Abstract
Acetylcholinesterase (AChE) is an essential enzyme that can be targeted by organophosphorus (OP) compounds, including nerve agents. Following exposure to OPs, AChE becomes phosphylated (inhibited) and undergoes a subsequent aging process where the OP–AChE adduct is dealkylated. The aged AChE is unable to hydrolyze acetylcholine, resulting in accumulation of the neurotransmitter in the central nervous system (CNS) and elsewhere. Current therapeutics are only capable of reactivating inhibited AChE. There are no known therapeutic agents to reverse the aging process or treat aged AChE. Quinone methides (QMs) have been shown to alkylate phosphates under physiological conditions. In this study, a small library of novel quinone methide precursors (QMPs) has been synthesized and examined as potential alkylating agents against model nucleophiles, including a model phosphonate. Computational studies have been performed to evaluate the affinity of QMPs for the aged AChE active site, and preliminary testing with electric eel AChE has been performed.
Keywords: Acetylcholinesterase, organophosphorus chemical nerve agents, quinone methide
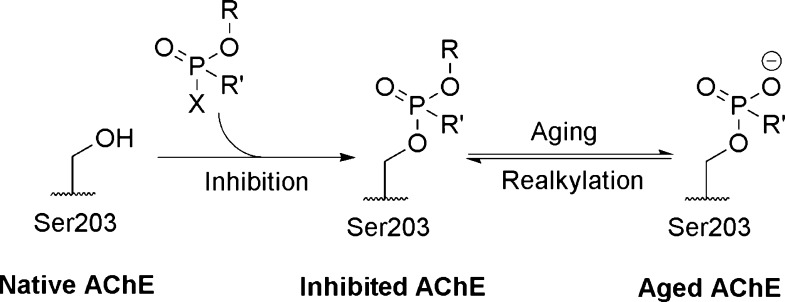
Organophosphorus (OP) compounds containing a phosphoryl group have been used as pesticides and chemical warfare agents.1−4 These compounds are toxic due to their inhibition of the enzyme acetylcholinesterase (AChE), a serine hydrolase typically found in the central and peripheral nervous system that regulates concentrations of the neurotransmitter acetylcholine.5,6 As illustrated in Scheme 1, when the central phosphorus atom is attacked by the catalytic serine, a leaving group is substituted, leaving a phosphyl group covalently attached to the serine, which blocks the active site. Inhibition of AChE can lead to death by respiratory failure due to overstimulation of muscarinic acetylcholine receptors at the neuromuscular junctions.7,8 There are known therapeutics, specifically oxime-containing compounds, to reverse OP inhibition of AChE.9−11
Scheme 1. Inhibition, Aging and Realkylation of AChE.
The leaving group X is substituted during inhibition. The alkyl group R is lost during aging.
If left untreated after OP inhibition, the O-alkyl group of the AChE-OP adduct can undergo a dealkylation reaction, leaving a phosphonate (or phosphate) anion in the AChE active site in a process known as “aging” (Scheme 1).11−14 The rate of aging varies from minutes to hours between different OPs.15−17 Currently there is no known therapeutic to reactivate aged AChE.18 Previous investigations utilized alkyl sulfonates19,20 and phenacyl bromides21 to alkylate a model phosphonate, but attempts to reactivate the aged enzyme were unsuccessful.
Thus, the need for an effective therapeutic to reverse the effects of aging in OP-inhibited AChE has not been met. Importantly, a single alkylating agent will treat the identical aged structures of each of the common OPs, except for tabun, which result in the same methylphosphonate structure after aging. Consequently, the same oxime should be effective for many realkylated OPs.
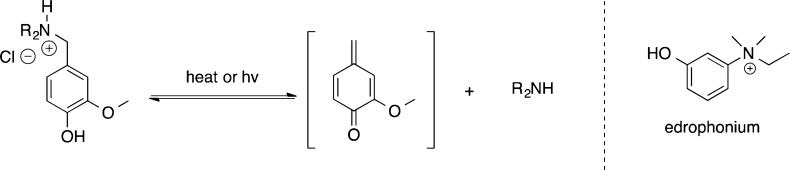
The preferred alkylating agent must be reactive enough to alkylate the phosphonate anion in the aged AChE enzyme, but selective enough to minimize alkylation of other biomolecules in vivo. Quinone methides (QMs) have been used as alkylating agents for other biological applications in vitro, including the alkylation of phosphodiesters at physiological conditions.22 QMs have also been shown to reversibly alkylate DNA and block transcription.23 Additionally, QMs have been shown to possess highly tunable reactivity through modification of substituents on the aromatic ring24 or the amine leaving group.25 The structure of potential quinone methide precursors (QMPs) also strongly mimics edrophonium, an oxyanilinium-based inhibitor of AChE that is known to bind in the (native) AChE active site.26 Another potentially useful aspect of QMs is their ability to be delivered in an unreactive prodrug form where a reactive QM could be generated selectively within the AChE active site (Scheme 2).
Scheme 2. Potential Quinone Methide Precursor (QMP) Prodrug and Quinone Methide Intermediate.
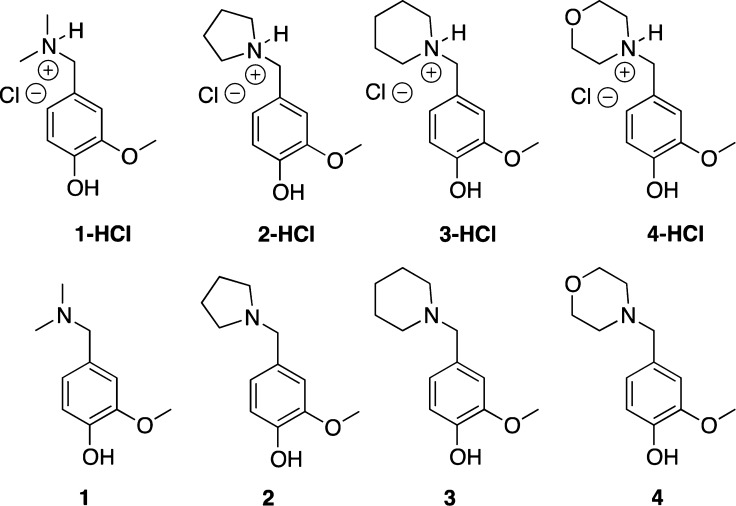
Recent studies have shown the generation of QMs from benzyl and naphthyl derivatives via silyl cleavage.27 QMs have predominantly been generated by oxidative28 or photochemical29 means due to higher yield of the reactive intermediate, but for in vivo applications, the QM intermediate must be generated thermally. Therefore, we decided to focus on a subset of para-QMPs as candidates for the realkylation of aged AChE (Figure 1). Initial computational modeling (see below) suggested that these QMPs would have some affinity for the active site of aged AChE. As a proof-of-concept, we will focus on finding the optimum QMP through quantitative alkylation experiments with a variety of nucleophiles, including a model phosphonate, in addition to molecular modeling and kinetic experiments with native and aged AChE.
Figure 1.
Target quinone methide precursors.
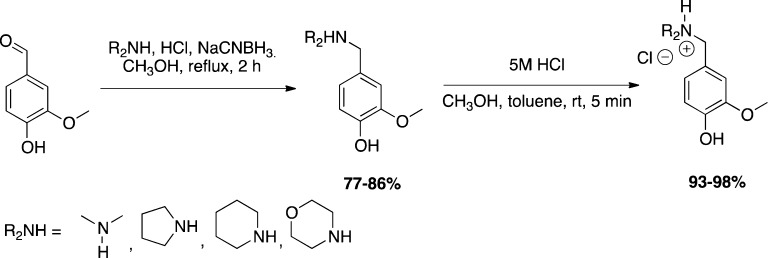
A reliable method for producing amines via reductive amination has been developed (Scheme 3). In the process, secondary amines were reacted with the aldehyde substrate to produce a conjugated iminium ion, which was reduced in situ by sodium cyanoborohydride (77–86%). The resulting amines (1–4) were isolated via column chromatography and then dissolved in methanol and toluene followed by slow addition of methanolic hydrochloric acid to yield the corresponding hydrochloride ammonium salts in 93–98% yield (1–4 HCl).
Scheme 3. Reductive Amination for the Preparation of QMPs.
Studies to evaluate the general effectiveness of the amines and ammonium salts toward nucleophilic substitution were undertaken. Each of the compounds shown in Figure 1 were separately subjected to reaction with 4-methylbenzenethiol, piperidine/pyrrolidine, and benzyl alcohol at 100 °C for 3 h. These nucleophiles were chosen as representative thiol, amines, and alcohol. We chose this high temperature because in the absence of assistance of desolvation and proximity, the energy barrier in bulk solvent could be higher than that at the active site of the enzyme. Both the neutral and the protonated forms of QMPs were tested because the active sites of enzymes are normally highly desolvated and can exhibit a different pH environment due to the surrounding dissociable residues compared to that in the bulk solvent. All compounds alkylated more than 65% of the thiol and the amine, as shown in Table 1. None alkylated more than 20% of the alcohol. This test implies that the candidate QMPs can react as alkylators of nucleophiles with moderate reactivity (Scheme 4).
Table 1. Percentage Yields of Alkylation of Model Nucleophiles by QMPs.
| nucleophile
(%) |
|||
|---|---|---|---|
| QMP | 4-methylbenzenethiol | piperidinea | benzyl alcohol |
| 1 | 84 | 80 | 0 |
| 2 | 87 | 77 | 8 |
| 3 | 83 | 81 | 11 |
| 4 | 83 | 79 | 13 |
| 1-HCl | 83 | 78 | 8 |
| 2-HCl | 83 | 78 | 12 |
| 3-HCl | 85 | 81 | 10 |
| 4-HCl | 84 | 69 | 16 |
Compounds 3 and 3-HCl were reacted with pyrrolidine because of their piperidinyl groups in the existing structure.
Scheme 4. Alkylation of Model Nucleophiles by QMPs.
In order to test the efficacy of our precursors against a model phosphonate as a simple model for the aged AChE active site, we sought to replicate a study from Steinberg21 to indirectly monitor alkylation by UV–vis spectroscopy. By appending a model phosphonate with a p-nitrophenol leaving group, alkylation could be indirectly monitored after addition of base to the synthesized alkylated product.
However, after following Steinberg’s protocol, our results proved unreliable over the course of replicate trials with large levels of background signal (Supporting Information). Thus, our efforts moved away from the indirect UV–vis screen and were refocused on confirming the alkylation of a model phosphonate by our QMPs.
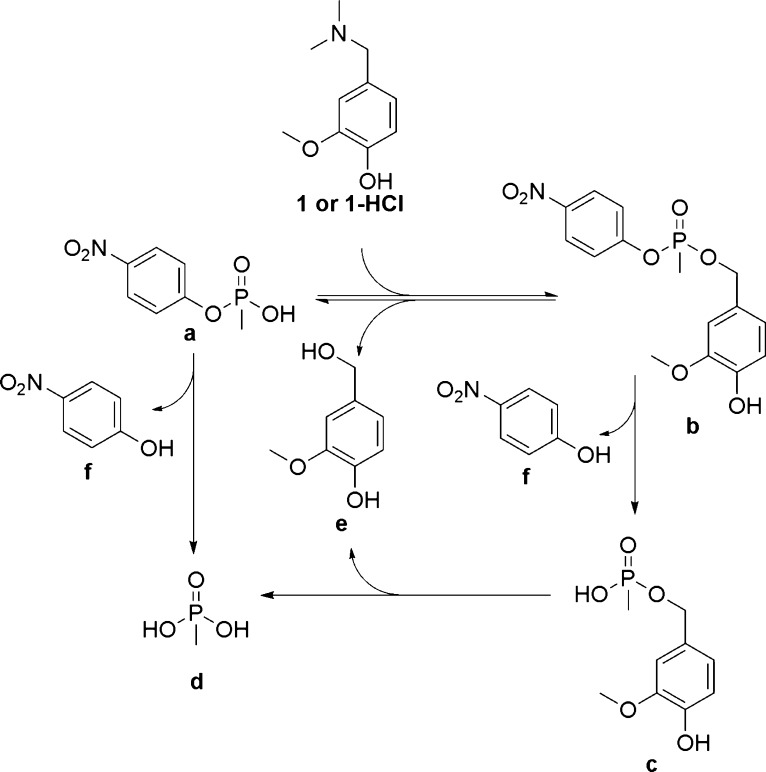
In order to confirm alkylation of the model phosphonate by QMPs, the reaction was monitored by tandem HPLC/ESI-MS-TOF. These techniques would allow for a better opportunity to monitor the potential products generated, and perhaps other previously unconsidered pathways that may be responsible for the degradation of the alkylated product. A representative QMP of the neutral (1) and protonated (1-HCl) forms were each reacted with the model phosphonate in water at 100 °C for 3 h and analyzed using the HPLC/ESI-MS-TOF to identify each of the species in solution after heating. The reaction pathways are illustrated in Scheme 5. For both 1 and 1-HCl, only trace amounts of the intact alkylated product (b) were observed. The major peaks observed in each reaction were a, c, e, and f. Species c indirectly implied the formation of b via alkylation.
Scheme 5. Reactions between 1 or 1-HCl and the Model Phosphonate (a).
Protonation states are not specified.
To further examine the alkylation of the model phosphonate by our QMPs, 31P NMR spectroscopy was used to follow the different phosphorus species generated from the same reactions (Scheme 5) in D2O. The results indicated the presence of all four different phosphorus species (a–d) after heating. The major species identified in the product were a (25.1 ppm) and d (20.6 ppm). Species b and c were also observed, indicating the occurrence of alkylation.
In parallel, we used computational approaches to evaluate this QMP approach. Past efforts toward understanding the effect of OP nerve agents by molecular modeling studies have been performed by our group.30 In order to better understand the potential interaction between our QMPs and the target aged AChE active site, molecular docking simulations were performed using AutoDock 4.0. The ligands were initially optimized using Gaussian 0931 at the B3LYP32,33/6-311+G** level of theory, and then submitted to a Merz–Kollman charge calculation to prepare the substrates for docking. In docking simulations with aged AChE, the receptor was kept mostly rigid and the ligand flexible, and each of the poses was analyzed to determine if it would present the potential for a favorable interaction between the aged serine residue and the benzylic carbon of our QMPs. Specifically, we evaluated docked poses in which the reactive benzylic carbon was within 5.0 Å of the anionic O-(P=O) of the aged serine residue. The aged AChE was prepared by in silico methods from the human isoform (PDB: 1B41; a newer structure (PDB ID: 4EY4), which is of higher resolution, was published after we initiated this study. However, alignment shows only trivial difference in coordinates (backbone RMSD = 0.708 Å)), and details are provided in the Supporting Information.
Examination of molecular docking snapshots revealed some variability in the specific orientation of the amine leaving group (Supporting Information). It was found that the pyrrolidine (2/2-HCl) and piperidine (3/3-HCl) amine leaving groups were the most promising of this library of ligands with the highest percentage of docking poses in which the benzylic carbon is within 5.0 Å of the aged serine residue (Figure 2). While the majority of poses are outside of this distance cutoff, a significant portion do exist within this distance criterion, suggesting that the ligands can access the aged active site. Thus, these QMPs may allow for a therapeutic reaction to occur whether alkylation were to take place through in situ generation of a quinone methide or by a direct displacement at the benzylic position. Also, among the poses not within the distance criterion, the vast majority were still within the enzyme itself, but further away from the aged serine toward the gorge bottleneck. Interestingly, the smaller dimethylamine group (1/1-HCl) and the similarly sized morpholine group (4/4-HCl) did not have as strong a recognition for the enzyme’s active site.
Figure 2.
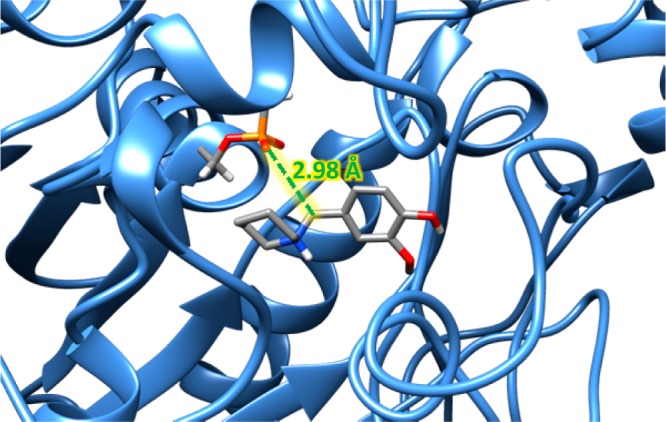
Docked pose of 3-HCl in the aged AChE active site, displaying the putative reactive orientation of the benzylic carbon being less than 5 Å from aged serine residue.
With molecular docking results in hand, molecular dynamics (MD) simulations were performed subsequently to examine how the ligands noncovalently interact with the enzyme over the course of time. For each ligand, the three lowest energy snapshots from each of the 13 aged AChE frames were chosen as the starting points for our MD simulations. Once again, of interest was the interaction between the reactive benzylic carbon of our QMPs and the target, phosphylated serine residue of aged AChE. Over the course of a brief 1 ns simulation for each starting orientation from docking, the QMP ligands could be further examined for their preference toward the aged active site versus other areas of the enzyme by establishing zoning criteria. Interestingly, once again the pyrrolidine (2-HCl) amine leaving group was the most promising ligand. Also, there was a strong preference for the protonated leaving groups to spend more time in the active site, while the neutral leaving groups rarely stayed in the enzyme’s active site (see Supporting Information).
We sought to evaluate whether our QMP ligands could bind into the active site of aged AChE; however, we were unable to develop a protocol to evaluate that binding as the aged AChE is not “active” to any known substrate. Instead, the binding affinity and selectivity of QMPs to the active site of AChE can be revealed by kinetics of reversible inhibition of native AChE and as measured by competition kinetics for AChE’s substrate. Indeed, the activity of electric eel AChE was monitored at room temperature using Ellman’s assay in the presence of QMPs at varied concentrations ranging from 0 to 1.5 mM.34 Electric eel AChE is a commercially available AChE homologue. Despite the difference between the gorge shapes of human and nonhuman AChE homologues, in this proof-of-concept study it is acceptable to qualitatively estimate the binding affinity using electric eel AChE. The concentrations of acetylthiocholine (ATC, used as the AChE substrate) were varied from 0 to 1.1 mM independently. Acquired data were processed with GraphPad Prism 6 for nonlinear regression. Details of the experimental procedure and calculations are included in the Supporting Information.
As illustrated in Table 2, the Ki values ranged from 0.08 to 1.57 mM indicate overall weak inhibition of native AChE. The constant α is the ratio between the dissociation equilibrium constants of the enzyme–inhibitor–substrate complex and the enzyme–inhibitor complex. The greater α is than 1, the closer the inhibition mechanism is to competitive inhibition.35 All QMPs showed α values greater than 1, especially 1-HCl and 2-HCl; thus, they are competitive inhibitors or near-competitive mix-type inhibitors35 that selectively bind to the active site of native AChE.
Table 2. Molecular Dynamics Results and Inhibition Kinetics of Electric Eel AChE by QMPs.
| QMP | 1-HCl | 2-HCl | 3-HCl | 4-HCl |
|---|---|---|---|---|
| MD AS%a | 44.8 (11.8) | 50.5 (24.1) | 44.6 (6.0) | 37.4 (8.9) |
| Vmax (nM/s) | 61.2 ± 2.2 | 104.9 ± 4.2 | 80.3 ± 3.9 | 141.0 ± 8.9 |
| Km (mM) | 0.032 ± 0.014 | 0.079 ± 0.014 | 0.062 ± 0.015 | 0.189 ± 0.037 |
| Ki (mM) | 0.080 ± 0.040 | 0.095 ± 0.024 | 0.106 ± 0.042 | 1.57 ± 0.83 |
| α | 34.9 ± 22.9 | 8.7 ± 4.0 | 3.1 ± 1.7 | 1.24 ± 0.94 |
| IC50 (mM)b | 0.919 | 0.403 | 0.227 | 1.83 |
Over a series of 1 ns simulations, the total percentage of time ligand spent in the aged AChE active site, defined as the benzylic carbon of the QMP being 0–5 Å from the phosphylated serine residue (Supporting Information). Values of the corresponding neutral compounds are displayed in parentheses.
[acetylthiocholine] = 0.5 mM.
The four hydrochloride compounds were subject to an in vitro test against methylphosphonate-aged AChE, which is the common aged product of many authentic V-agents and G-agents. Electric eel AChE (Sigma-Aldrich) was used in these studies. Each tested QMP (4 mM) was added to AChE that was aged with a soman analogue, originally reported by Amitai et al.36 The negative and the positive controls prepared in parallel were aged and native AChE without QMPs. To determine the amount of residual inhibited AChE after the aging process, a 2-pralidoxime chloride (2-PAM) control was also prepared by mixing aged AChE with 4 mM 2-PAM.
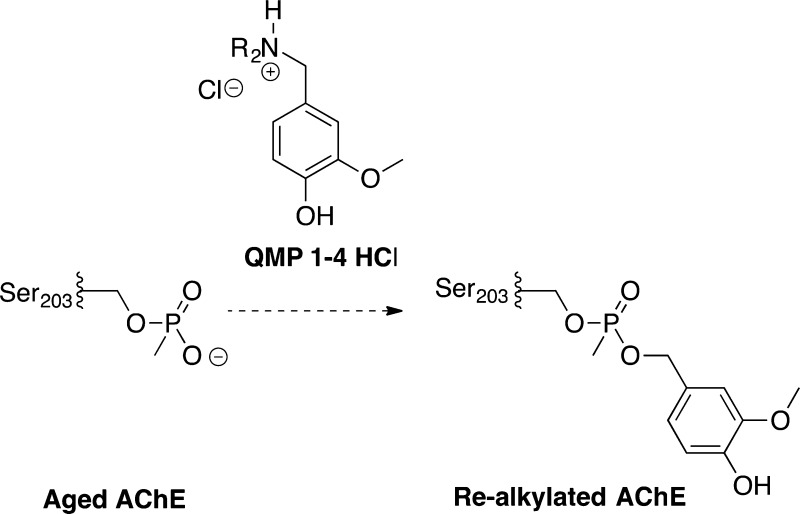
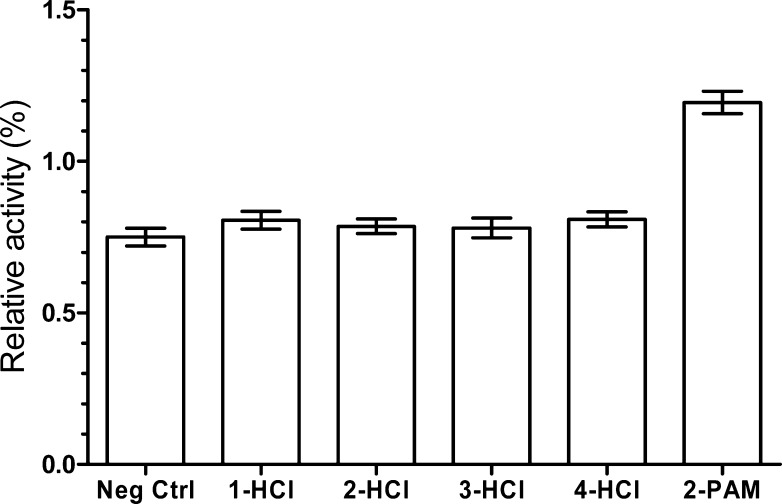
In a similar manner to 2-PAM, fluoride ion at high concentration can function as a reactivator of AChE.37,38 As an anion with simple structure, it is speculated to reactivate all inhibited/realkylated AChE without bias and not to compete with QMPs for AChE’s active site. Therefore, NH4F (4 mM) was also added to the reaction mixture to reactivate the realkylated AChE, in order to avoid reaging after alkylation. After reaction for 24 h at 37 °C and pH 8.0, all samples were treated with 4 mM of 2-PAM to ensure the reactivation of any aged AChE that was (hopefully) realkylated. The resulting AChE activity was determined with Ellman’s assay with ATC as a substrate. The relative activities of QMP-treated samples, the negative control, and the 2-PAM control compared to the positive control are displayed in Figure 3 (see the Supporting Information for specific procedures and additional details). Unfortunately, none of our QMP substrates appear to realkylate the aged form of AChE to any significant extent; indeed, the QMP-treated samples have similar activities in the Ellman’s assay and are less than 2-PAM by itself (Figure 3). (2-PAM is known not to reactivate the aged form of AChE.)11−14
Figure 3.
Result of AChE realkylation test. The relative activity of 2-PAM control was 1.2%, implying the near completeness of aging. Aged AChE samples treated with tested QMPs were not obviously more active than the negative control (Neg Ctrl).
In conclusion, we have shown the ability of a novel class of quinone methide precursors (QMPs) to act as alkylating agents with a variety of model nucleophiles, including a model phosphonate to mimic the aged AChE active site. Molecular modeling studies confirm that this moiety has some affinity for the aged AChE active site, with a significant contribution of poses oriented in a potentially reactive conformation. The varied results of our experiments also suggest the tunability within our initial scaffold to modulate reactivity. With confirmation of the alkylated model phosphonate and preliminary computational and kinetic data completed, the hydrochloride QMPs described herein were further investigated to determine their efficacy toward reactivating aged AChE. Though not effective at present, this proof-of-concept model demonstrates the feasibility to develop potentially effective countermeasures against OP exposure using QMP-based AChE realkylators. Further studies are in progress in order to optimize this potential strategy and to reverse the effects of aging of AChE by OP agents.
Acknowledgments
The authors would like to acknowledge financial support from the National Institutes of Health (U01–NS087983) and computing resources from the Ohio Supercomputer Center. Initial financial support of this project was obtained with resources from the US Army (W81XWH-10-2-0044).
Glossary
ABBREVIATIONS
- AChE
acetylcholinesterase
- ATC
acetylthiocholine
- MD
molecular dynamics
- OP
organophosphorus
- QM
quinone methide
- QMP
quinone methide precursor
- 2-PAM
2-pyridine aldoxime methyl chloride
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.7b00037.
Synthesis and characterization of QMPs; alkylation of model nucleophiles; UV–vis studies; HPLC data; computational methods; determination of inhibition characteristics of QMPs against native AChE; procedures for aging of AChE; procedures for realkylation and reactivation of aged AChE (PDF)
NMR data set (PDF)
NMR spectra (PDF)
Atom coordinates and parameters (Microsoft Word)
The authors declare no competing financial interest.
Supplementary Material
References
- Barr D. B.; Allen R.; Olsson A. O.; Bravo R.; Caltabiano L. M.; Montesano A.; Nguyen J.; Udunka S.; Walden D.; Walker R. D.; Weerasekera G.; Whitehead R. D. Jr.; Schober S. E.; Needham L. L. Concentrations of Selective Metabolites of Organophosphorus Pesticides in the United States Population. Environ. Res. 2005, 99, 314–326. 10.1016/j.envres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Bronstein A. C.; Spyker D. A.; Cantilena L. R.; Green J. L.; Rumack B. H.; Giffin S. L. 2008 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 26th Annual Report. Clin. Toxicol. 2009, 47, 911–1084. 10.3109/15563650903438566. [DOI] [PubMed] [Google Scholar]
- Delfino R. T.; Ribeiro T. S.; Figueroa-Villar J. D. Organophosphorus Compounds as Chemical Warfare Agents: a Review. J. Braz. Chem. Soc. 2009, 20, 407–428. 10.1590/S0103-50532009000300003. [DOI] [Google Scholar]
- Dolgin E. Syrian Gas Attack Reinforces Need for Better Anti-sarin Drugs. Nat. Med. 2013, 19, 1194–1195. 10.1038/nm1013-1194. [DOI] [PubMed] [Google Scholar]
- Quinn D. M. Acetylcholinesterase: Enzyme Structure, Reaction Dynamics, and Virtual Transition States. Chem. Rev. 1987, 87, 955–979. 10.1021/cr00081a005. [DOI] [Google Scholar]
- Sussman J. L.; Harel M.; Frolow F.; Oefner C.; Goldman A.; Toker L.; Silman I. Atomic Structure of Acetylcholinesterase from Torpedo californica: a Prototypic Acetylcholine-binding Protein. Science 1991, 253, 872–879. 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- Jokanovic M.; Prostran M. Pyridinium oximes as cholinesterase reactivators. Structure-activity Relationship and Efficacy in the Treatment of Poisoning with Organophosphorus Compounds. Curr. Med. Chem. 2009, 16, 2177–2188. 10.2174/092986709788612729. [DOI] [PubMed] [Google Scholar]
- Worek F.; Thiermann H.; Szinicz L.; Eyer P. Kinetic Analysis of Interactions between Human Acetylcholinesterase, Structurally Different Organophosphorus Compounds and Oximes. Biochem. Pharmacol. 2004, 68, 2237–2248. 10.1016/j.bcp.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Worek F.; Szinicz L.; Eyer P.; Thiermann H. Evaluation of Oxime Efficacy in Nerve Agent Poisoning: Development of a Kinetic-Based Dynamic Model. Toxicol. Appl. Pharmacol. 2005, 209, 193–202. 10.1016/j.taap.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Worek F.; Eyer P.; Aurbek N.; Szinicz L.; Thiermann H. Recent Advances in Evaluation of Oxime Efficacy in Nerve Agent Poisoning by in vitro Analysis. Toxicol. Appl. Pharmacol. 2007, 219, 226–234. 10.1016/j.taap.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Gorecki L.; Korabecny J.; Musilek K.; Malinak D.; Nepovimova E.; Dolezal R.; Jun D.; Soukup O.; Kuca K. SAR study to find optimal cholinesterase reactivator against organophosphorous nerve agents and pesticides. Arch. Toxicol. 2016, 90, 2831–2859. 10.1007/s00204-016-1827-3. [DOI] [PubMed] [Google Scholar]
- Michel H. O.; Hackley B. E. Jr.; Berkowitz L.; List G.; Hackley E. B.; Gillilan W.; Pankau M. Ageing and Dealkylation of Soman (Pinacolylmethylphosphonofluoridate)-inactivated Eel Cholinesterase. Arch. Biochem. Biophys. 1967, 121, 29–34. 10.1016/0003-9861(67)90006-9. [DOI] [PubMed] [Google Scholar]
- Millard C. B.; Kryger G.; Ordentlich A.; Greenblatt H. M.; Harel M.; Raves M. L.; Segall Y.; Barak D.; Shafferman A.; Silman I.; Sussman J. L. Crystal Structures of Aged Phosphonylated Acetylcholinesterase: Nerve Agent Reaction Products at the Atomic Level. Biochemistry 1999, 38, 7032–7039. 10.1021/bi982678l. [DOI] [PubMed] [Google Scholar]
- Wang J.; Gu J.; Leszczynski J. Phosphonylation Mechanisms of Sarin and Acetylcholinesterase: A Model DFT Study. J. Phys. Chem. B 2006, 110, 7567–7573. 10.1021/jp060370v. [DOI] [PubMed] [Google Scholar]
- Sidell F. R.; Groff W. A. The Reactivatibility of Cholinesterase Inhibited by VX and Sarin in Man. Toxicol. Appl. Pharmacol. 1974, 27, 241–252. 10.1016/0041-008X(74)90195-1. [DOI] [PubMed] [Google Scholar]
- Mager P. P.Multidimensional Pharmacochemistry; Academic Press: San Diego, 1984; pp 52–53. [Google Scholar]
- Barak D.; Ordentlich A.; Segall Y.; Velan B.; Benschop H. P.; De Jong L. P. A.; Shafferman A. Carbocation-Mediated Processes in Biocatalysts. Contribution of Aromatic Moieties. J. Am. Chem. Soc. 1997, 119, 3157–3158. 10.1021/ja963861+. [DOI] [Google Scholar]
- Zhuang Q.; Young A.; Callam C. S.; McElroy C. A.; Ekici Ö. D.; Yoder R. J.; Hadad C. M. Efforts toward Treatments against Aging of Organophosphorus-inhibited Acetylcholinesterase. Ann. N. Y. Acad. Sci. 2016, 1374, 94–104. 10.1111/nyas.13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumbergs P.; Ash A. B.; Daniher F. A.; Stevens C. L.; Michel H. O.; Hackley B. E. Jr.; Epstein J. Alkylating Agents Containing a Quaternary Nitrogen Group. J. Org. Chem. 1969, 34, 4065–4070. 10.1021/jo01264a067. [DOI] [Google Scholar]
- Ash A. B.; Blumbergs P.; Stevens C. L.; Michel H. O.; Hackley B. E. Jr.; Epstein J. Relative Nucleophilicity. Methylation of Anions in Aqueous Media. J. Org. Chem. 1969, 34, 4070–4072. 10.1021/jo01264a068. [DOI] [Google Scholar]
- Steinberg G. M.; Lieske C. N.; Boldt R. Model Studies for the Reactivation of Aged Phosphonylated Acetylcholinesterase. Use of Alkylating Agents Containing Nucleophilic Groups. J. Med. Chem. 1970, 13, 435–446. 10.1021/jm00297a024. [DOI] [PubMed] [Google Scholar]
- Zhou Q.; Turnbull K. D. Quinone Methide Phosphodiester Alkylations under Aqueous Conditions. J. Org. Chem. 2001, 66, 7072–7077. 10.1021/jo015792+. [DOI] [PubMed] [Google Scholar]
- Bakke B. A.; McIntosh M. C.; Turnbull K. D. Improved Alkylation and Product Stability in Phosphotriester Formation through Quinone Methide Reactions with Dialkyl Phosphates. J. Org. Chem. 2005, 70, 4338–4345. 10.1021/jo050050s. [DOI] [PubMed] [Google Scholar]
- Freccero M. Quinone Methides as Alkylating and Cross-linking Agents. Mini-Rev. Org. Chem. 2004, 1, 403–415. 10.2174/1570193043403091. [DOI] [Google Scholar]
- Weinert E. E.; Dondi R.; Colloredo-Melz S.; Frankenfield K. N.; Mitchell C. H.; Freccero M.; Rokita S. E. Substituents on Quinone Methides Strongly Modulate Formation and Stability of Their Nucleophilic Adducts. J. Am. Chem. Soc. 2006, 128, 11940–11947. 10.1021/ja062948k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove S. J.; Kaur J.; Muir A. W.; Pow E.; Tarver G. J.; Zhang M. Q. Oxyaniliniums as Acetylcholinesterase Inhibitors for the Reversal of Neuromuscular Block. Bioorg. Med. Chem. Lett. 2002, 12, 193–196. 10.1016/S0960-894X(01)00703-X. [DOI] [PubMed] [Google Scholar]
- Shaikh A.k.; Cobb A. J. A.; Varvounis G. Mild and Rapid Method for the Generation of ortho-(Naphtho)quinone Methide Intermediates. Org. Lett. 2012, 14, 584–587. 10.1021/ol203196n. [DOI] [PubMed] [Google Scholar]
- Bolton J. L.; Comeau E.; Vukomanovic V. The Influence of 4-Alkyl Substituents on the Formation and Reactivity of 2-Methoxy-Quinone Methides: Evidence that Extended Pi-Conjugation Dramatically Stabilizes the Quinone Methide Formed from Eugenol. Chem.-Biol. Interact. 1995, 95, 279–290. 10.1016/0009-2797(94)03566-Q. [DOI] [PubMed] [Google Scholar]
- Modica E.; Zanaletti R.; Freccero M.; Mella M. Alkylation of Amino Acids and Glutathione in Water by o-Quinone Methide. Reactivity and Selectivity. J. Org. Chem. 2001, 66, 41–52. 10.1021/jo0006627. [DOI] [PubMed] [Google Scholar]
- Beck J. M.; Hadad C. M. Reaction Profiles of the Interaction between Sarin and Acetylcholinesterase and the S203C Mutant: Model Nucleophiles and QM/MM Potential Energy Surfaces. Chem.-Biol. Interact. 2010, 187, 220–224. 10.1016/j.cbi.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam N. J.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas Ö.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J.. Gaussian 09, revision D.01; Gaussian, Inc.: Wallingford, CT, 2009. [Google Scholar]
- Lee C.; Yang W.; Parr R. G. Development of the Colle-Salvetti Correlation-energy Formula into a Functional of the Electron Density. Phys. Rev. B: Condens. Matter Mater. Phys. 1988, 37, 785–789. 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]
- Becke A. D. Density-Functional Thermochemistry. III. the Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. 10.1063/1.464913. [DOI] [Google Scholar]
- Ellman G. L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Copeland R. A.Evaluation of Enzyme Inhibitors in Drug Discovery, 2nd ed.; Wiley-Interscience: Hoboken, 2013; pp 57–121. [Google Scholar]
- Amitai G.; Adani R.; Yacov G.; Yishay S.; Teitlboim S.; Tveria L.; Limanovich O.; Kushnir M.; Meshulam H. Asymmetric Fluorogenic Organophosphates for the Development of Active Organophosphate Hydrolases with Reversed Stereoselectivity. Toxicology 2007, 233, 187–198. 10.1016/j.tox.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Heilbronn E. Action of Fluoride on Cholinesterase—II: in vitro Reactivation of Cholinesterases Inhibited by Organophosphorous Compounds. Biochem. Pharmacol. 1965, 14, 1363–1373. 10.1016/0006-2952(65)90120-6. [DOI] [PubMed] [Google Scholar]
- Hagstrom D.; Hirokawa H.; Zhang L.; Radic Z.; Taylor P.; Collins E. S. Planarian Cholinesterase: in vitro Characterization of an Evolutionarily Ancient Enzyme to Study Organophosphorus Pesticide Toxicity and Reactivation. Arch. Toxicol. 2016, 10.1007/s00204-016-1908-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.