Abstract
Hematopoietic stem cell transplantation (HSCT) is an established procedure for many acquired and congenital disorders of the hematopoietic system. A record number of 42 171 HSCT in 37 626 patients (16 030 allogeneic (43%), 21 596 autologous (57%)) were reported by 655 centers in 48 countries in 2015. Trends include continued growth in transplant activity over the last decade, with the highest percentage increase seen in middle-income countries but the highest absolute growth in the very-high-income countries in Europe. Main indications for HSCT were myeloid malignancies 9413 (25% 96% allogeneic), lymphoid malignancies 24 304 (67% 20% allogeneic), solid tumors 1516 (4% 3% allogeneic) and non-malignant disorders 2208 (6% 90% allogeneic). Remarkable is the decreasing use of allogeneic HSCT for CLL from 504 patients in 2011 to 255 in 2015, most likely to be due to new drugs. Use of haploidentical donors for allogeneic HSCT continues to grow: 2012 in 2015, a 291% increase since 2005. Growth is seen for all diseases. In AML, haploidentical HSCT increases similarly for patients with advanced disease and for those in CR1. Both marrow and peripheral blood are used as the stem cell source for haploidentical HSCT with higher numbers reported for the latter.
Introduction
Hematopoietic stem cell transplantation (HSCT) is an established procedure for many acquired and congenital disorders of the hematopoietic system, including disorders of the immune system, and as enzyme replacement in metabolic disorders.1, 2, 3, 4 The annual activity survey of the European Society of Blood and Marrow Transplantation (EBMT), describing the status of HSCT in Europe and affiliated countries, has become an instrument used to observe trends and to monitor changes in technology use.5, 6, 7, 8, 9, 10, 11, 12 The survey captures the numbers of HSCT performed in the preceding year by each participating team, divided by indication, donor type and stem cell source. The standardized structure of the survey over many years and the excellent commitment of the participating teams allow us to observe changes over time and to evaluate factors associated with these changes. More recently, the survey has included additional information on novel cell therapies with hematopoietic stem cells for non-hematopoietic use, as well as on the use of non-hematopoietic stem and progenitor cells. This coincides with the recent interest of the World Health Organization (www.who.org) in cell and tissue transplants, and further stresses the need for adequate and timely information.13 The analysis of the survey data spanning over 25 years and amassing data on more than 600 000 transplants in over 550 000 patients, has shown a continued and constant increase in the annual numbers of HSCT and transplant rates (number of HSCT/10 million inhabitants) for both allogeneic and autologous HSCT.
This report is based on the 2015 survey data. In addition to transplant rates and indications, this report focuses on the use of haploidentical donors for transplantation including disease entities and stem cell sources.
Patients and methods
Data collection and validation
Participating teams were invited to report data for 2015 by indication, stem cell source and donor type as listed in Table 1. The survey allows the possibility to report additional information on the numbers of subsequent transplants performed as a result of relapse, rejection or those that are part of a planned sequential transplant protocol. Supplementary Information on the numbers of donor lymphocyte infusions, reduced intensity HSCT and the numbers of pediatric HSCT is also collected. New in this year's survey is the more detailed report on cellular therapies (Table 2). Quality-control measures included several independent systems: confirmation of validity of the entered data by the reporting team, selective comparison of the survey data with minimum essential data (MED-A) data sets in the EBMT Registry database and cross-checking with the National Registries.
Table 1. Numbers of HSCTs in Europe 2015 by indication, donor type and stem cell source.
|
Transplant activity 2015 |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
No. of patients |
||||||||||||||||||
|
Allogeneic |
Autologous |
Total |
||||||||||||||||
|
Family |
Unrelated |
BM only | BM + PB | Cord | Allo | Auto | ||||||||||||
|
HLA-id |
Twin |
Haplo ⩾2MM |
Other family |
BM | PB | Cord | ||||||||||||
| BM | PB | Cord | All | BM | PB | BM | PB | Cord | ||||||||||
| Myeloid malignancies | 412 | 2439 | 5 | 9 | 310 | 603 | 28 | 68 | 0 | 525 | 4425 | 170 | 9 | 410 | 0 | 8994 | 419 | 9413 |
| AML | 290 | 1750 | 5 | 6 | 208 | 460 | 15 | 52 | 0 | 330 | 2940 | 133 | 9 | 404 | 0 | 6189 | 413 | 6602 |
| First complete remission | 201 | 1077 | 5 | 3 | 100 | 196 | 10 | 29 | 0 | 203 | 1537 | 79 | 6 | 349 | 0 | 3440 | 355 | 3795 |
| Not first complete remission | 64 | 485 | 0 | 3 | 81 | 199 | 4 | 21 | 0 | 90 | 864 | 37 | 3 | 50 | 0 | 1848 | 53 | 1901 |
| AML therapy related | 11 | 57 | 0 | 0 | 4 | 18 | 0 | 1 | 0 | 9 | 115 | 6 | 0 | 2 | 0 | 221 | 2 | 223 |
| AML from MDS/MPN | 14 | 131 | 0 | 0 | 23 | 47 | 1 | 1 | 0 | 28 | 424 | 11 | 0 | 3 | 0 | 680 | 3 | 683 |
| CML | 26 | 115 | 0 | 0 | 14 | 12 | 0 | 2 | 0 | 27 | 196 | 6 | 0 | 3 | 0 | 398 | 3 | 401 |
| Chronic phase | 14 | 48 | 0 | 0 | 2 | 5 | 0 | 1 | 0 | 14 | 78 | 1 | 0 | 1 | 0 | 163 | 1 | 164 |
| Not first chronic phase | 12 | 67 | 0 | 0 | 12 | 7 | 0 | 1 | 0 | 13 | 118 | 5 | 0 | 2 | 0 | 235 | 2 | 237 |
| MDS or MD/MPN | 87 | 442 | 0 | 3 | 68 | 108 | 12 | 9 | 0 | 146 | 971 | 28 | 0 | 3 | 0 | 1874 | 3 | 1877 |
| MPN | 9 | 132 | 0 | 0 | 20 | 23 | 1 | 5 | 0 | 22 | 318 | 3 | 0 | 0 | 0 | 533 | 0 | 533 |
| Lymphoid malignancies | 338 | 1410 | 5 | 11 | 222 | 356 | 16 | 41 | 1 | 392 | 1984 | 114 | 26 | 19424 | 0 | 4890 | 19450 | 24340 |
| Acute lymphatic leukemia | 264 | 641 | 5 | 2 | 101 | 185 | 15 | 27 | 1 | 309 | 851 | 93 | 3 | 81 | 0 | 2494 | 84 | 2578 |
| First complete remission | 164 | 457 | 0 | 2 | 49 | 92 | 9 | 12 | 1 | 146 | 556 | 43 | 1 | 65 | 0 | 1531 | 66 | 1597 |
| Not first complete remission | 100 | 184 | 5 | 0 | 52 | 93 | 6 | 15 | 0 | 163 | 295 | 50 | 2 | 16 | 0 | 963 | 18 | 981 |
| CLL | 9 | 83 | 0 | 0 | 7 | 13 | 0 | 3 | 0 | 7 | 131 | 2 | 0 | 36 | 0 | 255 | 36 | 291 |
| Plasma cell disorders: MM | 6 | 194 | 0 | 2 | 13 | 14 | 0 | 1 | 0 | 20 | 285 | 3 | 5 | 10856 | 0 | 538 | 10861 | 11399 |
| Plasma cell disorders: other | 0 | 15 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 2 | 9 | 0 | 0 | 326 | 0 | 29 | 326 | 355 |
| Hodgkin's lymphoma | 12 | 130 | 0 | 2 | 55 | 61 | 1 | 2 | 0 | 9 | 166 | 4 | 8 | 2062 | 0 | 442 | 2070 | 2512 |
| Non-Hodgkin's lymphoma | 47 | 347 | 0 | 3 | 46 | 82 | 0 | 8 | 0 | 45 | 542 | 12 | 10 | 6063 | 0 | 1132 | 6073 | 7205 |
| Solid tumors | 5 | 2 | 1 | 1 | 2 | 19 | 0 | 1 | 0 | 2 | 4 | 1 | 47 | 1430 | 1 | 38 | 1478 | 1516 |
| Neuroblastoma | 3 | 1 | 1 | 0 | 2 | 12 | 0 | 0 | 0 | 0 | 1 | 1 | 27 | 459 | 1 | 21 | 487 | 508 |
| Soft tissue sarcoma/Ewing | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 1 | 0 | 0 | 2 | 0 | 10 | 205 | 0 | 8 | 215 | 223 |
| Germinal tumors | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 350 | 0 | 0 | 351 | 351 |
| Breast cancer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 29 | 0 | 0 | 29 | 29 |
| Other solid tumors | 2 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 2 | 1 | 0 | 9 | 387 | 0 | 9 | 396 | 405 |
| Non-malignant disorders | 648 | 241 | 28 | 4 | 64 | 131 | 72 | 49 | 4 | 419 | 227 | 98 | 9 | 214 | 0 | 1985 | 223 | 2208 |
| BM failure: SAA | 185 | 105 | 5 | 3 | 16 | 22 | 11 | 3 | 1 | 124 | 79 | 12 | 0 | 0 | 0 | 566 | 0 | 566 |
| BM failure: other | 68 | 24 | 4 | 0 | 8 | 20 | 9 | 12 | 1 | 73 | 31 | 11 | 0 | 0 | 0 | 261 | 0 | 261 |
| Thalassemia | 148 | 66 | 9 | 1 | 5 | 12 | 17 | 7 | 1 | 55 | 19 | 1 | 1 | 3 | 0 | 341 | 4 | 345 |
| Sickle cell disease | 92 | 11 | 6 | 0 | 10 | 4 | 3 | 1 | 0 | 14 | 5 | 0 | 0 | 0 | 0 | 146 | 0 | 146 |
| Primary immune deficiencies | 130 | 26 | 3 | 0 | 19 | 65 | 27 | 22 | 1 | 120 | 77 | 37 | 2 | 6 | 0 | 527 | 8 | 535 |
| Inh. disorders of metabolism | 23 | 5 | 1 | 0 | 6 | 7 | 5 | 3 | 0 | 30 | 14 | 35 | 4 | 0 | 0 | 129 | 4 | 133 |
| Auto immune disease | 2 | 4 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 3 | 2 | 2 | 2 | 205 | 0 | 15 | 207 | 222 |
| Others | 31 | 10 | 1 | 0 | 8 | 16 | 5 | 1 | 0 | 17 | 27 | 7 | 0 | 26 | 0 | 123 | 26 | 149 |
| Total patients | 1434 | 4102 | 40 | 25 | 606 | 1125 | 121 | 160 | 5 | 1355 | 6667 | 390 | 91 | 21504 | 1 | 16030 | 21596 | 37626 |
| Re/additional transplants | 55 | 257 | 0 | 2 | 96 | 257 | 4 | 12 | 0 | 71 | 497 | 21 | 7 | 3266 | 0 | 1272 | 3273 | 4545 |
| Total transplants | 1489 | 4359 | 40 | 27 | 702 | 1382 | 125 | 172 | 5 | 1426 | 7164 | 411 | 98 | 24770 | 1 | 17302 | 24869 | 42171 |
Abbrveiations: BM=bone marrow; HSCT=hematopoietic stem cell transplant; MDS=myelodysplastic; MPN=myeloproliferative neoplasm; MM=multiple myeloma; PB=peripheral blood; SAA=severe aplastic anemia.
Bold text implies the subtotals.
Table 2. Numbers of cellular therapies in Europe 2015 by indication, donor type and cell source.
| Number of patients | DLI |
MSC |
NK cells |
Selected/expanded T cells or CIK |
TREGS |
Genetically modified T cells |
Dendritic cells |
Expanded CD34+ cells |
Genetically modified CD34+ cells |
Other |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allo | Auto | Allo | Auto | Allo | Auto | Allo | Auto | Allo | Auto | Allo | Auto | Allo | Auto | Allo | Auto | Allo | Auto | ||
| GvHD | 396 | 3 | 29 | 1 | 3 | ||||||||||||||
| Graft enhancement | 803 | 44 | 1 | 2 | 14 | 5 | 24 | ||||||||||||
| Autoimmune disease | 4 | 40 | |||||||||||||||||
| Genetic disease | 2 | 1 | 8 | ||||||||||||||||
| Infection | 4 | 119 | 6 | ||||||||||||||||
| Malignancy | 1 | 11 | 1 | 32 | 5 | 1 | 5 | 8 | 4 | 5 | 20 | 19 | 3 | ||||||
| DLI for residual disease | 410 | ||||||||||||||||||
| DLI for relapse | 1285 | ||||||||||||||||||
| DLI per protocol | 442 | ||||||||||||||||||
| Regenerative medicine | 16 | 7 | 1 | 3 | 94 | ||||||||||||||
| Total | 2940 | 467 | 48 | 13 | 1 | 168 | 5 | 30 | 5 | 9 | 5 | 5 | 20 | 9 | 0 | 19 | 8 | 33 | 97 |
Abbreviations: DLI=donor lymphocyte infusions; MSC=mesenchymal stem cells; NK=natural killer; TREGS=regulatory T cells. Bold text implies the totals.
Teams
Six hundred and eighty-seven centers from 48 countries were contacted for the 2015 survey (39 European and 9 affiliated countries), of which 655 teams reported. This corresponds to a 95% return rate and includes 552 active EBMT member teams. Thirty-two active teams failed to report in 2015.
Contacted teams are listed in the online Supplementary Appendix in alphabetical order by country, city and EBMT centre code, with their reported numbers of first and total HSCT, and of first allogeneic and autologous HSCT as Supplementary Material. The World Health Organization regional office definitions (www.who.org) were used to classify countries as European or Non-European. Nine non-European countries participated in the 2015 EBMT survey: Algeria, Iran, Israel, Jordan, Lebanon, Nigeria, Saudi Arabia, South Africa and Tunisia. Their data (2776 HSCT in 2657 patients) from 29 actively transplanting teams make up 6.6% of the total data set and is included in all analyses.13
Patient and transplant numbers
Wherever appropriate, patient numbers corresponding to the number of patients receiving a first transplant and transplant numbers reflecting the total number of transplants performed are listed.
The term sibling donor includes HLA identical siblings and twins, but not siblings with HLA mismatches. Unrelated donor transplants include HSCT from unrelated donors with peripheral blood and marrow as a stem cell source, but not cord blood HSCT. In the 2015 survey, we collected separately the numbers of haplo-identical and other family member HSCT. Haplo-identical being described as any family member with two or more loci mismatch within the loci HLA-A, -B, -C, -DRB1 and -DQB1 in GvH and/or HvG direction. Other family member donors are those related donors who are mismatched to a lesser degree than a full haplotype. Additional non-first transplants may include multiple transplants defined as subsequent transplants within a planned double or triple autologous or allogeneic transplant protocol, and retransplants (autologous or allogeneic) defined as unplanned HSCT for rejection or relapse after a previous HSCT.
Transplant rates
Transplant rates, defined as the total number of HSCT per 10 million inhabitants, were computed for each country without adjustments for patients who crossed borders and received their HSCT in a foreign country. Population numbers were obtained from Eurostats for 2015 for the European countries, (http://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=demo_urespop&lang=en) and the US census bureau database for the non-European countries (http://www.census.gov/population/international/data/idb/rank.php).
Analysis
Wherever appropriate, the absolute numbers of transplanted patients, transplants or transplant rates are shown for specific countries, indications or transplant techniques. Myeloid malignancies include AML, myelodysplastic or myelodysplastic/myeloproliferative neoplasm (MPN), MPN and CML. Lymphoid malignancies include ALL, CLL, Hodgkin's disease, non-Hodgkin lymphoma and plasma cell disorders. The non-malignant disorders include bone marrow failure, thalassemia, sickle cell disease, primary immune disease inherited disease of metabolism and autoimmune disease AID. Others include histiocytosis and other rare disorders not included in the above.
Results
2015 data
Participating teams in 2015
Of the 655 teams, 412 (62%) performed both allogeneic and autologous transplants; 227 (35%) restricted their activity to autologous HSCT and 11 teams (2%) to allogeneic transplants only. Five teams (1%) reported having performed no transplants in 2015 due to renovation or temporary closure of the transplant unit. Of the 655 active centers, 131 (20%) centers performed transplants on both adult and pediatric patients. An additional 106 (16%) centers were dedicated pediatric transplant centers and 417 (64%) centers performed transplants on adults only.
Numbers of patients and transplants
In 2015, 42 171 transplants were reported in 37 626 patients (first transplant); of these, 17 302 HSCT (41%) were allogeneic and 24 869 (59%) autologous (Table 1). When compared with that in 2014, the total number of transplants increased by 3.3% (2.1% allogeneic HSCT and 4.1% autologous HSCT).12 Furthermore, there were 4545 second or subsequent transplants, 1272 allogeneic and 3273 autologous. The total number of patients transplanted under the age of 18 in both dedicated and joint adult-pediatric units was 4490 (3338 allogeneic and 1152 autologous HSCT). Of these, 3015 patients (67%, 2570 allogeneic and 896 autologous) reporting a total of 3466 transplants were performed in dedicated pediatric centers. A total of 4545 transplants were second or multiple transplants. Among these, 3273 were autologous, the majority of which were probably part of multiple transplant programs, for example, as for plasma cell disorders. In total, 1272 were allogeneic HSCTs mainly to treat relapse or graft failure. In addition, 794 HSCTs were reported as allogeneic HSCT after a previous autologous HSCT and were mainly for lymphoma or plasma cell disorders.
Indications
Indications for HSCT in 2015 are listed in detail in Table 1. Main indications were myeloid malignancies (AML, CML, myelodysplastic/MPN and MPN): 9413 (25% of total; 96% of which were allogeneic); lymphoid malignancies (ALL, CLL, Hodgkin's disease, non-Hodgkin lymphoma and plasma cell disorder): 24 340 (65% 20% allogeneic); solid tumors: 1516 (4% 3% allogeneic); non-malignant disorders: 2208 (6% 90% allogeneic) and others: 149 (0.4%). As seen in previous years, the majority of HSCT for lymphoid malignancies were autologous, whereas most transplants for leukemia were performed using stem cells from allogeneic donors. Autologous HSCT for non-malignant disorders predominantly include patients with autoimmune disorders (207).
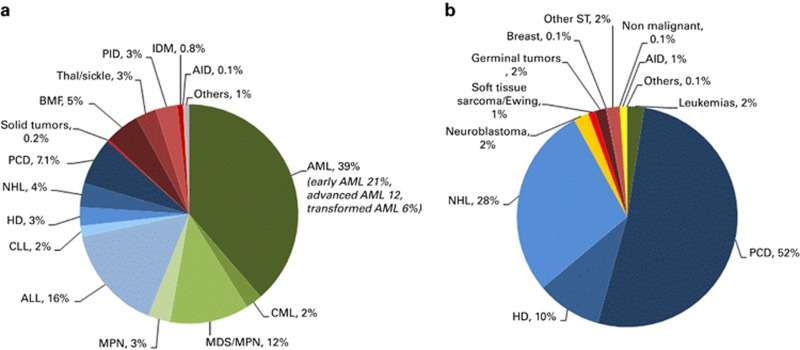
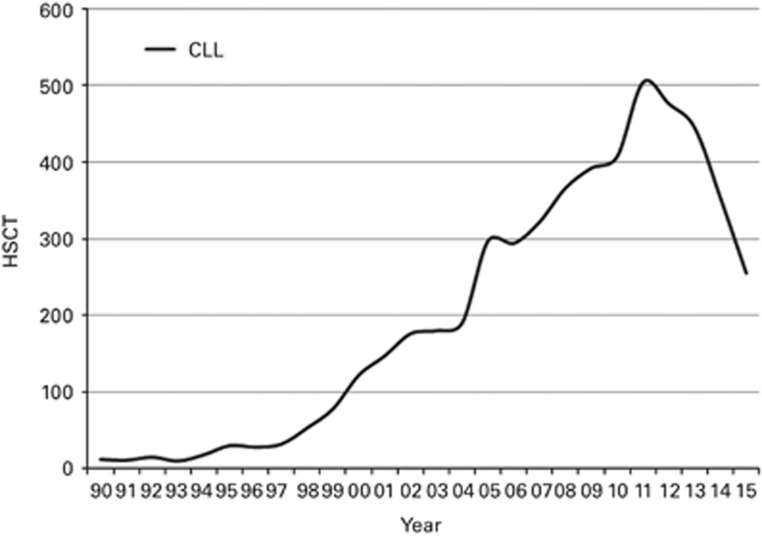
Figures 1a and b show as a pie graph the distribution of disease indications for allogeneic (Figure 1a) and autologous (Figure 1b), respectively. Of interest, we show that for allogeneic HSCT AML is the most frequent indication (39%); of these, 21% were for patients in CR1, 12% for patients with more advanced disease and 6% for patients with transformed AML, either therapy related or from myelodysplastic /MPN. Compared with that in 2014, there were increases in allogeneic HSCT for AML by 7.9% and MPN 3.5% and a major decrease by 28% was seen in allogeneic HSCT use for CLL (Figure 2), dropping from 504 patients in 2011 to 255 in 2015. Among allogeneic HSCT, 6933 were performed using non-myeloablative conditioning. This is an increase of 1% since in 2014 and is 40% of all allogeneic HSCT. For autologous HSCT, there was an increase in myeloma by 8.1% and Hodgkin lymphoma by 2.1% proportions for most other diseases remained stable.
Figure 1.
Relative proportion of indications for HSCT in Europe in 2015. (a) Proportions of disease indications for allogeneic HSCT in Europe in 2015. (b) Proportions of disease indications for autologous HSCT in Europe in 2015.
Figure 2.
The rise and fall in absolute numbers of allogeneic HSCT for CLL in Europe 1990–2015.
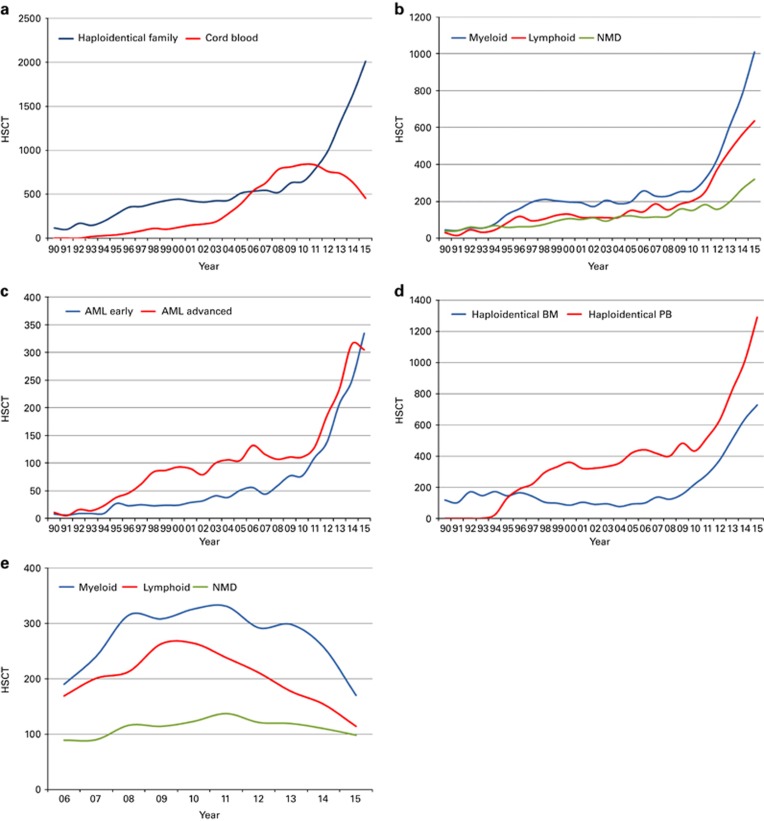
Important trends in 2015 include continued increase in patients treated with allogeneic and autologous HSCT as shown in Supplementary Figure 1, and increasing use among allogeneic HSCT recipients of unrelated donor transplantation,14 although it might appear that the growth rate is slowing down (Supplementary Figure 2). Figure 3a shows the continued use of alternative donor transplantation and among these an impressive increase of the use of haploidentical donors to 2012 patients in 2015 across Europe, an increase of 291% since 2005. The highest growth is seen in myeloid malignancies (1008), with lymphoid malignancies 636, nonmalignant disorders 316 and 52 others. Figure 3b shows that the growth of haploidentical donor HSCT is seen more in patients with myeloid malignancy, but also in lymphoid malignancy and non-malignant disorders, although to a lesser degree. Among myeloid malignancies, the majority (n=735) are patients with AML. Of note, there are equal proportions of patients with AML receiving haploidentical donor HSCT transplanted in CR1 and with more advanced disease (Figure 3c). Stem cell source for haploidentical donor HSCT is shown in Figure 3d, peripheral blood is used more frequently than marrow. The decreasing use of unrelated cord blood as donor source is shown in Figure 3a for total HSCT, or by main indication in Figure 3e. This decrease is in sharp contrast to the rise seen in haploidentical donor HSCT. As shown in Figure 3e, this decrease pertains to myeloid and lymphoid malignancies but not to nonmalignant disorders where the use of unrelated cord blood is stable over time.
Figure 3.
Change in the absolute numbers of haploidentical and cord blood HSCT in Europe 1990–2015. (a) Change in donor selection from cord blood HSCT to haploidentical HSCT. (b) Increase in the use of haploidentical donors by main indication group. (c) Haploidentical HSCT by AML early disease and advanced disease. (d) Haploidentical HSCT by cell source; bone marrow versus peripheral blood. (e) Trend in the use of unrelated cord blood HSCT by main indication group 2006–2015.
Transplant rates
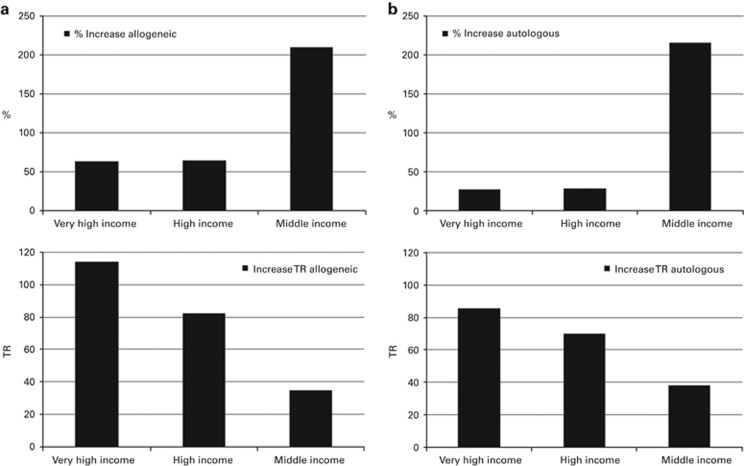
Supplementary Figures 3a and b show transplant rates by country for allogeneic and autologous HSCT comparing rates in 2015 on maps of Europe. Median transplant rates per 10 million inhabitants were 153.1 (range, 4.4–460.9) for allogeneic HSCT and 251.8 (range, 1.0–759.9) for autologous HSCT in 2015. For the purpose of this analysis, we have grouped countries according to World Bank income group gross national income per capita in USD in 2015, (http://data.worldbank.org/data-catalog/world-development-indicators). All European countries fall within the group of either middle-income or high-income category; thus, we created a third group to split the high-income countries into very wealthy countries defined as >40 000 USD gross national income per capita. Median transplant rates in 2015 for the 3 groups are as follows: 262, 179, and 34 for very-high-, high- and medium-income countries (allogeneic HSCT, transplants per 10 million inhabitants), and 396, 295 and 56 for autologous HSCT, respectively. Figure 4 shows growth rates in allogeneic and autologous HSCT use by income category as relative growth, that is, % increase from 2005 to 2015 or as absolute increase in transplant rates from 2005 to 2015 normalized for size of the population. Figure 4a and b shows that % increase is highest for middle income countries (gross national income per capita<12 500 USD) for both allogeneic and autologous HSCT, and lowest for the very-high-income countries. The higher income countries had already achieved a high level of transplant rates in 2005. To the contrary, the absolute growth, that is, increase in access of patients to transplant centers is highest in the very-high-income countries in the period of 2005–2015, again for both allogeneic and autologous HSCT.
Figure 4.
Effect of income group on changes in transplant activity and transplant rates 2005–2015. (a) Percentage increase in transplant activity for allogeneic HSCT (top left), increase in transplant rates for allogeneic HSCT (bottom left). (b) Percentage increase in transplant activity for autologous HSCT (top right), increase in transplant rates for autologous HSCT (bottom right).
Additional cellular therapies
A total of 35 countries (330 teams) reported having performed 3882 cellular therapies in 2015. Of these, 2940 patients received donor lymphocyte infusions (Table 2). Indications were graft enhancement: 803 (27%), residual disease: 410 (14%), relapsed disease: 1285 (44%), and per protocol 442 (15%).
Other cellular therapies were given either within the context of a HSCT or not. The majority were mesenchymal stem cells given for GvHD treatment (396) of for graft enhancement (45). Seventy-four patients received mesenchymal stem cells for various other indications. The largest additional group of cellular therapies were selected or expanded T cells given to treat infectious complications (119 patients) or for anti-malignant effects (37). Other cellular therapies including natural killer cells,14 regulatory T-cells (35), genetically modified T-cells,14 dendritic cells (25) and expanded or genetically modified hematopoietic stem cells (36) were reported more rarely. One hundred and twenty-one patients received cellular products for purposes of regenerative medicine.15, 16
Discussion
The EBMT activity survey has been conducted annually since 1990.6 The 2010 survey reported for the first time >30 000 patients transplanted in a given year17 and >40 000 transplants in 2014. Again, transplant numbers continue to increase unabated across Europe.
HSCT for some indications continues to increase but not for others. Of interest is the decreasing use of allogeneic HSCT for CLL and a growth in allogeneic HSCT using haploidentical donors, an increase over 200% in the last 5 years. The drop in allogeneic HSCT for CLL is remarkable and reminds us of the drop seen in CML transplants once kinase inhibitors became available.18, 19 Whether this drop is going to be permanent or whether this is temporary will depend on the long-term results of kinase inhibitors and possibly bcl2 inhibitors developed to treat CLL.
The continuing use of haploidentical donors is impressive and it becomes apparent that haploidentical donor HSCT is not only used for advance disease stages but also for early disease stages as exemplified by AML (Figure 3c). The use of peripheral blood as a stem cell source has surpassed the use of marrow (Figure 3d), although the original studies describing haploidentical donor HSCT with post-transplant cyclophosphamide as GvHD prophylaxis have been using mainly marrow.20 The use of unrelated cord blood, a competitor for alternative donor HSCT when identical siblings and well-matched unrelated donors are not available, continues to decrease but only for malignant disorders.21 Cord blood transplant rates for nonmalignant diseases remain stable, reflecting the practice mainly in pediatric centers.
We looked at development of transplant technology in countries in Europe by economic strength of the societies and, for this purpose, show growth of allogeneic and autologous HSCT as relative and as absolute growth for countries in the middle-, high- and very-high-income categories. We here confirm that the relative growth is more significant in middle income countries, but that the highest absolute growth over the last decade is seen in the category of very-high-income countries. This exemplifies that autologous and allogeneic HSCT remains an expensive technology broadly available in wealthy societies.
We have added data on the use of cellular therapies, most of which is donor lymphocyte infusions given to treat relapse or residual disease in over 2900 patients. Other cellular therapies have been given to over 900 patients, the largest group of which is mesenchymal stromal cells to treat GvHD. Although we have established confidence in the reported transplant numbers we cannot exclude a certain degree of underreporting, particularly in the most advanced fields of cellular therapies of patients included in studies across Europe.
In conclusion, this year's activity survey shows continued increase in the use of HSCT across Europe. Some trends are visible and are discussed here. The study reflects current practice and results may be useful to healthcare planning and health policy makers.
Acknowledgments
The cooperation of all participating teams and their staff (listed in the Supplementary Appendix), the EBMT Co-ordination offices; Barcelona, Paris, London (C Ruiz de Elvira), the Austrian Registry (ASCTR) (H Greinix, B Lindner and C Wagner), the Belgium Registry (Yves Beguin and M Van Spauwen), the Czech Registry (P Zak, M Trnkova and K Benesova), the French Registry (SFGM) (I Yakoub-Agha and N Raus), the German Registry (DRST) (H Ottinger, K Fuchs, C Müller, H Neidlinger and F Hanke), the Italian Registry (GITMO) (F Bonifazi, B Bruno and E Oldani), the Dutch Registry (JJ Cornelissen and M Groenendijk), the Spanish Registry (GETH) (C Solano and A Cedillo), the Swiss Registry (SBST) (U Schanz, H Baldomero and E Buhrfeind), the Turkish Registry (G Gurman and M Arat) and the British Registry (BSBMT) (J Perry) are greatly appreciated. We also thank D John for database support. EBMT is supported by grants from the corporate sponsors: Jazz Pharmaceuticals plc, Molmed S.p.A, Accord Biopharmaceuticals, Amgen Oncology GmbH, AstellasPharma Europe Ltd, Celgene International SARL, Clinigen Group Ltd, Gilead Sciences Europe Ltd, Janssen, Medac Hematology GmbH, MiltenyiBiotec GmbH, MSD Sharp&Dohme GmbH, Neovii Biotech GmbH, Pfizer Oncology, Sanofi Oncology, Takeda Pharmaceuticals, Therakos Photopheresis, Alexion, Apotex Advancing Generics, Basilea Pharaceutica, Bellicum Pharmaceuticals, Cell Medica, Eurocept International, Kiadis Pharma, Macropharma, Mundipharma Oncologie, Pierre Fabre Médicament and Terumo BCT.
Footnotes
Supplementary Information accompanies this paper on Bone Marrow Transplantation website (http://www.nature.com/bmt)
The authors declare no conflict of interest.
Supplementary Material
References
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med 2006; 354: 1813–1826. [DOI] [PubMed] [Google Scholar]
- Appelbaum FR. Hematopoietic-cell transplantation at 50. N Engl J Med 2007; 357: 1472–1475. [DOI] [PubMed] [Google Scholar]
- Ljungman P, Bregni M, Brune M, Cornelissen J, deWitte T, Dini G et al. European Group for Blood and Marrow. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe 2009. Bone Marrow Transplant 2010; 45: 219–234. [DOI] [PubMed] [Google Scholar]
- Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A et al. Hematopoietic stem cell transplantation: a global perspective. JAMA 2010; 303: 1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratwohl A, Pasquini MC, Aljurf M, Atsuta Y, Baldomero H, Foeken L et al. One million haemopoietic stem-cell transplants: a retrospective observational study. Lancet Haematol 2015; 2: e91–e100. [DOI] [PubMed] [Google Scholar]
- Gratwohl A, Baldomero H, Schwendener A, Gratwohl M, Apperley J, Frauendorfer K et al. The EBMT activity survey 2008 impact of team size, team density and new trends. Bone Marrow Transplant 2011; 46: 174–191. [DOI] [PubMed] [Google Scholar]
- Gratwohl A. Bone marrow transplantation activity in Europe 1990. Report from the European Group for Bone Marrow Transplantation (EBMT). Bone Marrow Transplant 1991; 8: 197–201. [PubMed] [Google Scholar]
- Gratwohl A, Baldomero H, Horisberger B, Schmid C, Passweg J, Urbano-Ispizua A. Accreditation Committee of the European Group for Blood and Marrow Transplantation (EBMT). Current trends in haematopoietic stem cell transplantation in Europe. Blood 2002; 100: 2374–2386. [DOI] [PubMed] [Google Scholar]
- Gratwohl A, Baldomero H, Schwendener A, Rocha V, Apperley J, Frauendorfer K et al. The EBMT activity survey 2007 with focus on allogeneic HSCT for AML and novel cellular therapies. Bone Marrow Transplant 2009; 43: 275–291. [DOI] [PubMed] [Google Scholar]
- Gratwohl A, Schwendener A, Baldomero H, Gratwohl M, Apperley J, Niederwieser D et al. Changes in use of hematopoietic stem cell transplantation; a model for diffusion of medical technology. Haematologica 2010; 95: 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passweg JR, Baldomero H, Peters C, Gaspar HB, Cesaro S, Dreger P et al. Hematopoietic SCT in Europe: data and trends in 2012 with special consideration of pediatric transplantation. Bone Marrow Transplant 2014; 49: 744–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P et al. Hematopoietic stem cell transplantation in Europe 2014: more than 40 000 transplants annually. Bone Marrow Transplant 2016; 51: 786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation. Transplantation. WHO: Geneva, Swtizerland, 2015. http://www.who.int/topics/transplantation/en/.
- Foeken LM, Green A, Hurley CK, Marry E, Wiegand T, Oudshoorn M. Monitoring the international use of unrelated donors for transplantation: the WMDA annual reports. Bone Marrow Transplant 2010; 45: 811–818. [DOI] [PubMed] [Google Scholar]
- Bonini C, Mondino A. Adoptive T-cell therapy for cancer: the era of engineered T cells. Eur J Immunol 2015; 45: 2457–2469. [DOI] [PubMed] [Google Scholar]
- Tolar J, Le Blanc K, Keating A, Blazar BR. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells 2010; 28: 1446–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passweg JR, Baldomero H, Gratwohl A, Bregni M, Cesaro S, Dreger P et al. The EBMT activity survey: 1990-2010. Bone Marrow Transplant 2012; 47: 906–923. [DOI] [PubMed] [Google Scholar]
- Jeyakumar D, O'Brien S. The next generation of targeted molecules for the treatment of chronic lymphocytic leukemia. Oncology (Williston Park) 2016; 30: 1008–1015. [PubMed] [Google Scholar]
- Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P et al. Impact of drug development on the use of stem cell transplantation: a report by the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant 2016; 52: 191–196. [DOI] [PubMed] [Google Scholar]
- Luznik L, O'Donnell PV, Symons, Chen AR, Leffell MS, Zahurak M et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2008; 14: 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J et al. Blood and Marrow Transplant Clinical Trials Network. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood 2011; 118: 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.