Abstract
A novel 2′,3′-dideoxy-2′-α-fluoro-2′-β-C-methyl-6-methoxy guanosine (8) and its phosphoramidate prodrug (1) have been designed and synthesized. Their biological activity was evaluated in both cytotoxicity and cell-based HCV replicon assays. Neither compounds exhibited cytotoxicity up to the highest concentration tested (100 μM) in the Huh-7 cell line. The prodrug (1) displayed nanomolar level antiviral activity (EC50 = 0.39–1.1 μM) against the HCV genotype (GT) 1a, 1b, 2a, and 1b S282T replicons.
Keywords: 2′,3′-Dideoxy guanosine; 2′-α-fluoro-2′-β-C-methyl; phosphoramidate prodrug; anti-HCV activity; NS5B RdRp inhibitor
Hepatitis C virus (HCV), a member of the Flaviviridea family, is a positive-sense single-stranded RNA virus. HCV infection affects over 170 million people worldwide1 and is a major cause of hepatocellular carcinoma (HCC). So far, a series of potential molecular targets have been identified for anti-HCV drug discovery, including NS2-NS3 autoprotease, the N3 protease, the N3 helicase, and the NS5B polymerase. The NS5B, one of nonstructural proteins serving as the HCV RNA-dependent RNA polymerase (RdRp), is essential for the replication of the viral RNA genome and has attracted significant interest among medicinal chemists.2−5 Nucleosides active against HCV are NS5B RdRp inhibitors, and they need to be converted to the corresponding triphosphates that can incorporate into viral RNA at the 3′-terminal so as to stop viral RNA elongation as chain terminators. Some nucleosides are weakly active because they cannot be efficiently phosphorylated by specific nucleoside kinases or are not substrates of the kinases at all. However, nucleoside phosphates (nucleotides) per se cannot be used as drugs very often because they are chemically less stable and/or too polar to enter the cells.6,7 In order to bypass the rate-limiting first-step phosphorylation as well as to improve the chemical stability and biological activity, various nucleoside phosphate prodrugs have been designed and synthesized,7−10 Recently, the phosphoramidate prodrug strategy has been demonstrated as an effective approach for intracellular delivery of the monophosphorylated nucleosides. The monophosphate can be further phosphorylated to di-, and then the biologically active triphosphate.
Among various antiviral nucleos(t)ide compounds, the 2′-C-methyl substituted analogues11−17 display potent anti-HCV activity in vitro, in vivo, and in clinic (Figure 1). These nucleotides act as nonobligate chain terminators of HCV RdRp as they all possess a 3′-OH moiety. 2′,3′-Dideoxy nucleosides, such as Zidovudine and Emtricitabine, exhibit good antiviral activity against HIV and HBV because they cannot support the elongation of the newly synthesized viral polynucleotide due to the lack of the 3′-hydroxy group. However, such analogues were rarely reported in the literature with good anti-HCV activity, a major reason for which could be ribo-nucleosides with 3′-OH may not be good substrates of phosphorylation kinases.18 Herein, we designed and synthesized such a novel 2′,3′-dideoxy-2′-α-fluoro-2′-β-C-methyl nucleoside analogue (8) and its phosphoramidate prodrug (1) for the treatment of HCV infection.19,20
Figure 1.
Representative 2′-C-methyl substituted nucleotide analogues with potent anti-HCV activity.
The synthesis of the target 2′,3′-dideoxy-2′-α-fluoro-2′-β-C-methyl-6-methoxy guanosine (8) is described in Scheme 1. The starting material 4 was readily obtained according to a previously reported synthetic route.13 Selective protection of the 5′-OH group in nucleoside 4 with tert-butyldimethylsilyl chloride in the presence of imidazole resulted in compound 5, which was further converted into intermediate 6 by the treatment with 1,1-thiocarbonyldiimidazole in acetonitrile. Bu3SnH/AIBN-mediated 3′-deoxylation of compound 6 and subsequent removal of the silyl protecting group with TBAF afforded the 2′,3′-dideoxy guanosine 8.
Scheme 1. Synthesis of 2′,3′-Dideoxy-2′-α-fluoro-2′-β-C-methyl-6-methoxy Guanosine 8.
Reagents and conditions: (a) TBDMSCl, imidazole, DMF, rt, 81%; (b) 1,1-thiocarbonyldiimidazole, MeCN, rt, 69%; (c) nBu3SnH, AIBN, toluene, 80 °C, 75%; (d) TBAF, THF, rt, 64%.
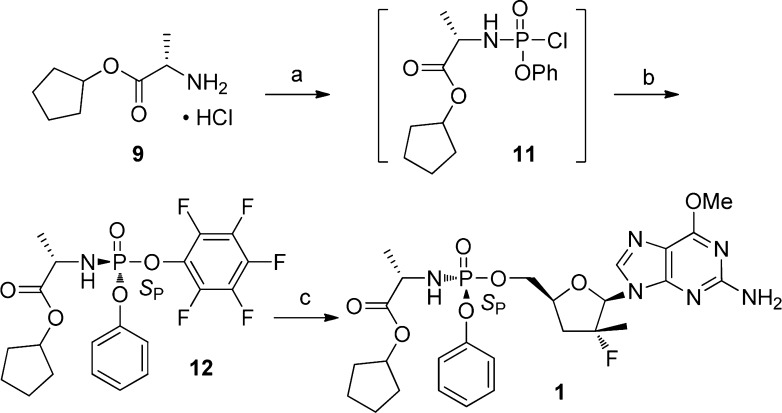
To synthesize the phosphoramidate prodrug 1, the corresponding perfluorophenyl phosphoramidate (12) was prepared (Scheme 2). Treatment of phenyl dichlorophosphate (10) with l-alanyl cyclopentyl ester hydrochloride (9) and triethylamine at −78 °C followed by reaction of the resulting monochlorophosphate 11 with pentafluorophenol and triethylamine gave the SP-cyclopentyl ester 12 after the recrystallization from a mixture of ethyl acetate and hexanes. Reaction of nucleoside 8 with compound 12 in the presence of tert-butyl magnesium chloride afforded the desired prodrug 1 as the pure SP-enantiomer.
Scheme 2. Synthesis of the Phosphoramidate Prodrug 1.
Reagents and conditions: (a) PO(OPh)Cl2 (10), NEt3, CH2Cl2, −78 °C ~ rt; (b) pentafluorophenol, NEt3, CH2Cl2, rt; then recrystallized in EtOAc/hexanes, 39% (over two steps); (c) guanosine 8, tBuMgCl, THF, rt, 83%.
The biological evaluation was carried out in cytotoxicity and cell-based HCV replicon assays, respectively. As summarized in Table 1, neither guanosine 8 nor its phosphoramidate prodrug (1) exhibited cytotoxicity at the highest concentration tested (100 μM) in Huh-7 cells. In the same cell line containing replicating HCV genotype (GT) 1b replicon, although the nucleoside 8 was inactive, its prodrug 1 inhibited the replication of the replicon with an EC50 of 0.58 μM. In light of these encouraging results, the antiviral activity of compound 1 was further profiled using different HCV replicon cells. This prodrug also displayed nanomolar antiviral activity (EC50 = 0.81 μM) against the HCV GT 1a, 2a, and 1b S282T replicons.
Table 1. Cytotoxicity and Anti-HCV Activity of Guanosine 8 and Its Phosphoramidate Prodrug 1a.
| compd | CC50 (μM) | HCV 1b EC50 (μM) | HCV 1a EC50 (μM) | HCV 2a EC50 (μM) | HCV 1b S282T EC50 (μM) |
|---|---|---|---|---|---|
| 8 | >100 | >100 | ND | ND | ND |
| 1 | >100 | 0.58 | 1.1 | 0.39 | 0.81 |
EC50: 50% effective concentration. CC50: 50% cytotoxic concentration.
In summary, we have designed and synthesized a novel 2′,3′-dideoxy-2′-α-fluoro-2′-β-C-methyl-6-methoxy guanosine (8) and its phosphoramidate prodrug (1). Neither compound is cytotoxic in the Huh-7 cell line at the highest tested concentration up to 100 μM. Although guanosine 8 is inactive, the corresponding prodrug (1) displayed potent anti-HCV activity against the genotype (GT) 1a, 1b, 2a, and 1b S282T replicons with similar EC50s (between 0.39 and 1.1 μM). For the first time, we have discovered a 2′,3′-dideoxy nucleotide analogue possessing potent antiviral activity against HCV infection. These encouraging results warrant further investigation toward the development of compound 1 as a potential anti-HCV agent.
Glossary
ABBREVIATIONS
- HCV
hepatitis C virus
- HCC
hepatocellular carcinoma
- RdRp
RNA-dependent RNA polymerase
- TBDMSCl
tert-butyldimethylsilyl chloride
- DMF
N,N-dimethylformamide
- AIBN
azobis(isobutyronitrile)
- TBAF
tetrabutylammonium fluoride
- THF
tetrahydrofuran
- GT
genotype
- EC50
50% effective concentration
- CC50
50% cytotoxic concentration
- PE
petroleum ether
- LTR
long terminal repeat
- DMEM
Dulbecco’s minimum essential medium
- FBS
fetal bovine serum
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.7b00174.
Experimental procedures, characterization data, and biological assays (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
We thank the National Natural Science Foundation of China (Nos. 81302637 and 81330075) and Outstanding Young Talent Research Fund of Zhengzhou University (No. 1521316004) for financial support.
The authors declare no competing financial interest.
Supplementary Material
References
- Gower E.; Estes C.; Blach S.; Razavi-Shearer K.; Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol. 2014, 61, S45–S57. 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Zhao C.; Wang Y. H.; Ma S. T. Recent advances on the synthesis of hepatitis C virus NS5B RNA-dependent RNA polymerase inhibitors. Eur. J. Med. Chem. 2015, 102, 188–214. 10.1016/j.ejmech.2015.07.046. [DOI] [PubMed] [Google Scholar]
- Zhao F. B.; Liu N.; Zhan P.; Jiang X. M.; Liu X. Y. Discovery of HCV NS5B thumb 1 inhibitors: Core-refining from benzimidazole to indole scaffold. Eur. J. Med. Chem. 2015, 94, 218–228. 10.1016/j.ejmech.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Caillet-Saguy C.; Lim S. P.; Shi P. Y.; Lescar J.; Bressanelli S. Polymerases of hepatitis C viruses and flaviviruses: structural and mechanistic insights and drug development. Antiviral Res. 2014, 105, 8–16. 10.1016/j.antiviral.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Mayhoub A. S. Hepatitis C RNA-dependent RNA polymerase inhibitors: a review of structure-activity and resistance relationships; different scaffolds and mutations. Bioorg. Med. Chem. 2012, 20, 3150–3161. 10.1016/j.bmc.2012.03.049. [DOI] [PubMed] [Google Scholar]
- Wagner C. R.; Iyer V. V.; McIntee E. J. Pronucleotides: toward the in vivo delivery of antiviral and anticancer nucleotides. Med. Res. Rev. 2000, 20, 417–451. . [DOI] [PubMed] [Google Scholar]
- Pradere U.; Garnier-Amblard E. C.; Coats S. J.; Amblard F.; Schinazi R. F. Synthesis of nucleoside phosphate and phosphonate prodrugs. Chem. Rev. 2014, 114, 9154–9218. 10.1021/cr5002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton P. J.; Kadri H.; Miccoli A.; Mehellou Y. Nucleoside phosphate and phosphonate prodrug clinical candidates. J. Med. Chem. 2016, 59, 10400–10410. 10.1021/acs.jmedchem.6b00523. [DOI] [PubMed] [Google Scholar]
- Sofia M. J. Nucleotide prodrugs for the treatment of HCV infection. Adv. Pharmacol. 2013, 67, 39–73. 10.1016/B978-0-12-405880-4.00002-0. [DOI] [PubMed] [Google Scholar]
- Bobeck D. R.; Schinazi R. F.; Coats S. J. Advances in nucleoside monophosphate prodrugs as anti-HCV agents. Antiviral Ther. 2010, 15, 935–950. 10.3851/IMP1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofia M. J.; Bao D.; Chang W.; Du J.; Nagarathnam D.; Rachakonda S.; Reddy P. G.; Ross B. S.; Wang P.; Zhang H. R.; Bansal S.; Espiritu C.; Keilman M.; Lam A. M.; Steuer H. M.; Niu C.; Otto M. J.; Furman P. A. Discovery of a β-D-2′-deoxy-2′-α-fluoro-2′-β-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus. J. Med. Chem. 2010, 53, 7202–7218. 10.1021/jm100863x. [DOI] [PubMed] [Google Scholar]
- McGuigan C.; Madela K.; Aljarah M.; Bourdin C.; Arrica M.; Barrett E.; Jones S.; Kolykhalov A.; Bleiman B.; Bryant K. D.; Ganguly B.; Gorovits E.; Henson G.; Hunley D.; Hutchins J.; Muhammad J.; Obikhod A.; Patti J.; Walters C. R.; Wang J.; Vernachio J.; Ramamurty C. V.; Battina S. K.; Chamberlain S. Phosphorodiamidates as a promising new phosphate prodrug motif for antiviral drug discovery: application to anti-HCV agents. J. Med. Chem. 2011, 54, 8632–8645. 10.1021/jm2011673. [DOI] [PubMed] [Google Scholar]
- Chang W.; Bao D.; Chun B. K.; Naduthambi D.; Nagarathnam D.; Rachakonda S.; Reddy P. G.; Ross B. S.; Zhang H. R.; Bansal S.; Espiritu C. L.; Keilman M.; Lam A. M.; Niu C.; Steuer H. M.; Furman P. A.; Otto M. J.; Sofia M. J. Discovery of PSI-353661, a novel purine nucleotide prodrug for the treatment of HCV infection. ACS Med. Chem. Lett. 2011, 2, 130–135. 10.1021/ml100209f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho A.; Zhang L.; Xu J.; Lee R.; Butler T.; Metobo S.; Aktoudianakis V.; Lew W.; Ye H.; Clarke M.; Doerffler E.; Byun D.; Wang T.; Babusis D.; Carey A. C.; German P.; Sauer D.; Zhong W.; Rossi S.; Fenaux M.; McHutchison J. G.; Perry J.; Feng J.; Ray A. S.; Kim C. U. Discovery of the first C-nucleoside HCV polymerase inhibitor (GS-6620) with demonstrated antiviral response in HCV infected patients. J. Med. Chem. 2014, 57, 1812–1825. 10.1021/jm400201a. [DOI] [PubMed] [Google Scholar]
- Jonckers T. H.; Vandyck K.; Vandekerckhove L.; Hu L.; Tahri A.; Van Hoof S.; Lin T. I.; Vijgen L.; Berke J. M.; Lachau-Durand S.; Stoops B.; Leclercq L.; Fanning G.; Samuelsson B.; Nilsson M.; Rosenquist Å.; Simmen K.; Raboisson P. Nucleotide prodrugs of 2′-deoxy-2′-spirooxetane ribonucleosides as novel inhibitors of the HCV NS5B polymerase. J. Med. Chem. 2014, 57, 1836–1844. 10.1021/jm4015422. [DOI] [PubMed] [Google Scholar]
- Clark J. L.; Hollecker L.; Mason J. C.; Stuyver L. J.; Tharnish P. M.; Lostia S.; McBrayer T. R.; Schinazi R. F.; Watanabe K. A.; Otto M. J.; Furman P. A.; Stec W. J.; Patterson S. E.; Pankiewicz K. W. Design, synthesis, and antiviral activity of 2′-deoxy-2′-C-methylcytidine, a potent inhibitor of hepatitis C virus replication. J. Med. Chem. 2005, 48, 5504–5508. 10.1021/jm0502788. [DOI] [PubMed] [Google Scholar]
- Eldrup A. B.; Allerson C. R.; Bennett C. F.; Bera S.; Bhat B.; Bhat N.; Bosserman M. R.; Brooks J.; Burlein C.; Carroll S. S.; Cook P. D.; Getty K. L.; MacCoss M.; McMasters D. R.; Olsen D. B.; Prakash T. P.; Prhavc M.; Song Q.; Tomassini J. E.; Xia J. Structure-activity relationship of purine ribonucleosides for inhibition of hepatitis C virus RNA-dependent RNA polymerase. J. Med. Chem. 2004, 47, 2283–2295. 10.1021/jm030424e. [DOI] [PubMed] [Google Scholar]
- Siddiqui M. A.; Hughes S. H.; Boyer P. L.; Mitsuya H.; Van Q. N.; George C.; Sarafinanos S. G.; Marquez V. E. A 4′-C-ethynyl-2′,3′-dideoxynucleoside analogue highlights the role of the 3′-OH in anti-HIV active 4′-C-ethynyl-2′-deoxy nucleosides. J. Med. Chem. 2004, 47, 5041–5048. 10.1021/jm049550o. [DOI] [PubMed] [Google Scholar]
- Chang J.; Huang Q.; Zhou S.. 2′,3′-Dideoxy-2′-alpha-fluoro-2′-beta-C-methylnucleosides and prodrugs thereof. CN Patent ZL 201280030844.X, July 19, 2012.
- Huang Q.; Zhou S.; Chang J.. 2′,3′-Dideoxy-2′-alpha-fluoro-2′-beta-C-methylnucleosides and prodrugs thereof. Pat. Appl. WO 2013013009 A3, January 24, 2013; US 20140315850 A1, October 23, 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.