with an initial aim of performing a meta-analysis of the effectiveness of various scaffold-/cell therapy-based treatments and of highlighting promising research avenues. Despite many high-quality individual papers reporting significant results on the basis of well-constructed experimental procedures, the heterogeneity in approaches and protocols prevents meta-analysis of the data.
Despite being unable to perform meta-analysis of wound-healing efficacy, many of the individual works still report findings that are of crucial importance to the progression of the wound-healing field. Some of these are highlighted in the relevant sections of this article.
Discussion
Experimental approaches
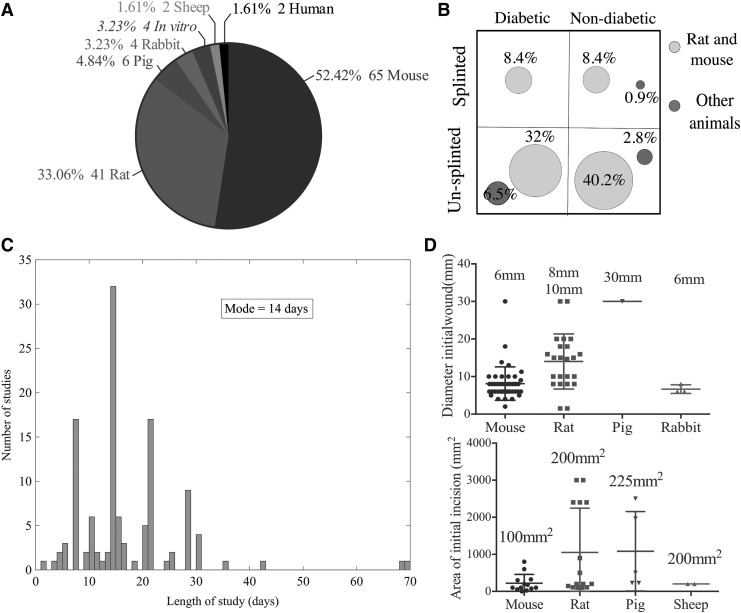
The heterogeneity and the lack of standardized protocol/approach to the reporting of results frustrated any meta-analysis of the systematic review data. This problem is well recognized and reported in other literature surveys involving in vivo animal studies.18–20 One such instance found was the lack of a standardized method or calculation for measuring the size of the wound and reporting the rate of healing. Another discrepancy of approach was the use of splints to prevent wound closure by contraction. This is of particular importance in rodent studies in which wound healing occurs predominantly via contraction rather than epithelial migration as it does in human wound healing.21 Great heterogeneity can also be found in the type of animal model used and in the use of diabetic or immunocompromised strains. Faster research progress in the field could be achieved by a more standardized approach to animal model use, which would allow for large meta-analyses. We report data gathered from the literature review to allow future researchers to standardize their methodology against the consensus in the field, where appropriate. Data are shown in Fig. 3 for the following: the proportions of different animal models, the use of splints with and without immunocompromised animals, the initial wound sizes created, and the length of the study.
Figure 3.
(A) Pie chart showing the number of studies performed in the various animal models. (B) The percentage of studies employing splints and either diabetic strains or inducing diabetes in the animal before wounding. (C) The number of days over which animal wound healing is measured, modal value found to be 14 days. (D) Top panel shows the diameter of the circular wound for various animal models; bottom panel shows the area in the case of rectangular wounds.
Role of cells in wound healing
Endogenous stem cells feature predominantly in the complex and coordinated signaling cascades of wound healing. The most abundant skin stem cells are the adnexal structures, particularly the hair follicle bulge stem cells, which represent the best characterized epidermal stem cell population. There are other stem cell populations described in the interfollicular epidermis and sebaceous glands22 (Fig. 1). Hair follicle bulge stem cells are the most commonly characterized by expression of Keratin 15, although other markers, including Lgr6 and MTS24, have been more recently identified.23 The seminal work of Ito et al. demonstrated that new skin cells arose from hair follicle bulge stem cells that had migrated to the epidermis after damage.24 Since this work, many other researchers have used a wider variety of markers to demonstrate the presence of these cells in the epidermis long after wound healing.25 However, recent controversy has emerged over the time course of the hair follicle bulge stem cells' involvement in wound healing. Langton et al. have shown that in the absence of these stem cells, the initial wound healing rate (4 days) is significantly reduced26; whereas more recently, Garcin et al. showed that these cells may, in fact, be excluded from the early stages of wound healing for excisional wounds.23 In the case of burns, the hair bulge's regenerative function has been particularly noted: Superficial burns, which leave the structures intact (Fig. 2), heal rapidly and regenerate epidermal appendages. With more severe burns in which the hair bulge is affected, the regenerated skin shows scarring and lacks adnexal structures.27
The use of cultured epithelial autografts (CEA) for the treatment of burns has been used to treat cutaneous defects since it was described by Green et al. in 1979.28 This was based on the hypothesis that delivery of the CEA would deliver the inherent skin stem cell population and would enhance wound healing. However, researchers were quick to realize that applying CEA alone into wounds did not achieve good clinical results.29 Clinically, they were cumbersome to use and patients experienced poor quality of healing with frequent blistering and wound contractures months after grafting.30,31 Since then, studies have shown that providing matrix support and a delivery system for CEA improves its in vivo success, leading to the development of bio-engineered cultured skin substitutes.32,33 There is still a role for cellular therapies, such as the commercially available Epicel® (Genzyme), through the instant replacement of lost cell mass in difficult-to-treat wounds, although its efficacy and economical benefits compared with other advanced therapies has yet to be determined.34
From our analysis of the literature, cellular therapies utilized a variety of cell sources. The predominant cells were stem cells (81.9%), which included bone marrow, adipose-derived stem cells, as well as umbilical cord, and Wharton's jelly mesenchymal stem cells (MSCs). Fibroblasts were the next most common cell type (7.1%). Table 4 lists all cell sources found in the reviewed literature. To understand the progression of various cellular therapies currently used for wound healing, the difference between stem cells and differentiated cells needs to be first appreciated. Differentiated cells, such as fibroblasts and keratinocytes, form the basis of commercially available autologous and allogeneic cell-based products. Some products have been on the market since the late 1990s. Their role in skin substitute products, such as Dermagraft® (Organogenesis, Inc.) and Apligraf® (Organogenesis, Inc. and Novartis), is to provide the necessary materials for wound closure via the laying down of matrix proteins and the production of growth factors.35,36 This stimulates healing by promoting host cell migration and infiltration, as well as neoangiogenesis into the wound bed, thereby enhancing rapid re-epithelialization and closure of the wound. However, there are inherent disadvantages with using allogeneic products. Although the risk is very low, there is a possibility of disease transmission and graft rejection.37 Conversely, it has been shown in several studies that allogeneic-differentiated cells delivered via the biological skin substitute Alipgraf® do not persist in the wound site beyond 6 weeks, which may explain why rejection is not commonly reported in literature.38,39 Despite such theoretical benefits, researchers and clinicians still experience limited success with its use. Reported clinical trial studies, using differentiated cell-based products, have shown a collective success rate of 35–56% in wound closure, leaving approximately half of the wounds ineffectively treated and vulnerable to the risk of infection and other complications.40 This has prompted researchers to consider stem cell-based therapies as a possible solution to further improve wound healing.
Table 4.
Cell types used in the studies systematically reviewed
| Cell Type | Number of Studies | Reference |
|---|---|---|
| Bone marrow-derived stem cells | 31 | 41–69 |
| Adipose-derived stem cells | 16 | 54,65,66,70–81 |
| Endothelial cells/endothelial progenitor cells | 5 | 53,75,82–84 |
| Umbilical cord MSCs | 4 | 65,85–87 |
| Wharton's jelly MSCs | 4 | 88–91 |
| Fibroblasts | 3 | 66,92,93 |
| Keratinocytes | 2 | 94,95 |
| Circulating cells | 2 | 96,97 |
| Skin-derived stem cells | 1 | 98,99 |
| Stromal vascular fraction | 1 | 100 |
| Amniotic fluid stem cells | 1 | 101 |
| Pluripotent stem cells | 1 | 102 |
| Human urine-derived stem cells | 1 | 103 |
| Myeloid cells | 1 | 104 |
| Pancreas or submandibular-derived stem cells | 1 | 105 |
| Endometrial regenerative cells | 1 | 106 |
MSC, mesenchymal stem cells.
Stems cells' capacity for self-renewal and their inherent clonogenicity and potency make them fundamental in the healing and regeneration of bodily tissues. From the wound-healing perspective, stems cells have the potential of correcting the biological deficiencies in chronic wounds, thereby offering the potential of complete skin regeneration, including the restoration of the skin appendages. Although embryonic and induced pluripotent stem cells have the most valuable potency of all cells to differentiate and regenerate, the ongoing issues around the ethics and safety of their use have prompted researchers to focus more on the other stem cell populations, such as MSCs instead.
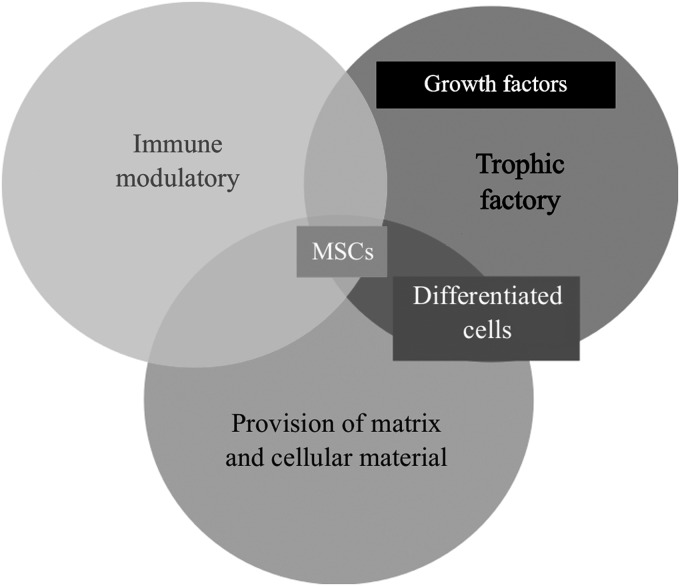
The delivery of MSCs to wounds is gaining popularity in this field. There are distinct advantages of the use of MSCs over differentiated cells. They are known to possess beneficial immunomodulatory effects, such as immune-suppressive and immune-privilege functions, theoretically making their allographic uses more suitable from that respect.107 They also have a strong trophic capability of releasing the necessary pro-regenerative cytokines and growth factors for regeneration,108,109 and their ability to differentiate provides a potential cell source for native tissue restoration (Fig. 4). All these help to provide further building blocks to the healing process.110
Figure 4.
Mesenchymal stem cells possess the right characteristics for use in tissue regeneration of skin.
Using a porcine model, Mansilla et al. investigated the use of bone marrow-derived MSCs (BMMSCs) seeded on an “intelligent” acellular dermal matrix in burns and found total regeneration of wounds with little scarring, including the regrowth of hair follicles as well as burned muscle and even ribs.111 Li et al. and Kataoka et al. also reported similar skin appendage formations on the addition of BMMSC in rat and mouse models, respectively. Promisingly in both studies, the labeled MSCs were found within regenerated hair follicles, sebaceous glands, and dermis, demonstrating MSCs' innate ability to contribute functionally to the wound-healing process.112,113 Conversely, there is also a growing body of evidence showing that the therapeutic effect of implanted MSCs comes from the release of necessary secretomes rather than long-term contribution to the structure and transdifferentiation.114–116 What is clear, however, is the fact that MSCs have the right regenerative characteristics and potential to improve wound healing where differentiated cells are not implanted. A direct comparison of the role of stem cells to commercially available differentiated cells would be helpful to both researchers and clinicians, but studies in this field are currently lacking. Interestingly, stem cells have been shown to display cellular cross-talk with differentiated cells when co-cultured together, improving and enhancing the therapeutic potential in wound healing. Aoki et al. demonstrated how BMMSCs interacted with keratinocytes such that rete ridge-like structures were created in the regenerated epidermis in its presence, indicating the benefits of cellular diversity within the wound-healing environment.117 However, there exists conflicting evidence on the importance of these cellular interactions, as shown in a study by Rodriguez-Menocal et al.118 Dose-dependent effects were reported in their in vitro study, where it was shown that higher levels of co-cultured MSCs inhibited fibroblast migration whereas lower doses of MSCs actually enhanced fibroblast migration patterns. Therefore, a better understanding of co-culture cellular dynamics, especially with the use of stem cells, is needed, and further research will be needed to address this void.
Delivery systems
There are four main mechanisms through which cells are delivered to wounds in vivo. These include delivery through topical spray, direct injection, systemic delivery, and cell-seeded scaffolds. Each delivery mode has its own advantages. The topical spray and direct injection are easy to administer but difficult to localize cells in the long term, as cells can “escape” the delivery site. As cells are not encapsulated within a material or matrix, these cells are also considered non-protective.110 With a systemic delivery of cells, there is reason to believe that stem cells, in particular, may “home-in” on the wound site; however, this is not certain and the localization of other cells at the wound is unlikely. Generally, it is accepted that 3D scaffolds (either native matrix rich or polymer) afford cells within them a protective environment and enable one to localize cells to a wound site.110 Modification of scaffolds' material properties, including degradation times, stiffness, porosity, and incorporation of growth factors and/or drugs, is also possible.
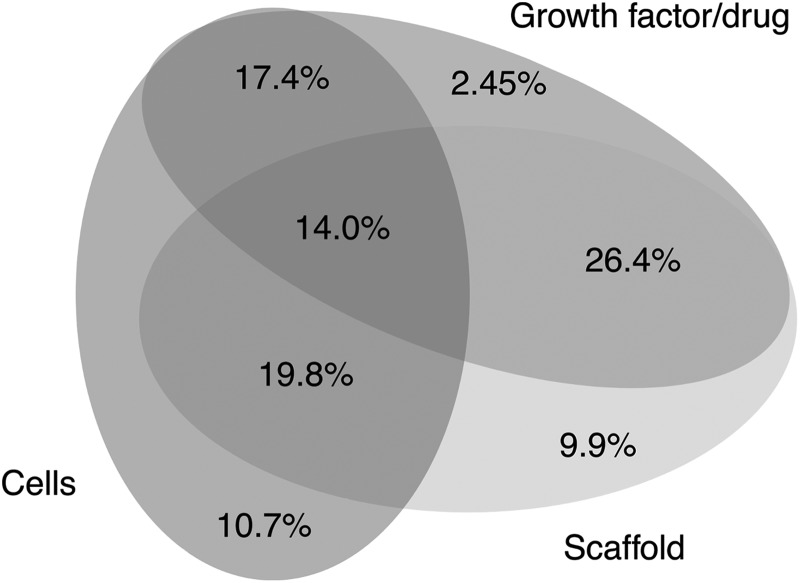
There is an emerging appreciation of supporting the stem cell niche in its 3D state, as it is known that maintenance of stem cell pluripotency or multipotency and differentiation are associated with specific microenvironmental cues. These niches provide a set of unique and specific features that help to maintain multipotency proliferation and differentiation and regulate stem cell maintenance. The specific microenvironmental features include chemical signals (including growth factors), cell–cell adhesion and interactions, cell–matrix attachment, mechanical features, stiffness, and oxygen environment.119,120 There is, thus, a need to understand which features can influence specific stem cell behaviors that we may wish to manipulate, in this case related to the wound-healing process. The systematic review data reflect the growing awareness of the importance of the biomimetic niche over the past 10 years. Figure 5 shows the relative proportions of studies that utilize scaffolds, cells, and growth factors and the combination thereof.
Figure 5.
Representation of the number of studies that adopt various approaches to wound healing. Percentage of studies applying either of the following: cells directly to the wound, growth factors directly, or a scaffold, as well as those that combine these approaches, that is, scaffold with encapsulated cells, scaffolds with encapsulated growth factors, and all three combined or cells with additional growth factors.
The importance of scaffolds in wound healing
As discussed earlier, cells in vivo reside within distinct microenvironments, which help to direct cell function, state, and signaling. Such microenvironments are critical to wound healing; therefore, it is crucial that any cell-based therapies support new or host cell populations by providing a suitable microenvironment. Only in such cases will cells contribute maximally to tissue regeneration and repair. Culturing cells within a 3D environment such as a scaffold is a method that aids in the creation and maintenance of specific microenvironments or biomimetic niches. In the case of delivering cell-seeded scaffolds into wound defects, this can allow for new tissue genesis.110 This greater appreciation of the native 3D microenvironment has brought about the emergence of biomimetic scaffolds that aim at creating a biomimetic cell niche, with particular attention to the stem cell niche. Biomimetic tissue-engineered scaffolds make use of biomaterials that mimic one or multiple characteristics of the native ECM.121 This can be in the form of its biodegradability, mechanical properties, matrix composition, and/or architecture. There are two main types of tissue-engineered scaffolds: biological and synthetic.
Biological scaffolds can exist as organic molecular polymer or as matrix protein, such as collagen and hyaluronic acid. These scaffolds tend to contain a maximum of three matrix components, so they are relatively simple in terms of composition. There is also the use of decellularized (acellular) allogeneic or xenographic-derived dermal matrices (artificial dermal matrix [ADM]), which are complex in terms of matrix composition and architecture. Using an ADM as a biological modulator is specifically believed to interrupt the continuous inflammatory process that is characteristic of chronic wounds and in so doing to lead to angiogenesis, cell infiltration, and re-epithelialization.122 However, only small numbers of good-quality clinical trials exist for supporting the usage of ADMs in chronic wounds, and the exact mechanism of action is not fully understood.122–124 The main processes used to decellularize tissues include detergents, hypo- and hypertonic solutions, enzymes, and chelating agents.124 The process by which tissues are decellularized is critical, as it determines (1) retention of matrix proteins, (2) maintenance of matrix architecture, and (3) retention of growth factors sequestered within the matrix. Overall, it seems that the decellularized acellular dermis potentially provides the most biomimetic matrix to host the all-important cellular niche for tissue engineering purposes.
Commercial wound dressing products commonly use molecular polymers and matrix proteins. Research on tissue-engineered wound-healing products has mostly moved away from the use of single biomolecular agents on wounds, favoring a more sophisticated biologically processed acellular matrix to provide the necessary ECM template for wound healing (Table 1). They can now be found in combination with other biomaterials, such as Apligraf (Organogenesis, Inc.), or as carriers for delivery of cellular products, such as Laserskin®/Vivoderm® (Fidia Advanced Biopolymers/ER Squibb & Sons, Inc.), to create more complex tissue-engineered products for wound healing.
An advantage of synthetic scaffolds is that they can be customized for purpose in a controlled environment to mimic the tissue architecture of interest. Another major advantage with synthetic scaffolds is the possibility of mass production, facilitating the key goal of providing a point-of-care product in tissue-engineered wound care. First described by Yannas and Burke in 1980, the design and use of artificial skin has been an evolving science.125
Currently, opinions in such research are turning toward the use of both organic and inorganic composite materials that are combined together to create a hybrid scaffold for skin tissue engineering.126 Organic scaffold components address the need for biological proteins to provide a favorable environment for cells to proliferate and differentiate; whereas inorganic components may facilitate manufacture process and quality control.
It is important to emphasize that scaffolds used alone without the addition of any other cellular or bioactive molecules only promote healing via secondary intention. Therefore, the scaffold influence is to mainly assist the in vivo host response in wound repair by provision of the right environment for cell and tissue adherence. Hence, these products are typically used in combination with the conventional gold-standard split-thickness skin grafts.
In addition, there is increasing use of scaffold-based delivery systems for stem cell transplantation over other delivery modalities. The driving hypothesis here is that the 3D environment provides cells with the necessary protection and matrix spatial cues for the seeded cells during the delivery process.110 There are also key advantages in the use of cell-seeded scaffolds over cell-only therapies in wound healing, especially for larger wounds. Three-dimensional scaffolds provide greater coverage as well as help to maintain the integrity of tissue architecture during wound healing.
Scaffolds may be engineered in a variety of ways. Despite decellularized skin being the most biomimetic of biological scaffolds, limited availability of donor skin and shelf life of such products would deny it from being a solution to the growing demand for tissue-engineered skin products. More recently, researchers have been turning to the use of 3D bioprinting technologies as another viable option for skin replacement.127–131 Bioprinting allows for precise and predefined positioning of living cells and other biological material, enabling the manufacture of customizable tissue constructs based on computer-generated designs.132 Bioprinting is a layer-by-layer process; hence, it allows for the creation of smaller functional tissue units that are made up of cells that can subsequently be assembled together like building blocks into larger, more complex organs.132 The skin as an organ, having a layered structure, is highly suited to this printing technology. Mini-tissue blocks with functional units, such as adenexal structures, can technically be recreated by using 3D bioprinting and using the relevant skin cell types.133 Among all the successful in vivo models, Cubo et al. have successfully bioprinted and transplanted human skin made up of cells from skin biopsy and have shown the resultant regenerated skin to be histologically similar to that of normal human skin.130 Hence, it is no surprise that the feasibility of this technology has caught the attention of the commercial industry, with bioprinting company Organovo collaborating with cosmetic giant L'Oreal US to invest and research into the bioprinting of skin in 2015.134 Despite its great potential, this field is still very much in its infancy and will require a few more years of fine tuning of its technology before it finds its way to the bedside.132
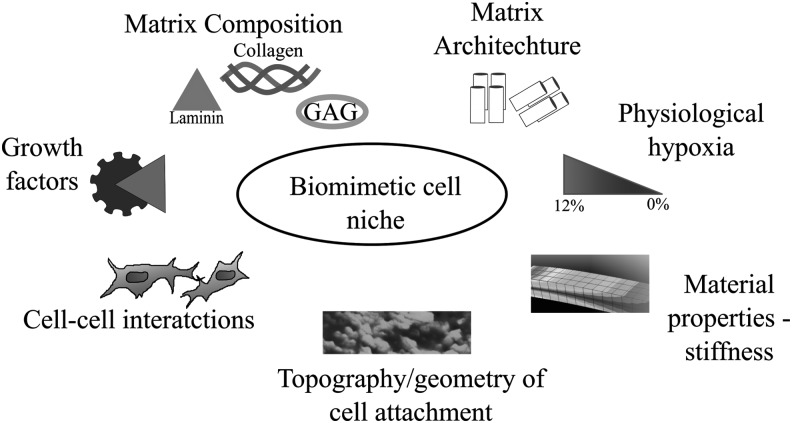
For all scaffolds, the manufacture process must take into account the specific tissue microenvironment that the scaffold aims to mimic. Mechanical, physical, and biochemical modifications are often introduced and applied to scaffolds to enhance their wound-healing potential.16 Figure 6 depicts seven features of the cellular microenvironment that should be considered when targeting a biomimetic cell niche.135
Figure 6.
Overview of a number of cell-scaffold interactions to re-create elements of the biomimetic niche. Re-capitulating elements of the biomimetic niche helps to direct cell behavior, response, and fate.135
Material properties
Recent studies have shown that the strength and type of intermolecular bonds within scaffolds may affect cell proliferation and differentiation.136 Work has also been done to show that the stiffness of the matrix in which cells are seeded can direct lineage specification.137 Where these cells are seeded in a “soft matrix” (Ebrain ∼0.1–1 kPa) they commit to a neurogenic lineage, compared with being seeded on a stiffer matrix (Emuscle ∼8–17 kPa) where MSCs commit to a myogenic lineage.137 It is imperative that where stem cells are being delivered to wounds within matrices, this aspect is considered, as MSC fate is influenced by a host of microenvironmental features and triggers.
Matrix composition
There has been much work done on the importance of integrin-mediated adhesion for stem cell maintenance. Although functional roles for β1 integrin are not completely understood in terms of stem cell maintenance, they are expressed across human MSCs of three different tissue origins, including bone marrow.138 α6β1 is an integrin that is associated with attachment to laminin, and the addition of laminin to scaffolds can enhance the ability of endothelial cells to fuse to form tube-like structures.139 Collagen/laminin scaffolds were used to deliver MSCs to an in vivo diabetic wound model, with significantly enhanced wound healing compared with collagen I only.59 This suggests that variations in MSCs' surface integrin expression and the ability to change integrin expression in 3D matrices can direct their behavior. More so, this highlights the importance of an appropriate choice of ECMs for a given cellular population.138 Within the literature reviewed here, a wide variety of scaffold materials were found, with 47.9% of studies using a natural scaffold material, 9.2% using a synthetic material, and 11.6% using a hybrid of synthetic and natural materials. The three most widely used materials were collagen or collagen:chitosan, fibrin, and alginate.
Topographic cues
There is an increasing body of work that shows that the topographic pattern onto which cells are seeded in vitro can direct stem cell behavior, including differentiation and commitment to different lineages.140 It has been found that cells attach to different nano-topographical features on material surfaces with specific integrin receptors and focal adhesions, which can contribute to cell fate through changes in both cell morphology and biochemistry.140
Hypoxia
Cells cultured in vitro are, in the main, exposed to atmospheric O2, which is far higher than physiological hypoxia, which ranges between 1% and 9%, and in some cases can be as low as 0%.120,141 It is known that oxygen tension can directly affect cell proliferation and differentiation, especially for BMMSCs.142 It is also appreciated that a hypoxic environment, even less than 1% oxygen, is critical for the maintenance of stem cell pluripotency and quiescence.143
Growth factors
Scaffolds have also been shown to be good vectors for delivery of growth factors and other necessary biomolecules that are beneficial to wound healing, such as anti-inflammatory and antioxidant substances. Growth factors are indicated in all tissue-healing cascades. They are key in coordinating the biological signaling component for cell function and tissue regeneration. They represent biological materials that can potentially be used to target distinct wound-healing phases. A variety of growth factors that attempt to target one or several of these phases have been investigated as found through the literature review conducted here and are summarized in Table 5. VEGF, stromal-derived growth factor 1-α (SDF-1-α), and FGF are the three most widely used factors.
Table 5.
Summary of all growth factors used in the studies systematically reviewed
| Growth Factor Drug/Growth Factor | Number of Studies | References |
|---|---|---|
| SDF-1-alpha | 8 | 42,50,51,65,74,102,144,145 |
| VEGF | 8 | 65,83,102,146–150 |
| bFGF/FGF | 11 | 55,65,83,102,147,151–156 |
| Human epidermal growth factor | 4 | 157–160 |
| Neutrophin-3 | 1 | 161 |
| Angiopoietin-1 | 1 | 43 |
| Conditioned media | 6 | 85,87,89–91,161,162 |
| Glucose oxidase | 1 | 163 |
| Platelet-rich plasma/platelet lysate | 4 | 46,164–166 |
| Hepatocyte growth factor | 1 | 166 |
| Platelet-derived growth | 4 | 55,65,83,167 |
| TNF | 2 | 65,102 |
| Thrombin | 1 | 168 |
| Substance P | 2 | 84,169 |
| Other | 14 | 45,56,72,84,97,102,103,152,170–175 |
bFGF, basic fibroblast growth factor; SDF, stromal-derived growth factor.
However, given the complexity of the wound-healing process, the exogenous use of growth factors does not usually produce results of satisfactory wound healing due to difficulties with mimicking the specific endogenous growth factor production. One effort to replicate this complexity is to control the release of the growth factor to create a more biomimetic environment and a longer-lasting therapeutic effect.135,176
There are two primary ways in which the release from scaffolds can be modulated, either by altering the porosity or surface area of the scaffold to control the rate of diffusion of the factors through and out of the scaffold or via chemical binding of the factor to the scaffold. In the former case, the rate of release of a factor depends on the factor's diffusivity through the scaffold, the relative concentration of the factor within the native tissue, and the rate of uptake or utilization of the factor by cells.
Control of the scaffold porosity is often done by altering the concentration of the scaffold's fibrous components. This effect is consistent across many types of scaffolds and growth factors, for example, for release of SDF-1 from a poly (polyethylene glycol citrate-co-N-isopropylacrylamide) (PPCN) scaffold,145 increasing the concentration of the polymer slows the release of SDF-1-α into the surrounding media. Similarly, increasing the chitosan proportion in a collagen:chitosan sponge produces a slower release of thymosin-β-4.170 In addition to controlling the initial scaffold density, controlling the scaffold's surface area will not only affect the diffusion of the factors through the scaffold surface but has also been shown to affect the scaffold's degradation rate. As the scaffold degrades, factors will be released at a greater rate. By controlling the degradation rate, the release of the scaffold may also be controlled. Fibrin microspheres within a fibrin scaffold described by Kulkarni et al.177 show some spatiotemporal control over the release of two factors through exploiting the differing degradation rates of microspheres as compared with larger fibrin gels. Although such approaches represent a highly interesting avenue of research, the temporal control over the release of such factors with these methods is highly simplified by a comparison to those released via cellular mechanisms.
The alternative method to controlling factor release is to bind the growth factor chemically or electrostatically to the scaffold. Two such systems were found in the literature reviewed. Fibrin-binding basic FGF (bFGF) was shown to increase angiogenesis of implanted scaffolds,154 as was fibrin-binding VEGF.149 Another approach is to bind cells into the scaffolds through similar techniques. Wang et al.172 utilized a collagen scaffold with a collagen-binding peptide with an affinity for MSCs. Using a porcine model, the authors report an increase in wound closure rate for the binding scaffold as compared with a non-binding scaffold as well as increased cell retention.172
Architecture
Tissue architecture dictates physical properties of tissues. For example, the orthogonal pattern of collagen fibrils in the cornea confer transparency to that tissue, whereas the parallel array of a bi-modally distributed collagen diameter size confers mechanical strength to tendons in one plane.178 Skin is a meshwork of interwoven ECM proteins that give the skin anisotropic properties as well as its flexibility. In regeneration of the skin, scarring can limit the flexibility of new skin; therefore, strategies to reproduce the normal architectural woven structure of the skin are desirable. This biomimetic feature may be introduced into a scar by grafting de-cellularized skin, with the hope that cells would use the biomimetic cues to align themselves and deposit matrix by using the scaffold cues.179 There is also an emerging view that although tissue architecture is seen as a consequence of cell behavior, mainly deposition of matrix protein and application of strain and tension applied to tissues, it is likely that tissue architecture itself may direct cell behavior and fate.180 So cells within an aligned tissue are likely to stress shield and align along the principal axis of strain. They are also more likely to deposit matrix along this alignment.
The interactions of cells with the native scaffold within tissues can direct and influence cell behavior. It is critical to decipher the specific components of the microenvironment that can successfully deliver cells to an injury site and direct them to aid tissue repair and re-growth.
Future of Tissue Engineering in Wound Repair
The end-goal for tissue engineering in wound repair is to be able to provide patients with high-quality, universal, and “off the shelf” skin substitutes that can regenerate skin in wounds as quickly as possible, with minimum scarring. There are still significant challenges in this rapidly evolving research field to be able to achieve this goal. The skin substitute products currently available to patients can only go so far as to partially replace the skin as a protective barrier. However, functional restoration, such as its innervation, thermoregulation, perspiration, melanin production, and aesthetic appearances, has yet to be achieved by current bioengineering techniques. It seems that the next steps for tissue-engineered skin products involve the marriage between the use of stem cells within a tissue-engineered scaffold to be able to achieve such a full regeneration of skin. Although there are several instances of pre-clinical animal research incorporating such techniques, there have been only a handful of clinical studies looking at the usage of MSCs in combination with a biological scaffold to aid skin wound healing in humans. This is summarized in Table 6.
Table 6.
Summary of the clinical trial data that use mesenchymal stem cells and extracellular matrix scaffolds
| Ref. | n | Wound Type | Scaffold | Cell Source | Treatment | Results |
|---|---|---|---|---|---|---|
| 181 | 1 | Idiopathic lower leg ulcer | Terudermis | Autologous BM aspirate | BM cell suspension in collagen matrix direct to wound. | Good granulation tissue at 2 weeks, at which point STSG with 100% take. |
| 182 | 20 | 4 trauma 2 venous ulcers |
Pelnac | Autologous BM aspirate | 9 cases: MSC+collagen matrix only direct to wound. | 7 healed within 8 weeks; 2 burns patients mostly healed. |
| 3 healed within 3 weeks after application of MSC+matrix; 2 healed after 2 applications. | ||||||
| 4 healed within 8 weeks; 2 died of unrelated pathology before end of study, but partially healed. | ||||||
| 3 burns, 11 decubitus ulcers | 5 cases: MSC+matrix, subsequent STSG. | |||||
| 6 cases: diced FTSG, then MSC+matrix on top. | ||||||
| 183 | 8 | All diabetic foot ulcers | Surgicoll | Autologous BM aspirate | BM cell suspension injected into a debrided wound bed. Suspension, platelet growth factors, and fibrin glue mixture applied and allowed to clot. Suspension-impregnated collagen matrix was placed on top. | 3 patients: complete healing of wound. 5 patients: significantly decreased in size (% decrease in wound area average: 57%; range 24–79% decrease) |
FTSG, full-thickness skin graft; STSG, split-thickness skin graft.
A 2005 study in Japan experimented on a murine model with a bovine-derived collagen sponge (Terudermis) impregnated with a suspension of cells derived from bone marrow.181 The study showed that the rate of angiogenesis in a healing wound was greater in the mice population implanted with collagen matrix containing bone marrow suspension compared with the control group. The study also presented a case report of a chronic leg ulcer that was treated with Terudermis impregnated with autologous bone marrow cell suspension. Two weeks after application, healthy granulation tissue formed and a split-thickness skin graft was performed, with successful outcome at long-term follow-up. Although the paper did not specifically mention MSCs, the BM-derived cell suspension would have contained some. In 2008, Yoshikawa et al. reported the use of artificial dermis (Pelnac) soaked with marrow MSC suspension on 20 subjects with chronic lower limb wounds.182 In nine cases, this composite graft was placed on the wound and allowed to heal secondarily. In five cases, the composite graft was followed by a split-thickness skin graft. Finally, in six cases, diced full-thickness skin graft pieces were placed on the wound before the composite graft was applied. Sixteen of the 20 cases demonstrated complete healing of the chronic defect. The remaining four cases were partially healed, among whom two subjects died before the conclusion of the study. Most recently, in 2011, Ravari et al. published data on eight patients who had chronic diabetic foot ulcers.183 The authors used a very different approach to the studies in Japan and utilized an intensive combined technique. Autologous BM aspirates were injected into a debrided wound bed. A mixture of more suspension, platelet growth factors, and fibrin glue was then applied and allowed to form a clot. Finally, BM aspirate-impregnated collagen matrix (Surgicoll) was placed on top. Three patients had complete wound closure, and the others significantly decreased in size (average wound area decrease was 57%, range 24–79%). Again, the paper did not specifically mention MSCs in the aspirate, but some would have been in the suspension.
There is a paucity of cutaneous wound-healing clinical studies that utilize both BMMSCs and dermal matrices. Preliminary data are from case reports and series, but, nonetheless, they seem to reveal real clinical potential. Further evidence from good-quality, well-powered, double-blinded randomized control trials is needed before its place can be established in the armament of options for chronic wound healing and reconstruction.
However, it is worth noting that there has been some degree of success of MSCs delivery to patients with wounds resulting from peripheral vascular disease, which have been demonstrated in several published clinical trials. In the double-blinded, randomized placebo-controlled trial by Powell et al., they found that an intramuscular injection of patient-specific, expanded bone marrow cells (CD90+ MSCs and CD14+ monocyte/macrophage subset of CD45+ hematopoietic cells) may have resulted in the prevention of wound area doubling, delayed time to treatment failure, and prolonged amputation-free survival in the legs of patients with baseline wounds from critical limb ischemia.184 Clinical trials have also shown that there is an advantage of using bone marrow-derived stem cells compared with bone marrow-derived mononuclear cells with regard to significantly enhancing limb perfusion and ulcer healing rates in patients with diabetes and peripheral vascular disease.185 These studies have found a potential for therapeutic angiogenesis through the use of MSC therapy. Due to the underlying pathology of chronic non-healing wounds, these findings can be easily translatable to the treatment of these wounds and beyond.
Despite such clinical possibilities of therapeutic success, there are still important questions that need addressing with regard to the use of stem cell-based therapies, such as which type of stem cell population would best serve for wound-healing therapies and what are the safety issues that could potentially arise from the autologous compared with allogeneic stem cell use in a clinical setting. There are very few studies addressing these specific issues, thus making it difficult for researchers and clinicians to have absolute confidence in its future clinical applications.40 It is very likely that potential complications and safety issues will surface in literature with time; an example could be seen in a paper published in 2004, almost three decades since the introduction of the use of CEA was advocated. It reported the first case of graft site malignancy in a patient who received CEA to his burns injury more than 13 years ago.186 Five separate localized skin cancer lesions were diagnosed and completely excised in different anatomical distributions of the body previously exposed to CEA, raising the safety concerns of the use of cellular therapies clinically. Although we must take into account that burns injuries themselves carry an innate risk of malignant transformations into squamous cell carcinomas, the author also noted the use of mitogenic stimulators and other chemicals during in vitro expansion, which may contribute to an increased risk of cancer.186 Therefore, such issues must be borne in mind when advocating cellular therapies to patients.
Take-Home Messages.
• Experimental measurements of animal models in wound healing should be standardized to facilitate study comparisons.
• It is important to take into account clinically different wound types when designing and applying tissue engineering strategies to the management of these wounds.
• The most widely used cell types for wound-healing applications are bone marrow-derived stem cells.
• The future of tissue engineering in wound regeneration lies with the use of scaffolds that provide a suitable stem cell environment by mimicking the biological architecture of the skin.
Summary
The field of tissue engineering has come through leaps and bounds over the past decade. There are still many challenges and limitations in the translation of cell therapies for wound healing, such as safety, cost, and efficacy of treatment. The delivery of stem cells in 3D scaffolds to wounds seems to be the most promising approach. It is safe to predict that as our understanding of stem cell biology improves along with technological advancements in bio-scaffold fabrication, the near future will see tissue-engineered techniques become a standard practice for wound regeneration.
Abbreviations and Acronyms
- 3D
three-dimensional
- ADM
artificial dermal matrix
- ADMSCs
adipose-derived mesenchymal stem cells
- bFGF
basic fibroblast growth factor
- BM
bone marrow
- BMMSCs
bone marrow-derived mesenchymal stem cells
- CEA
cultured epithelial autografts
- ECM
extracellular matrix
- EGF
epidermal growth factor
- FGF
fibroblast growth factor
- FTSG
full-thickness skin graft
- GF
growth factor
- hEGF
human epidermal growth factor
- IFN-α
interferon alpha
- IGF
insulin-like growth factor
- IL-1
interleukin 1
- MSCs
mesenchymal stem cells
- PDGF
platelet-derived growth factor
- PPCN
poly (polyethylene glycol citrate-co-N-isopropylacrylamide)
- SDF-1-α
stromal-derived growth factor 1-α
- STSG
split-thickness skin graft
- TE
tissue engineered
- TGF
transforming growth factor
- TNF
tumor necrosis factor
- TXA2
thromboxane
- VEGF
vascular endothelial-derived growth factor
Acknowledgments and Funding Sources
This work was supported in part by the Yale-UCL Medtech Initiative Flagship Project Vascular Engineering award.
Author Disclosure and Ghostwriting
The content of this article was written solely by the authors listed. The authors have no competing financial interests.
About the Authors
Jasmine Ho, MBBS, BSc, MSc, MRCS, is an MRC and Rosetrees Trust funded clinical research fellow at University College London, UK. Her research interests include vascularization of tissue-engineered constructs. Claire Walsh, MSci, MRes, PhD, is a post-doctoral researcher at University College London, UK. Her research interest include computational modeling of tissue-engineered constructs and bubble dynamics. Dominic Yue, MBBS, BSc, MRCS, is a specialist registrar in Plastic and Reconstructive Surgery at the Royal Stoke University Hospital, Stoke-on-Trent, UK. Alan Dardik, MD, PhD, is a professor of surgery and the vice-chairman of the Department of Surgery at Yale University as well as the chief of vascular surgery at VA Connecticut. He is a surgeon-scientist who seeks to understand the molecular mechanisms by which blood vessels, fistulae, and patches that are used to treat patients successfully adapt to their new environment, yet often proceed to failure. Umber Cheema, BSc (Hon), PhD, is a senior lecturer at University College London, UCL Institute for Orthopaedics and Musculoskeletal Sciences, UCL Division of Surgery and Interventional Sciences, UK. Her research interests include engineering vascular networks in 3D-engineered tissues to further understand the role that native matrix density and composition have in this biological process.
References
- 1.Guest JF, Ayoub N, McIlwraith T, et al. . Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open 2015;5:e009283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrientos S, Brem H, Stojadinovic O, et al. . Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen 2014;22:569–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brem H, Maggi J, Nierman D, et al. . High cost of stage IV pressure ulcers. Am J Surg 2010;200:473–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziegler TR, Pierce GF, Herndon DN. Growth Factors and Wound Healing: Basic Science and Potential Clinical Applications. New York: Springer, 2012 [Google Scholar]
- 5.Broughton G, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg 2006;117:12S–34S [DOI] [PubMed] [Google Scholar]
- 6.Minutti CM, Knipper JA, Allen JE, et al. . Tissue-specific contribution of macrophages to wound healing. Semin Cell Dev Biol 2017;61:3–11 [DOI] [PubMed] [Google Scholar]
- 7.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008;8:958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrientos S, Stojadinovic O, Golinko MS, et al. . Growth factors and cytokines in wound healing. Wound Repair Regen 2008;16:585–601 [DOI] [PubMed] [Google Scholar]
- 9.Tracy LE, Minasian RA, Caterson EJ. Extracellular matrix and dermal fibroblast function in the healing wound. Adv Wound Care 2016;5:119–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campos ACL, Groth AK, Branco AB. Assessment and nutritional aspects of wound healing. Curr Opin Clin Nutr Metab Care 2008;11:281–288 [DOI] [PubMed] [Google Scholar]
- 11.Madden JW, Peacock EE., Jr. Studies on the biology of collagen during wound healing. I. Rate of collagen synthesis and deposition in cutaneous wounds of the rat. Surgery 1968;64:288. [PubMed] [Google Scholar]
- 12.Delavary BM, van der Veer WM, van Egmond M, et al. . Macrophages in skin injury and repair. Immunobiology 2011;216:753–762 [DOI] [PubMed] [Google Scholar]
- 13.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83:835–870 [DOI] [PubMed] [Google Scholar]
- 14.Sussman C, Bates-Jensen BM. Wound Care: A Collaborative Practice Manual. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2007 [Google Scholar]
- 15.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res 2010;89:219–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horch RE, Kopp J, Kneser U, et al. . Tissue engineering of cultured skin substitutes. J Cell Mol Med 2005;9:592–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee KH. Tissue-engineered human living skin substitutes: Development and clinical application. Yonsei Med J 2000;41:774–779 [DOI] [PubMed] [Google Scholar]
- 18.Isakson M, de Blacam C, Whelan D, et al. . Mesenchymal stem cells and cutaneous wound healing: Current evidence and future potential. Stem Cells Int 2015;2015:831095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilkenny C, Parsons N, Kadyszewski E, et al. . Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS One 2009;4:e7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hooijmans CR, Leenaars M, Ritskes-Hoitinga M. A gold standard publication checklist to improve the quality of animal studies, to fully integrate the Three Rs, and to make systematic reviews more feasible. Altern Lab Anim 2010;38:167–182 [DOI] [PubMed] [Google Scholar]
- 21.Falanga V, Iwamoto S, Chartier M, et al. . Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 2007;13:1299–1312 [DOI] [PubMed] [Google Scholar]
- 22.Taylor G, Lehrer MS, Jensen PJ, et al. . Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 2000;102:451–461 [DOI] [PubMed] [Google Scholar]
- 23.Garcin CL, Ansell DM, Headon DJ, et al. . Hair follicle bulge stem cells appear dispensable for the acute phase of wound re-epithelialization. Stem Cells 2016;34:1377–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito M, Liu Y, Yang Z, et al. . Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 2005;11:1351–1354 [DOI] [PubMed] [Google Scholar]
- 25.Pastar I, Stojadinovic O, Yin NC, et al. . Epithelialization in wound healing: A comprehensive review. Adv Wound Care 2014;3:445–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langton AK, Herrick SE, Headon DJ. An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J Invest Dermatol 2008;128:1311–1318 [DOI] [PubMed] [Google Scholar]
- 27.Zhang CP, Fu XB. Therapeutic potential of stem cells in skin repair and regeneration. Chin J Traumatol 2008;11:209–221 [DOI] [PubMed] [Google Scholar]
- 28.Green H, Kehinde O, Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci U S A 1979;76:5665–5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones I, Currie L, Martin R. A guide to biological skin substitutes. Br J Plast Surg 2002;55:185–193 [DOI] [PubMed] [Google Scholar]
- 30.Hafemann B, Hettich R, Ensslen S, et al. . Treatment of skin defects using suspensions of in vitro cultured keratinocytes. Burns 1994;20:168–172 [DOI] [PubMed] [Google Scholar]
- 31.Woodley DT, Peterson HD, Herzog SR, et al. . Burn wounds resurfaced by cultured epidermal autografts show abnormal reconstitution of anchoring fibrils. JAMA 1988;259:2566–2571 [PubMed] [Google Scholar]
- 32.Fang T, Lineaweaver WC, Sailes FC, et al. . Clinical application of cultured epithelial autografts on acellular dermal matrices in the treatment of extended burn injuries. Ann Plast Surg 2014;73:509–515 [DOI] [PubMed] [Google Scholar]
- 33.Raghunath M, Meuli M. Cultured epithelial autografts: Diving from surgery into matrix biology. Pediatr Surg Int 1997;12:478–483 [DOI] [PubMed] [Google Scholar]
- 34.Rennert RC, Rodrigues M, Wong VW, et al. . Biological therapies for the treatment of cutaneous wounds: Phase III and launched therapies. Expert Opin Biol Ther 2013;13:1523–1541 [DOI] [PubMed] [Google Scholar]
- 35.Phillips TJ, Gilchrest BA. Cultured epidermal allografts as biological wound dressings. Prog Clin Biol Res 1991;365:77–94 [PubMed] [Google Scholar]
- 36.Hansbrough JF, Morgan J, Greenleaf G, et al. . Development of a temporary living skin replacement composed of human neonatal fibroblasts cultured in Biobrane, a synthetic dressing material. Surgery 1994;115:633–644 [PubMed] [Google Scholar]
- 37.Hart CE, Loewen-Rodriguez A, Lessem J. Dermagraft: Use in the treatment of chronic wounds. Adv Wound Care (New Rochelle) 2012;1:138–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu S, Kirsner RS, Falanga V, et al. . Evaluation of Apligraf persistence and basement membrane restoration in donor site wounds: A pilot study. Wound Repair Regen 2006;14:427–433 [DOI] [PubMed] [Google Scholar]
- 39.Griffiths M, Ojeh N, Livingstone R, et al. . Survival of Apligraf in acute human wounds. Tissue Eng 2004;10:1180–1195 [DOI] [PubMed] [Google Scholar]
- 40.Sorice S, Rustad KC, Li AY, et al. . The role of stem cell therapeutics in wound healing: Current understanding and future directions. Plast Reconstr Surg 2016;138:31S–41S [DOI] [PubMed] [Google Scholar]
- 41.Javazon EH, Keswani SG, Badillo AT, et al. . Enhanced epithelial gap closure and increased angiogenesis in wounds of diabetic mice treated with adult murine bone marrow stromal progenitor cells. Wound Repair Regen 2007;15:350–359 [DOI] [PubMed] [Google Scholar]
- 42.Nakamura Y, Ishikawa H, Kawai K, et al. . Enhanced wound healing by topical administration of mesenchymal stem cells transfected with stromal cell-derived factor-1. Biomaterials 2013;34:9393–9400 [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Zheng L, Xu X, et al. . Mesenchymal stem cells modified with angiopoietin-1 gene promote wound healing. Stem Cell Res Ther 2013;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chehelcheraghi F, Eimani H, Homayoonsadraie S, et al. . Effects of acellular amniotic membrane matrix and bone marrow-derived mesenchymal stem cells in improving random skin flap survival in rats. Iran Red Crescent Med J 2016;18:e25588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hou C, Shen L, Huang Q, et al. . The effect of heme oxygenase-1 complexed with collagen on MSC performance in the treatment of diabetic ischemic ulcer. Biomaterials 2013;34:112–120 [DOI] [PubMed] [Google Scholar]
- 46.Lian Z, Yin X, Li H, et al. . Synergistic effect of bone marrow-derived mesenchymal stem cells and platelet-rich plasma in streptozotocin-induced diabetic rats. Ann Dermatol 2014;26:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inoue H, Murakami T, Ajiki T, et al. . Bioimaging assessment and effect of skin wound healing using bone-marrow-derived mesenchymal stromal cells with the artificial dermis in diabetic rats. J Biomed Opt 2008;13:64036. [DOI] [PubMed] [Google Scholar]
- 48.Lu F, Mizuno H, Uysal CA, et al. . Improved viability of random pattern skin flaps through the use of adipose-derived stem cells. Plast Reconstr Surg 2008;121:50–58 [DOI] [PubMed] [Google Scholar]
- 49.Chehelcheraghi F, Eimani H, Sadraie SH, et al. . Improved viability of random pattern skin flaps with the use of bone marrow mesenchymal-derived stem cells and chicken embryo extract. Iran J Basic Med Sci 2015;18:764. [PMC free article] [PubMed] [Google Scholar]
- 50.Castilla DM, Liu Z-J, Tian R, et al. . A novel autologous cell based therapy to promote diabetic wound healing. Ann Surg 2012;256:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Z-J, Tian R, An W, et al. . Identification of E-selectin as a novel target for the regulation of post-natal neovascularization: Implications for diabetic wound healing. Ann Surg 2010;252:625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuo Y-R, Wang C-T, Cheng J-T, et al. . Bone marrow–derived mesenchymal stem cells enhanced diabetic wound healing through recruitment of tissue regeneration in a rat model of streptozotocin-induced diabetes. Plast Reconstr Surg 2011;128:872–880 [DOI] [PubMed] [Google Scholar]
- 53.Sukpat S, Isarasena N, Wongphoom J, et al. . Vasculoprotective effects of combined endothelial progenitor cells and mesenchymal stem cells in diabetic wound care: Their potential role in decreasing wound-oxidative stress. Biomed Res Int 2013;2013:459196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cerqueira MT, Pirraco RP, Santos TC, et al. . Human adipose stem cells cell sheet constructs impact epidermal morphogenesis in full-thickness excisional wounds. Biomacromolecules 2013;14:3997–4008 [DOI] [PubMed] [Google Scholar]
- 55.Lee EJ, Park H-W, Jeon H-J, et al. . Potentiated therapeutic angiogenesis by primed human mesenchymal stem cells in a mouse model of hindlimb ischemia. Regen Med 2013;8:283–293 [DOI] [PubMed] [Google Scholar]
- 56.Li M, Xu J, Shi T, et al. . Epigallocatechin-3-gallate augments therapeutic effects of mesenchymal stem cells in skin wound healing. Clin Exp Pharmacol Physiol 2016;43:1115–1124 [DOI] [PubMed] [Google Scholar]
- 57.Takeda K, Fukumoto S, Motoyama K, et al. . Injectable cell scaffold restores impaired cell-based therapeutic angiogenesis in diabetic mice with hindlimb ischemia. Biochem Biophys Res Commun 2014;454:119–124 [DOI] [PubMed] [Google Scholar]
- 58.Raheja LF, Genetos DC, Wong A, et al. . Hypoxic regulation of mesenchymal stem cell migration: The role of RhoA and HIF-1α. Cell Biol Int 2011;35:981–989 [DOI] [PubMed] [Google Scholar]
- 59.Assi R, Foster TR, He H, et al. . Delivery of mesenchymal stem cells in biomimetic engineered scaffolds promotes healing of diabetic ulcers. Regen Med 2016;11:245–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu K, Cantu DA, Fu Y, et al. . Thiol-ene Michael-type formation of gelatin/poly (ethylene glycol) biomatrices for three-dimensional mesenchymal stromal/stem cell administration to cutaneous wounds. Acta Biomater 2013;9:8802–8814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rustad KC, Wong VW, Sorkin M, et al. . Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials 2012;33:80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu J, Zgheib C, Hu J, et al. . The role of microRNA-15b in the impaired angiogenesis in diabetic wounds. Wound Repair Regen 2014;22:671–677 [DOI] [PubMed] [Google Scholar]
- 63.Öksüz S, Ülkür E, Öncül O, et al. . The effect of subcutaneous mesenchymal stem cell injection on statis zone and apoptosis in an experimental burn model. Plast Reconstr Surg 2013;131:463–471 [DOI] [PubMed] [Google Scholar]
- 64.Yeum CE, Park EY, Lee S-B, et al. . Quantification of MSCs involved in wound healing: Use of SIS to transfer MSCs to wound site and quantification of MSCs involved in skin wound healing. J Tissue Eng Regen Med 2013;7:279–291 [DOI] [PubMed] [Google Scholar]
- 65.Naaldijk Y, Johnson AA, Ishak S, et al. . Migrational changes of mesenchymal stem cells in response to cytokines, growth factors, hypoxia, and aging. Exp Cell Res 2015;338:97–104 [DOI] [PubMed] [Google Scholar]
- 66.Rettinger CL, Fourcaudot AB, Hong SJ, et al. . In vitro characterization of scaffold-free three-dimensional mesenchymal stem cell aggregates. Cell Tissue Res 2014;358:395–405 [DOI] [PubMed] [Google Scholar]
- 67.Tong C, Hao H, Xia L, et al. . Hypoxia pretreatment of bone marrow–derived mesenchymal stem cells seeded in a collagen-chitosan sponge scaffold promotes skin wound healing in diabetic rats with hindlimb ischemia. Wound Repair Regen 2016;24:45–56 [DOI] [PubMed] [Google Scholar]
- 68.Guo X, Xia B, Lu X-B, et al. . Grafting of mesenchymal stem cell-seeded small intestinal submucosa to repair the deep partial-thickness burns. Connect Tissue Res 2016;57:388–397 [DOI] [PubMed] [Google Scholar]
- 69.Peng Y, Xuan M, Zou J, et al. . Freeze-dried rat bone marrow mesenchymal stem cell paracrine factors: A simplified novel material for skin wound therapy. Tissue Eng Part A 2014;21:1036–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zonari A, Martins TMM, Paula ACC, et al. . Polyhydroxybutyrate-co-hydroxyvalerate structures loaded with adipose stem cells promote skin healing with reduced scarring. Acta Biomater 2015;17:170–181 [DOI] [PubMed] [Google Scholar]
- 71.Gao W, Qiao X, Ma S, et al. . Adipose-derived stem cells accelerate neovascularization in ischaemic diabetic skin flap via expression of hypoxia-inducible factor-1α. J Cell Mol Med 2011;15:2575–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trinh NT, Yamashita T, Tu TC, et al. . Microvesicles enhance the mobility of human diabetic adipose tissue-derived mesenchymal stem cells in vitro and improve wound healing in vivo. Biochem Biophys Res Commun 2016;473:1111–1118 [DOI] [PubMed] [Google Scholar]
- 73.Nie C, Zhang G, Yang D, et al. . Targeted delivery of adipose-derived stem cells via acellular dermal matrix enhances wound repair in diabetic rats. J Tissue Eng Regen Med 2015;9:224–235 [DOI] [PubMed] [Google Scholar]
- 74.Li Q, Guo Y, Chen F, et al. . Stromal cell-derived factor-1 promotes human adipose tissue-derived stem cell survival and chronic wound healing. Exp Ther Med 2016;12:45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verseijden F, Posthumus-van Sluijs SJ, van Neck JW, et al. . Vascularization of prevascularized and non-prevascularized fibrin-based human adipose tissue constructs after implantation in nude mice. J Tissue Eng Regen Med 2012;6:169–178 [DOI] [PubMed] [Google Scholar]
- 76.Feng J, Doi K, Kuno S, et al. . Micronized cellular adipose matrix as a therapeutic injectable for diabetic ulcer. Regen Med 2015;10:699–708 [DOI] [PubMed] [Google Scholar]
- 77.Sun B, Guo S, Xu F, et al. . Concentrated hypoxia-preconditioned adipose mesenchymal stem cell-conditioned medium improves wounds healing in full-thickness skin defect model. Int Sch Res Notices 2014;2014:652713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuo Y-R, Wang C-T, Cheng J-T, et al. . Adipose-derived stem cells accelerate diabetic wound healing through the induction of autocrine and paracrine effects. Cell Transplant 2016;25:71–81 [DOI] [PubMed] [Google Scholar]
- 79.Zeng Y, Zhu L, Han Q, et al. . Preformed gelatin microcryogels as injectable cell carriers for enhanced skin wound healing. Acta Biomater 2015;25:291–303 [DOI] [PubMed] [Google Scholar]
- 80.Park I-S, Chung P-S, Ahn JC. Enhancement of ischemic wound healing by spheroid grafting of human adipose-derived stem cells treated with low-level light irradiation. PLoS One 2015;10:e0122776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park I-S, Chung P-S, Ahn JC. Angiogenic synergistic effect of adipose-derived stromal cell spheroids with low-level light therapy in a model of acute skin flap ischemia. Cells Tissues Organs 2016;202:307–318 [DOI] [PubMed] [Google Scholar]
- 82.Caiado F, Carvalho T, Silva F, et al. . The role of fibrin E on the modulation of endothelial progenitors adhesion, differentiation and angiogenic growth factor production and the promotion of wound healing. Biomaterials 2011;32:7096–7105 [DOI] [PubMed] [Google Scholar]
- 83.Ackermann M, Pabst AM, Houdek JP, et al. . Priming with proangiogenic growth factors and endothelial progenitor cells improves revascularization in linear diabetic wounds. Int J Mol Med 2014;33:833–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tellechea A, Silva EA, Min J, et al. . Alginate and DNA gels are suitable delivery systems for diabetic wound healing. Int J Low Extrem Wounds 2015;14:146–153 [DOI] [PubMed] [Google Scholar]
- 85.Shrestha C, Zhao L, Chen K, et al. . Enhanced healing of diabetic wounds by subcutaneous administration of human umbilical cord derived stem cells and their conditioned media. Int J Endocrinol 2013;2013:Article ID 592454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Han K-H, Kim A-K, Kim M-H, et al. . Enhancement of angiogenic effects by hypoxia-preconditioned human umbilical cord-derived mesenchymal stem cells in a mouse model of hindlimb ischemia. Cell Biol Int 2016;40:27–35 [DOI] [PubMed] [Google Scholar]
- 87.Wang S, Yang H, Tang Z, et al. . Wound dressing model of human umbilical cord mesenchymal stem cells-alginates complex promotes skin wound healing by paracrine signaling. Stem Cells Int 2016;2016:3269267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Edwards SS, Zavala G, Prieto CP, et al. . Functional analysis reveals angiogenic potential of human mesenchymal stem cells from Wharton's jelly in dermal regeneration. Angiogenesis 2014;17:851–866 [DOI] [PubMed] [Google Scholar]
- 89.Tam K, Cheyyatraviendran S, Venugopal J, et al. . A nanoscaffold impregnated with human Wharton's jelly stem cells or its secretions improves healing of wounds. J Cell Biochem 2014;115:794–803 [DOI] [PubMed] [Google Scholar]
- 90.Arno AI, Amini-Nik S, Blit PH, et al. . Human Wharton's jelly mesenchymal stem cells promote skin wound healing through paracrine signaling. Stem Cell Res Ther 2014;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fong C-Y, Tam K, Cheyyatraivendran S, et al. . Human Wharton's jelly stem cells and its conditioned medium enhance healing of excisional and diabetic wounds. J Cell Biochem 2014;115:290–302 [DOI] [PubMed] [Google Scholar]
- 92.Kazemi-Darabadi S, Sarrafzadeh-Rezaei F, Farshid A-A, et al. . Allogenous skin fibroblast transplantation enhances excisional wound healing following alloxan diabetes in sheep, a randomized controlled trial. Int J Surg 2014;12:751–756 [DOI] [PubMed] [Google Scholar]
- 93.Hu G-L, Ma Y-G, Li Y-M. Transplantation of artificial gelatin-co-bletillastriata gelatin/salvia miltiorrhiza corium promotes dermal repair in rats. Trop J Pharm Res 2016;15:735–741 [Google Scholar]
- 94.Kairuz E, Upton Z, Dawson RA, et al. . Hyperbaric oxygen stimulates epidermal reconstruction in human skin equivalents. Wound Repair Regen 2007;15:266–274 [DOI] [PubMed] [Google Scholar]
- 95.Velander P, Theopold C, Bleiziffer O, et al. . Cell suspensions of autologous keratinocytes or autologous fibroblasts accelerate the healing of full thickness skin wounds in a diabetic porcine wound healing model. J Surg Res 2009;157:14–20 [DOI] [PubMed] [Google Scholar]
- 96.De Angelis B, Gentile P, Orlandi F, et al. . Limb rescue: A new autologous-peripheral blood mononuclear cells technology in critical limb ischemia and chronic ulcers. Tissue Eng Part C Methods 2015;21:423–435 [DOI] [PubMed] [Google Scholar]
- 97.O'Loughlin A, Kulkarni M, Vaughan EE, et al. . Autologous circulating angiogenic cells treated with osteopontin and delivered via a collagen scaffold enhance wound healing in the alloxan-induced diabetic rabbit ear ulcer model. Stem Cell Res Ther 2013;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Quan R, Zheng X, Xu S, et al. . Gelatin-chondroitin-6-sulfate-hyaluronic acid scaffold seeded with vascular endothelial growth factor 165 modified hair follicle stem cells as a three-dimensional skin substitute. Stem Cell Res Ther 2014;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ke T, Yang M, Mao D, et al. . Co-transplantation of skin-derived precursors and collagen sponge facilitates diabetic wound healing by promoting local vascular regeneration. Cell Physiol Biochem 2015;37:1725–1737 [DOI] [PubMed] [Google Scholar]
- 100.Jiang Y, Chen B, Liu Y, et al. . Effect of collagen scaffold with adipose-derived stromal vascular fraction cells on diabetic wound healing: A study in a diabetic porcine model. Tissue Eng Regen Med 2013;10:192–199 [Google Scholar]
- 101.Jun EK, Zhang Q, Yoon BS, et al. . Hypoxic conditioned medium from human amniotic fluid-derived mesenchymal stem cells accelerates skin wound healing through TGF-β/SMAD2 and PI3K/Akt pathways. Int J Mol Sci 2014;15:605–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shen Y-I, Cho H, Papa AE, et al. . Engineered human vascularized constructs accelerate diabetic wound healing. Biomaterials 2016;102:107–119 [DOI] [PubMed] [Google Scholar]
- 103.Yuan H, Guan J, Zhang J, et al. . Exosomes secreted by human urine-derived stem cells accelerate skin wound healing by promoting angiogenesis in rat. Cell Biol Int 2016. [Epub ahead of print]; DOI: 10.1002/cbin.10615 [DOI] [PubMed] [Google Scholar]
- 104.Tong X, Lv G, Huang J, et al. . Gr-1+ CD11b+ myeloid cells efficiently home to site of injury after intravenous administration and enhance diabetic wound healing by neoangiogenesis. J Cell Mol Med 2014;18:1194–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Egaña JT, Danner S, Kremer M, et al. . The use of glandular-derived stem cells to improve vascularization in scaffold-mediated dermal regeneration. Biomaterials 2009;30:5918–5926 [DOI] [PubMed] [Google Scholar]
- 106.Murphy MP, Wang H, Patel AN, et al. . Allogeneic endometrial regenerative cells: An “Off the shelf solution” for critical limb ischemia? J Transl Med 2008;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dazzi F, Lopes L, Weng L. Mesenchymal stromal cells: A key player in “innate tolerance”? Immunology 2012;137:206–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Caplan AI, Correa D. The MSC: An injury drugstore. Cell Stem Cell 2011;9:11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2016;98:1076–1084 [DOI] [PubMed] [Google Scholar]
- 110.Duscher D, Barrera J, Wong VW, et al. . Stem cells in wound healing: The future of regenerative medicine? A mini-review. Gerontology 2015;62:216–225 [DOI] [PubMed] [Google Scholar]
- 111.Mansilla E, Spretz R, Larsen G, et al. . Outstanding survival and regeneration process by the use of intelligent acellular dermal matrices and mesenchymal stem cells in a burn pig model. Transpl Proc 2010;42:4275–4278 [DOI] [PubMed] [Google Scholar]
- 112.Li H, Fu X, Ouyang Y, et al. . Adult bone-marrow-derived mesenchymal stem cells contribute to wound healing of skin appendages. Cell Tissue Res 2006;326:725–736 [DOI] [PubMed] [Google Scholar]
- 113.Kataoka K, Medina RJ, Kageyama T, et al. . Participation of adult mouse bone marrow cells in reconstitution of skin. Am J Pathol 2003;163:1227–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kurtz A. Mesenchymal stem cell delivery routes and fate. Int J stem cells 2008;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow—derived cells. Arch Dermatol 2003;139:510–516 [DOI] [PubMed] [Google Scholar]
- 116.Hocking AM, Gibran NS. Mesenchymal stem cells: Paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res 2010;316:2213–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Aoki S, Toda S, Ando T, et al. . Bone marrow stromal cells, preadipocytes, and dermal fibroblasts promote epidermal regeneration in their distinctive fashions. Mol Biol Cell 2004;15:4647–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rodriguez-Menocal L, Salgado M, Ford D, et al. . Stimulation of skin and wound fibroblast migration by mesenchymal stem cells derived from normal donors and chronic wound patients. Stem Cells Transl Med 2012;1:221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jones DL, Wagers AJ. No place like home: Anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol 2008;9:11–21 [DOI] [PubMed] [Google Scholar]
- 120.Stamati K, Mudera V, Cheema U. Evolution of oxygen utilization in multicellular organisms and implications for cell signalling in tissue engineering. J Tissue Eng 2011;2:2041731411432365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev 2008;60:184–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.International consensus. Acellular matrices for the treatment of wounds. An expert working group review. London: Wounds International, 2010 [Google Scholar]
- 123.Climov M, Bayer LR, Moscoso AV, et al. . The role of dermal matrices in treating inflammatory and diabetic wounds. Plast Reconstr Surg 2016;138:148S–157S [DOI] [PubMed] [Google Scholar]
- 124.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials 2011;32:3233–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yannas IV, Burke JF. Design of an artificial skin. I. Basic design principles. J Biomed Mater Res 1980;14:65–81 [DOI] [PubMed] [Google Scholar]
- 126.Ninan N, Muthiah M, Park I-K, et al. . Natural polymer/inorganic material based hybrid scaffolds for skin wound healing. Polym Rev 2015;55:453–490 [Google Scholar]
- 127.Skardal A, Mack D, Kapetanovic E, et al. . Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl Med 2012;1:792–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Koch L, Deiwick A, Schlie S, et al. . Skin tissue generation by laser cell printing. Biotechnol Bioeng 2012;109:1855–1863 [DOI] [PubMed] [Google Scholar]
- 129.Binder KW, Zhao W, Aboushwareb T, et al. . In situ bioprinting of the skin for burns. J Am Coll Surg 2010;211:S76 [Google Scholar]
- 130.Cubo N, Garcia M, del Cañizo JF, et al. . 3D bioprinting of functional human skin: Production and in vivo analysis. Biofabrication 2016;9:015006. [DOI] [PubMed] [Google Scholar]
- 131.Michael S, Sorg H, Peck C-T, et al. . Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS One 2013;8:e57741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol 2014;32:773–785 [DOI] [PubMed] [Google Scholar]
- 133.Ng WL, Wang S, Yeong WY, et al. . Skin bioprinting: Impending reality or fantasy? Trends Biotechnol 2016;34:689–699 [DOI] [PubMed] [Google Scholar]
- 134.Organovo Holdings I. L'Oreal USA announces research partnership with organovo to develop 3-D bioprinted skin tissue. http://ir.organovo.com/phoenix.zhtml?c=254194&p=irol-newsArticle&ID=2129344 (last accessed February3, 2017)
- 135.Hadjipanayi E, Brown RA, Mudera V, et al. . Controlling physiological angiogenesis by hypoxia-induced signaling. J Control Release 2010;146:309–317 [DOI] [PubMed] [Google Scholar]
- 136.Jeon O, Alsberg E. Regulation of stem cell fate in a three-dimensional micropatterned dual-crosslinked hydrogel system. Adv Funct Mater 2013;23:4765–4775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Engler AJ, Sen S, Sweeney HL, et al. . Matrix elasticity directs stem cell lineage specification. Cell 2006;126:677–689 [DOI] [PubMed] [Google Scholar]
- 138.Prowse ABJ, Chong F, Gray PP, et al. . Stem cell integrins: Implications for ex-vivo culture and cellular therapies. Stem Cell Res 2011;6:1–12 [DOI] [PubMed] [Google Scholar]
- 139.Stamati K, Priestley JV, Mudera V, et al. . Laminin promotes vascular network formation in 3D in vitro collagen scaffolds by regulating VEGF uptake. Exp Cell Res 2014;327:68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dalby MJ, Gadegaard N, Oreffo ROC. Harnessing nanotopography and integrin–matrix interactions to influence stem cell fate. Nat Mater 2014;13:558–569 [DOI] [PubMed] [Google Scholar]
- 141.Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol 2008;9:285–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ivanovic Z, Bartolozzi B, Bernabei PA, et al. . Incubation of murine bone marrow cells in hypoxia ensures the maintenance of marrow-repopulating ability together with the expansion of committed progenitors. Br J Haematol 2000;108:424–429 [DOI] [PubMed] [Google Scholar]
- 143.Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A 2005;102:4783–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rabbany SY, Pastore J, Yamamoto M, et al. . Continuous delivery of stromal cell-derived factor-1 from alginate scaffolds accelerates wound healing. Cell Transplant 2010;19:399–408 [DOI] [PubMed] [Google Scholar]
- 145.Zhu Y, Hoshi R, Chen S, et al. . Sustained release of stromal cell derived factor-1 from an antioxidant thermoresponsive hydrogel enhances dermal wound healing in diabetes. J Control Release 2016;238:114–122 [DOI] [PubMed] [Google Scholar]
- 146.Tan Q, Chen B, Yan X, et al. . Promotion of diabetic wound healing by collagen scaffold with collagen-binding vascular endothelial growth factor in a diabetic rat model. J Tissue Eng Regen Med 2014;8:195–201 [DOI] [PubMed] [Google Scholar]
- 147.Losi P, Briganti E, Errico C, et al. . Fibrin-based scaffold incorporating VEGF-and bFGF-loaded nanoparticles stimulates wound healing in diabetic mice. Acta Biomater 2013;9:7814–7821 [DOI] [PubMed] [Google Scholar]
- 148.Murali R, Thanikaivelan P. Bionic, porous, functionalized hybrid scaffolds with vascular endothelial growth factor promote rapid wound healing in Wistar albino rats. RSC Adv 2016;6:19252–19264 [Google Scholar]
- 149.Ehrbar M, Zeisberger SM, Raeber GP, et al. . The role of actively released fibrin-conjugated VEGF for VEGF receptor 2 gene activation and the enhancement of angiogenesis. Biomaterials 2008;29:1720–1729 [DOI] [PubMed] [Google Scholar]
- 150.Chen F, Wan H, Xia T, et al. . Promoted regeneration of mature blood vessels by electrospun fibers with loaded multiple pDNA-calcium phosphate nanoparticles. Eur J Pharm Biopharm 2013;85:699–710 [DOI] [PubMed] [Google Scholar]
- 151.Huang C, Orbay H, Tobita M, et al. . Proapoptotic effect of control-released basic fibroblast growth factor on skin wound healing in a diabetic mouse model. Wound Repair Regen 2016;24:65–74 [DOI] [PubMed] [Google Scholar]
- 152.Liem PH, Morimoto N, Ito R, et al. . Treating a collagen scaffold with a low concentration of nicotine promoted angiogenesis and wound healing. J Surg Res 2013;182:353–361 [DOI] [PubMed] [Google Scholar]
- 153.Park CJ, Clark SG, Lichtensteiger CA, et al. . Accelerated wound closure of pressure ulcers in aged mice by chitosan scaffolds with and without bFGF. Acta Biomater 2009;5:1926–1936 [DOI] [PubMed] [Google Scholar]
- 154.Zhao W, Han Q, Lin H, et al. . Improved neovascularization and wound repair by targeting human basic fibroblast growth factor (bFGF) to fibrin. J Mol Med 2008;86:1127–1138 [DOI] [PubMed] [Google Scholar]
- 155.Gérard C, Bordeleau L-J, Barralet J, et al. . The stimulation of angiogenesis and collagen deposition by copper. Biomaterials 2010;31:824–831 [DOI] [PubMed] [Google Scholar]
- 156.Wang W, Lin S, Xiao Y, et al. . Acceleration of diabetic wound healing with chitosan-crosslinked collagen sponge containing recombinant human acidic fibroblast growth factor in healing-impaired STZ diabetic rats. Life Sci 2008;82:190–204 [DOI] [PubMed] [Google Scholar]
- 157.Chu Y, Yu D, Wang P, et al. . Nanotechnology promotes the full-thickness diabetic wound healing effect of recombinant human epidermal growth factor in diabetic rats. Wound Repair Regen 2010;18:499–505 [DOI] [PubMed] [Google Scholar]
- 158.Hajimiri M, Shahverdi S, Esfandiari MA, et al. . Preparation of hydrogel embedded polymer-growth factor conjugated nanoparticles as a diabetic wound dressing. Drug Dev Ind Pharm 2016;42:707–719 [DOI] [PubMed] [Google Scholar]
- 159.Goh M, Hwang Y, Tae G. Epidermal growth factor loaded heparin-based hydrogel sheet for skin wound healing. Carbohydr Polym 2016;147:251–260 [DOI] [PubMed] [Google Scholar]
- 160.Kim HS, Yoo HS. In vitro and in vivo epidermal growth factor gene therapy for diabetic ulcers with electrospun fibrous meshes. Acta Biomater 2013;9:7371–7380 [DOI] [PubMed] [Google Scholar]
- 161.Shen L, Zeng W, Wu Y-X, et al. . Neurotrophin-3 accelerates wound healing in diabetic mice by promoting a paracrine response in mesenchymal stem cells. Cell Transplant 2013;22:1011–1021 [DOI] [PubMed] [Google Scholar]
- 162.Kwon SH, Bhang SH, Jang H-K, et al. . Conditioned medium of adipose-derived stromal cell culture in three-dimensional bioreactors for enhanced wound healing. J Surg Res 2015;194:8–17 [DOI] [PubMed] [Google Scholar]
- 163.Arul V, Masilamoni JG, Jesudason EP, et al. . Glucose oxidase incorporated collagen matrices for dermal wound repair in diabetic rat models: A biochemical study. J Biomater Appl 2012;26:917–938 [DOI] [PubMed] [Google Scholar]
- 164.Ito R, Morimoto N, Pham LH, et al. . Efficacy of the controlled release of concentrated platelet lysate from a collagen/gelatin scaffold for dermis-like tissue regeneration. Tissue Eng Part A 2013;19:1398–1405 [DOI] [PubMed] [Google Scholar]
- 165.Losi P, Briganti E, Sanguinetti E, et al. . Healing effect of a fibrin-based scaffold loaded with platelet lysate in full-thickness skin wounds. J Bioact Compat Polym Biomed Appl 2015;30:222–237 [Google Scholar]
- 166.Bhang SH, Park J, Yang HS, et al. . Platelet-rich plasma enhances the dermal regeneration efficacy of human adipose-derived stromal cells administered to skin wounds. Cell Transplant 2013;22:437–445 [DOI] [PubMed] [Google Scholar]
- 167.Sun W, Sun W, Lin H, et al. . Collagen membranes loaded with collagen-binding human PDGF-BB accelerate wound healing in a rabbit dermal ischemic ulcer model. Growth Factors 2007;25:309–318 [DOI] [PubMed] [Google Scholar]
- 168.Kulkarni M, O'Loughlin A, Vazquez R, et al. . Use of a fibrin-based system for enhancing angiogenesis and modulating inflammation in the treatment of hyperglycemic wounds. Biomaterials 2014;35:2001–2010 [DOI] [PubMed] [Google Scholar]
- 169.Park JH, Kim S, Hong HS, et al. . Substance P promotes diabetic wound healing by modulating inflammation and restoring cellular activity of mesenchymal stem cells. Wound Repair Regen 2016;24:337–348 [DOI] [PubMed] [Google Scholar]
- 170.Ti D, Hao H, Xia L, et al. . Controlled release of thymosin beta 4 using a collagen—chitosan sponge scaffold augments cutaneous wound healing and increases angiogenesis in diabetic rats with hindlimb ischemia. Tissue Eng Part A 2014;21:541–549 [DOI] [PubMed] [Google Scholar]
- 171.Chen H, Jia P, Kang H, et al. . Upregulating Hif-1α by hydrogel nanofibrous scaffolds for rapidly recruiting angiogenesis relative cells in diabetic wound. Adv Healthc Mater 2016;5:907–918 [DOI] [PubMed] [Google Scholar]
- 172.Wang H, Yan X, Shen L, et al. . Acceleration of wound healing in acute full-thickness skin wounds using a collagen-binding peptide with an affinity for MSCs. Burn Trauma 2015;2:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kobayashi H, Katakura O, Morimoto N, et al. . Effects of cholesterol-bearing pullulan (CHP)-nanogels in combination with prostaglandin E1 on wound healing. J Biomed Mater Res Part B Appl Biomater 2009;91:55–60 [DOI] [PubMed] [Google Scholar]
- 174.Romana-Souza B, Porto LC, Monte-Alto-Costa A. Cutaneous wound healing of chronically stressed mice is improved through catecholamines blockade. Exp Dermatol 2010;19:821–829 [DOI] [PubMed] [Google Scholar]
- 175.Nguyen PD, Tutela JP, Thanik VD, et al. . Improved diabetic wound healing through topical silencing of p53 is associated with augmented vasculogenic mediators. Wound Repair Regen 2010;18:553–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cheema U, Alekseeva T, Abou-Neel EA, et al. . Switching off angiogenic signalling: Creating channelled constructs for adequate oxygen delivery in tissue engineered constructs. Eur Cells Mater 2010;20:274–281 [DOI] [PubMed] [Google Scholar]
- 177.Kulkarni MM, Greiser U, O'Brien T, et al. . A temporal gene delivery system based on fibrin microspheres. Mol Pharm 2011;8:439–446 [DOI] [PubMed] [Google Scholar]
- 178.Cicchi R, Vogler N, Kapsokalyvas D, et al. . From molecular structure to tissue architecture: Collagen organization probed by SHG microscopy. J Biophotonics 2013;6:129–142 [DOI] [PubMed] [Google Scholar]
- 179.Zhang Q, Johnson JA, Dunne LW, et al. . Decellularized skin/adipose tissue flap matrix for engineering vascularized composite soft tissue flaps. Acta Biomater 2016;35:166–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xu R, Boudreau A, Bissell MJ. Tissue architecture and function: Dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev 2009;28:167–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ichioka S, Kouraba S, Sekiya N, et al. . Bone marrow-impregnated collagen matrix for wound healing: Experimental evaluation in a microcirculatory model of angiogenesis, and clinical experience. Br J Plast Surg 2005;58:1124–1130 [DOI] [PubMed] [Google Scholar]
- 182.Yoshikawa T, Mitsuno H, Nonaka I, et al. . Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg 2008;121:860–877 [DOI] [PubMed] [Google Scholar]
- 183.Ravari H, Hamidi-Almadari D, Salimifar M, et al. . Treatment of non-healing wounds with autologous bone marrow cells, platelets, fibrin glue and collagen matrix. Cytotherapy 2011;13:705–711 [DOI] [PubMed] [Google Scholar]
- 184.Powell RJ, Marston WA, Berceli SA, et al. . Cellular therapy with ixmyelocel-T to treat critical limb ischemia: The randomized, double-blind, placebo-controlled RESTORE-CLI trial. Mol Ther 2012;20:1280–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lu D, Chen B, Liang Z, et al. . Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: A double-blind, randomized, controlled trial. Diabetes Res Clin Pract 2011;92:26–36 [DOI] [PubMed] [Google Scholar]
- 186.Theopold C, Hoeller D, Velander P, et al. . Graft site malignancy following treatment of full-thickness burn with cultured epidermal autograft. Plast Reconstr Surg 2004;114:1215–1219 [DOI] [PubMed] [Google Scholar]