Abstract
Excellent medication adherence contributes to decreases in morbidity, mortality, and health care costs. Although researchers have tested many interventions to increase adherence, results are sometimes conflicting and often unclear. This systematic review applied meta-analytic procedures to integrate primary research that tested medication adherence interventions. Comprehensive searching completed in 2015 located 771 published and unpublished intervention studies with adherence behavior outcomes. Random-effects model analysis calculated standardized mean difference effect sizes. Meta-analytic moderator analyses examined the association between adherence effect sizes and sample, design, and intervention characteristics. Analyses were conducted in 2016. A standardized mean difference effect size of 0.290 comparing treatment and control groups was calculated. Moderator analyses revealed larger effect sizes for habit-based and behavioral-targeted (vs. cognitive-focused) interventions. The most effective interventions were delivered face-to-face, by pharmacists, and administered directly to patients. Effect sizes were smaller in studies with older and homeless participants. Risks of bias were common; effect sizes were significantly lower among studies with masked data collectors and intention-to-treat analyses. The largest effect sizes were reported by studies using medication electronic event monitoring and pill count medication adherence measures. Publication bias was present. This most comprehensive review to date documented that, although interventions can increase adherence, much room remains for improvement. Findings suggest health care providers should focus intervention content on behavioral strategies, especially habit-based interventions, more so than cognitive strategies designed to change knowledge and beliefs.
Keywords: medication adherence, intervention, meta-analysis
Introduction
Inadequate medication adherence contributes to increases in morbidity, mortality, and health care costs, as well as frustration among patients and providers (Christensen, 2004; World Health Organization, 2003). Many researchers have tested interventions for increasing medication adherence (henceforth adherence). Findings differ widely among studies, suggesting study-related differences (e.g., interventions with certain characteristics may be more effective than other interventions). The mixed findings among the many primary research studies combined with the importance of the topic justify a comprehensive meta-analysis to synthesize findings. Most previous meta-analyses have synthesized narrow sets of studies focused on specific patient populations and settings, certain health care provider interventionists, or specific interventions such as dose frequency, special packaging, reminder systems, and financial rewards. Moderator analyses in previous meta-analyses categorized intervention characteristics broadly (for example, educational versus non-educational interventions), which prevented adequate examination of intervention details. Other common limitations included narrow searching which led to limited samples, inclusion of pediatric and psychiatric primary research, and lack of attention to potential sample characteristic and design moderators.
Although researchers have tested many interventions to increase adherence, results are sometimes conflicting and often unclear, perhaps because interventions vary. These primary studies have not been adequately synthesized, which seriously impedes progress in both practice and research. This meta-analysis addresses the following research questions: 1) What are the average effects of interventions on adherence? 2) Do intervention effects vary depending on study design or sample characteristics? 3) Do intervention effects vary depending on intervention characteristics?
Materials and Methods
Standard systematic review and meta-analysis methods in accordance with PRISMA guidelines were used to conduct and report this project (Cooper et al., 2009; Liberati et al., 2009). The PRISMA checklist is available from the corresponding author. The protocol was not registered.
Information Sources and Search
To avoid the bias resulting from narrow searches, we used multiple comprehensive search strategies (Royle and Milne, 2003). An experienced reference librarian conducted searches in MEDLINE, PsycINFO, CINAHL, EBSCO, PubMED, Cochrane Central Trials Register, Cochrane Database of Systematic Reviews, PQDT, ERIC, IndMed, International Pharmaceutical Abstracts, EBM Reviews - Database of Abstracts of Reviews of Effects, and Communication and Mass Media. The primary MeSH terms upon which searches were constructed were Patient Compliance and Medication Adherence. Patient Compliance was used to locate studies published prior to 2009, which was the year Medication Adherence was introduced as a MeSH term. Medication Adherence was used to locate studies published after 2008. Other MeSH terms used were: pharmaceutical preparations, dosage forms, drugs, generic, or prescription drugs. Text words used in searches were: medication(s), regimen(s), prescription(s), prescribed, drug(s), pill(s), tablet(s), compliant, compliance, adherent, adherence, noncompliant, noncompliance, nonadherent, nonadherence, improve, promote, enhance, encourage, foster, advocate, influence, incentive, ensure, remind, optimize, increase, impact, prevent, address, decrease. The final search was conducted in 2015 with coding and analyses in 2016.
Nineteen research registries were searched (e.g., Research Portfolio Online Reporting tool, European Union Clinical Trials Register) (Easterbrook, 1992). Author searches were conducted for authors of more than one eligible study (Sindhu and Dickson, 1997). Abstracts from 48 conferences were searched (Sindhu and Dickson, 1997). Hand searches were conducted in 57 journals with more than three eligible studies (Dickersin et al., 1994). Ancestry searches were conducted on eligible studies and review papers.
Eligibility Criteria and Study Selection
Adherence refers to the extent to which patients’ medication-taking behavior is consistent with the prescribed regimen (Cramer et al., 2008b; Vrijens et al., 2012; World Health Organization, 2003). Common terms for adherence problems include inadequate adherence, poor adherence, lack of adherence, and nonadherence (World Health Organization, 2003). We included reports of adults’ adherence to medications that health care providers prescribed. We excluded studies of contraceptive/sexual function medications. We excluded samples with major psychiatric or substance abuse problems, as well as incarcerated/institutionalized persons. Studies of adults with predominantly physical health problems with limited mental health symptoms, such as depressive symptoms in cardiac populations, were included.
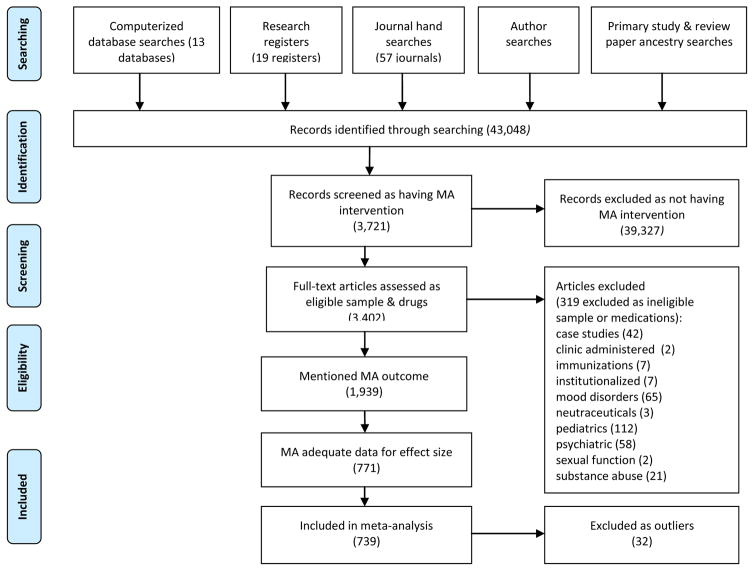
Diverse adherence interventions were eligible for inclusion (e.g., educational or motivational content). Studies with varied measures of adherence (e.g., electronic cap devices, pharmacy refills) were included. Corresponding authors were contacted to secure effect size data when necessary. Studies reported in English or Spanish were included. Both published and unpublished studies were included to reduce bias (Borenstein et al., 2009). Small-sample and pre-experimental studies were included (Borenstein et al., 2009). The flow of potential primary studies is displayed in PRISMA formatted Figure 1.
Figure 1.
Flow diagram of medication adherence (MA) primary studies
Note: Searching completed in 2015, analyses completed in 2016.
Data Collection Process and Data Items
We developed a coding frame based on extensive examination of primary studies, related meta-analyses, and review articles (Wilson, 2009), and piloted it with 50 studies. The coding frame was used to record results of primary studies, as well as characteristics of sources, primary study participants, research methods, and interventions. Distribution vehicle, year of distribution, and presence of funding were coded as source features. We coded mean age, gender and minority distribution, geographic location, selective inclusion of subjects with adherence problems, presence of cognitive impairment, health characteristics, and number of prescribed medications. Research design characteristics included adherence measures, nature of control groups, allocation procedures, masking of data collectors, and intention-to-treat analyses. We coded details about interventions, including involvement of health care providers in intervention delivery, dose, and intervention delivery mechanisms, location, and social setting. Intervention content (e.g., barriers management, prompts) were extensively coded. Online supplementary appendix A includes descriptions of intervention content reported by at least 30 primary studies.
Data were coded at a micro level to enhance validity (Wilson, 2009). To establish that data were coded reliably, two extensively trained coders independently extracted data, which were compared between coders to achieve 100% agreement.
Statistical Analyses
A standardized mean difference (d) effect size was calculated for each primary study comparison (Borenstein et al., 2009). This effect size is based on the treatment group outcome mean minus the control group outcome mean divided by the pooled standard deviation. A positive d indicates better adherence scores for the treatment group. Each effect size was adjusted for bias and weighted by the inverse of its sampling variance to give more precise effect sizes more weight in results (Hedges and Olkin, 1985). The standardized mean difference effect size was converted to the metric of percent of prescribed doses taken to enhance interpretation (Lipsey and Wilson, 2001).
Potential outliers were detected by omitting each effect size one at a time and checking for substantially reduced measures of heterogeneity or large externally standardized residuals. Homogeneity was assessed using a conventional heterogeneity statistic (Q) and computing the I2 index of heterogeneity beyond within-study sampling error. Publication bias was explored with funnel plots and Egger’s tests (Egger et al., 1997; Sterne et al., 2008; Sutton, 2009; Vevea and Hedges, 1995).
The random-effects model was selected for calculating effect sizes because it assumes individual effect sizes vary as a result of both study-level and subject-level variations. Thus, the model is consistent with this area of research, which includes common inclusion criteria differences, varied intervention characteristics, and study execution variations. The expected heterogeneity was handled in four ways. First, the random-effects model was used for analyses. Second, we report both a location and variability parameter. Third, sources of heterogeneity were explored with moderator analyses. Finally, findings were interpreted in the context of heterogeneity. These strategies allow us to interpret the extent to which heterogeneity affects conclusions.
Moderator Analyses
A meta-analytic analogue of ANOVA was used to examine categorical potential moderators using the between-groups heterogeneity statistic (QBetween). A conventional mixed-effects meta-regression procedure was used to estimate and test unstandardized regression coefficients for continuous moderators.
Source, sample, design, and individual intervention characteristics were explored as potential moderators. In addition to conducting moderator analyses on individual intervention content, analyses were conducted on groups of interventions. Behavioral interventions included automatic administration devices, behavior modification, behavioral rehearsal, contracting, consequences, self-administration practice, dose modification, feedback about adherence, financial assistance, habit modification, labeling, packaging, prompts to take medication or refill medications, refill synchronicity, self-monitoring, and skill development. Cognitive strategies included barriers management, cognitive modification, decision making, decisional balance, disease or drug education, emotional arousal, fear messages, health literacy, motivational interviewing, problem solving, and thought restructuring.
Results
The comprehensive searching yielded 771 treatment-versus-control comparisons. The list of primary studies appears in online supplementary appendix B. The earliest studies were published in 1971, the most recent in 2015. Forty studies were published in the 1970s, 60 in the 1980s, 104 in the 1990s, and the remainder in 2000 or more recently. Older studies reported slightly larger effect sizes than more recent studies (Qmodel = 13.30).
Most studies (k = 530) received some funding (k denotes the number of studies). Unfunded studies reported larger effect sizes (d = 0.404) than did funded studies (d = 0.254, Qbetween = 26.737). Two unpublished papers, 9 conference presentations, 49 dissertations or masters’ theses, and 711 published articles reported the eligible research. Publication status was not related to adherence effect sizes. Most studies were conducted in North America (411), and others were conducted in Europe (177), Asia (94), Africa (41), Australia (32), and South America (12). Effect sizes were significantly smaller for North American studies (d = 0.227) than for studies conducted elsewhere (d = 0.369, Qbetween = 32.831). Effect sizes were significantly larger for studies conducted in Asia (d = 0.488) than for studies conducted outside Asia (d = 0.262 Qbetween = 24.803). Eight reports in Spanish were included in the review.
Overall Effects of Interventions on Medication Adherence
The overall effects of interventions are presented in Table 1. The standardized mean difference effect size (d) comparing medication adherence between treatment and control subjects was 0.290. The effect size was significantly heterogeneous. Thirty-two primary studies were excluded as outliers based on examination of residuals; the effect size with the outliers included was 0.383. All subsequent analyses excluded outliers. Three studies each included over 10,000 participants. An effect size of 0.297 was calculated excluding these three large-sample studies. The large studies were included in subsequent analyses. The effect size of 0.290 is consistent with control subjects consuming 77% of prescribed doses and treatment subjects consuming 84% of doses.
Table 1.
Random-Effects Medication Adherence Outcome Estimates and Tests
| k | Effect size | p (ES) | 95% Confidence interval | SE | I2 | Q | p (Q) | |
|---|---|---|---|---|---|---|---|---|
| Treatment vs. controla | 739 | 0.290 | < .001 | 0.267, 0.312 | 0.011 | 77.610 | 3296.111 | < .001 |
| Treatment vs. controlb | 771 | 0.383 | < .001 | 0.356, 0.411 | 0.014 | 87.962 | 6396.362 | < .001 |
| Treatment vs. controlc | 736 | 0.297 | < .001 | 0.272, 0.322 | 0.013 | 73.213 | 2743.894 | < .001 |
| Cardiac samples treatment vs. controla,d | 215 | 0.247 | < .001 | 0.207, 0.286 | 0.020 | 67.634 | 661.196 | < .001 |
| HIV samples treatment vs. controla,d | 114 | 0.233 | < .001 | 0.170, 0.296 | 0.032 | 53.989 | 245.594 | < .001 |
| Pulmonary samples treatment vs. controla,d | 76 | 0.348 | < .001 | 0.283, 0.413 | 0.033 | 78.530 | 349.323 | < .001 |
| Diabetes samples treatment vs. controla.d | 45 | 0.283 | < .001 | 0.188, 0.378 | 0.048 | 75.977 | 183.155 | < .001 |
k denotes number of comparisons, Q is a conventional homogeneity statistic, I2 is the percentage of total variation among studies’ observed ES due to heterogeneity.
32 outliers excluded
32 outliers included
32 outliers excluded and three studies with over 10,000 subjects/study excluded
Includes only studies with 100% subjects having that category of disease
Final searching completed in 2015, analyses in 2016.
Sample Characteristics
The primary studies included 568,811 subjects. The median sample size was 98 participants. Attrition was modest in most studies (median attrition, 6.7%). Middle-aged and older adult samples were common (median of mean age, 55.8 years). Interventions delivered to older samples were associated with lower effect sizes than interventions for younger samples (Qmodel = 7.00, p = .008, k = 538).
Moderator analyses of dichotomous sample moderators are in Table 2. Six hundred and thirty-two studies reported sample gender distribution, 21 studies excluded women, and 39 excluded men. Gender distribution was unrelated to effect sizes (Qmodel = 0.23, k = 609). Forty-five of the 266 North American studies that mentioned minority inclusion reported more than 90% minority participants. The proportion of minority participants was unrelated to adherence outcomes (Qmodel = 0.83, k = 266).
Table 2.
Dichotomous Sample Moderator Results for Medication Adherence: Treatment vs. Control at Outcome
| Moderator | k | Effect size | Standard error | Qbetween | p (Qbetween) |
|---|---|---|---|---|---|
| Income | 0.400 | .527 | |||
| Sample included low income subjects | 79 | 0.265 | 0.041 | ||
| No low income subjects reported in sample | 660 | 0.293 | 0.012 | ||
|
| |||||
| Illiterate | 3.371 | .066 | |||
| Sample included illiterate subjects | 35 | 0.417 | 0.071 | ||
| Study did not report including illiterate subjects | 704 | 0.285 | 0.012 | ||
|
| |||||
| Substance abuse (including alcohol) | 0.927 | .336 | |||
| Sample included subjects with substance abuse problems | 58 | 0.346 | 0.066 | ||
| Study did not report including subjects with substance abuse problems | 681 | 0.282 | 0.011 | ||
|
| |||||
| Depressive symptoms | 0.248 | .619 | |||
| Sample included subjects with depressive symptoms | 49 | 0.326 | 0.082 | ||
| Study did not report including subjects with depressive symptoms | 690 | 0.285 | 0.011 | ||
|
| |||||
| Cognitive impairment | 0.182 | .670 | |||
| Sample included subjects with cognitive impairment | 18 | 0.252 | 0.092 | ||
| Study did not report including subjects with cognitive impairment | 721 | 0.291 | 0.011 | ||
|
| |||||
| Homelessness | 4.289 | .038 | |||
| Sample included subjects without homes | 17 | 0.160 | 0.063 | ||
| Study did not report including homeless subjects | 722 | 0.292 | 0.011 | ||
|
| |||||
| Targeted subjects with low adherence at baseline | 0.477 | .490 | |||
| Targeted low-adherence subjects | 53 | 0.328 | 0.057 | ||
| Study did not report targeting subjects with low adherence | 686 | 0.288 | 0.012 | ||
Final searching completed in 2015, analyses in 2016.
Several sample characteristics were not related to effect sizes: low-income, cognitive impairment, illiteracy, substance abuse (including alcohol), and depressive symptoms. The difference between studies which targeted participants with adherence problems and studies which did not target low-adherence subjects was not statistically significant. Few studies included homeless subjects, and they reported significantly smaller effect sizes than studies not including homeless subjects.
Samples with diverse health problems were included in the meta-analyses. The most common categories of illnesses were cardiac diseases, HIV, pulmonary diseases, and diabetes among primary studies with samples of specific diseases. Subgroup analyses documented a 0.247 effect size for cardiac sample primary studies, 0.233 for HIV samples, 0.348 for pulmonary studies, and 0.283 for diabetes samples. See table 1 for further details.
Medication Adherence Intervention Characteristics
Moderator analysis of intervention characteristics are presented in Table 3. Interventions were significantly more effective when delivered by pharmacists than by other health care professionals. Delivery of interventions by subjects’ own health care providers was not associated with effect-size differences. Interventions that targeted health care providers in hopes the providers would influence patient adherence were less effective than interventions delivered directly to subjects.
Table 3.
Dichotomous Intervention Moderator Results for Medication Adherence: Treatment vs. Control at Outcome
| Moderator | k | Effect size | Standard error | Qbetween | p (Qbetween) |
|---|---|---|---|---|---|
| Interventionist | |||||
|
| |||||
| Physician interventionist | 3.106 | .078 | |||
| Physician | 102 | 0.351 | 0.040 | ||
| Not physician | 637 | 0.279 | 0.012 | ||
|
| |||||
| Pharmacist interventionist | 4.633 | .031 | |||
| Pharmacist | 200 | 0.337 | 0.023 | ||
| Not pharmacist | 539 | 0.279 | 0.014 | ||
|
| |||||
| Nurse | 0.002 | .967 | |||
| Nurse | 179 | 0.292 | 0.026 | ||
| Not nurse | 560 | 0.291 | 0.013 | ||
|
| |||||
| Interventionist is patients’ personal health care provider | 1.586 | .206 | |||
| Personal health care provider | 57 | 0.241 | 0.041 | ||
| Not personal health care provider | 682 | 0.295 | 0.012 | ||
|
| |||||
| Delivery location and target | |||||
|
| |||||
| Intervention delivered in health care clinic | 1.897 | .168 | |||
| Clinic | 184 | 0.323 | 0.028 | ||
| Not clinic | 555 | 0.281 | 0.013 | ||
|
| |||||
| Intervention delivered in patients’ homes | 7.506 | .006 | |||
| Homes | 151 | 0.242 | 0.022 | ||
| Not homes | 588 | 0.315 | 0.015 | ||
|
| |||||
| Intervention delivered to individual patients/families | 0.009 | .923 | |||
| Individual patients/families | 612 | 0.290 | 0.012 | ||
| Groups of patients/families | 127 | 0.294 | 0.040 | ||
|
| |||||
| Intervention targets health care provider or health care system | 4.855 | .028 | |||
| Health care provider/system focus | 84 | 0.211 | 0.039 | ||
| Individual patient/family focus | 655 | 0.302 | 0.012 | ||
|
| |||||
| Intervention Delivery | |||||
|
| |||||
| Automated intervention delivery (e.g. automated phone messages) | 1.228 | .268 | |||
| Automated delivery | 34 | 0.242 | 0.044 | ||
| Not automated delivery | 705 | 0.292 | 0.012 | ||
|
| |||||
| Surface mail delivery | 4.529 | .033 | |||
| Intervention included surface mail delivery | 56 | 0.234 | 0.032 | ||
| Intervention did not include surface mail delivery | 683 | 0.307 | 0.014 | ||
|
| |||||
| Telephone intervention delivery | 0.732 | .392 | |||
| Telephone | 193 | 0.273 | 0.026 | ||
| Not telephone | 546 | 0.297 | 0.013 | ||
|
| |||||
| Text message intervention delivery | 0.007 | .953 | |||
| Text message delivery of at least part of the intervention | 28 | 0.285 | 0.061 | ||
| Not text message delivery of intervention | 711 | 0.290 | 0.012 | ||
|
| |||||
| Computer delivery of intervention | 33.141 | <.001 | |||
| Computer delivery of at least part of the intervention | 14 | 0.066 | 0.038 | ||
| Not computer delivery of intervention | 725 | 0.295 | 0.011 | ||
|
| |||||
| Written materials exclusively used to deliver intervention | 0.494 | 0.482 | |||
| Written intervention | 36 | 0.269 | 0.043 | ||
| Intervention not limited to written materials | 703 | 0.300 | 0.013 | ||
|
| |||||
| Face-to-face intervention | 18.832 | < .001 | |||
| Face-to-face intervention delivery | 564 | 0.331 | 0.016 | ||
| Not face-to-face delivery | 175 | 0.222 | 0.019 | ||
|
| |||||
| Standardized vs. targeted interventiona | |||||
|
| |||||
| Intervention varied based on sample attributes | 5.489 | .019 | |||
| Targeted intervention | 27 | 0.173 | 0.050 | ||
| Not targeted intervention | 712 | 0.294 | 0.012 | ||
|
| |||||
| Theory-based vs. not theory-based intervention | |||||
|
| |||||
| Interventions reported to be based on theories | 0.122 | 0.727 | |||
| Theory-based intervention | 140 | 0.302 | 0.033 | ||
| No theory included in report | 599 | 0.289 | 0.012 | ||
k denotes number of comparisons, effect size is standardized mean difference, Q is a conventional homogeneity statistic.
Only 9 studies individually tailored interventions so tailoring was not assessed as a moderating variable.
Final searching completed in 2015, analyses in 2016.
Interventions were less effective at improving adherence when they were delivered in subjects’ homes as compared to other locations such as clinics or pharmacies. No significant differences occurred between interventions delivered to individuals versus groups. Interventions delivered face-to-face were more effective than interventions delivered in other ways, such as by computer, telephone, surface mail, text messages, and written materials. Interventions delivered by surface mail were significantly less effective than those not delivered this way. Interventions delivered by computer were significantly less effective than interventions delivered by other means.
Most studies used standardized interventions across participants. Studies that targeted interventions for subsamples reported significantly smaller effect sizes compared to studies with standardized interventions. No significant differences were found between effect sizes of studies that reported interventions based on theories and studies that did not report interventions were based on theories.
Intervention strategies reported by at least 30 primary studies included written medication instructions, medication adherence problem solving, improvement of health care provider skills to enhance medication adherence, barriers management, self-monitoring of symptoms/signs influenced by medications, integration of health care, adherence goal setting, stimuli/prompts to take medications, social support formation, increased existing social support from significant others, special packaging of medications, succinct written medication instructions, self-monitoring of medication adherence, habit analysis and intervention, medication side-effect management, feedback about medication adherence, medication calendars, feedback about clinical signs/symptoms related to medication adherence, self-management skills, improving patients’ communication skills, providing consequences/rewards for medication adherence, behavior modification, motivational interviewing, self-efficacy enhancement, medication dose modification, improvement of health care provider communication, and stress management. Descriptions of interventions are found in online supplementary appendix A.
Moderator analyses examined possible associations between intervention strategies and medication adherence outcomes. The only intervention component associated with better adherence outcomes was habit analysis and linking medication adherence with existing habits. Interventions that included habit analysis were more effective (d = 0.369) than interventions that did not (d = 0.284). Interventions were more effective when some intervention strategies were absent: barriers management (p = .025), cognitive modification (p = .007), feedback about disease signs (p = .008), teaching health care providers to improve communication (p = .003), adherence problem solving (p = .006), and social support from investigators (p = .046). Other specific intervention strategies were not associated with differences in effect sizes.
The number of cognitive intervention strategies (those designed to change beliefs, knowledge, or attitudes) was a significant predictor of adherence (Qmodel = 14.26, k = 739). Interventions with a larger number of cognitive strategies were associated with lower adherence outcome effect sizes. The difference between interventions with any cognitive strategies (d = 0.265, k = 295) and interventions without cognitive strategies (d = 0.309, k = 444) was not statistically significant (p = .085).
In contrast, interventions with any behavioral strategies (d = 0.326, k = 298) were more effective than interventions without any behavioral strategies (d = 0.268, k = 441; Qbetween = 4.501). Interventions with a larger number of behavioral strategies were more effective in improving adherence than interventions with fewer behavioral strategies (Qmodel = 11.26, k = 739).
Among studies with complete intervention dose information, dose of the adherence intervention was not related to adherence effect size (k = 147, p = .493). The number of intervention sessions was unrelated to adherence effect size (k = 539, p = .077). The number of days over which the intervention was delivered was not related to adherence outcomes (k = 655, p = .332).
Methodological Characteristics and Risks of Bias
Random assignment to groups was reported in 499 studies (see Table 4). Allocation concealment was present in 177 studies. Neither random assignment nor allocation concealment was associated with effect-size differences. Studies with masked data collectors reported significantly smaller effect sizes than studies without them. Studies with intention-to-treat analyses reported significantly smaller effect sizes than studies without such analyses.
Table 4.
Moderator Analyses of Risks of Bias
| Moderator | k | Effect size | Standard error | Qbetween | p (Qbetween) |
|---|---|---|---|---|---|
| Assignment to groups | 0 | .996 | |||
| Random | 499 | 0.295 | 0.016 | ||
| Non-random | 240 | 0.295 | 0.018 | ||
|
| |||||
| Allocation to groups | 2.785 | .095 | |||
| Concealed | 177 | 0.255 | 0.026 | ||
| Not concealed | 562 | 0.304 | 0.013 | ||
|
| |||||
| Data collector masking | 6.102 | .014 | |||
| Masked | 166 | 0.232 | 0.027 | ||
| Not masked | 573 | 0.307 | 0.013 | ||
|
| |||||
| Attrition statistical management | 9.942 | .002 | |||
| Intention-to-treat analyses | 195 | 0.230 | 0.024 | ||
| No intention-to-treat analyses | 544 | 0.317 | 0.013 | ||
|
| |||||
| Adherence measured with medication electronic measuring caps (MEMS) | 3.236 | .072 | |||
| MEMS adherence measure | 96 | 0.349 | 0.035 | ||
| Non-MEMS adherence measures | 643 | 0.282 | 0.012 | ||
|
| |||||
| Adherence measured with pharmacy refill data | 2.787 | .095 | |||
| Pharmacy refill adherence measure | 104 | 0.261 | 0.024 | ||
| Non-pharmacy refill measure | 635 | 0.307 | 0.015 | ||
|
| |||||
| Adherence measured by research pill counts | 6.340 | .012 | |||
| Pill count adherence measure | 116 | 0.381 | 0.039 | ||
| Non-pill count measure | 623 | 0.279 | 0.012 | ||
|
| |||||
| Adherence measured by subjects self-report | 4.208 | .040 | |||
| Self-report adherence measure | 320 | 0.261 | 0.021 | ||
| Not self-report measure | 419 | 0.313 | 0.014 | ||
Final searching completed in 2015, analyses in 2016.
The analyses comparing four measures of adherence (electronic medication event monitoring, pill counts, pharmacy refill, self-report) documented significant differences in effect sizes across the measures. Adherence measures used in studies with the largest effect sizes were medication event monitoring system and pill counts. The measures used in studies with the smallest effect size were self-report and pharmacy refill data. Effect sizes were significantly smaller for studies with self-report and pharmacy refill adherence data than for other measures.
The funnel plot demonstrated evidence of potential publication bias (see online supplementary appendix C), which was confirmed with Egger’s regression test (t = 4.379, p < .001).
Discussion
This review of 771 adherence intervention studies is the most comprehensive investigation of extant adherence research to date. Regarding intervention characteristics, significantly larger effect sizes were reported among studies with habit-focused interventions and standardized (vs. targeted) content. Interventions that focused on behavioral strategies were significantly more effective than those designed to change knowledge, beliefs, or attitudes (cognitive strategies). On average, interventions delivered by pharmacists were the most effective. Interventions that targeted health care providers in hopes that they would then increase patients’ adherence were less effective than interventions delivered directly to patients. Interventions delivered in subjects’ homes were less effective than those delivered elsewhere. Delivering interventions face-to-face was most effective. Interventions delivered by surface mail were associated with lower effect sizes. Primary study characteristics linked with higher and lower adherence are summarized in Table 5.
Table 5.
Summary of Primary Study Characteristics Linked with Medication Adherence Outcomes
| Higher Adherence | Lower Adherence |
|---|---|
| Intervention Content | |
| Behaviorally focused interventions (e.g., prompts, behavioral goal setting or contracts, rewards) | Cognitive focused interventions to change knowledge, beliefs, or attitudes |
| Habit-based interventions (e.g., link medication taking with existing habits) | |
| Standardized interventions | Individualized interventions |
| Intervention Delivery | |
| Pharmacist interventionists | |
| Intervention delivered directly to patients | Interventions delivered to health care providers with the intent that they will affect patient adherence |
| Face-to-face interventions | Surface-mail interventions |
| Interventions delivered in patients’ homes | |
| Computer-based interventions | |
| Intervention Recipients | |
| Younger patients | Older patients |
| Homeless patients | |
| Research Methods | |
| Pill count adherence measurements | Self-report adherence measurements |
| Masked data collectors | |
| Intention to treat analyses | |
Statistically significant moderators of medication adherence effect sizes.
Overall Effects of Interventions
Multiple possible explanations exist for the modest magnitude 0.290 standardized mean difference effect size, which is consistent with effect sizes reported by previous smaller and more narrowly focused meta-analyses (Demonceau et al., 2013; Mullen et al., 1985; Peterson et al., 2003; Roter et al., 1998). Adherence is difficult to change. Entirely different interventions may be needed than the ones tested to date. Although comparison groups are described as control, attention control, or usual care, subjects in those groups may receive some form of intervention, perhaps to avoid ethical concerns, thus diminishing treatment-versus-control comparisons (Demonceau et al., 2013). People with poor adherence may also be less likely to enroll in studies, which leads to selection bias and may influence outcomes (Demonceau et al., 2013).
The overall effect size was consistent with control subjects consuming 77% of prescribed doses and treatment subjects consuming 84% of doses. We do not know the adherence level required to achieve therapeutic outcomes for each drug, disease, and patient characteristic (Cramer et al., 2008a; Cutrona et al., 2010). Nor do we fully understand the individual and combined effects of adherence and other health behaviors for many health outcomes. It is possible that effectiveness of intervention strategies may be somewhat disease-specific; the best intervention approaches may vary somewhat for the particular diseases, drug class, or patient population. Despite these limitations, the call to improve adherence is widespread because many pharmaceutical agents can profoundly affect disease trajectories and because nonadherence rates are so high.
Links Between Adherence Outcomes and Intervention Characteristics
This study’s detailed coding of intervention characteristics is another way it moved beyond previous reviews (Demonceau et al., 2013; Mullen et al., 1985; Roter et al., 1998; van Dulmen et al., 2007). Micro-level coding allowed moderator analyses of specific intervention characteristics. The superiority of habit-based and behavioral-focused interventions compared to cognitive-based interventions was the most interesting finding of the study. Both habit-based interventions and behavioral-focused interventions remove medication-taking from the realm of daily deliberate decision making. In contrast, cognitive-based interventions focus on knowledge and beliefs that would inform medication-administration decisions. Cognitive interventions might be useful for health behaviors such as cancer screening in which important decisions are infrequent and deliberate. However, activities requiring repeated behavior over years may require behavioral interventions. Health care providers emphasize patient education, but evidence suggests knowledge is inadequate to change adherence (Conn et al., 2009; Mazzuca, 1982). This review-generated evidence suggests the value of future direct comparisons of behavioral-focused and cognitive-focused interventions. These findings across hundreds of completed primary studies suggest health care providers should emphasize strategies that link medication administration with existing daily routines and that focus on behavioral strategies such as prompts to take medications, self-administration practice, special packaging and labeling, behavioral contracts, and self-monitoring of adherence.
Although others have suggested that interventions be modified to match individual subject characteristics (Demonceau et al., 2013), this review found standardized interventions most effective. Standardized interventions may be easier to deliver and result in better treatment integrity. It is also possible that individualized interventions may allow interventionists to focus more on cognitive interventions, which are less effective.
Pharmacists were the most effective interventionists. Their specialized training may best prepare them to improve adherence (Cutrona et al., 2010). Patients may be especially likely to believe pharmacists have expert medication knowledge and thus follow their suggestions. Although mediated delivery of interventions may be low-cost, face-to-face delivery was most effective in improving adherence. The potential costs of poor health outcomes from inadequate adherence may justify the personnel time to deliver interventions face-to-face. Interventions targeting health care providers may have focused on cognitive strategies and thus had limited effects.
Methodological Variations and Adherence Outcomes
Risks of bias are common in this area of science. Two common methodological biases — lack of data collector masking and absence of intention-to-treat analyses — were linked with larger effect sizes. This study confirmed publication bias reported in other reviews (Demonceau et al., 2013). Studies using event monitoring and pill count adherence measures reported the largest effect sizes. Although measuring adherence is challenging, the continued use of self-report measures is unfortunate because their imprecise nature may obscure group differences (Culig and Leppee, 2014; Haynes et al., 2008; Lehmann et al., 2014). Future adherence intervention research should randomize subjects, conceal allocation, mask data collectors, conduct intention-to-treat analyses, avoid self-report adherence measures, and report findings regardless of statistical significance.
Study Limitations and Strengths
This synthesis was limited to the outcome of adherence behavior, which is a process measure. Future primary research should include not only adherence behavior but also health outcomes (Chisholm-Burns et al., 2010; Haynes et al., 2008). Future research should address cost-effectiveness of interventions. The synthesis was limited to studies comparing treatment and control subjects. Too few studies directly compared similar treatment groups for quantitative syntheses. This project did not address interventions for predominantly psychiatric populations, as these populations have unique factors influencing adherence behavior. Meta-analytic moderator analyses are observational findings; direct comparisons in future primary research can confirm findings. Descriptive statistics and moderator analyses of intervention and sample characteristics were limited by information provided in reports. Although we used very comprehensive search strategies, it is possible a few adherence studies were not retrieved.
Despite its limitations, this meta-analysis moved far beyond previous reviews. This is the most comprehensive review of medication adherence interventions reported. The review quantified the effect of interventions on patient adherence. The micro-level coding allowed examination of multiple intervention characteristics such as content, interventionist, and delivery mechanism and site as potential moderators of adherence outcomes. The links between risks of bias and effect sizes were explored.
Conclusion
In conclusion, the findings from this most comprehensive review to date document that, although interventions can improve adherence, much room remains for improvement. Meta-analytic moderator analyses suggest health care providers should focus intervention content on behavioral strategies, especially habit-based interventions, over cognitive strategies designed to change knowledge and beliefs. Future research designs should strive to incorporate fewer threats of bias. Finally, we recommend that more adherence research report outcomes of health, quality of life, and health care costs as they seek to fully evaluate the impact of adherence interventions.
Supplementary Material
Highlights.
We retrieved 771 comparisons that tested interventions to improve adherence.
The mean difference medication adherence effect size was of 0.290.
Habit-based and behavioral (vs. cognitive) interventions were most effective.
Face-to-face and pharmacist delivered interventions improved adherence.
Medication adherence effect sizes were related to some common risks of bias.
Acknowledgments
Funding
The project was supported by the National Institutes of Health (grant number R01NR011990). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The sponsor had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Conflicts of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Vicki S. Conn, S317 Sinclair Building University of Missouri Columbia MO 65211 USA.
Todd M. Ruppar, University of Missouri Columbia MO.
References
- Borenstein M, Hedges L, Higgins JPT, Rothstein H. Introduction to Meta-Analysis. John Wiley & Sons, Ltd; West Sussex, England: 2009. [Google Scholar]
- Chisholm-Burns MA, Kim Lee J, Spivey CA, Slack M, Herrier RN, Hall-Lipsy E, Graff Zivin J, Abraham I, Palmer J, et al. US pharmacists’ effect as team members on patient care: systematic review and meta-analyses. Med Care. 2010;48:923–33. doi: 10.1097/MLR.0b013e3181e57962. [DOI] [PubMed] [Google Scholar]
- Christensen AJ. Patient Adherence to Medical Treatment Regimens. Yale; New Haven: 2004. [Google Scholar]
- Conn VS, Hafdahl AR, Cooper PS, Ruppar TM, Mehr DR, Russell CL. Interventions to improve medication adherence among older adults: meta-analysis of adherence outcomes among randomized controlled trials. Gerontologist. 2009;49:447–62. doi: 10.1093/geront/gnp037. [DOI] [PubMed] [Google Scholar]
- Cooper H, Hedges LV, Valentine JC. The Handbook of Research Synthesis and Meta-Analysis. 2. Russell Sage Foundation; New York: 2009. p. 615. [Google Scholar]
- Cramer JA, Benedict A, Muszbek N, Keskinaslan A, Khan ZM. The significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a review. Int J Clin Pract. 2008a;62:76–87. doi: 10.1111/j.1742-1241.2007.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication compliance and persistence: terminology and definitions. Value Health. 2008b;11:44–7. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- Culig J, Leppee M. From Morisky to Hill-bone; self-reports scales for measuring adherence to medication. Collegium Antropol. 2014;38:55–62. [PubMed] [Google Scholar]
- Cutrona SL, Choudhry NK, Stedman M, Servi A, Liberman JN, Brennan T, Fischer MA, Brookhart MA, Shrank WH. Physician effectiveness in interventions to improve cardiovascular medication adherence: a systematic review. J Gen Intern Med. 2010;25:1090–96. doi: 10.1007/s11606-010-1387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonceau J, Ruppar T, Kristanto P, Hughes DA, Fargher E, Kardas P, De Geest S, Dobbels F, Lewek P, et al. Identification and assessment of adherence-enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: a systematic literature review and meta-analysis. Drugs. 2013;73:545–62. doi: 10.1007/s40265-013-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ. 1994;309:1286–91. doi: 10.1136/bmj.309.6964.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterbrook PJ. Directory of registries of clinical trials. Statistics in Medicine. 1992;11:363–423. [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes R, Ackloo E, Sahota N, McDonald H, Yao X. Interventions for enhancing medication adherence. Cochrane Db Syst Rev. 2008;2008(2):CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- Hedges L, Olkin I. Statistical Methods for Meta-analysis. Academic Press; Orlando, FL: 1985. [Google Scholar]
- Lehmann A, Aslani P, Ahmed R, Celio J, Gauchet A, Bedouch P, Bugnon O, Allenet B, Schneider MP. Assessing medication adherence: options to consider. Int J Clin Pharm-Net. 2014;36:55–69. doi: 10.1007/s11096-013-9865-x. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsey M, Wilson D. Practical Meta-analysis. Sage; Thousand Oaks, CA: 2001. [Google Scholar]
- Mazzuca SA. Does patient education in chronic disease have therapeutic value? J Chron Dis. 1982;35:521–9. doi: 10.1016/0021-9681(82)90071-6. [DOI] [PubMed] [Google Scholar]
- Mullen P, Green LW, Persintger G. Clinical trials of patient education for chronic conditions: a comparative meta-analysis of intervention types. Prev Med. 1985;14:753–581. doi: 10.1016/0091-7435(85)90070-2. [DOI] [PubMed] [Google Scholar]
- Peterson AM, Takiya L, Finley R. Meta-analysis of trials of interventions to improve medication adherence. Am J Health-Syst Ph. 2003;60:657–65. doi: 10.1093/ajhp/60.7.657. [DOI] [PubMed] [Google Scholar]
- Roter DL, Hall JA, Merisca R, Nordstrom B, Cretin D, Svarstad B. Effectiveness of interventions to improve patient compliance: a meta-analysis. Med Care. 1998;36:1138–61. doi: 10.1097/00005650-199808000-00004. [DOI] [PubMed] [Google Scholar]
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. Int J Technol Assess. 2003;19:591–603. doi: 10.1017/s0266462303000552. [DOI] [PubMed] [Google Scholar]
- Sindhu F, Dickson R. The complexity of searching the literature. Int J Nurs Pract. 1997;3:211–7. doi: 10.1111/j.1440-172x.1997.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Sterne J, Egger M, Moher D. Addressing reporting biases. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Wiley-Blackwell; West Sussex, England: 2008. pp. 297–334. [Google Scholar]
- Sutton AJ. Publicaton bias. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2. Russell Sage Foundation; New York: 2009. pp. 435–52. [Google Scholar]
- van Dulmen S, Sluijs E, van Dijk L, de Ridder D, Heerdink R, Bensing J. Patient adherence to medical treatment: a review of reviews. BMC Health Services Research. 2007;7:55. doi: 10.1186/1472-6963-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vevea JL, Hedges LV. A general linear model for estimating effect size in the presence of publication bias. Psychometrika. 1995;60:419–35. [Google Scholar]
- Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, Dobbels F, Fargher E, Morrison V, et al. A new taxonomy for describing and defining adherence to medications. Brit J Clin Pharmaco. 2012;73:691–705. doi: 10.1111/j.1365-2125.2012.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. Systematic coding. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2. Russell Sage Foundation; New York: 2009. pp. 159–76. [Google Scholar]
- World Health Organization. Adherence to long-term therapies: evidence for action. Geneva, Switzerland: 2003. pp. 1–198. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.