Abstract
Monoclonal antibody 10-1074 targets the V3 glycan supersite on the HIV-1 envelope protein. It is among the most potent anti-HIV-1 neutralizing antibodies isolated to date. Here we report on its safety and activity in 33 subjects who received a single intravenous infusion of the antibody. 10-1074 was well tolerated with a half-life of 24.0 days in uninfected and 12.8 days in HIV-1-infected subjects. 13 viremic subjects received the highest dose of 30 mg/kg 10-1074. 11 of these participants were 10-1074-sensitive and showed a rapid decline of viremia by a mean of 1.52 log10 copies/ml. Virologic analysis revealed the emergence of multiple independent 10-1074-resistant viruses within the first weeks after infusion. Emerging escape variants were generally resistant to the related V3-specific antibody PGT121, but remained sensitive to antibodies targeting non-overlapping epitopes, such as the anti-CD4 binding site antibodies 3BNC117 and VRC01. The results demonstrate the safety and activity of 10-1074 in humans and support the idea that antibodies targeting the V3 glycan supersite may be useful for treatment and prevention of HIV-1 infection.
Introduction
A small fraction of HIV-1-infected individuals develop antibodies that effectively neutralize the majority of existing HIV-1 isolates1–7. Single cell antibody cloning methods revealed that this serum neutralizing activity is due to one or a combination of monoclonal antibodies that target different non-overlapping epitopes on the HIV-1 envelope spike1,3,5,6. These sites of vulnerability include the membrane proximal region8–10, the base of the V3 loop and surrounding glycans11–14, the V1/V2 loops at the apex15,16, the CD4 binding site17–19, and a series of epitopes that span gp120 and gp4120,21.
When passively transferred, many of these newly discovered antibodies protect against infection in humanized mice and macaques, even when present at very low concentrations22–25. In addition, combinations of antibodies targeting non-overlapping epitopes can control active infection in humanized mice and macaques26–29. Finally, when they are administered together with agents that induce viral transcription to activate latently infected cells, antibodies decrease the incidence of viral rebound from the latent reservoir in HIV-1-infected humanized mice30. These effects are in part dependent on the ability of antibodies to engage the host immune system by binding to Fc receptors expressed on a variety of host leukocytes30–33.
These preclinical findings were extended to humans in two separate phase 1 clinical trials. A single intravenous injection of an anti-CD4 binding site antibody, 3BNC117 or VRC01, was generally safe and suppressed viremia by 0.8 to 2.5 log10 in participants infected with a virus that was sensitive to the antibody34–36. Moreover, 3BNC117 infusion was associated with enhanced Fc receptor-dependent clearance of infected cells and increased breadth and potency of host anti-HIV-1 antibody responses33,37. Finally, in participants that carried sensitive viruses, administration of 2 or 4 3BNC117 30mg/kg infusions significantly delayed viral rebound during interruption of antiretroviral therapy (ART) and resulted in viral suppression for 6.7 or 9.9 weeks, respectively38. However, both 3BNC117 and VRC01 recognize the same target on the HIV-1 envelope protein, and whether bNAbs that target additional epitopes on the HIV-1 spike are safe and clinically effective has not been determined.
Results
10-1074 shows a favorable pharmacokinetic profile and is well tolerated
10-1074 is a highly potent anti-HIV-1 antibody isolated from an HIV-1 clade A-infected individual11. It targets a carbohydrate-dependent epitope in the V3 loop of the HIV-1 envelope spike11,14. When tested against large panels of HIV-1 pseudoviruses in TZM.bl neutralization assays in vitro, 10-1074 neutralizes 60.5% of 306 strains comprising 13 subtypes and 88.5% of 26 clade B strains at an average 80% inhibitory concentration (IC80) of 0.18 μg/ml and 0.13 μg/ml, respectively (Supplementary Fig. 1a, c)39. When tested against primary HIV-1 isolates from 179 HIV-1-infected individuals (77 off and 102 on antiretroviral therapy, ART) living in the United States or in Germany, 77.7% of cultures were neutralized with a mean IC80 of 0.67 μg/ml (Supplementary Fig. 1b,c).
To determine whether 10-1074 is safe and has antiviral activity in humans, we performed an open label phase 1 first-in-human clinical trial. 14 uninfected and 19 HIV-1-infected individuals (3 on ART, 16 off ART) received a single intravenous infusion of 10-1074 at doses of 3, 10, or 30 mg/kg (Fig. 1a, Table 1, Supplementary Fig. 2 and Supplementary Table 1). The antibody was generally safe and well tolerated in all participants. No grade 3, 4, or serious treatment-related adverse events, or significant laboratory abnormalities were observed during a follow-up period of up to 168 days (total of 5,447 patient-days, Supplementary Table 2).
Figure 1. Study design and pharmacokinetics of 10-1074 in healthy and HIV-1-infected individuals.

(a) Schematic representation of the study design. (b) Serum levels of 10-1074 as determined by TZM.bl assay. Mean values for each dose group with SEM for uninfected (left) and HIV-1-infected individuals (right). Dotted grey lines at the bottom indicate lower limit of detection of the assays when performed with HIV-1 strains Du422.1 (top, 1.59 μg/ml), 3103.v3.c10 (middle, 0.43 μg/ml), and 3103.v3.c10 in an antiretroviral therapy-resistant backbone (bottom 0.26 μg/ml). Each sample was measured in duplicate. Serum half-life of 10-1074 between HIV-1-uninfected and -infected individuals was significantly different (p<0.0001). Samples demonstrating nonspecific activity were excluded from the analysis (Supplementary Table 3).
Table 1.
Study participant demographics
| Uninfected (n = 14) | HIV-1-infected (n = 19) | |
|---|---|---|
| Gender (% male) | 71% | 84% |
| Mean Age (range) | 43 (25 – 60) | 39 (24 – 53) |
| Race/Ethnicity | ||
| White | 36% | 47% |
| Black or African American | 43% | 42% |
| Hispanic | 7% | 11% |
| Multiple | 7% | – |
| Unknown | 7% | – |
| ART status at enrollment | ||
| On ART n (%) | – | 3 (16%) |
| Off ART n (%) | – | 16 (84%) |
|
| ||
| CD4+ T cell count (day 0) | ||
| Mean Absolute (cells/μl) | – | 593 (289 – 880) |
| Mean Relative (%) | – | 30% (11 – 49%) |
|
| ||
| HIV-1 RNA levels (day 0)* | ||
| Geometric Mean (copies/ml) | – | 12,851 (840 – 77,610) |
| Mean Log | – | 4.11 (2.92 – 4.89) |
HIV-1 RNA levels in individuals off ART on day 0.
10-1074 serum levels were determined by TZM.bl neutralization assay, which measures the amount of active antibody in the serum (Supplementary Table 3)34,40–42. Similar to 3BNC11734,35, 10-1074 was eliminated more rapidly in HIV-1-infected individuals than in uninfected participants (Fig. 1B), resulting in a t1/2 of 24.0 and 12.8 days in uninfected and HIV-1-infected subjects, respectively (Supplementary Table 4). The differences in antibody half-life may be due to the presence of the target antigen in the HIV-1-infected individuals and accelerated clearance of antigen-antibody immune complexes34–36,43. Moreover, HIV-1-infection is associated with increased levels of immunoglobulins potentially resulting in reduced antibody half-lives44.
10-1074 reduces viremia in individuals with sensitive HIV-1 strains
As expected, no changes in viral load were detected after infusion in 3 ART suppressed individuals (VL < 20 copies/ml) (Table 1, Supplementary Table 1 and Supplementary Table 3a). At the time of enrollment, HIV-1-RNA levels in the 16 HIV-1-infected individuals who were not on ART ranged from 840 – 77,610 copies/ml (geometric mean of 12,851 copies/ml, Table 1, Supplementary Table 1, and Supplementary Table 3a). The 3 individuals that received a dose of 10 mg/kg of the antibody showed a rapid 1.08–1.56 log10 copies/ml decrease in viremia, with a nadir at 7 – 9 days (Fig. 2a, Supplementary Fig. 3a and Supplementary Table 3a) and a return of viral RNA copy number to baseline levels within 3 to 4 weeks after infusion (Fig. 2a, Supplementary Fig. 3a and Supplementary Table 3a). Out of 13 viremic participants administered a dose of 30 mg/kg, 11 experienced a rapid decrease in their HIV-1-RNA levels. The 2 individuals that failed to respond, 1HD2 and 1HD9K, were infected with 10-1074-resistant HIV-1 variants before 10-1074 infusion (Fig. 2a and 3b, Supplementary Fig. 4). The average drop in viremia in individuals that received 30 mg/kg of 10-1074 and harbored sensitive strains was 1.52 log10 copies/ml (range 0.9 to 2.06 log10 copies/ml). The nadir was reached after an average of 10.3 days (range 7–25). When compared to pre-treatment HIV-1-RNA levels (day 0), the decrease in viremia in this group was significant from about day 3 to day 27 post-infusion (Fig. 2a and Supplementary Table 3a, Supplementary Figure 5). The average 10-1074 serum concentrations at viral rebound (defined as an increase of at least 0.5 log10 copies/ml above nadir and confirmed in the follow-up visit) were 23.7 and 76.9 μg/ml in the 10 and 30 mg/kg dose groups, respectively (Fig. 2b). The most sustained response, 58 days, occurred in individual 1HD6K, who exhibited a 10-1074 concentration of 6.5 μg/ml at rebound (Fig. 2b). Maximum drop in viral load (Δlog10 copies/ml) was not correlated with the 10-1074 neutralization sensitivity (IC80) of primary virus cultures obtained before infusion (Spearman’s rho: −0.05) or with baseline viremia (Spearman’s rho = −0.19) (Fig. 2c, d). Relative T cell subsets remained unchanged (Supplementary Fig. 6 and Supplementary Table 3a). We conclude that 10-1074 administration rapidly decreases viremia in individuals infected with sensitive HIV-1 strains.
Figure 2. Viral load dynamics after 10-1074 infusion in HIV-1-infected participants.
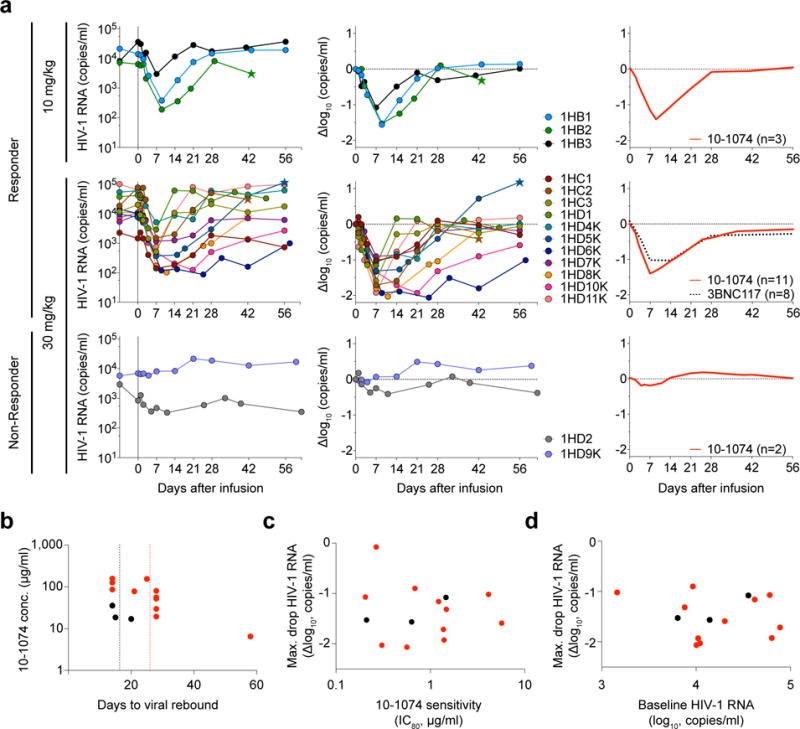
(a) 10-1074 dose of either 10 or 30 mg/kg is indicated on the left. Left graphs show absolute viral loads in HIV-1 RNA copies/ml (y-axis) vs. time in days after infusion (x-axis). Middle graphs demonstrate log10 changes in HIV-1 RNA levels from day 0. Right graphs show average log10 change in viremia after 10-1074 (red line) or 3BNC117 infusion34 (dotted black; curves were fitted by robust lowess regression with 40% of the data using MATLAB_R2016a). At the 30 mg/kg dose level, viremia was significantly suppressed from about day 3 to day 27 post infusion compared to viral load at day 0. The window of significant viral suppression was assessed by computing simultaneous confidence bands and determined when these excluded zero (Supplementary Figure 5). Stars indicate initiation of antiretroviral therapy. (b) Plot shows 10-1074 serum levels at the time of rebound for individuals receiving 10 or 30 mg/kg of 10-1074 (black or red circles, respectively). Dotted lines indicate mean time to rebound after 10-1074 infusion (black, 10 mg/kg, and red, 30 mg/kg). (c) Maximum log10 decline in viremia as measured by RNA copies/ml vs. 10-1074 IC80 (Spearman coefficient rho = −0.05, p=0.88) of primary culture virus from samples obtained 557 to 61 days prior to infusion as determined by TZM.bl assay. No sensitivity data were obtained from 1HC2, 1HD2 and 1HD10K prior to enrollment. Colors as in (b). (d) Maximum log10 decline in viremia in 10-1074-sensitive individuals vs. initial viral load as measured by RNA copies/ml (Spearman coefficient rho = −0.19, p=0.52). Colors as in (b).
Figure 3. Viral evolution after 10-1074 infusion in HIV-1-infected participants.
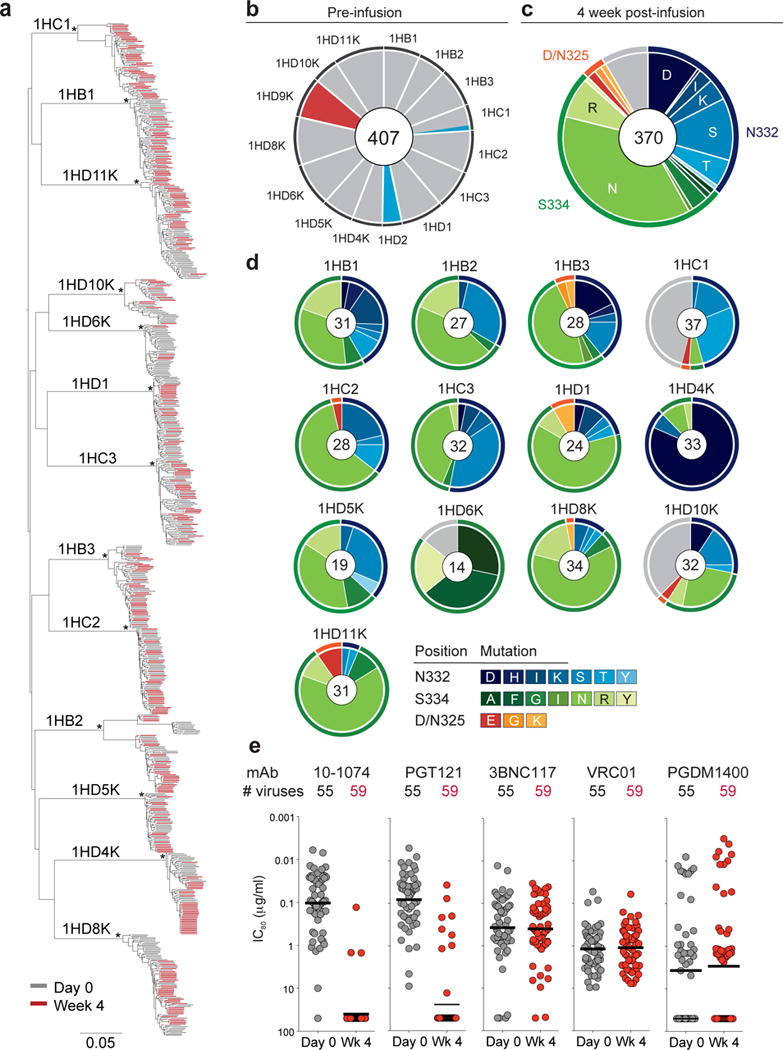
(a) Maximum-likelihood phylogenetic tree of single genome-derived env gene sequences obtained from plasma before (day 0, grey) and 4 weeks after 10-1074 infusion (Week 4, red). Asterisks indicate nodes with bootstrap support of 100% (100 replicates). (b) Pie chart showing the frequency of resistance mutations found in circulating viruses by SGS before infusion for each subject. Grey indicates absence of potential resistance mutation at positions 325, 332 and 334. Colors correspond to mutations indicated in (c). For all pie charts the number of analyzed sequences is shown in the center. (c) Pie chart showing amino acid frequencies at three recurrently mutated 10-1074 contact sites for all pooled circulating virus sequences obtained by SGS 4 weeks after infusion. Outer rings indicate position of mutation (orange, 325; blue, 332; green, 334; grey, unmutated). (d) As in (c) but for each individual. Colors indicate the type of mutation. For 1HD6K and 1HD10K both week 4 and week 8 were included in (c) and (d). (e) Graph showing sensitivity to the indicated anti-HIV-1 antibodies of 114 different viral isolates obtained from 11 individuals before (grey, 55 isolates) and 4 weeks after 10-1074 infusion (red, 59 isolates) with IC80 values (μg/ml) on the y-axis (log10 scale). Each dot represents one viral isolate. Lines indicate geometric mean. Samples were run in duplicate.
Viremia at rebound contains a mixture of different escape variants
To examine the precise nature of the virologic effects of 10-1074 infusion, we performed single genome sequencing (SGS) on circulating viruses from 15 out of 16 viremic individuals before and after infusion, retrieving a total of 1,111 full length envelope sequences (Supplementary Table 5). Study subjects were infected with epidemiologically distinct Clade B viruses (Fig. 3a, Supplementary Fig. 4). Consistent with different durations of HIV-1 infection, day 0 env diversity and phylogenetic complexity varied between participants (Fig. 3a and Supplementary Fig. 4).
The 10-1074 antibody makes critical contacts with Env glycans at a potential N-linked glycosylation site (PNGS) at position N332 and with the 324G(D/N)IR327 motif at the base of the V3 loop11,14,45. With the exception of the 2 individuals that were resistant to 10-1074 (1HD2 and 1HD9K) and subject 1HC1, all day 0 plasma env sequences in the remaining 10 individuals displayed a PNGS at position N332 and an intact 324G(D/N)IR327 motif, suggesting that the corresponding viruses are sensitive to 10-1074 (Fig. 3b, Supplementary Fig. 4 and Supplementary Table 5). Subject 1HC1, who responded to 10-1074 infusion with only a 1.0 log10 copies/ml drop in viremia, carried an N332T mutation in 2 of 19 viruses sequenced before infusion (Fig. 3b and Supplementary Fig. 4). The 2 individuals that did not respond carried single amino acid mutations at either the PNGS (N332T in 1HD2) or a 10-1074 contact residue in the protein-binding motif (D325E in 1HD9K) in 100% of day 0 plasma env sequences (Fig. 3b and Supplementary Fig. 4).
Four weeks after infusion, 370 intact envelope sequences were obtained from 13 subjects that responded to 10-1074. Over 91% of sequences showed recurrent amino acid (AA) mutations, most of which (97%) eliminated the PNGS at position 332 by mutating either N332 or S334 (Fig. 3c, d). 3% of mutated sequences showed changes at D/N325 in the 324G(D/N)IR327 motif (Fig. 3c,d, Supplementary Table 5). Viral sequences comprised a mixture of different variants, all of which were closely related to pre-infusion day 0 viruses, highlighting the multi-clonal origin of rebound viremia (Fig. 3a and Supplementary Fig. 4). Mutations at AA positions 325, 332 and 334 were mutually exclusive with only one of three positions being mutated in any given virus (Supplementary Fig. 4). Two of the most frequent mutations, S334N and N332S, were found in 12/13 (S334N) and 11/13 (N332S) of the individuals analyzed (Fig 3c, d and Supplementary Fig. 4).
On the nucleotide level, 99% (319 out of 322) of mutations affecting the PNGS at N332 were due to a single change relative to day 0 (Supplementary Fig. 7). The majority of these mutations were transitions, consistent with reverse transcriptase (RT) errors46. Day 0 viruses from 10 out of 13 individuals that responded to 10-1074 infusion carried the triplets AAC-ATT-AGT at positions 332–334 in 99% of their pre-infusion sequences (Supplementary Fig. 7). Interestingly, these individuals exhibited a very similar spectrum of amino acid escape mutations at week 4 (Fig. 3d, Supplementary Fig. 7). In contrast, subject 1HD6K, who maintained viral suppression for over 8 weeks, exhibited a different set of codons, i.e., AAT-ATT-TCT, in the antibody target region. This likely resulted in a different spectrum of escape mutations indicating that the codon composition in this region may influence viral escape from 10-1074 (Fig. 3d, Supplementary Fig. 7).
To determine whether loss of the PNGS at position N332 or 324G(D/N)IR327 mutation is associated with resistance to 10-1074, we performed neutralization assays on 114 pseudoviruses expressing envelope proteins derived from circulating viruses on day 0 (55 pseudoviruses) and 4 weeks (59 pseudoviruses) after 10-1074 infusion (Fig. 3e, Supplementary Figure 4, Supplementary Table 6). The pseudoviruses were tested against antibodies currently in, or being considered for, clinical testing. These include 3BNC117 and VRC01 that target the CD4 binding site34–36,38; 10-1074 and PGT121 that recognize the base of the V3 loop and surrounding glycans11,12; and PGDM1400, which recognizes a conformational epitope at the top of the envelope spike16. PGT121 differs from 10-1074 in that it interacts more strongly with glycans at positions N137, N156, and N301, and as a result PGT121 is believed to be less dependent on the glycan at N332 than 10-107413,14.
As expected, there was no correlation between emergence of resistance to 10-1074 and resistance to antibodies targeting non-overlapping sites on HIV-1 Env (3BNC117, VRC01, or PGDM1400, Fig. 3e). Consistent with data obtained by examining large panels of HIV-1 pseudoviruses, there was no significant difference in sensitivity against 10-1074 and PGT121 (MeanGEO IC80 = 0.10 vs. 0.08 μg/ml; p=0.19, respectively; Fig. 3e) and 3BNC117 was generally more potent than VRC01 (MeanGEO IC80 = 0.37 vs. 1.18 μg/ml; p=5.1 × 10−6; (Fig. 3e). In 10 out of 11 individuals from whom envelopes were tested, HIV-1 strains that developed resistance to 10-1074 were also resistant to PGT121 (Supplementary Table 6). Thus, the interaction between PGT121 and additional glycans on the Env protein had little impact on the neutralizing potency of PGT121 on the naturally arising N332 or 324G(D/N)IR327 viral variants tested.
We also analyzed changes in the composition of observed escape mutations in HIV-1 variants in 6 individuals over time. In all 6 individuals, the relative abundance of escape variants changed over time (Fig. 4). In addition, in 5 out of 6 subjects, strains carrying the intact 324G(D/N)IR327 protein motif and a PNGS at N332 re-emerged at 12, 16 or 24 weeks after infusion at a time when 10-1074 levels approached or were below the limit of detection (Fig. 4). This indicates ongoing selection for both resistance and/or viral fitness after 10-1074 infusion.
Figure 4. Temporal evolution of escape from 10-1074 over time in subjects 1HB1, 1HB3, 1HC1, 1HD1, 1HD6K, and 1HD10K.

Plots display relative frequencies of escape mutations observed in SGS data at envelope positions 325, 332 and 334 as shaded areas over time. Sequencing was performed on day 0 (all subjects), and at week 1 (1HB3, 1HD1), week 2 (1HB3), week 4 (all subjects), week 8 (1HD6K, 1HD10K), week 12 (1HD6K, 1HD10K), week 16 (1HB3), week 20 (1HD6K) and week 24 (1HB1, 1HC1, 1HD1) (see Supplementary Table 5 for absolute numbers). White line indicates serum concentration of 10-1074 as determined by TZM.bl assay (Supplementary Table 3). White circles without border depict 10-1074 serum levels below the limit of detection.
Escape variants are pre-existing or rapidly generated
Previous studies suggest that high viral turnover in HIV-1 infection results in a multitude of circulating mutant strains in any given individual47,48. To examine the dynamics of early viral escape, we sequenced viruses present in the plasma 1 week after 10-1074 administration in 7 individuals (Supplementary Fig. 8 and Supplementary Table 5). We found a mixture of unmutated and N332-PNGS- or 324G(D/N)IR327-mutant viruses. Moreover, the frequency of escape variants at this early time point after infusion was inversely correlated with the duration of viral suppression; R2 = 0.73 (Supplementary Fig. 8).
The kinetics of escape are consistent with pre-existing resistant viral variants in circulation at the time of infusion and/or rapid de novo generation of these mutations. To estimate the frequency of pre-existing variants and to assess how they contribute to 10-1074 escape, we performed primer-ID based deep-sequencing (PIDS) of the V3-loop region49,50 on 5 study participants. A total of 2,077 and 1,844 viral consensus sequences were obtained at day 0 and week 4, respectively (Supplementary Table 7). The obtained sampling depth was powered to detect a mutation present in 1.0% (range: 0.5%–2.4%) of the viral population with 95% confidence. While mutations at positions 325, 332 or 334 were found in 99.8% of the sequences in the week 4 samples, none were observed at day 0 (Supplementary Table 7). This indicates that potential pre-existing escape mutations are likely less frequent than 1.0%.
We also performed full-length envelope deep-sequencing (SMRT [Single molecule real time] sequencing) of day 0 and week 4 samples from 3 of the same subjects (Fig. 5 and Supplementary Table 8)51. Frequencies of detected variants carrying a 325 or 332–334 mutation agreed well among SMRT sequencing, PIDS, and SGS (Supplementary Table 8). A phylogenetic tree of SMRT sequences confirmed that escape variants arose from multiple independent HIV-1 viruses (Fig. 5 and Supplementary Fig. 9). Out of a total of 30,711 day 0 sequences obtained by SMRT sequencing, we found D/N325K and S334R mutations in subject 1HD1 at a rate of 0.15% and 0.11%, respectively, and the S334N mutation at a rate of 0.34% in subject 1HB3. No 325 or 332–334 mutants were detected in subject 1HC2 (Fig. 5 and Supplementary Fig. 9). We conclude that circulating pre-existing variants carrying mutations at key positions associated with resistance to 10-1074 can be detected at very low frequency in individuals considered sensitive to the antibody and that resistance originates from multiple viral variants.
Figure 5. SMRT sequencing analysis.

(a) Maximum-likelihood phylogenetic tree of full-length plasma envelope sequences obtained on day 0 and week 4 after 10-1074 infusion from subject 1HD1. Branches show high-quality consensus sequences (HQCSs) with their respective copy number visualized as the size of the colored circle (day 0, grey, and week 4, red). (b) Insets highlight two day 0 minority variants that carry 10-1074 escape mutations 324GKIR327 (blue, mutation in yellow) and 332NIR334 (green, mutation in yellow). (c) Table shows the number of filtered reads for the indicated sequence variants at positions 324–334 and the relative frequency of each variant. Residues 325 and 332–334 are shaded in grey. Deviations from the day 0 majority variant are highlighted in bold.
Discussion
Antibodies play a unique role in the therapeutic armamentarium against human cancers and inflammatory diseases because they engage the host immune system to attack tumor cells or modify inflammatory responses by binding to Fc receptors on host leukocytes. Similarly, in HIV-1-infected individuals, passively transferred antibodies accelerate the clearance of infected cells, and induce host immunity against HIV-130,32,33,37. These unique features of immunotherapy suggest that antibodies should be further explored as adjuncts to conventional ART for prevention or treatment of HIV-1 infection.
Anti-CD4 binding site antibodies 3BNC117 and VRC01 have been generally safe and effective in decreasing plasma HIV-1-RNA levels34,35, and preventing rebound viremia during analytical treatment interruption in humans38,52. The two antibodies differ in their relative potency and half-lives, with 3BNC117 having a somewhat longer half-life and greater potency34,35,38. As expected, these properties are reflected in the relative ability of the two antibodies to prevent infection in macaques, and to prolong viral suppression in humans undergoing analytical treatment interruption38,52. The finding that 10-1074 has favorable safety and pharmacokinetics profiles and is effective in decreasing viremia extends these observations to an additional non-overlapping target of vulnerability on the HIV-1 spike.
10-1074 is more potent, and has a comparable half-life, but has a narrower spectrum of activity than either of the two CD4 binding site antibodies. Its effect on viremia is similar to 3BNC117, but a higher frequency of fully resistant escape variants was detected for 10-1074 compared to 3BNC11734,37,38. We speculate that this difference in occurrence of escape may be due to the relative cost of altering the CD4 binding site, making viable escape from antibodies that target this site more difficult as has been shown in vitro53. As a consequence, the number of distinct escape variants that can give rise to high-level viremia may be reduced. This idea is consistent with the relative paucity of naturally arising viruses that are resistant to 3BNC117 in comparison to 10-1074 or all other glycan patch bNAbs54. However, we did observe that 10-1074 escape mutations are selected against in several individuals when antibody levels drop. This finding suggests that these mutations are also associated with a fitness cost in vivo.
In humanized mice and in humans, a single antibody, like a single small molecule drug, is insufficient to prevent the emergence of resistant viral variants because the infection produces a swarm of related mutant viruses26,34,35,55. Similar to what has been described for small molecule drugs, resistance to antibodies seems to arise from pre-existing minority variants and/or de-novo mutations produced during rapid HIV-1 turnover. Our findings underscore some of the similarities in antiviral activity between small molecule drugs and antibodies, and emphasize that combinations of antibodies targeting non-overlapping epitopes will be required for effective therapy and possibly prevention.
10-1074 targets an epitope that is distinct from other second-generation bNabs that have been tested in humans34,35. It has favorable clinical characteristics and potent anti-viral activity and therefore, 10-1074 is a promising candidate for antibody-mediated combination immunotherapy and prevention of HIV-1 infection.
Data availability
HIV-1 envelope SGS data can be downloaded from GenBank (accession numbers XX-XX). Raw data of Primer-ID sequencing and Pacific Biosciences SMRT have been deposited at the NCBI Short Read Archive BioProject accession number PRJNA356756.
Online Methods
10-1074 Study Drug
10-1074 is a recombinant, fully human IgG1λ mAb recognizing the base of the V3 loop and surrounding glycans on the HIV-1 envelope11. 10-1074 was cloned from an African donor (Patient 10)56 infected with an HIV-1 clade A virus11. It was expressed in Chinese hamster ovary cells (clone 3G4), and purified using standard methods. The 10-1074 drug substance was produced at Celldex Therapeutics’ Fall River (MA) GMP facility, and the drug product was fill-finished at Althea Technologies, Inc. (CA). The resulting purified 10-1074 was supplied as a single use sterile 20 mg/ml solution for intravenous injection in a 5.0 ml volume of buffered solution composed of sodium phosphate, potassium phosphate, potassium chloride, sodium chloride, and polysorbate 80 with a pH of 7.0. 10-1074 vials were shipped and stored at 4°C.
Study design
An open-label, dose-escalation phase 1 study (ClinicalTrials.gov: NCT02511990, EudraCT: 2015-004574-15) was conducted in HIV-1-infected (Group 1) and uninfected subjects (Group 2) to evaluate the safety, pharmacokinetics and antiviral activity of 10-1074. Study participants were enrolled sequentially according to eligibility criteria. A standard “3+3” phase I trial design was used in the dose-escalation phase of the study. 10-1074 was administered as a single intravenous infusion over 60 minutes at three dose levels: 3mg/kg (subjects 1HA1–1HA3, 2HA1–2HA3), 10 mg/kg (subjects 1HB1–1HB3, 2HB1–2HB3), or 30 mg/kg (subjects 1HC1- 1HC3, 1HD1, 1HD2, 1HD4K-1HD11K, 2HC1–2HC3, 2HD1–2HD5). Study participants were followed for 84–168 days (24 weeks) after infusion. All participants provided written informed consent before participation in the study and the study was conducted in accordance with Good Clinical Practice. The protocol was approved by the Federal Drug Administration in the USA, the Paul-Ehrlich-Institut in Germany, and the Institutional Review Boards at the Rockefeller University and the University of Cologne.
Study Participants
All study participants were recruited at the Rockefeller University Hospital, New York, USA, at the University Hospital Cologne, Cologne, Germany, or at Montefiore Medical Center, New York, USA. Eligible subjects were adults aged 18 – 65 years, HIV-1-infected or uninfected, and without concomitant hepatitis B or C infections. HIV-1-infected subjects enrolled in study group 1A were on ART with plasma HIV-1 RNA levels < 20 copies/ml, and received a single 3 mg/kg 10-1074 infusion. In groups 1B through 1D, subjects were off ART (ART-experienced or ART-naïve) for at least 8 weeks before participation in the study and had plasma HIV-1 RNA levels < 100,000 copies/ml, measured on two separate occasions at least 1 week apart, and received a single 10 mg/kg (1B) or 30 mg/kg (1C-1D) 10-1074 infusion. Baseline sensitivity of viruses from outgrowth cultures to 10-1074 was known for all subjects enrolled in groups 1A-D with the exception of 1HC2, 1HD2, and 1HD10K. Subjects with CD4+ T-cell counts < 300 cells/μl, clinically relevant deviations from normal physical findings, and/or laboratory examinations were excluded. Screening period was from day -49 to day -7 prior to infusion, and the window for the pre-infusion visit was day -42 to day -1 prior to infusion. Race was recorded as self-reported. Women of childbearing age were required to have a negative serum pregnancy test on the day of 10-1074 infusion. HIV-1-infected individuals, who were not on ART at enrollment, were encouraged to initiate ART 6 weeks after 10-1074 infusion.
Study Procedures
The appropriate volume of 10-1074 was calculated according to study dose group, diluted in sterile normal saline to a total volume of 100 or 250 ml, and administered intravenously over 60 minutes. Study participants received 10-1074 on day 0 and remained under close monitoring in the infusion unit of the Rockefeller University Hospital or the University Hospital Cologne for 24 hours. Participants returned for frequent follow up visits for safety assessments that included physical examination and measurement of clinical laboratory parameters such as hematology, chemistries, urinalysis, coagulation times, pregnancy tests, as well as HIV-1 viral load and CD4+ and CD8+ T cell counts (Fig. 1a). Adverse events were graded according to the DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (version 2.0, November 2014) (HIV-1-infected groups) or the Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials (September 2007) (uninfected groups). Blood samples (30 to 120 ml) were collected before and at multiple times after 10-1074 infusion. Samples were processed within 4 h of collection, and serum and plasma samples were stored at −80°C. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation. The absolute number of PBMCs was determined by an automated cell counter (Vi-Cell XR; Beckman Coulter) or manually, and cells were cryopreserved in fetal bovine serum plus 10% DMSO.
Plasma HIV-1 RNA Levels
Plasma was collected for measuring HIV-1 RNA levels at screening (from d −49 to d −7), the pre-infusion visit (from d -42 to d -1), day 0 (before infusion), and on days 1, 2, 4, 7, 14, 21, 28, 42, 56, 84, 112, 140 and 168. HIV-1 RNA levels were determined using the Roche COBAS AmpliPrep/COBAS TaqMan HIV-1 Assay, Version 2.0, or the Roche Cobas 6800 HIV-1 kit, which detect 20–10×106 copies/ml.
CD4+ and CD8+ T cells
CD4+ and CD8+ T-cell counts were determined at screening, on day 0 (before infusion), days 7, 14, 28, 56, 112, and 168 by a clinical flow cytometry assay, performed at LabCorp or at the University Hospital Cologne.
Measurement of 10-1074 serum levels by TZM.bl
Blood samples were collected immediately before, at the end, 24 hours after completion of the 10-1074 infusion, and on days 2, 4, 7, 14, 21, 28, 42, 56, 84, 112, 140 and 168. Levels of active 10-1074 were determined by a TZM.bl neutralization assay34,40–42. Serum samples were heat-inactivated for 1h at 56°C and measured for neutralizing activity against an HIV-1 strain that was highly sensitive to 10-1074 but resistant to autologous HIV-1 neutralizing serum activity. In non-infected subjects, serum samples were tested against Du422 (clade C, tier 2) and ID50 values were derived using 5-parameter curve fitting. The serum concentration of active 10-1074 was calculated by taking into account the sera ID50 titers multiplied by the known IC50 of 10-1074 for Du422. In HIV-1-infected subjects, pre-infusion samples were first tested against a panel of 10-1074-sensitive HIV-1 strains. All subjects showed no or only minimal background activity to 3103.v3.C10 (clade ACD, tier 2), so this strain was used in this study group. ID50 values were used to determine 10-1074 serum concentration in the same way as described above. Murine leukemia virus (MuLV)-pseudotyped viruses were used to detect unspecific serum activity and serum samples displaying unspecific activity were excluded from analyses (Supplementary Table 3). Serum samples from individuals on ART were tested for neutralizing activity against pseudoviruses produced using an ART-resistant backbone. Briefly, mutations associated with resistance to integrase inhibitors and non-nucleoside reverse transcriptase inhibitors (Q148H, and K101P and Y181C, respectively) were co-introduced into the SG3ΔEnv vector using site-directed mutagenesis. This triple mutant backbone vector was found to significantly reduce or eliminate ART-associated inhibition against a MuLV control when tested against a large panel of serum samples obtained from individuals on a variety of ART regimens (M.S.S., unpublished data). Testing of serum samples from study subjects on ART included an MuLV-pseudotyped triple mutant virus to detect any residual ART-associated inhibition in the assay.
Pharmacokinetic analysis
Pharmacokinetic parameters were estimated by performing a non-compartmental analysis (NCA) using WinNonlin 6.3.
Virus cultures from HIV-1-infected individuals
Autologous virus was grown from PBMCs of 179 HIV-1 infected individuals under a separate IRB-approved protocol, as previously described34,37. Individuals from whom virus was isolated were recruited in New York, USA, and Cologne, Germany, suggesting that the majority are infected with Clade B viruses. Culture supernatants were tested for neutralization by 10-1074 as previously described34,40–42.
Statistical analyses
Pseudovirus neutralization data of 10-1074 was downloaded from the CATNAP database39 (Supplementary Fig. 1). A virus was considered neutralized by 10-1074, if the antibody reached an 80% inhibitory concentration (IC80) for a given virus within the range of up to 20 μg/ml. Any virus for which 10-1074 did not reach an IC80 within this concentration was considered not neutralized. Differences in serum half-life of 10-1074 between HIV-1-infected and uninfected individuals (Fig. 1) were compared by an unpaired two-tailed Student’s t-test with equal variances using GraphPad Prism. The sample size to detect > 0.6 log10 decline in viremia with 80% power at significance level α of 0.05 was determined to be 10 HIV-1-infected individuals, not on ART, infected with 10-1074-sensitive viruses assuming that the standard deviation would be similar to 10-1074 effects in humanized mice27. Curves illustrating average change in viremia after 10-1074 infusions were fitted by robust lowess regression with 40% of the data using MATLAB_R2016a (Fig. 2a). The significance of the effect of 10-1074 on viral load was assessed by computing simultaneous confidence bands, and noting when the simultaneous confidence band did exclude a viral load difference of zero (Supplementary Fig. 5). Simultaneous confidence bands were computed with the R package locfit (version 1.5–9.1) for each patient subgroup using the Gaussian family for the local likelihood function (Supplementary Fig. 5). Nonparametric Spearman coefficients were calculated to assess the correlation between maximum drop in viremia after 10-1074 infusion and baseline sensitivity of autologous viruses to 10-1074 or baseline HIV-1 viral load (Fig. 2c, d). CD4+ and CD8+ T cell counts before and after 10-1074 infusion were compared, respectively, by repeated measures one-way ANOVA and post-hoc Dunnett’s multiple comparisons test using GraphPad Prism (Supplementary Fig. 6). The neutralization potency of different antibodies against patient-derived pseudoviruses (10-1074 vs. PGT121, 3BNC117 vs. VRC01) was compared using a model built on generalized estimating equations (Figure 3e)57. Comparisons were drawn on the basis of all day 0 pseudoviruses (n=55). The model was built with intercept and bNAb group as the only covariate. Measurements within a cluster (patient) were assumed to be equicorrelated and normal distribution was used. p-values were estimated for the null-hypothesis that the weight parameter of the group covariate is zero. GEEQBOX version 1.0 was used for calculations57.
Single genome sequencing (SGS) of viral env genes
Single genome amplification and sequencing of HIV-1 env genes was performed as described previously37,58. Sequences containing premature stopcodons or large internal deletions that would compromise env functionality were excluded from downstream analyses. Sequences were aligned using CLUSTALW version 259 and regions that could not be unambiguously aligned were removed from subsequent phylogenetic relationships. Phylograms were constructed using PhyML version 3.160 using evolutionary models favored by AICc with jModelTest version 2.1.461 or, for the large dataset in Fig. 3A, RAxML using a GTRGAMMA model62. To delineate 10-1074 escape mutations, SGS was performed on day 0 and week 4 plasma samples of 13/14 viremic individuals that responded to the antibody. Plasma samples from non-responders 1HD2 and 1HD9K were sequenced on day 0 to determine resistance mutations. Week 1 sequencing was only performed on samples with higher viral loads for which sufficient plasma was available. Follow-up sequencing (week 12, week 16, week 20, week 24) was performed on samples from subjects that remained off-ART for more than 12 weeks post-infusion and for which sufficient plasma material was available.
Pseudovirus neutralization assays
CMV promoter-based pseudoviruses were generated as previously described63. Pseudovirus supernatants were tested for neutralization by 10-1074, PGT121, 3BNC117, VRC01, and PGDM1400 in a TZM.bl assay as previously described34,40–42. Neutralization testing was performed up to 50 μg/ml.
Primer ID-based deep-sequencing of the V3 loop
A Primer-ID deep-sequencing protocol of a ~500 bp region encompassing the base of the V3 loop (HxB2 positions 6854 – 7356) of HIV-1 env was designed based on two recently published protocols49,50. Five subjects were chosen for analysis based on higher viral load and different times of rebound. Primers were designed to bind all 5 subjects’ env based on previously obtained SGS sequences. All primers were ordered from IDT using standard desalting as purification and the cDNA-primer was ordered using hand-mixing of all 10 random nucleotides. RNA was extracted from plasma using the Qiagen MinElute Virus Spin Kit. cDNA synthesis was performed using Superscript III and primer 5′-GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTNNNNN-NNNNNCAGTTTTACAGTAGAAAAATTCCCCTCCACA-3′. cDNA was purified using Agencourt SPRIselect beads with a bead to reaction ratio of 0.6. All purified cDNA was amplified using primers V3-Forward 5′GCCTCCCTCGCGCCATCAGAGATGTGTATAAGAGACAGNNNNTGAGCCAATTCCCATA-CATTATTGTGC and ADPT_2a 5′-GTGACTGGAGTTCAGACGTGTGCTC-3′. PCR was performed using Phusion Polymerase and a cycling protocol of initial denaturation at 98°C for 30 s, 25 cycles of 98°C for 10 s and 72°C for 1 min, and then a final extension at 72°C for 5 min. First-round PCR products were again purified using Agencourt SPRIselect and eluted in a 50 μl volume. 2 μl of the first-round PCR product was then used in a second PCR using primers Universal Adapter 5′-AATGATACGGCGACCACCGAGATCTACACGCCTCCCTCGCGCCATCAGAGATGTG-3′ and and Indexed adapter 5′-CAAGCAGAAGACGGCATACGAGATNNNNNNGTG-ACTGGAGTTCAGACGTGTGCTC-3′ that carried a six-nucleotide fixed barcode that allowed for the identification of specific samples. Second-round PCR was performed using KAPA HiFi Polymerase 2× Ready Mix and cycling conditions of initial denaturation at 95°C for 2 min, followed by 25 cycles of 98°C 20s, 63°C 15s, 72°C 30s, and final elongation of 3 min at 72°C. Second-round PCR product was again purified with Agencourt SPRIselect beads, visualized on a 2% agarose gel, and gel purified using the Macherey-Nagel Gel and PCR Purification Kit. Final products were analyzed using an Agilent Tapestation and Qubit fluorophotomer. Final products were multiplexed and sequenced paired-end on Illumina MiSeq using 2× 300 bp v3 chemistry. Reads were trimmed using trimfq from seqtk toolkit with error rate threshold of 1% (−q 0.01) and sequences shorter than 200nt were removed. Primer IDs were defined as the first 10nt at the 5′ of each read containing the 5′ cDNA primer. SMALT aligner (v. 0.76) was used to align the primer sequence with up 3 mismatches or one gap. Primer IDs containing 10 or more sequences were assembled using MIRA (v. 4.0.2) to yield consensus sequences. Consensus sequences containing ambiguity codes were removed for the final analysis. Cross-contamination between subjects was checked by phylogenetic analysis and contaminants were discarded from the analysis. The limit-of-detection of the Primer-ID protocol was estimated for each sequenced patient separately using a power calculation as described previously64. MATLAB R2016a was used for power calculations using the sampsizepwr function with the binomial distribution option.
Single molecule real time sequencing (SMRT sequencing) on Pacific Biosciences RS II
Three subjects were selected for SMRT sequencing analysis based on time of rebound, high viral load, and sample availability. Full-length HIV-1 env was amplified and sequenced on the Pacific Biosciences RS-II as described previously with two minor modifications51. First, primers were designed specifically for sequenced subjects using available SGS sequences that spanned the primer regions. Primers used for amplification were 5′-GAGCAGARGACAGTGGCAATGA-3′ and 5′-GGAGAATAGCTCTACGAGCTCTTTG-3′ for 1HB3, 5′GAGCAGAAGACAGTGGCAATGA-3′51 and 5′TGACCATTTATTGCCCATCTTATAGC-3′ for 1HC2 and 5′-GAGCAGAAGACAGTGGCAATGA-3′51 and 5′-CCACTTGCCACCCATTTTATAGGA-3′ for 1HD1. Second, the more recent PacBio P6-C4 chemistry was used. Data was pre-processed using the PacBio CCS2 algorithm, generating single molecule circular consensus sequences. The Full-Length Envelope Analysis (FLEA) pipeline51 was used for further processing and visualization. MAFFT65 with manual curation was used to align amino acid sequences, and fastree266 was used to infer maximum likelihood phylogenies.
Supplementary Material
Acknowledgments
We thank all study participants for devoting their time to support our research. We thank the Clinical Research Support Teams of the Rockefeller University Hospital and the Infectious Disease Division at the University Hospital Cologne, in particular G. Kremer, S. Margane, and E. Thomas. We thank L. Burke, S. Durant, I. Suárez, and the nursing staff for patient care and recruitment, and all members of the laboratories of M. Nussenzweig and F. Klein for helpful discussions. We thank P. Fast and H. Park for clinical monitoring, A. Louie, D. Jordan, C. Conrad, and D. Adzic for regulatory support, C. Anthony and S. Zhou for help in establishing Primer-ID sequencing, C. Ruping, K. Jain, M. Ercanoglu, R. Patel, and J. Dizon for sample processing, U. Kerkweg, R. Macarthur, and A. Johnson for pharmacy services, A. Germann and H. von Briesen for HIV culture analyses, R. Kaiser for p24 measurements, and D. Sok for providing PGT121 and PGDM1400 for neutralization assays. Amplification and library preparation for SMRT sequencing was performed with the support of the Translational Virology Core at the UC San Diego Center for AIDS Research (P30 AI036214). SMRT sequencing was conducted at the IGM Genomics Center, University of California, San Diego, La Jolla, CA. Computational analysis of sequence data was performed, in part, on a cluster which was supported by U01 GM110749 (NIH/NIGMS). This work was supported in part by the Bill and Melinda Gates Foundation Collaboration for AIDS Vaccine Discovery (CAVD) Grants OPP1032144 (M.S.S.), OPP1092074 and OPP1124068 (M.C.N.), a BEAT-HIV Delaney grant UM1 AI126620 (B.H.H.), the Robertson Foundation to M.C.N., NIH Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery (CHAVI-ID) 1UM1 AI100663-01 (M.C.N.). T.S. was supported by a German Research Foundation postdoctoral fellowship (SCHO 1612/1-1) and is currently supported in part by grant #UL1 TR001866 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program. H.G. is supported by a fellowship from the German Center for Infection Research (DZIF). T.K. is an HHMI Medical Research Fellow. E.F.K. is supported by a Ruth L. Kirschstein National Research Service Award (F30 AI112426). B.M. was supported by grant number R00 AI120851 from the National Institute of Allergy and Infectious Diseases. K.E. was supported by T15 LM007092 from the National Library of Medicine. F.K. is supported by the Heisenberg-Program of the DFG (KL 2389/2-1), the European Research Council (ERC-StG639961), and the German Center for Infection Research (DZIF), partner site Bonn-Cologne, Cologne, Germany. M.C.N. is a Howard Hughes Medical Institute Investigator. Aspects of this work are encompassed by patent application PCT/US2013/065696.
Footnotes
Author Contributions:
M.C. (principal investigator, U.S.), M.C.N., and F.K. (principal investigator, Germany) designed the trial; M.C., T.S., H.G., M.C.N., and F.K. analyzed the data and wrote the manuscript; R.M.G., G.F., and S.J.S. contributed to study design and implementation. M.C., H.G., A.S., Y.Z.C., R.L., M.W.P., and F.K. implemented the study. C.L., D.G., T.K., C.W., S.K., B.S.Z., and G.F. contributed to participant recruitment and clinical assessments. I.S., C.U.B., and D.W. coordinated sample processing. T.S., T.K. and L.N., performed viral culture, SGS, and Primer-ID sequencing work. T.Y.O. performed Primer-ID analyses and bioinformatics processing of SGS data. A.R. and M.S.S. performed TZM.bl neutralization assays. B.M., K.E., and C.I. carried out SMRT Sequencing and analysis. E.F.K., G.H.L., and B.H.H. analyzed SGS data. N.P. performed statistical analysis. H.M., A.P.W., and P.J.B. contributed to data analysis. T.K. was responsible for 10-1074 manufacture and provided regulatory guidance. All authors read and contributed to the writing of the manuscript.
Competing Financial Interests Statement
The authors declare no competing financial interests.
References
- 1.Klein F, et al. Antibodies in HIV-1 vaccine development and therapy. Science. 2013;341:1199–1204. doi: 10.1126/science.1241144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hraber P, et al. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. AIDS. 2014;28:163–169. doi: 10.1097/QAD.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.West AP, Jr, et al. Structural insights on the role of antibodies in HIV-1 vaccine and therapy. Cell. 2014;156:633–648. doi: 10.1016/j.cell.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mikell I, et al. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog. 2011;7:e1001251. doi: 10.1371/journal.ppat.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton DR, Mascola JR. Antibody responses to envelope glycoproteins in HIV-1 infection. Nat Immunol. 2015;16:571–576. doi: 10.1038/ni.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haynes BF, et al. HIV-Host Interactions: Implications for Vaccine Design. Cell Host Microbe. 2016;19:292–303. doi: 10.1016/j.chom.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore PL, Williamson C, Morris L. Virological features associated with the development of broadly neutralizing antibodies to HIV-1. Trends Microbiol. 2015;23:204–211. doi: 10.1016/j.tim.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchacher A, et al. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retroviruses. 1994;10:359–369. doi: 10.1089/aid.1994.10.359. [DOI] [PubMed] [Google Scholar]
- 9.Muster T, et al. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang J, et al. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mouquet H, et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc Natl Acad Sci U S A. 2012;109:E3268–3277. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker LM, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sok D, et al. Promiscuous glycan site recognition by antibodies to the high-mannose patch of gp120 broadens neutralization of HIV. Sci Transl Med. 2014;6:236ra263. doi: 10.1126/scitranslmed.3008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garces F, et al. Structural evolution of glycan recognition by a family of potent HIV antibodies. Cell. 2014;159:69–79. doi: 10.1016/j.cell.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker LM, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sok D, et al. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proc Natl Acad Sci U S A. 2014;111:17624–17629. doi: 10.1073/pnas.1415789111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheid JF, et al. Sequence and Structural Convergence of Broad and Potent HIV Antibodies That Mimic CD4 Binding. Science. 2011 doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu X, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao HX, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scharf L, et al. Antibody 8ANC195 reveals a site of broad vulnerability on the HIV-1 envelope spike. Cell Rep. 2014;7:785–795. doi: 10.1016/j.celrep.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J, et al. Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface. Nature. 2014;515:138–142. doi: 10.1038/nature13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pietzsch J, et al. A mouse model for HIV-1 entry. Proc Natl Acad Sci U S A. 2012;109:15859–15864. doi: 10.1073/pnas.1213409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moldt B, et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci U S A. 2012;109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gautam R, et al. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature. 2016;533:105–109. doi: 10.1038/nature17677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shingai M, et al. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J Exp Med. 2014;211:2061–2074. doi: 10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein F, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492:118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horwitz JA, et al. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc Natl Acad Sci U S A. 2013;110:16538–16543. doi: 10.1073/pnas.1315295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barouch DH, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shingai M, et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013;503:277–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halper-Stromberg A, et al. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014;158:989–999. doi: 10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hessell AJ, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 32.Bournazos S, et al. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell. 2014;158:1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu CL, et al. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science. 2016;352:1001–1004. doi: 10.1126/science.aaf1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caskey M, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522:487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lynch RM, et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med. 2015;7:319ra206. doi: 10.1126/scitranslmed.aad5752. [DOI] [PubMed] [Google Scholar]
- 36.Ledgerwood JE, et al. Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin Exp Immunol. 2015;182:289–301. doi: 10.1111/cei.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schoofs T, et al. HIV-1 therapy with monoclonal antibody 3BNC117 elicits host immune responses against HIV-1. Science. 2016;352:997–1001. doi: 10.1126/science.aaf0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheid JF, et al. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature. 2016 doi: 10.1038/nature18929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon H, et al. CATNAP: a tool to compile, analyze and tally neutralizing antibody panels. Nucleic Acids Res. 2015;43:W213–219. doi: 10.1093/nar/gkv404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li M, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seaman MS, et al. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol. 2009;84:1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarzotti-Kelsoe M, et al. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J Immunol Methods. 2014;409:131–146. doi: 10.1016/j.jim.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:493–507. doi: 10.2165/11531280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 44.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9:235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gristick HB, et al. Natively glycosylated HIV-1 Env structure reveals new mode for antibody recognition of the CD4-binding site. Nature Structural & Molecular Biology. 2016 doi: 10.1038/nsmb.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abram ME, Ferris AL, Shao W, Alvord WG, Hughes SH. Nature, position, and frequency of mutations made in a single cycle of HIV-1 replication. J Virol. 2010;84:9864–9878. doi: 10.1128/JVI.00915-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coffin J. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995;267:483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 48.Maldarelli F, et al. HIV populations are large and accumulate high genetic diversity in a nonlinear fashion. J Virol. 2013;87:10313–10323. doi: 10.1128/JVI.01225-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou S, Jones C, Mieczkowski P, Swanstrom R. Primer ID Validates Template Sampling Depth and Greatly Reduces the Error Rate of Next-Generation Sequencing of HIV-1 Genomic RNA Populations. J Virol. 2015;89:8540–8555. doi: 10.1128/JVI.00522-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhiman JN, et al. Viral variants that initiate and drive maturation of V1V2-directed HIV-1 broadly neutralizing antibodies. Nat Med. 2015;21:1332–1336. doi: 10.1038/nm.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laird Smith M, et al. Virus Evolution. Oxford University Press; 2016. Rapid Sequencing of Complete env Genes from Primary HIV-1 Samples. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bar KJ, et al. Effect of HIV Antibody VRC01 on Viral Rebound after Treatment Interruption. N Engl J Med. 2016;375:2037–2050. doi: 10.1056/NEJMoa1608243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lynch RM, et al. HIV-1 fitness cost associated with escape from the VRC01 class of CD4 binding site neutralizing antibodies. J Virol. 2015;89:4201–4213. doi: 10.1128/JVI.03608-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.West AP, Jr, et al. Computational analysis of anti-HIV-1 antibody neutralization panel data to identify potential functional epitope residues. Proc Natl Acad Sci U S A. 2013;110:10598–10603. doi: 10.1073/pnas.1309215110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trkola A, et al. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med. 2005;11:615–622. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- 56.Simek MD, et al. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol. 2009;83:7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ratcliffe SJ, Shults J. GEEQBOX: A MATLAB toolbox for generalized estimating equations and quasi-least squares. Journal of Statistical Software. 2008;25:1–14. [Google Scholar]
- 58.Salazar-Gonzalez JF, et al. Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. J Virol. 2008;82:3952–3970. doi: 10.1128/JVI.02660-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 60.Guindon S, et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 61.Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kirchherr JL, et al. High throughput functional analysis of HIV-1 env genes without cloning. J Virol Methods. 2007;143:104–111. doi: 10.1016/j.jviromet.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keele BF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Price MN, Dehal PS, Arkin AP. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
HIV-1 envelope SGS data can be downloaded from GenBank (accession numbers XX-XX). Raw data of Primer-ID sequencing and Pacific Biosciences SMRT have been deposited at the NCBI Short Read Archive BioProject accession number PRJNA356756.


