Abstract
It has been hypothesized that some antibiotic resistance genes (ARGs) found in pathogenic bacteria derive from antibiotic-producing actinobacteria. Here we provide bioinformatic and experimental evidence supporting this hypothesis. We identify genes in proteobacteria, including some pathogens, that appear to be closely related to actinobacterial ARGs known to confer resistance against clinically important antibiotics. Furthermore, we identify two potential examples of recent horizontal transfer of actinobacterial ARGs to proteobacterial pathogens. Based on this bioinformatic evidence, we propose and experimentally test a ‘carry-back' mechanism for the transfer, involving conjugative transfer of a carrier sequence from proteobacteria to actinobacteria, recombination of the carrier sequence with the actinobacterial ARG, followed by natural transformation of proteobacteria with the carrier-sandwiched ARG. Our results support the existence of ancient and, possibly, recent transfers of ARGs from antibiotic-producing actinobacteria to proteobacteria, and provide evidence for a defined mechanism.
Some antibiotic resistance genes found in pathogenic bacteria might derive from antibiotic-producing actinobacteria. Here, Jiang et al. provide bioinformatic and experimental evidence supporting this hypothesis, and propose a specific mechanism for the transfer of these genes between bacterial phyla.
The discovery of antibiotics from microorganisms and their development into clinical drugs constitutes one of the greatest advances in medical history, but the acquisition and dissemination of genes that confer antibiotic resistance among pathogens has severely curtailed the effectiveness of many of these compounds. Elucidating the origins of these antibiotic resistance genes (ARGs) and the mechanisms mediating their spread to pathogens is consequently a public health priority1. Actinobacteria, especially of the genus Streptomyces, produce many clinically important antibiotics. In most cases, the gene clusters encoding the biosynthesis of these compounds also contain resistance genes as a self-protecting mechanism towards these compounds2 or to modulate their signalling activity3. As early as 1973, it was hypothesized that the enzymes found in Gram-negative pathogens that inactivate aminoglycoside antibiotics could have originated from the ARGs of actinobacteria that produce this class of antibiotics through ancient horizontal gene transfer (HGT), based on the discovery that they employ the same enzymatic mechanisms4. This ‘producer hypothesis' was subsequently proposed for additional ARGs, for example, some class-A β-lactamases5 in Gram-negative pathogens, and erythromycin6 and vancomycin resistance genes7 in Gram-positive pathogens. However, in these cases the sequence similarities between ARG proteins in actinobacteria and Gram-negative pathogens are low, making it difficult to distinguish if they result from ancient HGT or from other types of evolutionary processes8.
Compared with ancient transfers, recent ARG dissemination from actinobacteria to pathogens may pose an even more urgent threat to human health9, as actinobacterial ARGs make up a large portion of the environmental resistome. In addition to self-protecting ARGs, most actinobacteria also carry ARGs horizontally obtained from other actinobacteria6,10. In an investigation of actinomycetes from soil, isolates were on average resistant towards seven to eight antibiotics from a collection of 21 representative antibiotics11. In a functional metagenomics study using Escherichia coli as an expression host, actinobacteria were found to be the most enriched source of resistance-conferring DNA fragments relative to their abundance12. However, since no recent transfer from actinobacteria to Gram-negative pathogens has been discovered and many recent studies showed that phylogenetic and ecological boundaries are two major ARG transfer barriers12,13,14,15, the clinical relevance of this large resistome remains unclear16,17.
Here, we examine a large collection of known Streptomyces ARGs, and find more examples supporting the ‘producer hypothesis'. Specifically, we provide evidence that two ARGs conferring resistance against chloramphenicol and lincomycin might have been recently transferred from actinobacteria to human and animal pathogens. Based on their surrounding sequence, we propose and test a potential mechanism mediating gene transfer from Gram-positive actinobacteria to Gram-negative pathogens.
Results
Actinobacterial ARGs have related proteins in proteobacteria
Proteobacteria encompass many important human pathogens such as Escherichia coli, Pseudomonas aeruginosa and others. We therefore sought to examine the similarity between corresponding ARGs in actinobacteria and proteobacteria. First, we extracted all experimentally validated Streptomyces ARG proteins from ARDB18 and CARD19 databases. A majority (39 of 57) of them are reported to have self-protecting roles or are located within antibiotic biosynthesis gene clusters as analysed by antiSMASH20 (Supplementary Data 1). Their most similar homologues in proteobacteria were subsequently identified from the NCBI non-redundant protein database by BLASTP, with sequence identities ranging from 23 to 68%. Seven of these proteobacterial proteins have sequences more similar to actinobacterial proteins than to proteins from any other phyla, including proteobacteria (Supplementary Data 1). Furthermore, phylogenetic trees were constructed and showed that 12 (including the above seven) of the proteobacterial proteins may have originated from actinobacteria by interphylum HGT (Fig. 1 and Supplementary Fig. 1)21. Potential HGTs in the opposite direction, from proteobacteria to actinobacteria, were also noticed in some trees. In particular, the Streptomyces ARG sul1 (AFN41071.1) shares 95% sequence identity with a proteobacterial gene (Supplementary Fig. 1).
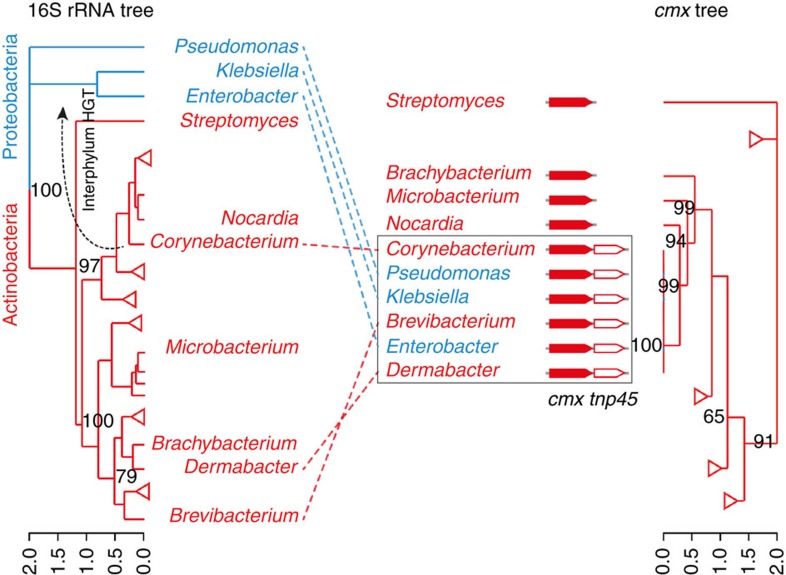
Figure 1. Proteobacterial proteins related to Streptomyces ARG proteins.
Relationships between all experimentally validated Streptomyces ARG proteins and their most similar homologues in proteobacteria were analysed by phylogenetic analysis (Supplementary Fig. 1). Selected pairs that might be connected via interphylum HGT are summarized here, with the proposed transfer direction indicated by an arrow. Protein sequence identity was determined by BLASTP. aThe proteins were BLASTed against pathogen genome database PATRIC to see if they have close homologues (sequence identity threshold of 90%) in pathogens.
By examining neighbouring genes, we also found evidence for potential cotransfer of additional genes in the case of the ARG pac (conferring resistance to puromycin). The products of six neighbouring genes also show higher similarity to actinobacterial proteins than to proteins from any other phyla, suggesting that they might have been transferred together from actinobacteria to proteobacteria (Supplementary Fig. 2).
Of the 12 proteobacterial proteins that might have originated from actinobacteria, nine are encoded in genomes of environmental species, one of which has close homologues encoded in the genomes of pathogens from the database PATRIC22. The other three proteins are harboured by pathogens, including the well-known aminoglycoside-inactivating phosphotransferase APH(3″) (WP_031942890.1)4.
Recent ARG transfers from actinobacteria to proteobacteria
By analysing DNA sequence composition signatures using RAIphy23, we found that two of the proteobacterial proteins, chloramphenicol exporter Cmx (WP_005297378.1) and lincomycin exporter LmrA (WP_038989331.1), may have been recently transferred from actinobacteria, since the proteobacterial genes still retain actinobacterial sequence signatures (Supplementary Data 2)8. Cmx is found in clinical isolates of P. aeruginosa, Klebsiella oxytoca and Enterobacter asburiae (Supplementary Data 3). It is 63% identical (100% coverage) to the chloramphenicol resistance protein (P31141) from Streptomyces lividans 66 (ref. 24), and 52% identical (99% coverage) to the self-protecting resistance protein (WP_015032122.1) from chloramphenicol producer Streptomyces venezuelae (Supplementary Fig. 3). Furthermore, its gene was found to be identical or almost identical (identity over 99%) to genes from many non-Streptomyces actinobacteria (Fig. 2), further supporting that the interphylum gene transfer happened recently.
Figure 2. A tanglegram between the phylogeny of the genus representative hits of cmx and the host phylogeny of their corresponding 16S rRNA sequences.
The red labels correspond to Gram-positive actinobacteria, while the blue labels correspond to Gram-negative proteobacteria. Genomes were selected if they have BLASTP hits against the Cmx protein (WP_005297378.1). The best hit per genus is taken as the representative hit. Hits with over 99% identity to WP_005297378.1 are framed. These cmx genes are located in transposons together with a transposase gene tnp45. Triangles represent collapsed branches. The full trees are provided in Supplementary Fig. 4.
Possible mechanisms of the ARG transfers
Conjugation or transduction from actinobacteria to proteobacteria has so far not been described. Many proteobacteria can take up free DNA through natural transformation, but because of low sequence similarity, actinobacterial DNA has a low probability of being incorporated into proteobacterial genomes by homologous recombination25. In theory, the DNA can be inserted randomly by non-homologous end joining, but the frequency is extremely low26. Transposase/integrase-mediated recombination after natural transformation is possible if a transposase or integrase is encoded in the DNA and can be expressed before the DNA is degraded27. We noticed that cmx is colocalized with an actinobacterial transposase gene tnp45 forming a transposon28, and the intact transposon can be found in both actinobacteria and proteobacteria (Fig. 2). Initially, we hypothesized that a DNA fragment containing the transposon released from dead actinobacteria could be taken up by proteobacteria, and the transposon could insert itself into the new genome by the activity of its transposase. To test this hypothesis, we examined if the transposition activity, which has been experimentally validated in Corynebacterium glutamicum29, was also functional in proteobacteria. However, no transposition was detected in our experimental set-up (details are described in Methods section).
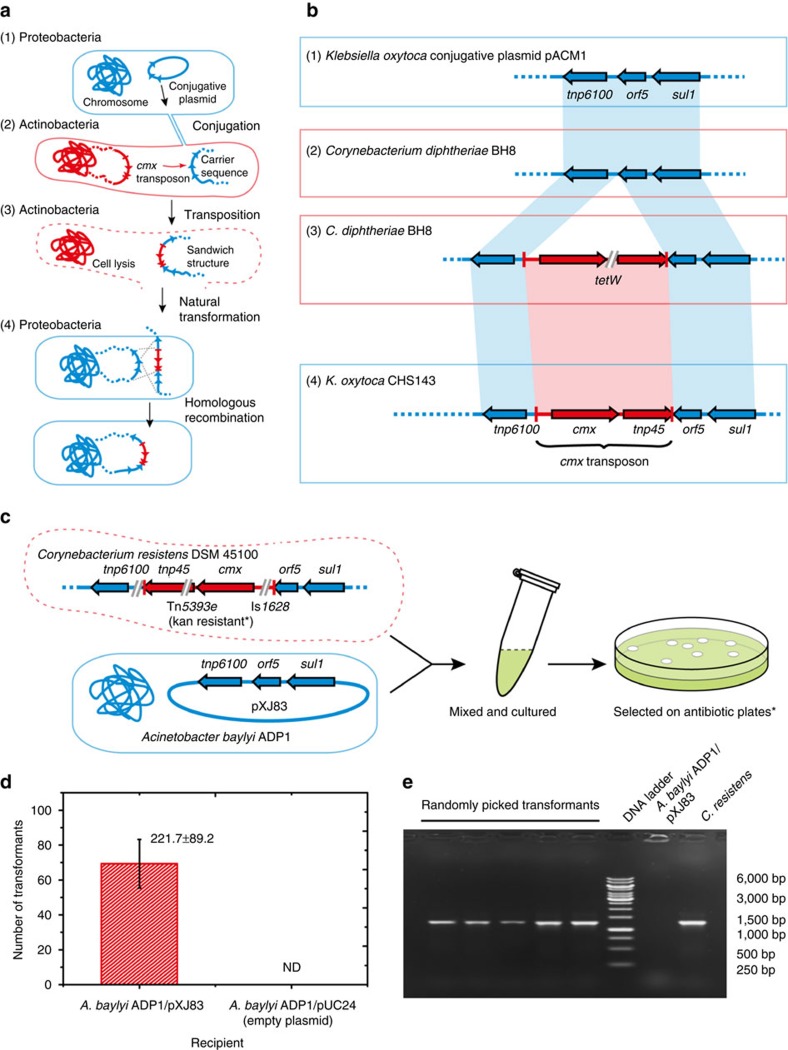
However, by further analysing the DNA sequence flanking the cmx transposon in both phyla, we arrived at a different hypothesis to explain the interphylum HGT, which we call the ‘carry-back' model (Fig. 3a). First, a proteobacterial sequence is transferred from proteobacteria into actinobacteria by conjugation, a mechanism known to be highly efficient30. Next, it recombines with actinobacterial DNA, for example, by the transposition of cmx transposon, forming a sandwich structure of actinobacterial DNA flanked by proteobacterial DNA. Then, the sandwich structured DNA released from dead actinobacterial cells is taken up by nearby proteobacteria through natural transformation and incorporated via homologous recombination. In support of this ‘carry-back' model, we identified DNA sequences representing all the proposed intermediates (Fig. 3b and Supplementary Data 4). The carrier sequence is a fragment from class 1 integron In4, composed of an IS6100 insertion sequence, an acyltransferase gene (orf5) and sul1 (ref. 31). This sequence is widely distributed among proteobacteria, and frequently found on conjugative plasmids (Fig. 3b). The same carrier sequences can be also found in actinobacteria, for example, in Corynebacterium diphtheriae BH8. Part of the carrier sequence, the sul1 gene, can be found in Streptomyces sp. 1AL4 (ref. 32). The sandwich structure with cmx transposon inserted between IS6100 and orf5 is found in C. diphtheriae BH8 (Fig. 3b) and Corynebacterium resistens plasmid pJA144188 (Fig. 3c), although with additional resistance genes and mobile elements inserted as well. The final product of the interphylum transfer, the sandwich structure in proteobacterial genome, is found in E. asburiae 35642 and K. oxytoca CHS143 (Fig. 3b). All these intermediates fit the proposed ‘carry-back' model, and appear to be stably maintained in their respective hosts, thus potentially providing sufficient time for the multistep process to be accomplished.
Figure 3. Transfer of a resistance gene from actinobacteria to proteobacteria by the ‘carry-back' model.
Actinobacterial and proteobacterial cells are marked in red and blue, respectively. DNA sequences of actinobacterial and proteobacterial origins are likewise labelled in red and blue. Dashed line represents DNA that can be either a fragment of a chromosome or a plasmid. The terminal inverted repeats of cmx transposon are represented by red bars. (a) The proposed ‘carry-back' interphylum gene transfer mechanism. (b) Examples of actual sequences found in different bacteria that resemble the proposed intermediates: (1) carrier sequence in proteobacteria; (2) carrier sequence in actinobacteria; (3) sandwich structure with cmx transposon flanked by carrier sequences in actinobacteria; (4) cmx transposon incorporated into genome in proteobacteria. Double-slash indicates additional resistance genes and mobile elements inserted into the proposed intermediate structures. NCBI accession numbers of these intermediate sequences are provided in Supplementary Data 4. (c) Experimental reconstruction of the interphylum gene transfer of cmx from actinobacteria to proteobacteria. C. resistens DSM 45100 naturally carries the sandwich structure. Plasmid pXJ83 is constructed by cloning the carrier sequence from P. aeruginosa BM4530 onto pUCP24 vector. *Kanamycin (kan) instead of chloramphenicol was used for the selection plates. Kanamycin resistance is coded by Tn5393e inserted in the cmx transposon. (d) Efficiency of natural transformation using A. baylyi ADP1/pXJ83 as recipient strain (error bar: s.d.; n=3). A. baylyi ADP1/pUCP24 was used as a negative control. The detection limit is one transformant per 109 colony-forming unit (c.f.u.) of recipient cells. ND, not detected. (e) Colony PCR of transformants using primers that target cmx. C. resistens DSM 45100 was used as a positive control. A. baylyi ADP1/pXJ83 was used as a negative control.
The ‘carry-back' model is also supported by the second case of potential recent transfer that we have identified in the in silico studies. LmrA (WP_038989331.1), found in Salmonella enterica and Escherichia coli isolated from farm animals, is 50% identical to the self-protecting lincomycin pump CAA42550 from Streptomyces lincolnensis. Its gene and an adjacent regulator gene still retain actinobacterial sequence signatures (Supplementary Data 3), suggesting that they may have been transferred from actinobacteria recently. Interestingly, they are located in an RSF1010-like plasmid (Supplementary Fig. 5), which could have served as the carrier sequence as the ability of RSF1010 (NC_001740.1) to be mobilized by conjugation from E.coli into Streptomyces and Mycobacterium has been documented33.
Experimental reconstruction of the interphylum transfer of cmx
To provide further evidence for the hypothesis, we reproduced the interphylum gene transfer of cmx experimentally. C. resistens DSM 45100, which naturally has the sandwich structure sequence, was used as the actinobacterial cmx donor. Acinetobacter baylyi ADP1, a model strain for HGT studies27,34 (Fig. 3c) with a cloned IS6100-orf5-sulI sequence, was used as the cmx acceptor to mimic proteobacterial pathogens with the same sequence, such as Acinetobacter baumannii D4 (Supplementary Data 4). Heat-killed and lysed C. resistens cells were added into A. baylyi ADP1 culture, incubated and followed by selection on kanamycin plate (kanamycin resistance was coded by the Tn5393e inserted between cmx and tnp45 in C. resistens, see Fig. 3c). The obtained colonies showed no chloramphenicol resistance, probably because the promoter activity was disrupted by the IS1628 inserted in front of cmx (Fig. 3c). However, the existence of cmx in these colonies was confirmed by colony PCR and sequencing (Fig. 3e), demonstrating that the cmx gene was transferred to A. baylyi. The negative control, an A. baylyi ADP1 strain without IS6100-orf5-sulI sequence, did not generate any colonies after the same treatment (Fig. 3d), confirming the involvement of the carrier sequence in the ARG transfer.
To confirm the resistance activity of cmx in proteobacteria, we cloned an intact cmx transposon free of IS1628 into E. coli and observed increased chloramphenicol tolerance when promoters were provided (Supplementary Fig. 6)29.
Possible dissemination route of cmx
Cmx family proteins are widely spread among Streptomyces and other actinobacteria. Its recruitment into tnp45 transposon may have occurred in soil environment, as both free cmx and transposon-carried cmx have been found in soil actinobacteria. For example, C. glutamicum 1014 contains cmx on the tnp45 transposon, whereas Arthrobacter sp. 161MFSha2.1 contains cmx but not tnp45. The two cmx genes are 93% identical (Supplementary Fig. 7). We hypothesize that the cmx transposon found in commensal and pathogenic actinobacteria was probably obtained from the related strains from soil. Then, the cmx transposon might have been transferred into Gram-negative pathogens by the ‘carry-back' mechanism. Currently, cmx transposon-harbouring pathogens have been isolated in Asia, Europe, United States of America and especially South America (Supplementary Data 3).
Discussion
Clinical ARGs have complex and diversified origins6,16, and our results indicate that some of them might have originated from actinobacteria. Ancient ARG transfers are proposed to have occurred in soil environments and then passed on to pathogens in modern times35. Recent transfers from actinobacteria to pathogens might also be possible, as suggested by the examples of cmx and lmrA.
Proteobacteria are well known to be able to transfer DNA to organisms from other phyla and even other kingdoms by conjugation34. A recent study suggested that conjugation from proteobacteria to actinobacteria might happen frequently in soil30. Thus, the ‘carry-back' mechanism might have mediated the HGTs from actinobacteria to proteobacteria in soil using conjugative plasmids as the carrier sequence. In modern times, likely caused by increased selection pressure due to the extensive use of antibiotics, mobile genetic elements including conjugative plasmids, integrons and transposons tend to be clustered together with ARGs forming mobile multidrug-resistant units. These units showed extraordinary capability of spreading among commensals, pathogens and even environmental bacteria in water and soil36. Not surprisingly, the cmx carrier sequence was from a widespread conjugative class I integron and is composed of the IS6100 transposon and another resistance gene sul1. Accordingly, the cmx carried back was incorporated into such units, facilitating further dissemination.
In addition to being a gene transfer barrier, different phyla also mean distinct cell environments for gene expression, regulation and protein function37. For example, due to the different cell membrane structure in proteobacteria, drug efflux pumps like Cmx and LmrA will export compounds into the periplasm instead of the extracellular environment as in actinobacteria. Future studies are required to understand how newly obtained resistance genes and their new hosts will evolve after the gene transfer.
Methods
Homologues of Streptomyces ARG proteins in proteobacteria
All 87 ARG proteins of the genus Streptomyces in ARDB18 and CARD19 databases were downloaded (October 2016). They were further manually curated by removing artificial constructs (ARGs used as selective marker during Streptomyces genetic engineering) and dereplicating proteins with high sequence similarity (identity over 95% with each other). Then, they were used as queries in BLASTP analyses against the NCBI non-redundant protein database of proteobacteria to find their most similar homologues. Some of the best hits were found to be caused by sequence contamination (by the following method), that is, they were actually from actinobacteria but mislabelled as proteobacterial proteins in the database. In this case, the next best hit was analysed by the same method until a true proteobacterial protein was found. In addition, the identified proteobacterial proteins were searched (BLASTP) against the non-redundant protein database of all phyla to see if they were more similar to actinobactieral proteins than to proteins from other phyla.
Sequence contamination check
Because HGT studies are very sensitive to cross-contaminations in the sequence data, a manual check of possible contaminations was performed: if the best BLAST hit was from a fully assembled genome or circular plasmid sequence with experimentally determined source organism to be proteobacteria, then the hit was considered as a true proteobacterial protein. In cases where the best hit was a contig instead of a full genome, then the protein was considered as a true proteobacterial protein only if the contig was 10 kbp or larger and the contig had the best sequence match to other proteobacterial sequences in the ‘nt' database. If the contig had best nucleotide matches against ‘nt' from actinobacteria, it was considered an artefact caused by contamination.
BLAST against PATRIC
The proteobacterial proteins were BLASTed against pathogen genome database PATRIC (https://www.patricbrc.org/app/BLAST, with sequence identity threshold of 90%) to see if they have close homologues in pathogens.
Comparison of the neighbouring area of the ARGs
In search of cotransfer of addition genes, neighbouring area of the ARGs were compared between the Streptomyces genomes and proteobacterial genomes by MultiGeneBlast using amino-acid translation sequences38.
Bacterial genome databases for phylogenetic tree construction
All protein data sets of bacterial genomes were downloaded from the NCBI RefSeq database available at ftp://ftp.ncbi.nlm.nih.gov/genomes/refseq/bacteria/ (October 2016). The collection covers 64,580 genomes and 55,667,859 unique proteins. To avoid contaminated genomes, we have excluded genomes that either lack the 16S rRNA gene or have multiple copies of 16S rRNA genes from multiple species.
Construction of phylogenetic trees
BLAST analysis for each ARG protein was performed using blastp command of NCBI BLAST version 2.2.31+, with the following parameters: the maximum target sequence of 55 million sequences and E value below 1E−50. A genome was considered if it has a hit with a minimum 30% identity and 80% coverage where coverage is defined as (number of matched amino acid+number of mismatched amino acid+gaps)/(query protein length). To simplify the phylogenetic tree, we normalized the number of hits to 100 genomes, considering the best hit per species, genus or maximum order. We then constructed phylogenetic trees for these genomes based on their 16S rRNA and constructed phylogenetic trees for the ARGs based on their protein sequences. Sequences were multiply aligned using MAFFT39, considering the local alignment option with default settings for the other parameters, then optimized and phylogenetic trees were generated, using the FastTree methods40. The generated host and ARG trees were visualized using ETE 3 package41.
DNA sequence signature analysis
RAIphy23 was used to analyse gene sequences to find their most likely taxonomic source. Relative abundance index (RAI) is calculated from the over- and underabundance statistics collected for each taxon. The set of RAI profiles was extracted from 2,773 complete bacterial genomes downloaded from NCBI ftp://ftp.ncbi.nlm.nih.gov/genomes/archive/old_genbank/Bacteria/ (March 2016). To avoid false taxonomic assignment, we excluded plasmids and genomes below 1 Mbp.
Strains and media
P. aeruginosa PA7, C. urealyticum DSM 7109 and C. resistens DSM 45100 were obtained from DSMZ and cultured in DSMZ media. E. coli DH5α and A. baylyi ATCC33305 cultures were cultured in Luria-Bertani (LB) medium. Antibiotics were added at the following concentrations: ampicillin, 50 μg ml−1; kanamycin, 50 μg ml−1; gentamicin, 20 μg ml−1. Chloramphenicol was added at indicated concentrations.
Cloning of cmx transposon and the carrier sequence
The cmx transposon was PCR amplified from P. aeruginosa PA7 by primer xj143 (as both forward and reverse primer). Plasmid backbone was amplified from plasmid pKD46 by primer xj144 and xj145. The two PCR products were assembled by Gibson reaction, transferred into E. coli DH5α and selected on Amp plates. Insertion direction of cmx transposon was determined by sequencing with primer xj146. The resulted plasmids with cmx transposon in the same or opposite direction with the plasmid repA gene were named as pXJ79 and pXJ80, respectively. pXJ79cu and pxj80cu were constructed likewise except that the cmx transposon was amplified from C. urealyticum DSM 7109. pXJ79 was amplified with primer xj177and xj178, the product was recircularised by Gibson reaction, generating pXJ79a. By this way, promoter pLac was inserted in front of the cmx transposon. The carrier sequence was amplified from P. aeruginosa BM4530 with primer xj173 and xj174.1 and cloned on a plasmid backbone amplified from pUCP24 with primer xj171.1 and xj172, generating pXJ83. Phusion Hot Start II DNA Polymerase for PCR was from Thermo Fisher. Gibson Assembly Master Mix was from New England Biolabs. Primer sequences are listed in Supplementary Table 1.
Test of transposition activity in E.coli
E.coli DH5α/pXJ79cu and E.coli DH5α/pXJ80cu were cultured in LB containing ampicillin and chloramphenicol, respectively, at 30 °C. Subculturing was repeated five times at 1:100 dilution. After that, 0.1 ml was inoculated into 10 ml fresh LB and cultured at 42 °C overnight to eliminate the plasmids, and then spread on LB agar containing 2.5 μg ml−1 chloramphenicol and culture at 37 °C until colonies appeared. The colonies were checked by colony PCR using primer xj144 and xj145 to see if they still had the plasmids. PCR-positive colonies were subjected to another round of plasmid elimination and chloramphenicol-sensitive test as described above.
Natural transformation of A. baylyi
Fifty microlitres overnight culture of A. baylyi ATCC33305/pXJ83 was inoculated into 1 ml fresh LB and incubated for 2 h at 120 r.p.m. C. resistens DSM 45100 cells from 1 ml overnight culture was washed with water, boiled for 15 min and mixed with the fresh A. baylyi culture. Two hundred micolitres of the mixture was spread on top of 1 ml LB agar (in 12-well plate) and cultured overnight. Then, cells were transferred to LB plates containing kanamycin. Colonies were randomly picked and checked by colony PCR using primer xj130 and xj131 for the presence of cmx.
Data availability
The authors declare that all the relevant data are provided in this published article and its Supplementary Information files, or are available from the corresponding authors on request.
Additional information
How to cite this article: Jiang, X. et al. Dissemination of antibiotic resistance genes from antibiotic producers to pathogens. Nat. Commun. 8, 15784 doi: 10.1038/ncomms15784 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary Figures and Supplementary Table
Streptomyces ARGs and their closest homologs in proteobacteria.
Sequence signature analysis of the Streptomyces ARG-like proteobacterial proteins.
Host information of Cmx and LmrA.
Genomes and plasmids containing DNA sequences that represent the proposed intermediates of cmx "carry back".
Acknowledgments
We thank the lab of Prof Jeff Dangl and the DOE-JGI for making their genome sequences available before publication. We thank Prof Justin Nodwell and Dr Günther Muth for discussion. We also thank Prof Patrice Courvalin for kindly providing total DNA of P. aeruginosa BM4530 and Prof John C. Alverdy for providing vector pucp24. M.M.H.E. acknowledges the help and assistance of Ali Syed and other members of computerome, Danish National Supercomputer for Life Sciences, in using the HPC facilities. This work was funded by the Novo Nordisk Foundation. M.O.A.S., C.M. and M.M.H.E. acknowledge funding from the Lundbeck foundation. S.Y.L. acknowledges additional funding from the Korean Ministry of Science, ICT and Future Planning (NRF-2012M1A2A2026556).
Footnotes
The authors declare no competing financial interests.
Author contributions X.J. and S.Y.L. conceived the project. T.W., X.J., M.O.A.S. and K.B. planned the study. X.J. executed experiments and analysed data. M.M.H.E. and X.J. performed bioinformatic analyses. All authors discussed results and wrote the manuscript together.
References
- Wright G. D. The antibiotic resistome: the nexus of chemical and genetic diversity. Nat. Rev. Microbiol. 5, 175–186 (2007). [DOI] [PubMed] [Google Scholar]
- Thaker M. N. et al. Identifying producers of antibacterial compounds by screening for antibiotic resistance. Nat. Biotechnol. 31, 922–927 (2013). [DOI] [PubMed] [Google Scholar]
- Yim G., Wang H. H. & FRS J. D. Antibiotics as signalling molecules. Philos. Trans. R. Soc. Lond. B 362, 1195–1200 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. & Davies J. Aminoglycoside antibiotic-inactivating enzymes in actinomycetes similar to those present in clinical isolates of antibiotic-resistant bacteria. Proc. Natl Acad. Sci. USA 70, 2276–2280 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsman M., Häggström B., Lindgren L. & Jaurin B. Molecular analysis of β-lactamases from four species of Streptomyces: comparison of amino acid sequences with those of other β-clactamases. Microbiology 136, 589–598 (1990). [DOI] [PubMed] [Google Scholar]
- Wright G. in Antibiotic Resistance Vol. 211 Handbook of Experimental Pharmacology ed. Anthony R. M. C. Ch. 2, 13–30Springer (2012). [DOI] [PubMed] [Google Scholar]
- Marshall C. G., Lessard I. A. D., Park I.-S. & Wright G. D. Glycopeptide antibiotic resistance genes in glycopeptide-producing organisms. Antimicrob. Agents Chemother. 42, 2215–2220 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravenhall M., Škunca N., Lassalle F. & Dessimoz C. Inferring horizontal gene transfer. PLoS Comput. Biol. 11, e1004095 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J. L., Coque T. M. & Baquero F. What is a resistance gene? Ranking risk in resistomes. Nat. Rev. Microbiol. 13, 116–123 (2015). [DOI] [PubMed] [Google Scholar]
- Egan S., Wiener P., Kallifidas D. & Wellington E. M. H. Phylogeny of Streptomyces species and evidence for horizontal transfer of entire and partial antibiotic gene clusters. Antonie Van Leeuwenhoek 79, 127–133 (2001). [DOI] [PubMed] [Google Scholar]
- D'Costa V. M., McGrann K. M., Hughes D. W. & Wright G. D. Sampling the antibiotic resistome. Science 311, 374–377 (2006). [DOI] [PubMed] [Google Scholar]
- Forsberg K. J. et al. Bacterial phylogeny structures soil resistomes across habitats. Nature 509, 612–616 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehrsson E. C. et al. Interconnected microbiomes and resistomes in low-income human habitats. Nature 533, 212–216 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer M. O. Microbiology: barriers to the spread of resistance. Nature 509, 567–568 (2014). [DOI] [PubMed] [Google Scholar]
- Munck C. et al. Limited dissemination of the wastewater treatment plant core resistome. Nat. Commun. 6, 8452 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas G. & Sommer M. O. A. Context matters—the complex interplay between resistome genotypes and resistance phenotypes. Curr. Opin. Microbiol. 15, 577–582 (2012). [DOI] [PubMed] [Google Scholar]
- Forsberg K. J. et al. The shared antibiotic resistome of soil bacteria and human pathogens. Science 337, 1107–1111 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B. & Pop M. ARDB—antibiotic resistance genes database. Nucleic Acids Res. 37, D443–D447 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur A. G. et al. The comprehensive antibiotic resistance database. Antimicrob. Agents Chemother. 57, 3348–3357 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T. et al. antiSMASH 3.0—a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 43, W237–W243 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boc A., Philippe H. & Makarenkov V. Inferring and validating horizontal gene transfer events using bipartition dissimilarity. Syst. Biol. 59, 195–211 (2010). [DOI] [PubMed] [Google Scholar]
- Wattam A. R. et al. PATRIC, the bacterial bioinformatics database and analysis resource. Nucleic Acids Res. 42, D581–D591 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbantoglu O., Way S., Hinrichs S. & Sayood K. RAIphy: phylogenetic classification of metagenomics samples using iterative refinement of relative abundance index profiles. BMC Bioinform. 12, 41 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich W., Betzler M. & Schrempf H. An amplifiable and deletable chloramphenicol-resistance determinant of Streptomyces lividans 1326 encodes a putative transmembrane protein. Mol. Microbiol. 5, 2789–2797 (1991). [DOI] [PubMed] [Google Scholar]
- Johnston C., Martin B., Fichant G., Polard P. & Claverys J.-P. Bacterial transformation: distribution, shared mechanisms and divergent control. Nat. Rev. Microbiol. 12, 181–196 (2014). [DOI] [PubMed] [Google Scholar]
- Chayot R., Montagne B., Mazel D. & Ricchetti M. An end-joining repair mechanism in Escherichia coli. Proc. Natl Acad. Sci. USA 107, 2141–2146 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingues S. et al. Natural transformation facilitates transfer of transposons, integrons and gene cassettes between bacterial species. PLoS Pathogens 8, e1002837 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z. et al. Complete nucleotide sequence analysis of two corynebacterial plasmids pXZ10142 and pXZ10145 containing a transposon-like sequence Tn45. Journal of Fudan University, Natural Science 43, 200–205 (2004). [Google Scholar]
- Tauch A., Zheng Z., Pühler A. & Kalinowski J. Corynebacterium striatum chloramphenicol resistance transposon Tn5564:genetic organization and transposition in Corynebacterium glutamicum. Plasmid 40, 126–139 (1998). [DOI] [PubMed] [Google Scholar]
- Klumper U. et al. Broad host range plasmids can invade an unexpectedly diverse fraction of a soil bacterial community. ISME J. 9, 934–945 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge S. R., Recchia G. D., Stokes H. W. & Hall R. M. Family of class 1 integrons related to In4 from Tn1696. Antimicrob. Agents Chemother. 45, 3014–3020 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli M. et al. Class 1 integrons in environments with different degrees of urbanization. PLoS ONE 7, e39223 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley E. P. & Davies J. Transfer of plasmid RSF1010 by conjugation from Escherichia coli to Streptomyces lividans and Mycobacterium smegmatis. J. Bacteriol. 173, 6705–6708 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. J., Fry J. C., Rogers H. J. & Day M. J. Transformation of Acinetobacter baylyi in non-sterile soil using recombinant plant nuclear DNA. Environ. Biosafety Res. 6, 101–112 (2007). [DOI] [PubMed] [Google Scholar]
- Perry J. & Wright G. The antibiotic resistance ‘mobilome': searching for the link between environment and clinic. Front. Microbiol. 4, 138 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillings M. R. Integrons: past, present, and future. Microbiol. Mol. Biol. Rev. 78, 257–277 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa O. & Dagan T. Trends and barriers to lateral gene transfer in prokaryotes. Curr. Opin. Microbiol. 14, 615–623 (2011). [DOI] [PubMed] [Google Scholar]
- Medema M. H., Takano E. & Breitling R. Detecting sequence homology at the gene cluster level with MultiGeneBlast. Mol. Biol. Evol. 30, 1218–1223 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K. & Standley D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M. N., Dehal P. S. & Arkin A. P. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS ONE 5, e9490 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta-Cepas J., Serra F. & Bork P. ETE 3: reconstruction, analysis, and visualization of phylogenomic data. Mol. Biol. Evol. 33, 1635–1638 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures and Supplementary Table
Streptomyces ARGs and their closest homologs in proteobacteria.
Sequence signature analysis of the Streptomyces ARG-like proteobacterial proteins.
Host information of Cmx and LmrA.
Genomes and plasmids containing DNA sequences that represent the proposed intermediates of cmx "carry back".
Data Availability Statement
The authors declare that all the relevant data are provided in this published article and its Supplementary Information files, or are available from the corresponding authors on request.