Abstract
Translation of therapeutic interventions for spinal cord injury (SCI) from laboratory to clinic has been historically challenging, highlighting the need for robust models of injury that more closely mirror the human condition. The high prevalence of acute, naturally occurring SCI in pet dogs provides a unique opportunity to evaluate expeditiously promising interventions in a population of animals that receive diagnoses and treatment clinically in a manner similar to persons with SCI, while adhering to National Institutes of Health guidelines for scientific rigor and transparent reporting. In addition, pet dogs with chronic paralysis are often maintained long-term by their owners, offering a similarly unique population for study of chronic SCI. Despite this, only a small number of studies have used the clinical dog model of SCI. The Canine Spinal Cord Injury Consortium (CANSORT-SCI) was recently established by a group of veterinarians and basic science researchers to promote the value of the canine clinical model of SCI. The CANSORT-SCI group held an inaugural meeting November 20 and 21, 2015 to evaluate opportunities and challenges to the use of pet dogs in SCI research. Key challenges identified included lack of familiarity with the model among nonveterinary scientists and questions about how and where in the translational process the canine clinical model would be most valuable. In light of these, we review the natural history, outcome, and available assessment tools associated with canine clinical SCI with emphasis on their relevance to human SCI and the translational process.
Keywords: : animal model, clinical trial, dog, spinal cord injury
Introduction
Traumatic spinal cord injury (SCI) affects 250,000–500,000 persons around the world each year,1 and for those with the most severe injuries, significant recovery is rare.2 The ensuing physical consequences result in high long-term medical costs ($1.5–4.5M per person over a lifetime).3 Laboratory science has provided a plethora of treatments that improve outcomes in rodent models of SCI in a homogenous animal population enhancing the basic knowledge about this injury; to date, however, those evaluated in human clinical trials do not show an unequivocal improvement. Performing human clinical trials is expensive and time consuming. We propose that the inefficiency of translating rodent therapies to human use could be improved by screening them with an animal model that more closely aligns with human SCI.4,5
Spontaneous dog models have proven useful in pioneering and rigorously testing new treatments for human diseases such as Addison's disease,6 retinal degeneration,7 and malignant glioma.8 The prevalence of naturally occurring traumatic SCI is high within the general population of pet dogs with approximately 20,000 new cases presenting to veterinary spinal surgeons each year,9 almost twice the incidence of human SCI in the United States.3 This makes this population of dogs a potential tool through which to screen new interventions for persons with SCI. To this effect, the model has been used as part of the SCI drug or device development process and as second-species confirmation of treatment effects observed in the laboratory setting in a number of studies.10–15
The Canine Spinal Cord Injury Consortium (CANSORT-SCI) was established recently by a group of veterinarians and basic science researchers to promote the value of the canine clinical model of SCI. This group brings together expertise in the dog model of SCI, basic neuroscience, and human SCI. Members of our international group span eight veterinary institutions, three renowned basic neuroscience programs, and one industry entity (Appendix 1; see online supplementary material at ftp.liebertpub.com). Members were invited based on history of research focus in dog models and expertise in SCI.
The aim of this consortium is to conduct treatment trials in naturally occurring canine SCI to generate relevant translational data for humans. Specific goals include performing rigorous multicenter studies in canine clinical SCI, developing and maintaining a common data registry for dogs with acute SCI managed at all member institutions, establishing a tissue bank to facilitate histopathological studies of naturally occurring SCI in dogs, and maintaining a network of clinical trial sites that provide standardized assessments and care for spinal cord-injured dogs involved in research protocols. The CANSORT-SCI group held an inaugural meeting November 20 and 21, 2015 to evaluate the strengths, weaknesses, opportunities, and challenges to the use of pet dogs in SCI research.
Key identified potential strengths of the model include:
(1) The ability to study naturally occurring injury, which shares many pathological aspects with certain human SCI cases, in a population of animals with diverse genetic backgrounds, an assortment of health co-morbidities, and other confounding clinical factors encountered in human clinical trials. Detecting a therapeutic effect in a heterogeneous cohort of dogs with clinical SCI (as opposed to the very homogeneous population of laboratory rodents used for “proof-of-principle” demonstration), while not assuring translational success, would increase confidence in the robustness of a given therapy and provide a stronger basis for translation to human SCI.
(2) The high prevalence makes the model highly amenable to conducting large-scale veterinary clinical trials that meet all National Institutes of Health/National Institute of Neurological Disorders and Stroke guidelines for scientific rigor and transparent reporting.16 For example, a study involving only the authors of this manuscript could be anticipated to enroll approximately 100 dogs with sensorimotor complete SCI within a year. A precedent for this approach using clinical dog models has recently been set in the field of malignant glioma.8
(3) Treatment of pet dogs with SCI (imaging, surgery, rehabilitation) relies on identical techniques and equipment used for humans, meaning that new therapies can be tested in a scenario that simulates a human clinical trial using treatment protocols that can be standardized across veterinary trial centers.
(4) Dogs that fail to recover after an acute injury are often managed long-term by their owners, making them available for study of interventions aimed at chronic SCI. Chronic SCI and its long-term consequences have been identified as a research priority by the human SCI community,17 yet laboratory studies of chronic injury are expensive and logistically challenging.
(5) Physical size and metabolism of dogs facilitates “scaling up” of therapeutics for human trials.
The group also identified several challenges to the pet dog model of SCI for use in translational research. Two key challenges included lack of familiarity with the model among nonveterinary scientists and questions about how and where in the translational process the canine clinical model would be most valuable. To address these concerns, we review here the natural history, pathobiology, outcome, and available assessment tools associated with canine clinical SCI in light of how they compare with human SCI and complement laboratory models of injury. We also review recent studies using canine clinical SCI and, where relevant, how their findings have impacted the field.
Clinical Characteristics of SCI in Dogs
Acute SCI in pet dogs has two major etiologies, both of which cause a mixed contusive-compressive lesion: (1) fracture or luxation of the vertebral column; and (2) spontaneous intervertebral disc herniation (IVDH) secondary to degeneration and calcification of the nucleus pulposus, which predominantly affects small breed dogs such as dachshunds, beagles and Pekingese.18,19 The IVDH is by far the more common cause and results in a mixed compressive and contusive SCI most frequently in the thoracolumbar region.20 The anatomy of the canine spinal cord differs from humans in that the spinal cord of dogs terminates at approximately the sixth lumbar vertebra in most animals (there are 13 thoracic vertebrae and seven lumbar vertebrae in dogs). As such, herniation of intervertebral disc material in the thoracolumbar spine is akin to a midthoracic injury in a person.
Severity of neurologic injury caused by IVDH-associated SCI spans a spectrum up to and including sensorimotor complete injury, with predictable patterns of recovery across different severities.18,19 For dogs with incomplete injuries, significant neurological recovery is often observed; however, for those with sensorimotor complete injuries (equivalent to Abbreviated Injury Scale [AIS-A] status in humans), 40–50% experience permanent paralysis, somatosensory dysfunction, and incontinence.21 A recent review describes the prevalence of AIS-A equivalent injuries in dogs with IVDH to be approximately 15%,22 which translates to approximately 2000–3000 cases per year in the United States alone.9 This provides a convenient clinical animal model for study of SCI. For the purposes of this review, the term SCI, as used in the context of dogs, will refer specifically to IVDH-associated thoracolumbar SCI.
Histopathology of Canine SCI
Because dogs with IVDH-associated SCI represent a clinical population of companion animals, the spinal cord is not routinely available for histopathology, mirroring the human condition in which histopathological studies are also limited. Several studies, however, describe the histopathological changes associated with naturally occurring IVDH-associated SCI in dogs. Lesions typically consist of varying degrees of gray matter hemorrhage and necrosis, inflammation and gliosis, axonal swelling, sparing of peripheral and small diameter axons, and variable but mild degrees of demyelination, thus corresponding to a mixture of contusion and compression injury, while complete laceration injuries are rare.20,23–25 These findings reflect histopathological features of moderate contusive/compressive types of human SCI, which have been detailed in several excellent publications.26,27 Histopathological studies of chronic canine IVDH-associated SCI are limited, preventing a detailed comparative discussion of similarities and potential differences in the nature and severity of histopathological lesions after canine and human SCI. Based on available histopathological data, a time course for tissue responses in the injured canine spinal cord is as follows:
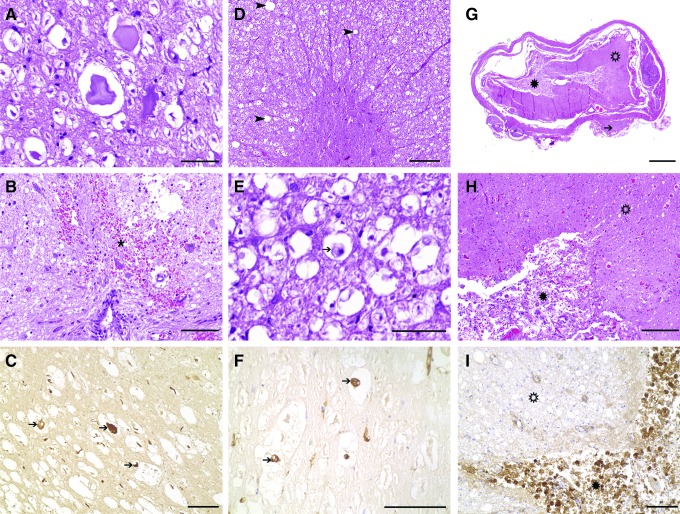
Acute changes (0–3 days; Fig. 1A-C)
FIG. 1.
Histopathology of representative dogs with acute (0–3 days) (A–C), subacute (3 days–3 weeks) (D–F), and chronic (>3 weeks) (G–I) spinal cord injury (SCI) because of intervertebral disk herniation (IVDH). (A) Overview of a case of acute SCI, demonstrating relatively preserved morphology. Several swollen axons within moderately dilated myelin sheaths are observed throughout the white matter, accompanied by mild to moderate gray matter hemorrhage. (B) Closer view of extravasated erythrocytes (hemorrhage) in close proximity to the central canal. Some axons appear hypereosinophilic and swollen (spheroids); 10 × objective. (C) Axonal damage is a consistent feature of IVDE-induced SCI, as demonstrated by intense axonal immunoreactivity for nonphosphorylated neurofilament (arrows); 20 × objective. (D) Overview of a case of subacute SCI, demonstrating preserved architecture but diffuse pallor of the white matter, indicating myelin sheath edema and swelling. (E) Within dilated myelin sheaths, which are commonly affected by axonal dropout, phagocytic cells with foamy, vacuolated cytoplasm and excentric nuclei are commonly observed (arrow), indicating myelino- and axonophagia; 40 × objective. (F) Cells intensely labeled by a lectin of Bandeiraea simplicifolia (arrows) are indicative of the microglia/macrophage origin of these cells; 40 × objective. (G) Overview of a case with chronic SCI with severe destruction of the organotypic architecture of white matter (open asterisk) and gray matter (asterisk). There is necrosis (myelomalacia) as well as glial scarring, which predominantly affects the gray matter. Note degenerated intervertebral disk material, which is attached to the dura mater in this case (arrow) (H) Closer view of the white (open asterisk) and gray matter (asterisk) of the same case. While there is intense diffuse hyperemia of blood vessels, the gray matter is characterized by severe proliferation of partly spindloid glial cells and capillaries, consistent with glial scar tissue formation; 10 × objective. (I) White matter (open asterisk) and gray matter (asterisk) of another case with chronic SCI. Besides myelin sheath swelling, the white matter is relatively preserved, while there is severe diffuse infiltration of myriads of phagocytic macrophages within the gray matter, as demonstrated by positivity with the lectin of Bandeiraea simplicifolia, indicating severe necrosis of the gray matter; 20 × objective. Hematoxylin and eosin staining (A, B, D, E, G, H) and immuno-(C) and lectin histochemistry (F, I) using the avidin-biotin-peroxidase complex method and 3,3′-diaminobenzidine as chromogen.
Foci of edema, gray matter hemorrhage, necrosis, and cavitation are apparent. Axonal swelling indicating primary axonal damage is a consistent observation in acute cases, while there is preferential sparing of peripheral axons and small (<5 μm diameter) axons of the dorsal funiculus.20,24 Both Wallerian-like degeneration and occasional severe segmental paranodal myelin disruption are observed, as are swollen astrocytes and neuronal changes.20,24
Data from studies evaluating cerebrospinal fluid (CSF) biomarkers in SCI indicate that neutrophils are an important component of the acute response to injury, as evidenced by significant upregulation of matrix metalloproteinase-9 (MMP-9) early after injury and the predominance of neutrophils in the CSF of acutely injured dogs.28 These findings closely mimic observations in acute stages of human SCI.29 An influx of MAC-387-positive blood-derived macrophages is observed in the acute stage of injury,30 and perivascular cuffing may occur in conjunction with infiltration of the meninges with leukocytes.20 Phagocytic cells are observed diffusely throughout the gray and white matter, primarily in areas of hemorrhage. Significant upregulation of pro-inflammatory cytokines such as interleukin (IL)-6, IL-8, IL-1β, and tumor necrosis factor is observed within the injured cord at this stage,30,31 paralleling rodent and human findings.32,33
Subacute (3 days–3 weeks; Fig. 1D–F)
Myelin edema is observed diffusely throughout the spinal cord parenchyma, and axonal degeneration and swelling extend both cranial and caudal to the lesion epicenter.24 Large numbers of debris-filled microglia/macrophages are observed within the cord, paralleled by marked upregulation of major histocompatibility complex class II, with roughly similar timing to peaks in microglial activation noted in humans with SCI.29,30 Enhanced numbers of glial fibrillary acidic protein-positive cells can be identified within the spinal ependymal lining of the central canal, consistent with astrocytic differentiation of endogenous neural precursor cells.34
Chronic changes (>3 weeks; Fig. 1G–I)
In rare reports of chronic cases, the spinal cord may show severe reduction of the cross-sectional area at the lesion epicenter. In parallel to marked gliosis, extensive oligodendrocyte remyelination of preserved axons has been reported in a case of chronic SCI.20 After severe injuries, there may be total loss of the organotypic architecture with complete dissolution of the spinal cord.35,36 Accentuated within the gray matter, there may be severe necrosis (malacia) with complete destruction of original tissue structure, which is replaced by abundant phagocytic microglia/macrophages and glial scar tissue. Histopathological studies focusing on chronic canine SCI lesions are so far limited and are thus highly needed to reveal commonalities and potential differences to advanced stages of naturally occurring human cases and experimental models, which, for instance, report formation of cystic cavities.26,27 The formation of cysts after SCI is highly variable across species including rats, mice, pigs, and nonhuman primates.37–41 In humans, a variable proportion of chronic cases of SCI is accompanied by the formation of cysts.26,27,29 Cystic cavities have been reported in dogs with chronic SCI20; however, the prevalence of this finding, particularly in the context of IVDH-associated SCI, requires further exploration.
Imaging
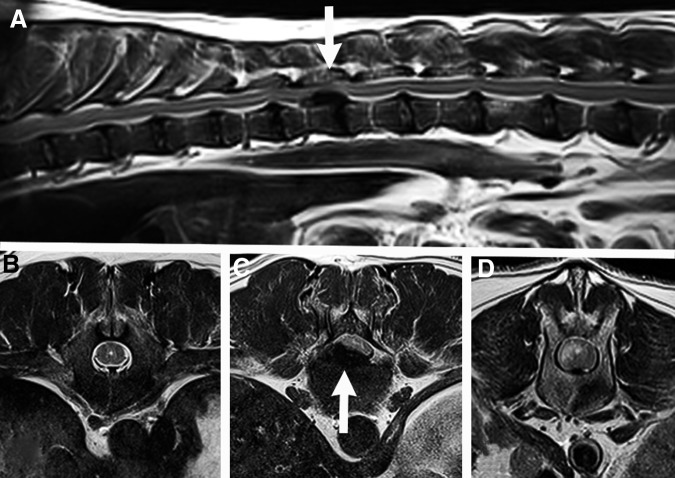
Magnetic resonance imaging (MRI) and computed tomography are routinely used in dogs with SCI to identify sites of spinal cord compression and parenchymal changes before surgical decompression paralleling the diagnostic approach used for humans with SCI (Fig. 2).42,43 While it is currently routine to use MRI to locate lesions and define compression severity, the role of MRI as a biomarker of disease/predictor of outcome is not yet well-defined.44,45 As in humans with traumatic myelopathies, hyperintensity within the canine spinal cord on T2-weighted images correlates with neurologic dysfunction measured by ordinal locomotor scores.44,46,47 Several studies have likewise found length of T2-hyperintensity within the spinal cord is negatively correlated with long-term recovery of ambulation.44,46,48,49 Further optimization of techniques is needed to enhance repeatability of MRI quantification of spinal cord lesions.47 Along with protocol refinement, the application of newer MRI sequences may be useful; the feasibility of DTI has been demonstrated in several recent studies.50–52
FIG. 2.
Sagittal (A) and transverse (B, C, D) T2-weighted 3.0T magnetic resonance images of the thoracolumbar spinal cord from a dog with an acute sensorimotor complete injury caused by an intervertebral disc herniation. At the T13-L1 vertebral junction (white arrows), there is severe extradural spinal cord compression caused by herniated intervertebral disc material. There is normal spinal cord proximal to the lesion (B), severe spinal cord compression at the lesion epicenter (C), and T2 hyperintensity within the spinal cord immediately caudal to the lesion (midbody of L1) (D).
Outcome Assessment
Most assessment tools used in dogs recapitulate the techniques that are used for humans including ordinal locomotor scales, kinematic assessments, quantitative sensory testing, and urodynamic studies.
Ordinal gait scales
Most basically, dogs can be categorized as “ambulatory” or “nonambulatory,” where the term ambulatory indicates that the animal can take at least 10 consecutive voluntary weight-bearing steps in both hind limbs without assistance and without falling.15,45 This provides a simple assessment of locomotor recovery, but does not discriminate between dogs who walk normally and those with a profoundly abnormal gait. It is most useful when applied to dogs with sensorimotor complete injuries, because a significant proportion (40–50%) of these animals do not regain the ability to walk with current standard of care therapy, which includes laminectomy to decompress the spinal cord.9,21,45,53 While not sensitive to small changes in locomotor recovery, it is possible that this scheme could be used as a primary outcome in a veterinary clinical trial. An intervention expected to result in an extra 15% of dogs with complete injuries converting to an ambulatory status would require a group size of approximately 160 (90% power, alpha 0.05).
Several ordinal scales have been adapted from the Frankel and Tarlov scales.54–56 These divide recovery into broad categories based on the presence or absence of pain perception, nonweight- and weight-bearing movement, and gait. These scales are easy to apply, but fail to discriminate phases of recovery once consistent weight-bearing steps are established, reaching a ceiling effect early in recovery after incomplete injury and providing little benefit over categorization as ambulatory or not for dogs that have complete injuries.
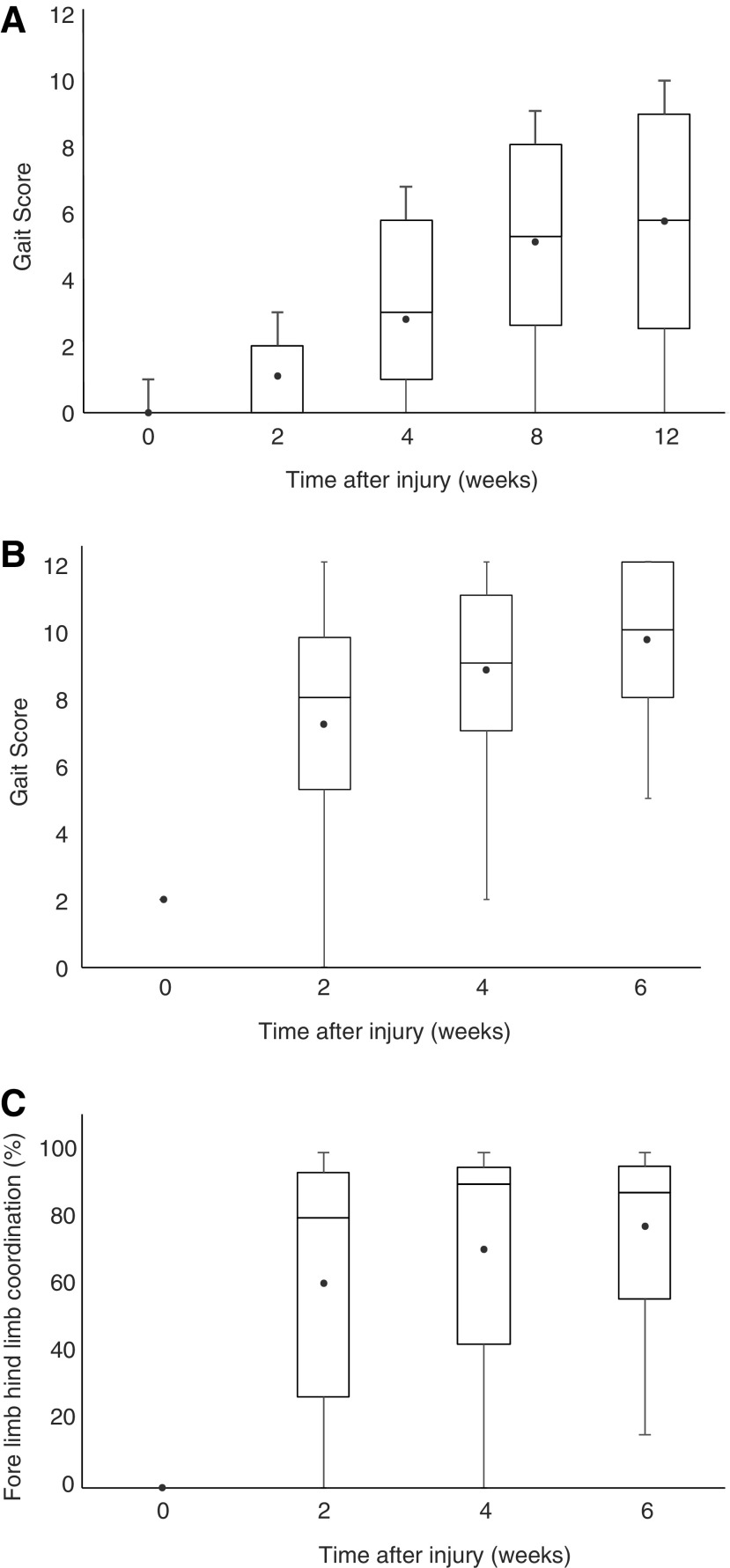
More complex ordinal locomotor scales have been developed and validated for dogs with clinical SCI and assist in discriminating recovery of dogs with incomplete SCI. These include the Texas SCI scale (TSCIS), the Open Field Score (OFS), and a modification of the Basso-Beattie-Bresnahan (BBB) scale—the canine BBB (cBBB).56–58 From a historical perspective, it should be noted that the BBB itself is a modified Tarlov scale, which was originally derived in dogs.54 Each of these locomotor assessments has been used in the canine clinical setting in single- or multi-institutional veterinary clinical studies in SCI. The OFS was recently used in a large-scale trial involving 13 clinical centers that evaluated the effects of several interventions in dogs with complete SCI.59 A goal of the consortium is to institute standardized training programs across centers to ensure strong interrater reliability for locomotor assessments in consortium-run trials. A wealth of historical data provides a basis for sample size determinations using ordinal gait scales as a primary outcome measure for future veterinary clinical trials (Fig. 3). For dogs with complete injuries, detecting a 20% improvement in locomotor score (on any of the three scales) by 6–12 weeks requires group sizes of approximately 40 dogs (90% power, alpha 0.05). The 6–12 week time frame in dogs is roughly equivalent to humans at 6 months after injury. If smaller but still clinically relevant improvements are to be detected, large sample sizes are required.
FIG. 3.
Box and Whiskers plots of hindlimb functional recovery after surgical decompression in dogs with clinical spinal cord injury, as measured by the Open Field Score (OFS). The upper and lower limits of the boxes represent the 25% and 75% quartiles, with the median transecting the box. The whiskers represent the maximum and minimum values and the circular point represents the mean. (A) Dogs with complete injuries show an incomplete recovery of ambulation, plateauing by 3 months after injury. (B) Dogs with incomplete injuries show robust recovery within two weeks. (C) In spite of motor recovery, dogs with incomplete injuries show an incomplete recovery of forelimb-hindlimb coordination quantified using the regularity index to express the number of steps occurring in a normal step cycle sequence. A score of 12 indicates “normal” using the OFS and normal dogs score 100 on the regularity index assessing forelimb-hindlimb coordination.
Treadmill-assisted walking and kinematics
A limitation of ordinal gait scales is that data are not continuous; meaning, intervals of improvement in function may not be equidistant from each other. An inexpensive method of generating continuous data on hindlimb function after SCI involves the evaluation of treadmill-assisted stepping and coordination scores. This technique has been described in dogs with various degrees of clinical SCI, with sling support provided for dogs with motor function to the hindlimbs that are unable to take unassisted weight-supported steps.60–62 It has also been used as a primary outcome measure in a recent canine clinical trial.63 Treadmill walking is videotaped at a standardized speed, and an observer scores a total of 50 step cycles. A “stepping score” is calculated as the ratio of hindlimb steps to forelimb steps, and the coordination can be assessed by calculating from the ratio of coordinated hindlimb steps to total hindlimb steps, or in the manner of Koopmans and associates,64 as a regulatory index, expressed as the percentage of total steps that are taken within a normally coordinated step cycle. There is a strong correlation between stepping score and the OFS, suggesting its utility to generate continuous locomotor data in dogs with clinical injury,62 and the incomplete recovery of forelimb-hindlimb coordination in dogs with an excellent recovery of stepping can be clearly documented using this technique.65
Kinematic assessment
Kinematic analysis provides an objective method for quantifying various aspects of limb movement in dogs that are able to step either with or without external weight support. To increase reproducibility and minimize inherent experimental variability, it is useful to assess dog ambulation on a treadmill.60 This allows observation of hundreds of step cycles in only a few minutes with cameras capturing the gait at >100 Hz. The use of a treadmill can induce stepping by stimulating intact central pattern generators,66 but kinematic evaluation of parameters such as forelimb-hindlimb coordination and lateral stability help differentiate “spinal walking” from true recovery of coordination.61,67
Kinematic outcomes after interventions in SCI-affected dogs have focused on thoracolumbar dysfunction, because this region is most commonly affected in canine clinical cases. Motion capture analysis provides a means by which incoordination can be objectively quantified. It also allows detection of more subtle changes in function that might arise as a result of a putative therapeutic intervention, but may not be observed by semi-quantitative or qualitative methods.60 This type of analysis is performed by attaching reflective markers to strategically important landmarks on the fore- and hindlimbs. The resulting numerical data can then be analyzed to produce summary values for the time delay between components of gait (such as the moment of lifting of the paw from the treadmill surface) between specific pairs of limbs. Normal dogs have extremely tight regulation of these temporal relationships, which is lost after SCI and then regained during spontaneous recovery after incomplete lesions.60 Kinematic assessment of gait has been used successfully to analyze the effect of cell transplantation into dogs with chronic SCI.14
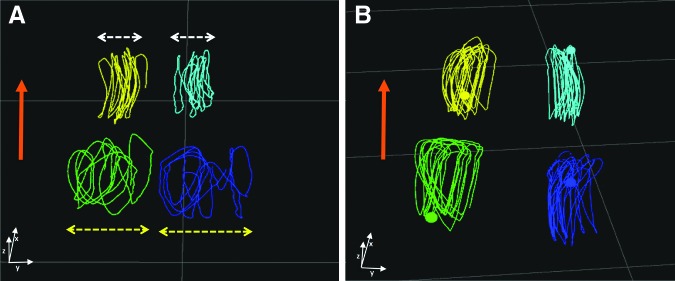
Kinematic assessments provide a menu of possibilities for quantification of subtle aspects of gait dysfunction after SCI. Even relatively mild spinal cord injuries will impair accuracy of paw placement in the lateral plane—i.e., paws of limbs of one girdle tend to be placed at an inconsistent distance from each other when viewed over a series of steps.61 This can be readily quantified using kinematics, where the distance between paws of consecutive steps can be measured and the coefficient of variation (standard deviation/mean) provides a simple summary measure of the variability (Fig. 4). Recovery of this variable to normal values is not always achieved even in dogs that recover independent ambulation after incomplete SCI. This permits objective analysis of quality of recovery after even mild injuries, thereby extending the population of injured dogs that can be used for future assessment of the impact of putative therapies.
FIG. 4.
Kinematic assessment of dogs with clinical spinal cord injury. Stick diagrams obtained from a dog during treadmill walking and filmed with infrared cameras detecting position of paw markers placed on the dog's fur; this dog had acute thoracolumbar spinal cord injury caused by intervertebral disc herniation; the dog is moving forward in the direction of the orange arrows and the three planes of space (x, y, z) are depicted at the bottom of (A) and (B); the thoracic limb step cycles appear in yellow and light blue and the pelvic limb step cycles appear in green and dark blue; this method allows kinematic assessment of gait in three dimensions. (A) is a recording 48 h after the onset of SCI: the degree of lateral instability (yellow double head arrows) can be summarized using a coefficient of variation compared with the thoracic limbs coefficient of variation that have a normal pattern in this dog with a thoracolumbar lesion (white double head arrows), 1.64 in this case. (B) is showing the recording of the same dog 2 weeks after onset of signs with improved pelvic lateral stability and a coefficient of variation ratio between pelvic and thoracic limbs of 0.9, returning to normal.
Urodynamic assessment
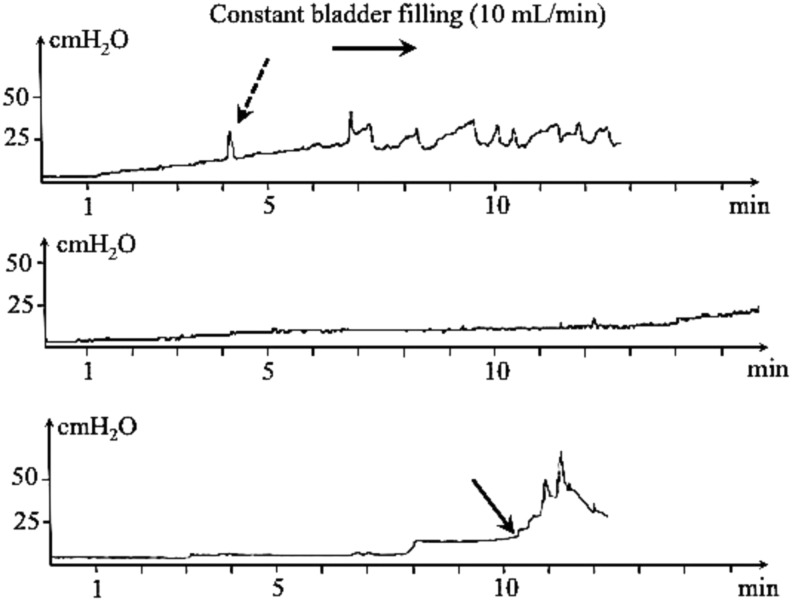
Similar to humans, severe SCI in pet dogs leads to dysfunction of urine voiding and storage.68–70 After recovery from the acute phase of injury, urinary incontinence in persistently paralyzed dogs is characterized by detrusor overactivity during bladder filling, lack of voluntary control of voiding, and detrusor-sphincter dyssynergia (Fig. 5). These result in frequent urination, large bladder residual volumes, and increased risk of urinary tract infection.
FIG. 5.
Cystometry curves obtained during bladder filling in dogs with chronic sensorimotor complete spinal cord injury (top and middle trace) and in a normal dog; the infused solution is sterile saline at a constant rate (10 mL/min, x-axis). The y-axis represents the bladder pressure (cmH20) measured simultaneously during bladder filling. The top trace depicts the recording in a dog with poor bladder compliance; involuntary detrusor contractions are detectable (dashed arrow) for small volumes. The middle trace represents bladder atony in a chronically paraplegic dog in which poor bladder management has led to distension of the detrusor and high compliance; the pressure remains low for a high infused saline volume. The bottom trace represents a normal cystometry curve in which bladder pressure slowly increases during bladder filling up to the micturition threshold (black arrow); this is followed by urination visible as a steep rise of pressure then declined as the bladder is emptied.
Bladder urodynamic function is monitored in humans because it improves clinical decision-making for management of incontinence and long-term outcome.71 In dogs with neurologically complete injuries, it can be performed without sedation using urodynamic equipment identical to that used in humans.72 Cystometry, urethral profilometry and sphincter electromyogram give access to a range of data in SCI-affected dogs, mirroring the basic urodynamic dataset proposed for humans.73 This includes detrusor activity such as number of involuntary detrusor contractions, bladder compliance, and sphincter activity during bladder filling and voiding, leak pressure point pressure, maximum detrusor pressure, and post-residual volume.
Cystometric characteristics of neurogenic bladder dysfunction in dogs with SCI are similar to those of spinal cord injured humans and consist of reduced cystometric bladder capacity, abnormally low compliance, and involuntary detrusor contractions.74 This suggests that the clinical dog model is suitable to test therapies directed at resolving incontinence after SCI, as evidenced by the testing of the Brindley system using a canine version of the sacral anterior root stimulator.75
Assessments of sensory function
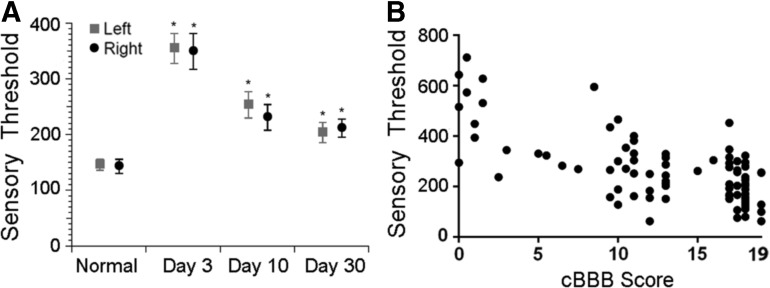
The canine clinical model of SCI may also provide a valuable model to study interventions targeted at somatosensory disturbances, the existence of which has been documented even in early studies of canine SCI by Tarlov.76 Investigation of neuropathic pain in dogs with SCI has hitherto been limited, likely because the phenomenon in humans typically requires self-reports on sensation. In addition, because pet dogs with SCI are clinical “patients,” an acclimation period to techniques and investigators as is suggested in rodents is not feasible. Two modalities for quantitative sensory testing have recently been validated in canine clinical models of neuromusculoskeletal disease: measurement of mechanical sensory threshold using various algometers, and thermal threshold testing.77,78 Specifically, both have been explored in acute moderate to severe SCI, revealing significant differences between normal and SCI-affected dogs, and recovery of sensory threshold toward normal as locomotor status improves (Fig. 6). Future studies focusing on the natural history of chronic somatosensory dysfunction in dogs with SCI will be instrumental in defining the role of dogs as a translational model of neuropathic pain.
FIG. 6.
Quantitative sensory testing using an electronic von Frey anesthesiometer in dogs with incomplete thoracolumbar spinal cord injury. (A) Comparison of left and right hindlimb sensory threshold (grams) between normal dogs (n = 20) and dog with incomplete spinal cord injury (n = 29) at days 3, 10, and 30 after injury. Sensory threshold is significant higher in SCI-affected dogs at all three time points evaluated, and sensory threshold decreases over time, consistent with either sensory recovery or the development of hyperesthesia. Mean ± standard error of the mean are presented and asterisk denotes p < 0.05. Image reprinted with permission.77 (B) Sensory thresholds (grams) in SCI-affected dogs are also inversely correlated with locomotor score as measured by the canine Basso-Beattie-Bresnahan (BBB) (cBBB) scale (r = - 0.68; p < 0.0001). Image reprinted with permission.58
Blood and Cerebrospinal Fluid Biomarkers
Serum and CSF biomarkers have been identified in canine SCI using targeted approaches based on data from human and rodent studies. These closely parallel the human and experimental neurotrauma literature (Appendix 2; see online supplementary material at ftp.liebertpub.com). Much of this work has focused on CSF acquired from the cerebellomedullary cistern (above the lesion site) at the time of SCI and has shown that a variety of structural, inflammatory, protein, cellular, and metabolite markers correlate with injury severity and long-term locomotor recovery. Blood-based biomarkers of injury hold tremendous promise because of the ease of sample acquisition and the ability to obtain longitudinal measures to correlate with outcome. Early work has shown correlation between serum phosphorylated neurofilament, injury severity, and outcome.79 Serial measurement of serum MMP-2/MMP-9 activity was also recently used to assess the pharmacodynamics of a broad-spectrum MMP inhibitor in dogs with SCI.15 Thus, there is an opportunity to use biomarkers for stratification of enrollment in clinical trials, serial measurement of pathologic processes, pharmacodynamics, and even as surrogate therapeutic trial end-points.
Canine Spinal Cord Injury Trials
The notion that pet dogs can be used in SCI research is not a new one. In fact, veterinary SCI studies have played an important role in early development of treatments such as polyethylene glycol (PEG),13 oscillating electrical fields,11,12 and 4-aminopyridine (4-AP).10 Most notably, dogs with SCI played a pivotal role in translation of 4-AP to the human market. A phase I veterinary clinical trial of 4-AP was originally completed in 39 dogs comprising both chronic complete and incomplete SCI, most caused by acute IVDH.10 The trial supported the utility of the drug to treat conduction block in certain dogs with chronic paralysis, and the drug subsequently moved on to human clinical trials.80–86 Although the drug ultimately failed to meet primary end-points in larger randomized trials in SCI,85,86 it was eventually approved for persons with multiple sclerosis based on two phase III trials demonstrating a significant effect on walking speed in that population.87,88
More recently, several randomized, placebo-controlled studies have demonstrated that it is feasible to use the clinical dog model for large-scale translational studies to pursue second-species confirmation of promising laboratory interventions by exploiting the added benefits of the veterinary clinical setting to mirror a human clinical trial. The types of interventions have ranged from acute neuroprotective strategies to pharmacological interventions in chronic injury to cell-based therapies.
Two acute neuroprotective studies have been completed recently using the clinical dog model. The first was a randomized, placebo-controlled study that followed the Consolidated Standards of Reporting Trials—known as CONSORT; see www.consort-statement.org—guidelines and assessed a metalloproteinase inhibitor (GM6001) in a population of dogs with both complete and incomplete injuries of fewer than 48 h duration.15 Dogs received a single subcutaneous injection of GM6001 in dimethyl sulfoxide (DMSO) to achieve blood drug levels required to inhibit metalloproteinase activity for a brief period. Control groups consisted of dogs receiving a saline injection and those receiving a DMSO injection at the same time points. The primary outcome measure was the TSCIS score at 42 days after injury. A significant reduction in serum MMP-2/MMP-9 activity was observed in the GM6001 group at three days after injury, and there was improved functional recovery for dogs with complete injuries that received both DMSO alone and GM6001/DMSO. While different from saline placebo, outcome in these two groups did not differ from each other, suggesting that DMSO was likely the mechanism by which recovery was enhanced.
The second was a multi-institutional placebo-controlled, randomized veterinary clinical trial evaluating the effects of PEG and methylprednisolone sodium succinate (MPSS) on outcome in acute SCI.59 The study enrolled dogs with sensorimotor complete injuries of less than 24 h duration from 13 participating trial centers. Dogs were randomized to one of three treatment groups (placebo, MPSS, PEG), and locomotor function was assessed at 2, 4, 8, and 12 weeks post-operatively using the OFS. Primary outcomes measures were OFS score at 12 weeks and ability to walk (“yes” or “no”) at 12 weeks. Secondary outcomes such as presence of pain sensation, OFS score, and ability to walk at 2, 4, and 8 weeks were also evaluated. The study failed to detect a treatment effect of either PEG or MPSS at 12 weeks after injury, and there was no difference between groups with respect to secondary outcome measures. The study was terminated after an interim analysis and conditional power calculations suggested futility of continued case recruitment to show significant treatment effects.
There are also several recent studies that have used a population of chronically injured dogs to model the situation of humans with chronic injury.14,63,75,89 One such study evaluated the use of two pharmacological interventions—4-AP and the t-butyl carbamate derivative of 4-AP in dogs with chronic SCI using a blinded, placebo-controlled crossover design.63 All dogs enrolled in this study had static paraplegia or nonambulatory paraparesis for a duration of at least three months. The primary outcome measures were OFS score and a treadmill-based stepping and coordination scores. Secondary outcomes such as blinded owner assessments of function and neurological examination findings were also evaluated. Statistically significant improvements in supported, but not unsupported, treadmill stepping and in OFS scores were noted in the dogs during treatment with both 4-AP and the t-butyl derivative. Interestingly, while the generation of unsupported steps was not statistically different from placebo, three dogs improved from being unable to generate unsupported weight-bearing steps to independent stepping with 4-AP (n = 3) or the t-butyl derivative (n = 2), highlighting the potential value of these drugs in a subset of chronically paralyzed persons.
Last, a well-publicized, randomized, double-blind trial that evaluated the effects of intraspinal olfactory ensheathing cell (OEC) transplantation on locomotor outcome was completed recently in dogs.14 In this study, dogs with sensorimotor complete injuries of greater than 3 months received intraparenchymal injections of OECs or the cell transport media alone. The primary outcome measure was kinematic assessment of forelimb-hindlimb coordination over 50 steps during treadmill walking. A variety of secondary outcomes were also assessed, including lateral stability during treadmill walking, somatosensory-evoked potentials, transcranial magnetic motor-evoked potentials, and bladder compliance.
Based on the primary outcome measure, a significant treatment effect was observed in the OEC treatment group, but other assessments of long tract function such as lateral stability, continence, and evoked potentials did not improve with treatment. The authors suggest that these results reflect plastic changes in propriospinal connections and do not imply restoration of brain control over hindlimb motion. This study underscores the feasibility of conducting investigations of cell-based therapies in dogs with clinical SCI. It also suggests that OECs are efficacious to restore motor function that is not under brain control, but used alone are unlikely to provide meaningful return of complex functions such as balance or continence. This suggests that a combinatorial strategy is necessary to repair the spinal cord and improve long tract function in the chronic phase of injury. Several other transplantation studies using cell-based therapies of various types have also been completed using the clinical dog model of SCI.90–96 The results of these transplantation studies have been summarized recently elsewhere.97
Conclusion
Dogs with clinical SCI can address an important translational gap in the field of SCI research. The clinical dog model of SCI parallels the human condition with respect to patient and lesion heterogeneity, clinical management, available outcome assessment tools, and spinal cord histopathology. Compared with most laboratory animals, the dog spinal cord is of more comparable size to that of humans, allowing for the study of interventions at a relevant scale and with outcome assessments similar to those used in human trials. Moreover, there is a large group of chronically paralyzed pet dogs available for study of chronic injury. Despite being considered a research priority by the human SCI community, experimental studies specifically aimed at the chronic injury state are costly and logistically challenging. The canine clinical model of SCI presents a unique opportunity to study chronic injury in a group of pet dogs living with SCI, and treatment effects in this model may be more predictive of outcome in human trials than laboratory studies.
Our multicenter international consortium, CANSORT-SCI, manages a high volume of both acute and chronic clinical SCI in dogs. This offers the opportunity to efficiently conduct rigorous “clinical trials” in the veterinary setting before taking an intervention into humans. The significant body of published work reviewed above indicates that the strength of the model lies in its utilization for translational studies to provide important assessments of promising laboratory interventions. Further, CANSORT-SCI is an example of the One Health Initiative, a worldwide strategy for expanding interdisciplinary collaborations and communications in all aspects of health care for humans, animals, and the environment. The studies reviewed here support continued and expanded use of dogs with clinical SCI to enhance translation from benchtop to the human bedside with the overall goal being to improve functional outcome for persons and animals affected by SCI.
Supplementary Material
Acknowledgments
Support for the inaugural CANSORT-SCI meeting was provided by Mission Connect, grant #015-105. Additional support was provided by R01 NS074882-06 and R01 NS39278. The authors gratefully acknowledge Dr. John Steeves for his valuable perspective on translational SCI models.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.World Health Organization. Available at: www.who.int/mediacentre/factsheets/fs384/en/ Accessed March7, 2017
- 2.Curt A., Van Hedel H.J., Klaus D., Dietz V., and EM-SCI Study Group. (2008). Recovery from a spinal cord injury: significance of compensation, neural plasticity, and repair. J. Neurotrauma 25, 677–685 [DOI] [PubMed] [Google Scholar]
- 3.National Spinal Cord Injury Statistical Center, Spinal Cord Injury (SCI) Facts and Figures at a Glance. Available at: www.nscisc.uab.edu Accessed March7, 2017
- 4.Kwon B.K., Hillyer J., and Tetzlaff W. (2010). Translational research in spinal cord injury: a survey of opinion from the SCI community. J. Neurotrauma 27, 21–33 [DOI] [PubMed] [Google Scholar]
- 5.Kwon B.K., Streijger F., Hill C.E., Anderson A.J., Bacon M., Beattie M.S., Blesch A., Bradbury E.J., Brown A., Bresnahan J.C., Case C.C., Colburn R.W., David S., Fawcett J.W., Ferguson A.R., Fischer I., Floyd C.L., Gensel J.C., Houle J.D., Jakeman L.B., Jeffery N.D., Jones L.A., Kleitman N., Kocsis J., Lu .P, Magnuson D.S., Marsala M., Moore S.W., Mothe A.J., Oudega M., Plant G.W., Rabchevsky A.S., Schwab J.M., Silver J., Steward O., Xu X.M., Guest J.D., and Tetzlaff W. (2015). Large animal and primate models of spinal cord injury for the testing of novel therapies. Exp. Neurol. 269, 154–168 [DOI] [PubMed] [Google Scholar]
- 6.Chase K., Sargan D., Miller K., Ostrander E.A., and Lark K.G. (2006). Understanding the genetics of autoimmune disease: two loci that regulate late onset Addison's disease in Portuguese Water Dogs. Int. J. Immunogenet. 33,179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beltran W.A., Cideciyan A.V., Lewin A.S., Iwabe S., Khanna H., Sumaroka A., Chiodo V.A., Fajardo D.S., Roman A.J., Deng W.T., Swider M., Aleman T.S., Boye S.L., Genini S., Swaroop A., Hauswirth W.W., Jacobson S.G., and Aquirre G.D. (2012). Gene therapy rescues photoreceptor blindness in dogs and paves the way for treating human X-linked retinitis pigmentosa. Proc. Natl. Acad. Sci. U. S. A. 109, 2132–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeBlanc A.K., Mazcko C., Brown D.E., Koehler J.W., Miller A.D., Miller C.R., Bentley R.T., Packer R.A., Breen M., Boudreau C.E., Levine J.M., Simpson R.M., Halsey C., Kisseberth W., Rossmeisl J.H., Jr., Dickinson P.J., Fan T.M., Corps K., Aldape K., Puduvalli V., Pluhar G.E., and Gilbert M.R. (2016). Creation of an NCI comparative brain tumor consortium: informing the translation of new knowledge from canine to human brain tumor patients. Neuro. Oncol. 14, pii: now051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore S.A., Early P.J., and Hettlich B.F. (2016). Practice patterns in the management of acute intervertebral disc herniation in dogs. J. Small Anim. Pract. 57, 409–415 [DOI] [PubMed] [Google Scholar]
- 10.Blight A.R., Toombs J.P., Bauer M.S., and Widmer W.R. (1991). The effects of 4-aminopyridine on neurological deficits in chronic cases of traumatic spinal cord injury in dogs: a phase I clinical trial. J. Neurotrauma 8, 103–119 [DOI] [PubMed] [Google Scholar]
- 11.Borgens R.B., Toombs J.P., Blight A.R., McGinnis M.E., Bauer M.S., Widmer W.R., and Cook J.R., Jr. (1993). Effects of applied electric fields on clinical cases of complete paraplegia in dogs. Restor. Neurol. Neurosci. 5, 305–322 [DOI] [PubMed] [Google Scholar]
- 12.Borgens R.B., Toombs J.P., Breur G., Widmer W.R., Waters D., Harbath A.M., March P., and Adams L.G. (1999). An imposed oscillating electrical field improves the recovery of function in neurologically complete paraplegic dogs. J. Neurotrauma 16, 639–657 [DOI] [PubMed] [Google Scholar]
- 13.Laverty P.H., Leskovar A., Breur G.J., Coates J.R., Bergman R.L., Widmer W.R., Toombs J.P., Shapiro S., and Borgens R.B. (2004). A preliminary study of intravenous surfactants in paraplegic dogs: polymer therapy in canine clinical SCI. J. Neurotrauma 21, 1767–1777 [DOI] [PubMed] [Google Scholar]
- 14.Granger N., Blamires H., Franklin R.J., and Jeffery N.D. (2012). Autologous olfactory mucosal cell transplants in clinical spinal cord injury: a randomized double-blinded trial in a canine translational model. Brain 135, 3227–3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine J.M., Cohen N.D., Heller M., Fajt V.R., Levine G.J., Kerwin S.C., Trivedi A.A., Fandel T.M., Werb Z., Modestino A., and Noble-Haeusslein L.J. (2014). Efficacy of a metalloproteinase inhibitor in spinal cord injured dogs. PLoS One 9, e96408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rigor and Reproducibility. Available at: www.nih.gov/research-training/rigor-reproducibility Accessed March6, 2017
- 17.van Middendorp J.J., Allison H.C., Ahuja S., Bracher D., Dyson C., Fairbank J., Gall A., Glover A., Gray L., Masri W.E., Uttridge A., and Cowan K. (2016). Top ten research priorities for spinal cord injury: the methodology and results of a British priority setting partnership. Spinal Cord 54, 341–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olby N., Harris T., Burr J., Munana K., Sharp N., and Keene B. (2004). Recovery of pelvic limb function in dogs following acute intervertebral disc herniations. J. Neurotrauma 21, 49–59 [DOI] [PubMed] [Google Scholar]
- 19.Bergknut N., Egenvall A., Hagman R., Gustas P., Hazewinkel H.A., Meij B.P., and Lagerstedt A.S. (2012). Incidence of intervertebral disk degeneration-related diseases and associated mortality rates in dogs. J Am. Vet. Med. Assoc. 240, 1300–1309 [DOI] [PubMed] [Google Scholar]
- 20.Smith P.M., and Jeffery N.D. (2006). Histological and ultrastructural analysis of white matter damage after naturally-occurring spinal cord injury. Brain Pathol. 16, 99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olby N., Levine J., Harris T., Munana K., Skeen T., and Sharp N. (2003). Long-term functional outcome of dogs with severe injuries of the thoracolumbar spinal cord: 87 cases (1996–2001). J. Am. Vet. Med. Assoc. 222, 762–769 [DOI] [PubMed] [Google Scholar]
- 22.Granger N., and Carwardine D. (2014). Acute spinal cord injury: tetraplegia and paraplegia in small animals. Vet. Clin. North Am. Small Anim. Pract. 44, 1131–1156 [DOI] [PubMed] [Google Scholar]
- 23.Griffiths I.R. (1972). Some aspects of the pathology and pathogenesis of the myelopathy caused by disc protrusions in the dog. J. Neurol. Neurosurg. Psychiatry 35, 403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bock P., Spitzbarth I., Haist V., Stein V.M., Tipold A., Puff C., Beineke A., and Baumgartner W. (2013). Spatio-temporal development of axonopathy in canine intervertebral disc disease as a translational large animal model for nonexperimental spinal cord injury. Brain Pathol. 23, 82–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henke D., Gorgas D., Doherr M.G., Howard J., Forterre F., and Vandevelde M. (2016). Longitudinal extension of myelomalacia by intramedullary and subdural hemorrhage in a canine model of spinal cord injury. Spine J. 16, 82–90 [DOI] [PubMed] [Google Scholar]
- 26.Bunge R.P., Puckett W.R., Becerra J.L., Marcillo A., and Quencer R.M. (1993). Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic compression with extensive focal demyelination. Adv. Neurol. 59, 75–89 [PubMed] [Google Scholar]
- 27.Kakulas B.A. (1999). A review of the neuropathology of human spinal cord injury with emphasis on special features. J. Spinal Cord Med. 22, 119–124 [DOI] [PubMed] [Google Scholar]
- 28.Levine J.M., Ruaux C.G., Bergman R.L., Coates J.R., Steiner J.M., and Williams D.A. (2006). Matrix metalloproteinase-9 activity in the cerebrospinal fluid and serum of dogs with acute spinal cord trauma from intervertebral disk disease. Am. J. Vet. Res. 67, 283–287 [DOI] [PubMed] [Google Scholar]
- 29.Fleming J.C., Norenberg M.D., Ramsay D.A., Dekaban G.A., Marcillo A.E., Saenz A.D., Pasquale-Styles M., Dietrich W.D., and Weaver L.C. (2006). The cellular inflammatory response in human spinal cords after injury. Brain 129, 3249–3269 [DOI] [PubMed] [Google Scholar]
- 30.Spitzbarth I., Bock P., Haist V., Stein V.M., Tipold A., Wewetzer K., Baumgartner W., and Beineke A. (2011). Prominent microglial activation in the early proinflammatory immune response in naturally occurring canine spinal cord injury. J. Neuropathol. Exp. Neurol. 70, 703–714 [DOI] [PubMed] [Google Scholar]
- 31.Moore S.A., and Oglesbee M.J. (2012). Involvement of the choroid plexus in the inflammatory response after acute spinal cord injury in dogs: an immunohistochemical study. Vet. Immunol. Immunopathol. 148, 348–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Popovich P.G., Wei P., and Stokes B.T. (1997) Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J. Comp. Neurol. 377, 443–464 [DOI] [PubMed] [Google Scholar]
- 33.Schmitt A.B., Buss A., Breuer S., Brook G.A., Pech K., Martin D., Schoenen J., Noth J., Love S., Schroder J.M., Kreutzberg G.W., and Nacimiento W. (2000). Major histocompatibility complex class II expression by activated microglia caudal to lesions of descending tracts in the human spinal cord is not associated with a T cell response. Acta Neuropathol. 100, 528–536 [DOI] [PubMed]
- 34.Moore S.A., and Oglesbee M.J. (2015). Spinal cord ependymal responses to naturally occurring traumatic spinal cord injury in dogs. Vet. Pathol. 52, 1108–1117 [DOI] [PubMed] [Google Scholar]
- 35.Lee J.H., Choi C.B., Chung D.J., Kang E.H., Chang H.S., Hwang S.H., Han H., Choe B.Y., Sur J.H., Lee S.Y., and Kim H.Y. (2008). Development of an improved canine model of percutaneous spinal cord compression injury by balloon catheter. J. Neurosci. Methods 167, 310–316 [DOI] [PubMed] [Google Scholar]
- 36.Henke D., Vandevelde M., Doherr M.G., Stockli M., and Forterre F. (2013). Correlations between severity of clinical signs and histopathological changes in 60 dogs with spinal cord injury associated with acute thoracolumbar intervertebral disc disease. Vet. J. 198, 70–75 [DOI] [PubMed] [Google Scholar]
- 37.Sroga J.M., Jones T.B., Kigerl K.A., McGaughy V.M., and Popovich P.G. (2003). Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J. Comp. Neurol. 462, 223–240 [DOI] [PubMed] [Google Scholar]
- 38.Iwanami A., Yamane J., Katoh H., Nakamura M., Momoshima S., Ishii H., Tanioka Y., Tamaoki H., Nomura T., Toyama Y., and Okano H. (2005). Establishment of granded spinal cord injury model in a nonhuman primate: the common marmoset. J. Neurosci. Res. 80, 172–181 [DOI] [PubMed] [Google Scholar]
- 39.Lee J.H., Jones C.F., Okon E.B., Anderson L., Tigchelaar S., Kooner P., Godbey T., Chua B., Gray G., Hildebrandt R., Cripton P., Tetzlaff W., and Kwon B.K. (2013). A novel porcine model of traumatic thoracic spinal cord injury. J. Neurotrauma 30, 142–159 [DOI] [PubMed] [Google Scholar]
- 40.Salegio E.A., Bresnahan J.C., Sparrey C.J., Camisa W., Fischer J., Leasure J., Buckley J., Nout-Lomas Y.S., Rosenzweig E.S., Moseanko R., Strand S., Hawbecker S., Lemoy M.J., Haefeli J., Ma X., Nielson J.L., Edgerton V.R., Ferguson A.R., Tuszynski M.H., and Beattie M.S. (2016). A unilateral cervical spinal cord contusion injury model in non-human primates (Macaca mulatta). J. Neurotrauma 33, 439–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma Z., Zhang Y.P., Liu W., Yan G., Li Y., Shields L.B., Walker M., Chen K., Huang W., Kong M., Lu Y., Brommer B., Chen X., Xu X.M., and Shields C.B. (2016). A controlled spinal cord contusion for the rhesus macaque monkey. Exp. Neurol. 279, 261–273 [DOI] [PubMed] [Google Scholar]
- 42.Alexander M.S., Anderson K.D., Biering-Sorensen F., Blight A.R., Brannon R., Bryce T.N., Creasey G., Catz A., Curt A, Donovan W., Ditunno J., Ellaway P., Finnerup N.B., Graves D.E., Haynes B.A., Heinemann A.W., Jackson A.B., Johnston M.V., Kalpakjian C.Z., Kleitman N., Krassioukov A., Krogh K., Lammertse D., Magasi S., Mulcahey M.J., Schurch B., Sherwood A., Steeves J.D., Stiens S., Tulsky D.S., van Hedel H.J., and Whiteneck G. (2009). Outcome measures in spinal cord injury: recent assessments and recommendations for future directions. Spinal Cord 47, 582–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cooper J.J., Young B.D., Griffin J.F., 4th, Fosgate G.T., and Levine J.M. (2014). Comparison between noncontrast computed tomography and magnetic resonance imaging for detection and characterization of thoracolumbar myelopathy caused by intervertebral disk herniation in dogs. Vet. Radiol. Ultrasound 55, 182–189 [DOI] [PubMed] [Google Scholar]
- 44.Levine J.M., Fosgate G.T., Chen A.V., Rushing R., Nghiem P.P., Platt S.R., Bagley R.S., Kent M., Hicks D.G., Young B.D., and Schatzberg S.J. (2009). Magnetic resonance imaging in dogs with neurologic impairment due to acute thoracic and lumbar intervertebral disk herniation. J. Vet. Intern. Med. 23, 1220–1226 [DOI] [PubMed] [Google Scholar]
- 45.Jeffery N.D., Barker A.K., Hu H.Z., Alcott C.J., Kraus K.H., Scanlin E.M., Granger N., and Levine J.M. (2016). Factors associated with recovery from paraplegia in dogs with loss of pain perception in the pelvic limbs following intervertebral disk herniation. J. Am. Vet. Med. Assoc. 248, 386–394 [DOI] [PubMed] [Google Scholar]
- 46.Ito D., Matsunaga S., Jeffery N.D., Sasaki N., Nishimura R., Mochizuki M., Kasahara M., Fujiwara R., and Ogawa H. (2005). Prognostic value of magnetic resonance imaging in dogs with paraplegia caused by thoracolumbar intervertebral disk extrusion: 77 cases (2000–2003). J. Am. Vet. Med. Assoc. 227, 1454–1460 [DOI] [PubMed] [Google Scholar]
- 47.Griffin J.F., Davis M.C., Ji J.X., Cohen N.D., Young B.D., and Levine J.M. (2015). Quantitative magnetic resonance imaging in a naturally occurring canine model of spinal cord injury. Spinal Cord 53, 278–284 [DOI] [PubMed] [Google Scholar]
- 48.Boekhoff T.M., Flieshardt C., Ensinger E.M., Fork M., Kramer S., and Tipold A. (2012). Quantitative magnetic resonance imaging characteristics: evaluation of prognostic value in the dog as a translational model for spinal cord injury. J. Spinal Disord. Tech. 25, E81–E87 [DOI] [PubMed] [Google Scholar]
- 49.Okada M., Kitagawa M., Ito D., Itou T., Kanayama K., and Sakai T. (2010). Magnetic resonance imaging features and clinical signs associated with presumptive and confirmed progressive myelomalacia in dogs: 12 cases (1997–2008). J. Am. Vet. Med. Assoc. 237, 1160–1165 [DOI] [PubMed] [Google Scholar]
- 50.Pease A., Miller R. (2011). The use of diffusion tensor imaging to evaluate the spinal cord in normal and abnormal dogs. Vet. Radiol. Ultrasound 52, 492–497 [DOI] [PubMed] [Google Scholar]
- 51.Griffin J.F., 4th, Cohen N.D., Young B.D., Eichelberger B.M., Padua A., Jr., Purdy D., and Levine J.M. (2013). Thoracic and lumbar spinal cord diffusion tensor imaging in dogs. J. Magn. Reson. Imaging 37, 632–641 [DOI] [PubMed] [Google Scholar]
- 52.Hobert M.K., Stein V.M., Dziallas P., Ludwig D.C., and Tipold A. (2013). Evaluation of normal appearing spinal cord by diffusion tensor imaging, fiber tracking, fractional anisotropy, and apparent diffusion coefficient measurement in 13 dogs. Acta Vet. Scand. 55, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aikawa T., Fujita H., Kanazono S., Shibata M., and Yoshigae Y. (2012). Long-term neurologic outcome of hemilaminectomy and disk fenestration for treatment of dogs with thoracolumbar intervertebral disk herniation: 831 cases (2000–2007). J. Am. Vet. Med. Assoc. 241, 1617–1626 [DOI] [PubMed] [Google Scholar]
- 54.Tarlov I.M., and Klinger H. (1954). Spinal cord compression studies. II. Time limits for recovery after acute compression in dogs. AMA Arch. Neurol. Psychiatry 71, 271–290 [PubMed] [Google Scholar]
- 55.Frankel H.L., Hancock D.O., Hyslop G., Melzak J., Michaelis L.S., Ungar G.H., Vernon J.D., and Walsh J.J. (1969). The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia 7, 179–192 [DOI] [PubMed] [Google Scholar]
- 56.Levine G.J., Levine J.M., Budke C.M., Kerwin S.C., Au J., Vinayak A., Hettlich B.F., and Slater M.R. (2009). Description and repeatability of a newly developed spinal cord injury scale for dogs. Prev. Vet. Med. 89, 121–127 [DOI] [PubMed] [Google Scholar]
- 57.Olby N.J., De Risio L., Munana K.R., Wosar M.A., Skeen T.M., Sharp N.J., and Keene B.W. (2001). Development of a functional scoring system in dogs with acute spinal cord injuries. Am. J. Vet. Res. 62, 1624–1628 [DOI] [PubMed] [Google Scholar]
- 58.Song R.B., Basso D.M., da Costa R.C., Fisher L.C., Mo X., and Moore S.A. (2016). Adaptation of the Basso-Beattie-Bresnahan locomotor rating scale for use in a clinical model of spinal cord injury in dogs. J. Neurosci. Methods 268, 117–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olby N.J., Muguet-Chanoit A.C., Lim J.H., Davidian M., Mariani C.L., Freeman A.C., Platt S.R., Humphrey J., Kent M., Giovanella C., Longshore R., Early P.J., and Munana K.R. (2016). A placebo-controlled, prospective, randomized clinical trial of polyethylene glycol and methylprednisolone sodium succinate in dogs with intervertebral disk herniation. J. Vet. Intern. Med. 30, 206–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamilton L., Franklin R.J., and Jeffery N.D. (2007). Development of a universal measure of quadrupedal forelimb-hindlimb coordination using digital motion capture and computerised analysis. BMC Neurosci. 8:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamilton L., Franklin R.J., and Jeffery N.D. (2008). Quantification of deficits in lateral paw positioning after spinal cord injury in dogs. BMC Vet. Res. 4, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olby N.J., Lim J.H., Babb K., Bach K., Domaracki C., Williams K., Griffith E., Harris T., and Muguet-Chanoit A. (2014). Gait scoring in dogs with thoracolumbar spinal cord injuries when walking on a treadmill. BMC Vet Res 10, 6148-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lim J.H., Muguet-Chanoit A.C., Smith D.T., Laber E., and Olby N.J. (2014). Potassium channel antagonists 4-aminopyridine and the T-butyl carbamate derivative of 4-aminopyridine improve hind limb function in chronically non-ambulatory dogs; a blinded, placebo-controlled trial. PLoS One 9, e116139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koopmans G.C., Deumens R., Honig W.M., Hamers F.P., Steinbusch H.W., and Joosten E.A. (2005). The assessment of locomotor function in spinal cord injured rats: the importance of objective analysis of coordination. J. Neurotrauma 22, 214–225 [DOI] [PubMed] [Google Scholar]
- 65.Rousse C.A., Olby N.J., Williams K., Harris T.L., Griffith E.H., Mariani C.L., Munana K.R., and Early PJ. (2016). Recovery of stepping and coordination in dogs following acute thoracolumbar intervertebral disc herniations. Vet. J. 213, 59–63 [DOI] [PubMed] [Google Scholar]
- 66.Grillner S., and Wallen P. (1985). Central pattern generators for locomotion, with special reference to vertebrates. Annu. Rev. Neurosci. 8, 233–261 [DOI] [PubMed] [Google Scholar]
- 67.Leblond H., L'Esperance M., Orsal D., and Rossignol S. (2003). Treadmill locomotion in the intact and spinal mouse. J. Neurosci. 23, 11411–11419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu C.W., Attar K.H., Gall A., Shah J., and Craggs M. (2010). The relationship between bladder management and health-related quality of life in patients with spinal cord injury in the UK. Spinal Cord 48, 319–324 [DOI] [PubMed] [Google Scholar]
- 69.Freeman P., Holmes M., Jeffery N., and Granger N. (2013). Time requirement and effect on owners of home-based management of dogs with severe chronic spinal cord injury. J. Vet. Behav. 8, 439–443 [Google Scholar]
- 70.Lidal I.B., Snekkevik H., Aamodt G., Hjeltnes N., Biering-Sorensen F., and Stanghelle J.K. (2007). Mortality after spinal cord injury in Norway. J. Rehabil. Med. 39, 145–151 [DOI] [PubMed] [Google Scholar]
- 71.Linsenmeyer T.A., and Linsenmeyer M.A. (2013). Impact of annual urodynamic evaluations on guiding bladder management in individuals with spinal cord injuries. J. Spinal Cord Med. 36, 420–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jeffery N.D., Hamilton L., and Granger N. (2011). Designing clinical trials in canine spinal cord injury as a model to translate successful laboratory interventions into clinical practice. Vet. Rec. 168, 102–107 [DOI] [PubMed] [Google Scholar]
- 73.Biering-Sorensen F., Craggs M., Kennelly M., Schick E., and Wyndaele J.J. (2008). International urodynamic basic spinal cord injury data set. Spinal Cord 46, 513–516 [DOI] [PubMed] [Google Scholar]
- 74.Allio B.A., and Peterson A.C. (2016). Urodynamic and physiologic patterns associated with the common causes of neurogenic bladder in adults. Transl. Androl. Urol. 5, 31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Granger N., Chew D., Fairhurst P., Fawcett J.W., Lacour S.P., Craggs M., Mosse C.A., Donaldson N., and Jeffery N.D. (2013). Use of an implanted sacral nerve stimulator to restore urine voiding in chronically paraplegic dogs. J. Vet. Intern. Med. 27, 9–-105 [DOI] [PubMed] [Google Scholar]
- 76.Tarlov I.M. (1954). Spinal cord compression studies. III. Time limits for recovery after gradual compression in dogs. AMA Arch. Neurol. Psychiatry 71, 588–597 [PubMed] [Google Scholar]
- 77.Song R.B., Basso D.M., da Costa R.C., Fisher L.C., Mo X., and Moore S.A. (2016). von Frey anesthesiometry to assess sensory impairment after acute spinal cord injury caused by thoracolumbar intervertebral disc extrusion in dogs. Vet. J. 209, 144–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gorney A.M., Blau S.R., Dohse C.S., Griffith E.H., Williams K.D., Lim J.H., Knazovicky D., Lascelles B.D., and Olby N.J. (2016). Mechanical and thermal sensory testing in normal chondrodystrophoid dogs and dogs with spinal cord injury caused by thoracolumbar intervertebral disc herniations. J. Vet. Intern. Med. 30, 627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nishida H., Nakayama M., Tanaka H., Kamishina H., Izawa T., Hatoya S., Sugiura K., Suzuki Y., Ide C., and Inaba T. (2014). Evaluation of serum phosphorylated neurofilament subunit NF-H as a prognostic biomarker in dogs with thoracolumbar intervertebral disc herniation. Vet. Surg. 43, 289–293 [DOI] [PubMed] [Google Scholar]
- 80.Hansebout R.R., Blight A.R., Fawcett S., and Reddy K. (1993). 4-Aminopyridine in chronic spinal cord injury: a controlled, double-blind, crossover study in eight patients. J. Neurotrauma 10, 1–18 [DOI] [PubMed] [Google Scholar]
- 81.Hayes K.C., Blight A.R., Potter P.J., Allatt R.D., Hsieh J.T., Wolfe D.L., Lam S., and Hamilton J.T. (1993). Preclinical trial of 4-aminopyridine in patients with chronic spinal cord injury. Paraplegia 31, 216–224 [DOI] [PubMed] [Google Scholar]
- 82.Donovan W.H., Halter J.A., Graves D.E., Blight A.R., Calvillo O., McCann M.T., Sherwood A.M., Castillo T., Parsons K.C., and Strayer J.R. (2000). Intravenous infusion of 4-AP in chronic spinal cord injured subjects. Spinal Cord 38, 7–15 [DOI] [PubMed] [Google Scholar]
- 83.Halter J.A., Blight A.R., Donovan W.H., and Calvillo O. (2000). Intrathecal administration of 4-aminopyridine in chronic spinal injured patients. Spinal Cord 38, 728–732 [DOI] [PubMed] [Google Scholar]
- 84.Hayes K.C., Potter P.J., Hansebout R.R., Bugaresti J.M., Hsieh J.T., Nicosia S., Katz M.A., Blight A.R., and Cohen R. (2003). Pharmacokinetic studies of single and multiple oral doses of fampridine-SR (sustained-release 4-aminopyridine) in patients with chronic spinal cord injury. Clin. Neuropharmacol. 26, 185–192 [DOI] [PubMed] [Google Scholar]
- 85.Cardenas D.D., Ditunno J., Graziani V., Jackson A.B., Lammertse D., Potter P., Sipski M., Cohen R., and Blight A.R. (2007). Phase 2 trial of sustained-release fampridine in chronic spinal cord injury. Spinal Cord 45, 158–168 [DOI] [PubMed] [Google Scholar]
- 86.Cardenas D.D., Ditunno J.F., Graziani V., McLain A.B., Lammertse D.P., Potter P.J., Alexander M.S., Cohen R., and Blight A.R. (2014). Two phase 3, multicenter, randomized, placebo-controlled clinical trials of fampridine-SR for treatment of spasticity in chronic spinal cord injury. Spinal Cord 52, 70–76 [DOI] [PubMed] [Google Scholar]
- 87.Goodman A.D., Brown T.R., Krupp L.B., Schapiro R.T., Schwid S.R., Cohen R., Marinucci L.N., and Blight AR. (2009). Sustained-release oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. Lancet 373, 732–738 [DOI] [PubMed] [Google Scholar]
- 88.Goodman A.D., Brown T.R., Edwards K.R., Krupp L.B., Schapiro R.T., Cohen R., Marinucci L.N., and Blight A.R. (2010). A phase 3 trial of extended release oral dalfampridine in multiple sclerosis. Ann. Neurol. 68, 494–502 [DOI] [PubMed] [Google Scholar]
- 89.Nishida H., Nakayama M., Tanaka H., Kitamura M., Hatoya S., Sugiura K., Suzuki Y., Ide C., and Inaba T. (2011). Evaluation of transplantation of autologous bone marrow stromal cells into the cerebrospinal fluid for treatment of chronic spinal cord injury in dogs. Am. J. Vet. Res. 72, 1118–1123 [DOI] [PubMed] [Google Scholar]
- 90.Jeffery N.D., Lakatos A., and Franklin R.J. (2005). Autologous olfactory glial cell transplantation is reliable and safe in naturally occurring canine spinal cord injury. J Neurotrauma 22, 1282–1293 [DOI] [PubMed] [Google Scholar]
- 91.Chung W.H., Park S.A., Lee J.H., Chung D.J., Yang W.J., Kang E.H., Choi C.B., Chang H.S., Kim D.H., Hwang S.H., Han H., and Kim H.Y. (2013). Percutaneous transplantation of human umbilical cord-derived mesenchymal stem cells in a dog suspected to have fibrocartilaginous embolic myelopathy. J. Vet. Sci. 14, 495–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Penha E.M., Meira C.S., Guimaraes E.T., Mendonca M.V., Gravely F.A., Pinheiro C.M., Pinheiro T.M., Barrouin-Melo S.M., Ribeiro-Dos-Santos R., and Soares M.B. (2014). Use of autologous mesenchymal stem cells derived from bone marrow for the treatment of naturally injured spinal cord in dogs. Stem Cells Int. 2014, 437521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tamura K., Harada Y., Kunimi M., Takemitsu H., Hara Y., Nakamura T., and Tagawa M. (2015). Autologous bone marrow mononuclear cell transplant and surgical decompression in a dog with chronic spinal cord injury. Exp. Clin. Transplant. 13, 100–105 [DOI] [PubMed] [Google Scholar]
- 94.Sarmento C.A., Rodrigues M.N., Bocabello R.Z., Mess A.M., and Miglino M.A. (2014). Pilot study: bone marrow stem cells as a treatment for dogs with chronic spinal cord injury. Regen. Med. Res. 2, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim Y., Jo S.H., Kim W.H., and Kweon O.K. (2015). Antioxidant and anti-inflammatory effects of intravenously injected adipose derived mesenchymal stem cells in dogs with acute spinal cord injury. Stem Cell Res. Ther. 6, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McMahill B.G., Spriet M., Siso S., Manzer M.D., Mitchell G., McGee J., Garcia T.C., Borjesson D.L., Sieber-Blum M., Nolta J.A., and Sturges B.K. (2015). Feasibility study of canine epidermal neural crest stem cell transplantation in the spinal cords of dogs. Stem Cells Transl. Med. 4, 1173–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McMahill B.G., Borjesson D.L., Sieber-Blum M., Nolta J.A., and Sturges B.K. (2015). Stem cells in canine spinal cord injury—promise for regenerative therapy in a large animal model of human disease. Stem Cell Rev. 11, 180–193 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.