Abstract
Purpose
Individuals with hyperimmunoglobulin E Syndrome (HIES) have central nervous system abnormalities, including focal white matter hyperintensities (WMH), or unidentified bright objects. This cross-sectional study aimed to describe the cognitive and emotional functioning and quality of life of people with HIES. We also sought to explore the relationship between cognitive functioning and WMHs in this population.
Methods
Twenty-nine individuals (13 males) with autosomal-dominant HIES (mean age=35.1 years, range 16–55) were administered a comprehensive psychological assessment as part of a natural history protocol. The assessment included measures of global cognitive functioning (Wechsler Adult Intelligence Scale-III), memory (California Verbal Learning Test-II, Wechsler Memory Scale-III), executive skills (Delis Kaplan Executive Function System), and attention (Test of Everyday Attention). Emotional symptoms and quality of life also were assessed.
Results
All mean cognitive scores were within normal limits. Mean scores on memory and executive functioning measures were significantly lower than Full Scale IQ scores (ps<.05). Substantial percentages of patients self-reported executive skills to be in the clinical range. Patients with fewer (1–20) versus more (21+) WMHs scored significantly better on measures of global cognitive skills, visual-perceptual skills, and working memory. Mean scores on emotional symptom and quality of life measures were in the average range and unrelated to WMHs.
Conclusions
Global cognitive functioning was average to high average in our sample of individuals with HIES. However, focal brain lesions were associated with lower scores in specific domains. Emotional functioning and quality of life are within normal limits in this sample.
Keywords: Hyperimmunoglobulin E Syndrome, Job’s Syndrome, cognitive functioning, psychological functioning, quality of life
Autosomal-dominant hyperimmunoglobulin E Syndrome (HIES), or Job’s Syndrome, is a primary immunodeficiency disorder characterized by elevated serum IgE, frequent infections of the skin, lungs, and connective tissue, dental and skeletal abnormalities [1–3], and a characteristic facial appearance. Autosomal-dominant HIES results from mutations in STAT3, a widely expressed signal transduction molecule [4, 5].
Previous work has examined the central nervous system (CNS) imaging abnormalities in patients with HIES. The most distinct abnormality was the age-disproportionate appearance of focal punctate lesions in the white matter, which demonstrate high signal intensity on FLAIR and T2-weighted sequences by Magnetic Resonance Imaging (MRI) in 70 % of the patients [6]. These white matter hyperintensities (WMHs) are non-specific, but the extent and number apparently increase with age. Specific lesions persist over time and are stable in their location. The presence of WMHs in individuals with HIES is described in much younger ages than expected in healthy individuals [6]. Figure 1 shows four sequential FLAIR-weighted images from a 22 year-old patient with AD-HIES. Note the presence of multiple bilateral foci of abnormally increased signal intensity in the frontal and parietal white matter bilaterally, some of which appearing to be subcortical in location.
Fig. 1.
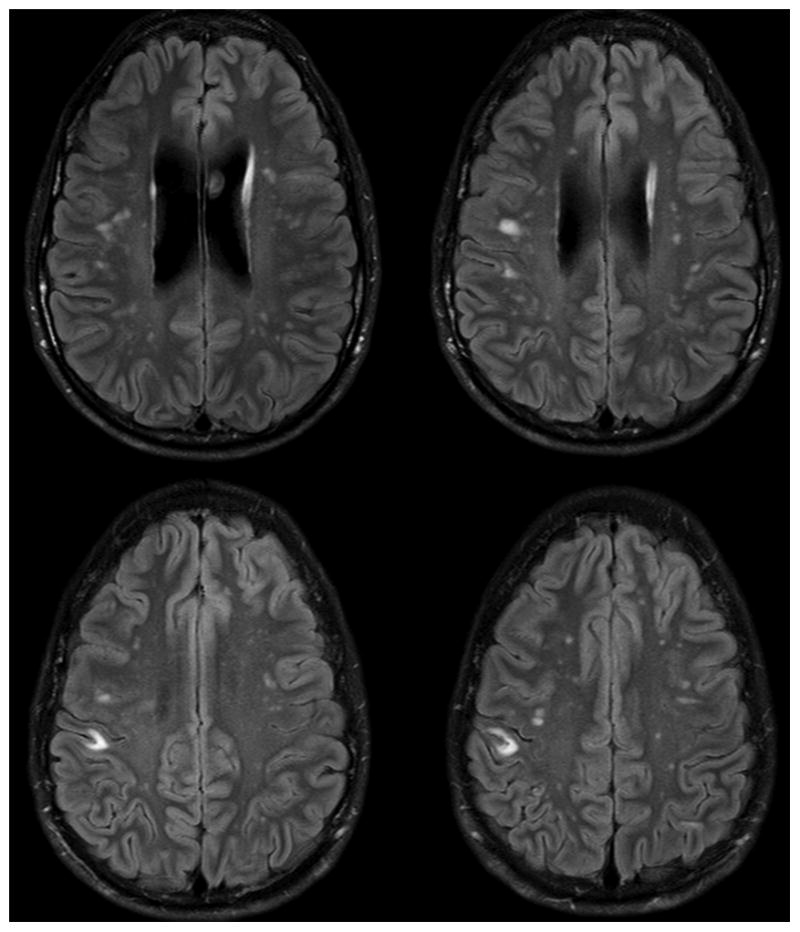
Four sequential FLAIR-weighted images from a 22 year-old patient with AD-HIES
The impact of WMHs on neurobehavioral functioning in HIES patients has not been explored. However, research from other groups has suggested a link between WMHs and specific neurological and cognitive variables. For example, WMHs are frequent incidental findings in the elderly [7]. In this age group, WMHs are associated with increased blood pressure, evidence of silent stroke, smoking, other vascular risk factors, and can be associated with cognitive decline [8, 9]. Individuals with HIES are known to have other vascular abnormalities such as coronary arterial tortuosity and aneurysm [10]. WMHs also have been described in patients with migraines [11], vasculitis [12], infectious diseases such as Lyme disease [13], old trauma (diffuse axonal injury) [14], and neurofibromatosis type 1 (NF1) [15, 16]. White matter changes are also the hallmark of demyelinating diseases such as multiple sclerosis (MS); however, those appear distinct from the typical age-related WMHs, both in shape (oval shaped in MS) and location (corpus callosum, periventricular).
Study Objectives
The two primary objectives of this cross-sectional study are (1) to describe the cognitive abilities, emotional functioning, and quality of life of a group of patients with HIES and (2) to explore the relationship between the aforementioned domains and WMHs in this population. Since no published reports have described these domains in individuals with HIES, our first objective was approached in an exploratory fashion. Regarding our second objective, given the research on WMHs in the other populations described above, we hypothesized that patients with more WMHs would perform lower on cognitive measures than patients with fewer WMHs. Our primary outcome measure was the WAIS-III Full Scale IQ. Secondary measures included tests of memory, executive functioning, attention, emotional symptoms (depression and anxiety), and quality of life.
Methods
Participants
Eligible participants for this study included individuals with HIES who were enrolled on a longitudinal natural history protocol at the National Institutes of Health. The natural history study was approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases (NIAID), and all participants or their guardian provided informed consent. To be deemed eligible to participate in the psychological substudy, patients must have been at least 16 years of age and had to have a previous MRI brain scan showing at least one WMH. In addition, patients with an acute infection or who were recently prescribed a medication that might influence cognitive functioning (e.g., a narcotic for pain management) were ineligible.
The HIES cohort from which we were able to recruit included 44 patients. Eleven of these patients were excluded due to prior CNS injury (e.g., cerebrovascular accident or infection), medications that we thought would interfere with performance, additional neurologic diagnosis (n =1), or inability to schedule the studies. Three of the 33 remaining individuals were excluded due to absence of WMH. Therefore, 91 % of the evaluable cohort had WMH present; this is somewhat higher than a prior report [6] that cited approximately 70 % of individuals, although that report included children, who likely have a lower incidence. In sum, thirty patients met eligibility criteria and were approached to participate in the psychological evaluation between 2006 and 2012. One of these patients declined the psychological evaluation, stating that he was too tired.
Measures
Background Information
Participants completed a questionnaire asking about demographic information as well as educational and psychiatric history. Specifically, they were asked about the number of years of formal education they had completed and any psychiatric or cognitive disorders with which they had been formally diagnosed.
Global Cognitive Functioning
The Wechsler Adult Intelligence Scale, Third Edition (WAIS-III) [17] is a test designed to assess the intellectual ability of adults aged 16 through 89 years. It is comprised of 14 subtests, which combine to yield composite scores including the Full Scale IQ (FSIQ), Verbal IQ (VIQ), Performance IQ (PIQ), Verbal Comprehension Index (VCI), Perceptual Organizational Index (POI), Working Memory Index (WMI), and Processing Speed Index (PSI). Each composite score is based on a mean of 100 and a standard deviation of 15. Scores between 90 and 109 are considered to be in the Average range. Subtest scores on the WAIS-III are based on a mean of 10 and a standard deviation of 3. For this study, patients were administered 11 of the 14 subtests: Vocabulary, Similarities, Information, Picture Completion, Block Design, Matrix Reasoning, Arithmetic, Digit Span, Letter-Number Sequencing, Coding and Symbol Search. In addition to the Digit Span total score, standard scores representing Digits Forward and Digits Backward were calculated based on span length [17]. Because the Picture Arrangement and Comprehension subtests were not administered, the VIQ and PIQ were pro-rated in accordance with instructions in the WAIS-III manual. The WAIS-III has been widely used and is considered reliable and valid [17, 18].
Memory
The California Verbal Learning Test, Second edition (CVLT-II) [19] is a widely-used measure of verbal learning and memory for individuals ages 16 to 89 years. Examinees are read a list of words and are asked to recall them across a series of trials. The CVLT-II produces scores representing overall learning (Trials 1–5), short delay free recall (SDFR), and long delay free recall (LDFR), among others. The reliability and validity of this measure are strong [20, 21].
The Logical Memory subtests I and II were administered from the Wechsler Memory Scale, Third edition (WMS-III) [22]. On these tasks, participants are required to retell two short stories immediately after they are read aloud by the examiner (Logical Memory I) and again after a 20-min delay (Logical Memory II). The WMS-III and the Logical Memory subtest in particular are reliable and valid [23].
Executive Functioning
The Delis-Kaplan Executive Function System (D-KEFS) [24] consists of nine subtests that reliably measure ability to plan and organize thoughts and behaviors and to shift between tasks. The subtests include reliable measures of both verbal and nonverbal executive function tasks for individuals ages 8 to 89 years [25]. The Verbal Fluency subtest’s Category Switching task and the Trail Making sub-test’s Number-Letter Switching task were chosen for this study, as they evaluate verbal and nonverbal executive domains, respectively.
The Behavior Rating Inventory of Executive Function—Adult Version (BRIEF-A) [26] is a self-report questionnaire comprised of 75 items that measure various aspects of everyday executive functioning. Adults ages 18 and older respond based on how often the items were a problem for them in the past month, with choices of Never, Sometimes, and Often. T-scores of ≥65 are considered “clinically significant” and indicative of a problem area. The BRIEF-A is considered a reliable and valid measure [26, 27]. Three adolescent participants were ineligible for this questionnaire due to the lower age limit of 18 years.
Attention
The Test of Everyday Attention (TEA)[28] consists of five subtests that measure various components of attention for adults 18 to 80 years of age. The Elevator Counting subtest administered in this study is a measure of sustained auditory attention that requires the examinee to listen to seven series of tones and state how many tones were heard in each series. A raw score of seven out of seven indicates normal attention, six out of seven indicates a possible attention problem, and less than six correct suggests an attention problem. The TEA’s reliability and validity are acceptable [29]. Three participants were too young for this test.
Emotional Functioning
The Hospital Anxiety and Depression Scale [30] is a self-report screen used to detect symptoms of depression and anxiety. The scale consists of 14 items that are scored on a four-point scale from 0 to 3, with higher scores representing greater symptomology. Item responses are added together to produce scores on the Depression and Anxiety subscales, which are categorized as “normal” (0–7), “mild” (8–10), “moderate” (11–14), or “severe” (15–21). This measure has been found to be reliable and valid in numerous studies with various populations ages 16 to 65 years [31].
Quality of Life
The Functional Assessment of Cancer Therapy—General [32] is a quality of life instrument intended for use with a variety of chronic illness populations. It is a 27-item self-report measure with responses given on a 5-point Likert scale ranging from 0 (not at all) to 4 (very much). Raw scores are transformed to T-scores (which have a mean of 50 and standard deviation of 10). Responses produce scores on sub-scales assessing aspects of physical, social/family, emotional, and functional well-being, and a Total score thought to represent overall quality of life. Higher scores indicate better quality of life; given the mean of 50 for the T-scores, scores below 40 can be considered to represent poor quality of life. Reliability and validity have been established [32, 33].
Neuroimaging Variables
Brain MRI imaging was obtained as part of the natural history protocol. MRIs were performed on either 1.5 or 3 Tesla magnets. Imaging included the following sequences: 3D sagittal T1, axial T1, axial T2, axial Proton density and axial FLAIR weighted images. Two authors (DLH and AFF) reviewed the MRI images obtained closest in time to the neuropsychological evaluation as long as the scan was obtained within one year of the neuropsychological testing. One reviewer was blinded to the clinical and neuropsychological status of the patients and the other reviewer was blinded to the neuropsychological test results but was familiar with the patients’ general clinical status. The number of WMHs were counted and grouped from 0–5, 5–10, 10–15, 15–20, 25–30, and >30 lesions, and the location of the lesions was recorded. The two reviewers obtained 100 % agreement when categorizing the scans. One patient could not be scanned due to an intracranial aneurysm clip placed following a subarachnoid hemorrhage, and two patients did not have readable scans close enough to the test date. Thus, valid neuroimaging data were obtained for 26 of the 29 patients in the total sample.
Statistical Analyses
For consistency across tests, all cognitive scores were converted to standard scores with a mean of 100 and standard deviation of 10. Pearson correlations or analyses of variance were used to assess the relationships between demographic variables with cognitive and neuroimaging variables. Analyses of covariance (ANCOVAs) were run to assess the relationship between cognitive and neuroimaging (WMH) variables, with gender effects held constant. To explore the relationship between WMHs and cognitive functioning, patients were grouped according to whether they had fewer (1 to 20; n =13) or more (21+; n =13) WMHs, since there was a natural split in the data at this point. Independent t-tests were used to compare the Low WMH and High WMH groups on cognitive variables. On two variables that showed a non-normal distribution, non-parametric statistics (Kruskal-Wallis) were computed. Follow-up exploratory analyses were conducted to examine the relationship of memory and executive skills with global cognitive functioning. Specifically, t-tests were performed on the differences between the memory scores from the CVLT-II, WMS-III, and DKEFS and the Full Scale IQ scores. Alpha was set at .05 due to the exploratory nature of the study.
Procedures
The psychological evaluation was part of the natural history protocol, and so consent for testing was obtained when the patients enrolled on that study. Verbal assent for the psychological assessment also was obtained from all 29 participants. The assessment battery typically took about 3 to 3.5 h and was administered during one session in an outpatient clinic at the National Institutes of Health Clinical Center. Patients were not tested if febrile, and were monitored for fatigue and given breaks if necessary.
Results
Demographic Characteristics
Demographic data is presented in Table I. Our complete sample ranged in age from 16 to 55 years (mean age=35.1 years± 10.9). Most patients were Caucasian (83 %, n =24), had completed some post-high school education (mean=15.4 years), and were working full-time (59 %, n =16).
Table I.
Demographic characteristics of the total sample (N =29)
| n | % | |
|---|---|---|
| Gender | ||
| Male | 13 | 45 |
| Female | 16 | 55 |
| Race | ||
| Caucasian | 24 | 83 |
| Hispanic | 2 | 7 |
| African-American | 1 | 3 |
| Asian | 1 | 3 |
| Biracial | 1 | 3 |
| Employment status | ||
| Working full-time | 16 | 59 |
| Working part-time | 5 | 19 |
| Unemployed | 5 | 19 |
| Past or current diagnosis | ||
| ADHD | 2 | 7 |
| Learning disability | 1 | 3 |
Cognitive Functioning for Entire Sample
Patients were asked if they had ever been diagnosed with a cognitive disorder. Two patients (7 %) had been diagnosed with Attention Deficit Hyperactivity Disorder (ADHD), and one patient (3 %) reported having been diagnosed with a learning disability. As shown in Table II, global cognitive functioning on the WAIS-III was in the High Average range (mean FSIQ=111.0±15.0). Verbal skills were at the high end of the Average range (mean VIQ=108.6±14.4) and nonverbal, visual-spatial skills were high average (mean PIQ=112.0±16.0). Scores on the WAIS-III indices were similar, with the VCI and POI in the High Average range and the WMI and PSI in the Average range. Among subtests, most of the mean scores fell within the Average range. The mean scores on the Vocabulary subtest and the Matrix Reasoning subtest were on the border between the Average and Above Average ranges. None of the mean scores were below average.
Table II.
Mean WAIS-III Composite and subtest scores for the total sample and Low and High WMH groups
| Scale | Total sample
|
Low WMHs | High WMHs | ||
|---|---|---|---|---|---|
| Mean (SD) | Median | Range | Mean (SD) | Mean (SD) | |
| Composite | |||||
| Verbal comprehension index | 111.9 (14.6) | 112.0 | 78–134 | 115.4 (12.8) | 105.2 (14.7) |
| Perceptual organization index* | 112.3 (12.6) | 109.0 | 93–133 | 117.9 (13.0) | 104.6 (5.8) |
| Working memory index | 103.8 (11.6) | 103.0 | 84–126 | 103.5 (12.2) | 100.6 (9.6) |
| Processing speed index | 103.1 (13.7) | 103.0 | 79–140 | 105.5 (16.3) | 99.7 (11.2) |
| Subtest | |||||
| Vocabulary | 114.7 (12.2) | 110.0 | 95–135 | 116.5 (12.0) | 109.6 (10.9) |
| Similarities | 109.8 (15.9) | 105.0 | 70–140 | 114.2 (13.4) | 102.7 (16.1) |
| Information | 107.4 (14.7) | 110.0 | 75–135 | 110.8 (12.7) | 101.5 (16.0) |
| Picture completion* | 109.5 (15.8) | 110.0 | 80–140 | 116.5 (17.5) | 100.0 (10.0) |
| Block design | 106.6 (14.1) | 110.0 | 75–130 | 110.8 (13.7) | 101.2 (12.4) |
| Matrix reasoning | 114.7 (12.2) | 115.0 | 80–140 | 115.8 (13.0) | 112.7 (9.9) |
| Arithmetic | 101.0 (12.8) | 100.0 | 75–140 | 102.7 (15.4) | 98.8 (11.0) |
| Digit span total | 103.3 (12.4) | 105.0 | 80–130 | 103.1 (10.9) | 99.2 (11.0) |
| Digits forward | 107.2 (19.1) | 104.4 | 81–127 | 110.2 (24.2) | 100.5 (11.7) |
| Digits backward* | 104.8 (14.8) | 101.4 | 81–143 | 108.1 (13.6) | 97.5 (10.6) |
| Letter-number sequencing | 106.3 (12.7) | 105.0 | 80–135 | 103.5 (13.4) | 105.8 (11.4) |
| Coding | 101.2 (15.1) | 100.0 | 75–140 | 102.3 (15.2) | 97.7 (16.0) |
| Symbol search | 104.8 (12.6) | 100.0 | 80–130 | 103.1 (12.8) | 106.3 (12.7) |
p <.05
WMH white matter hyperintensities
Table III presents results of the memory and executive function measures. All mean scores on the CVLT-II and WMS-III were within the Average range. Mean scores on the DKEFS Verbal Fluency Category Switching and Trail Making Switching tasks also were within normal limits. On the TEA Elevator Counting task, 81 % of patients responded correctly on 7 out of 7 items and were considered “within normal limits.” The remaining 19 % obtained a raw score of 6 out of 7, falling in the “possibly abnormal” range.
Table III.
Mean standard scores on the memory and executive function measures
| Mean (SD) | Median | Range | |
|---|---|---|---|
| CVLT-II | |||
| Trials 1–5 total | 103.1 (15.9) | 104.5 | 73–137 |
| Short delay free recall | 98.7 (14.6) | 100.0 | 77.5–122.5 |
| Long delay free recall | 101.0 (13.8) | 100.0 | 77.5–122.5 |
| WMS-III | |||
| Logical memory I | 102.7 (13.1) | 105.0 | 65–130 |
| Logical memory II | 104.3 (14.9) | 105.0 | 75–135 |
| DKEFS | |||
| Verbal fluency category switching | 100.2 (19.2) | 100.0 | 55–145 |
| Trailmaking switching | 102.2 (11.6) | 105.0 | 75–120 |
CVLT-II California verbal learning test, second edition; WMS-III Wechsler memory scale, third edition; DKEFS Delis-Kaplan executive function system
All scores on the BRIEF-A self-report questionnaire were within normal limits. However, the percentage of patients who obtained clinically significant scores on some of the subscales was substantial (see Table IV). For example, 27 % and 23 % of patients scored in the clinical range on the Working Memory and Initiate subscales, respectively.
Table IV.
Mean BRIEF-AT-scores and percent of patients with clinically elevated scores
| Subscale | Mean | SD | % Clinically elevated |
|---|---|---|---|
| Inhibit | 50.9 | 11.3 | 12 |
| Shift | 52.6 | 8.1 | 8 |
| Emotional control | 48.1 | 8.0 | 8 |
| Self-monitor | 48.5 | 11.2 | 8 |
| Initiate | 56.5 | 12.2 | 23 |
| Working memory | 56.7 | 12.6 | 27 |
| Plan/organize | 54.4 | 11.6 | 15 |
| Task monitor | 53.8 | 10.5 | 15 |
| Organization of materials | 52.9 | 10.9 | 19 |
| BRI | 49.7 | 9.3 | 8 |
| MI | 56.3 | 13.1 | 19 |
| GEC | 53.3 | 11.0 | 12 |
BRIEF-A Behavior rating inventory of executive function—adult. BRI Behavioral regulation index; MI Metacognition index; GEC Global executive composite. T-scores ≥65 suggest a “clinically significant” problem
No significant correlations emerged between age or years of education and any of the cognitive measures. However, there were several gender differences that were significant at the .05 level. Males scored higher than females on the WAIS-III Vocabulary subtest, a measure of verbal expression (males=119.6 ±11.8, females=110.6±11.4). Females scored significantly higher than males on the WAIS-III Coding subtest, a measure of nonverbal processing speed (females=106.6±14.8, males= 94.6±13.1), and on the DKEFS Verbal Fluency Category Switching task (females=107.2±18.3, males=90.8±16.8).
Follow-up Analyses: Relationship Between Global IQ and Specific Cognitive Domains
Because mean scores on supplemental cognitive tests were lower than what would be expected based on the mean IQ scores, we conducted follow-up analyses to explore this finding further. Results indicated that the Full Scale IQ scores were significantly higher than the WMS-III Logical Memory I (t = 2.9, p <.01) and II (t =2.06, p <.05) scores, as well as the CVLT-II Trials 1–5 (t =2.2, p <.05), short-delay free recall (t =3.4, p <.01) and long-delay free recall (t =2.6, p <.05) scores. Full Scale IQ scores also were significantly higher than the DKEFS Verbal Fluency Category Switching score (t =3.09, p <.01) and the Trail Making Switching score (t =3.54, p >.01).
Neuroimaging Data
Of the 26 participants with usable scan data, the number of WMHs ranged from 1 to >30; more specifically, 50 % (n =13) of these patients had WMHs ranging from 1 to 17, and 50 % had WMHs ranging from 27 to 30+. Number of WMHs was not systematically related to patient gender or age. However, the mean number of years of education was significantly higher in the Low WMH (1–20) group than the High (>20) WMH group (16.7 years versus 14.7 years). Number of WMHs was not related to employment status. The field strength of MRI (1.5 vs 3.0 Tesla) was unrelated to the number of WMHs found (p >.05).
Scans for all 26 patients showed WMHs present in frontal white matter. Twenty-one (81 %) had WMHs evident in the white matter of the parietal region, while four scans (15 %) showed WMHs in temporal white matter. Much less frequently, WMHs were present in parietal (8 %) and temporal (4 %) gray matter, and in the pons (4 %). Most patients (81 %) had WMHs in more than one location. Because of the heterogeneous distribution of patients with WMHs in various brain regions, it was not possible to examine statistically the relationship between cognitive functioning and WMH location.
Cognitive Functioning in Patients with High versus Low WMHs
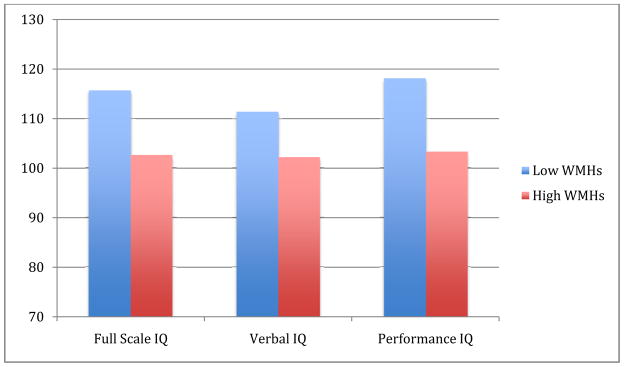
When patients in the Low WMH and High WMH groups were compared, statistically significant differences emerged on several of the cognitive scores (see Fig. 2 and Table II). First, the Low WMH group achieved higher Full Scale (115.8 versus 102.6) and Performance (118.2 versus 103.4) IQ scores than the High WMH group (ps<.05). Within WAIS-III sub-tests, the Low WMH group scored significantly higher on the Picture Completion subtest (116.5 versus 100.0) and the Digits Backward task (108.1 versus 97.5; ps<.05). Most other WAIS-III mean scores were higher in the group with fewer WMHs but not at a statistically significant level. Due to the significant gender differences described earlier, analyses of covariance were run. Holding gender constant, the relationships between WMH group and the above WAIS-III variables remained significant (ps<.05). None of the scores on the other cognitive measures were significantly different across the two WMH groups (ps>.05; results not shown).
Fig. 2.
Full scale, verbal, and performance IQ scores in the low and high WMH groups
Due to the relatively small samples in the two WMH groups, the primary variables of interest were examined to determine whether they followed a normal distribution. For two variables that were non-normal (WAIS-III Arithmetic and Matrix Reasoning subtests), the Kruskal-Wallis non-parametric statistic was calculated. In both cases, the obtained value did not exceed the critical value and results were therefore consistent with those from the t-tests presented above.
Emotional Functioning/Psychiatric Disorders
Nine participants (31 %) reported having been diagnosed with at least one psychiatric disorder. Depression was the most common diagnosis cited (17 %), followed by anxiety (14 %), Attention Deficit Hyperactivity Disorder (ADHD; 7 %), and Obsessive-Compulsive Disorder (3 %). Six patients (21 %) reported taking a psychiatric medication at the time of the evaluation; five were taking a medication for depression and one for comorbid depression and anxiety. On the HADS, the mean score on the Depression subscale was 3.2 (± 3.2), and the mean score on the Anxiety subscale was 5.5 (± 2.2), both of which fall in the “normal” range. On the Depression subscale, 90 % of patients scored within normal limits, while 3 % scored in the “Mild” range and 7 % scored in the “Moderate” range of symptomatology; no patients scored in the “Severe” range. On the Anxiety subscale, 83 % of patients scored within normal limits, and the remaining 17 % scored in the “Mild” range.
Quality of Life
The mean Total T-score on the FACT-G quality of life measure was 53.2 (± 10.0 SD), and individual T-scores ranged from 34 to 83. Mean T-scores were within one standard deviation of the mean for the Physical Well Being (44.6±13.2), Social Well Being (51.1±10.6), Emotional Well Being (47.2± 10.0), and Functional Well Being (49.7±11.0) subscales. Despite these mean scores in the average range, more than 20 % of the participants’ T-scores fell more than one standard deviation below the mean (suggesting poor quality of life) on the Physical Well Being (32 %) and Emotional Well Being sub-scales. Percentages who scored one standard deviation or more below the mean on the Total Scale and the Social and Functional Well Being subscales were 12, 12, and 16, respectively. The relatively small number of patients in some of the cells prevented further analyses of these subgroups (that is, those patients scoring outside versus within normal limits).
Emotional Functioning and Quality of Life in Patients with High versus Low WMHs
On the HADS, the Depression and Anxiety scores were unrelated to WMH group classification. Likewise, neither the FACT-G total score nor the subscale scores were related to WMH group. Thus, WMHs do not seem to be a factor in the emotional functioning or quality of life of individuals with HIES.
Discussion
HIES is a multi-system disorder reflecting the widespread expression and functions of STAT3. Although CNS imaging findings of HIES patients have been studied previously, cognitive functioning and its relationship with WMHs have not. Further, gross neurologic deficits have not been noted despite the frequent presence of WMHs, but the question of whether more subtle impairments may exist has not been investigated. As the vast majority of adults with HIES have WMHs, we compared the cognitive functioning of those with HIES and WMHs to normative data and also examined the aforementioned domains in relation to the number of WMHs.
Overall, the cognitive functioning of the patients with HIES in our study was in the average to high average range. Most patients scored within normal limits on global measures of cognitive ability, verbal memory and learning, working memory, executive functioning, and attention. However, percentages of patients who scored in the clinically significant problem range on the executive functioning questionnaire (BRIEF-A) were substantial. For example, compared to the 27 % and 23 % of our participants who scored in the clinical range on the Working Memory and Initiate scales, respectively, none of the “normal control” sample scored in the clinical range on these subscales according to the BRIEF-A manual [34].
When examining functioning across subgroups based on the number of WMHs (0–20 versus 21+), several important differences emerged. Patients with fewer WMHs performed significantly better on scores of global cognitive ability and overall visual-spatial skills (Full Scale IQ and Performance IQ, respectively) as well as specific cognitive tasks (Picture Completion and Digits Backward). The Picture Completion subtest is an indicator of visual perceptual skills, while Digits Backward measures working memory, a key component of executive functioning. Also, quite a few of the other subtests showed the same trend, with patients in the Low WMH group scoring higher than those in the High WMH group. It may be that with a larger sample, more of these differences would have reached statistical significance.
WMHs are not fully understood. They can be detected incidentally in healthy individuals, and prevalence increases with age [9]. The pathology associated with incidental WMHs appears to reflect non-specific reduced myelination with atrophy of the neuropil around fibrohyalinotic arteries as well as different stages of perivenous damage, suggesting minor perivascular damage rather than frank infarction [35]. Depending on the associated disease entity, the pathology of WMHs probably differs; however, there is a lack of post mortem evaluation of the associated histopathologies.
WMHs have been linked to cognitive dysfunction in a variety of populations. For example, a recent systematic review and meta-analysis of WMHs in the elderly suggested an association of lesions with a faster decline in global cognitive performance, executive function, and processing speed [36]. Similarly, in individuals with neurofibromatosis type 1 (NF1), data suggest that WMHs are associated with impairments in global cognitive functioning and visual-spatial skills [37, 38]. These findings are concordant with the relationships detected in our patients, whereby scores of global cognitive ability, overall visual-spatial skills, and specific cognitive tasks were associated with increasing number of WMHs. While the location of WMHs in patients with NF1 has been found to relate to specific cognitive deficits [15, 39], the variation in locations of WMHs in our sample of patients with HIES prevented us from being able to investigate this factor. With more data this question could be addressed with future research.
Another notable observation found in our sample was that, despite their average to high average cognitive abilities, only 59 % of our patients were employed full-time and nearly a quarter was unemployed. In our sample, employment status was unrelated to WMH group. The lack of full-time employment could be from a variety of issues, such as frequent or chronic skin and/or pulmonary infections, or chronic pain from the musculoskeletal defects. One study of Danish patients with eczema and other atopic dermatitis disorders found that nearly 6 days of work were missed over a 6-month period due to eczema and that over a third of respondents chose not to pursue education or work due to dermatological disease [40]. There is a need for more research on the frequency of HIES patients who are unemployed or disabled and contributory factors.
The percentage of individuals in our sample with a psychiatric diagnosis according to patient report was 31 %. National Institute of Mental Health statistics cite a 26 % 12-month prevalence rate of a psychiatric diagnosis for adults in the U.S. population [41]. Our results are just above the national average. Studies of patients with chronic itch-related skin disorders have reported elevated levels of somatization, obsessive-compulsive behavior, depression, anxiety, hostility and sleeping disorders [42] as well as an increased likelihood of schizophrenic and affective disorders [43]. In our study, mean scores on the Depression and Anxiety subscales of the HADS were within the “normal” range and similar to mean scores reported in healthy control groups from other studies [44, 45]. No patients scored in the “severe” range on either subscale. A recent study of patients with inflammatory bowel disease reported that 11 % and 41 % scored in the severe range on the HADS Depression and Anxiety subscales, respectively [46], suggesting that our sample of individuals with HIES are faring better with respect to emotional well-being than at least some other chronic illness groups.
With respect to quality of life, our sample had results well within normal limits on the FACT-G Total score and all of the domains. Cella and colleagues [47] provided normative data from the general U.S. adult population on the FACT-G. Mean raw scores are similar to those obtained among our sample on all domains and the total score. For example, the mean Total raw score in our patient group was 83.4±13.1, compared to 80.1±18.1 in the general population. This suggests that quality of life among individuals with HIES is comparable to typical adults, but this observation is speculative since our study lacked a healthy comparison group. However, it also should be noted that nearly one third of our sample (32 %) scored lower than one standard deviation of the mean (suggesting worse quality of life) on the Physical Well Being scale, and 12 to 20 % scored outside the normal range on the other scales. Thus, subgroups of patients with HIES seem to be experiencing disturbances in one or more aspects of their quality of life. Further, our results tentatively suggest that WMHs do not have a detrimental impact on emotional functioning or quality of life in individuals with HIES. However, additional exploration of psychological functioning in the HIES population is needed, as our measure (the HADS) was meant to screen for psychiatric symptoms rather than serve as an extensive diagnostic tool.
Limitations of the current study include a small sample size and lack of longitudinal neuropsychological testing in relationship to the baseline number of lesions and progression of lesions over time. Nonetheless, our data provide an initial picture of the cognitive profiles of adults with HIES and the relationship between their cognitive functioning and WMHs. It is recommended that future research examine the cognitive and psychiatric status of youth with HIES, as well as the impact of the unique features of this disease, including recurrent infections and characteristic facial features. Also, we chose to set our alpha level at .05 due to the exploratory nature of the study. Given the number of analyses, the results should be interpreted with this caution in mind. Finally, our study would have benefitted from a comparison group of healthy individuals or another patient group with WMHs. The pathophysiology underlying WMHs in our patient population remains elusive and could reflect some type of vasculopathy, in view of the known vasculopathic changes identified in larger vessels such as the coronaries and the higher incidence of hypertension [10, 48]. The exact pathophysiology could be further evaluated in postmortem brain MR imaging in correlation with detailed histopathology.
Conclusions
This study offers an initial overview of the cognitive functioning of individuals with HIES and WMHs. Our results suggest that, while cognitive domains are generally intact for most patients, focal brain lesions may be a risk factor for relative weakness in specific areas, such as visual-spatial skills and working memory. It may be worthwhile to monitor individuals with HIES for the presence and number of WMHs beginning in adolescence. Those individuals with more WMHs could be targeted early for cognitive interventions aimed at teaching compensatory strategies. These adolescents also may be advised to obtain a neurocognitive evaluation and seek vocational or career counseling based on their pattern of strengths and weaknesses. However, the full impact of the WMHs and associated lower cognitive scores is unclear. Since WMHs are unrelated to employment status, and the lower cognitive scores in the High WMH group still lie in the Average range, it is difficult to discern the clinical significance of these factors on the daily functioning of individuals with HIES.
Future studies using longitudinal designs, with more patients, and with comparison groups including those without WMHs, are needed to answer remaining questions about the relationship between cognitive functioning and WMHs, and to explore underlying neurological mechanisms that may play a role. Knowing more about the impact of WMHs on neurocognitive outcomes may lead the way toward preventive and rehabilitative cognitive interventions.
Acknowledgments
The authors wish to extend their appreciation to the individuals with HIES who participated in this research. We also thank Brittany Abel, M.A. for her assistance. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, by the National Institute of Allergy and Infectious Diseases, and by federal contract HHSN261200477004C. In addition, this project was funded in whole or in part with federal funds from the Frederick National Laboratory for Cancer Research and National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Contributor Information
Staci Martin, Pediatric Oncology Branch, National Cancer Institute, Bethesda, MD 20892, USA. 9030 Old Georgetown Road, #107, Bethesda, MD 20892-8200, USA.
Pamela Wolters, Pediatric Oncology Branch, National Cancer Institute, Bethesda, MD 20892, USA.
Nia Billings, Clinical Research Directorate/CMRP, SAIC-Frederick, Inc, Frederick National Laboratory for Cancer Research, Frederick, MD 21702, USA.
Mary Anne Toledo-Tamula, Clinical Research Directorate/CMRP, SAIC-Frederick, Inc, Frederick National Laboratory for Cancer Research, Frederick, MD 21702, USA.
Dima A. Hammoud, Center for Infectious Disease Imaging, Radiology and Imaging sciences, Clinical Center, National Institutes of Health, Bethesda, MD 20892, USA
Pamela Welch, Clinical Research Directorate/CMRP, SAIC-Frederick, Inc, Frederick National Laboratory for Cancer Research, Frederick, MD 21702, USA.
Dirk Darnell, Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, Bethesda, MD 20892, USA.
Steven M. Holland, Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, Bethesda, MD 20892, USA
Alexandra F. Freeman, Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, Bethesda, MD 20892, USA
References
- 1.Zhang Q, Su HC. Hyperimmunoglobulin E syndromes in pediatrics. Curr Opin Pediatr. 2011;23(6):653–8. doi: 10.1097/MOP.0b013e32834c7f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman AF, Holland SM. The hyper-IgE syndromes. Immunol Allergy Clin N Am. 2008;28(2):277–91. viii. doi: 10.1016/j.iac.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sowerwine KJ, Holland SM, Freeman AF. Hyper-IgE syndrome update. Ann N Y Acad Sci. 2012;1250:25–32. doi: 10.1111/j.1749-6632.2011.06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448(7157):1058–62. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 5.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, et al. STAT3 mutations in the hyper-IgE syndrome. New Engl J Med. 2007;357(16):1608–19. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 6.Freeman AF, Collura-Burke CJ, Patronas NJ, Ilcus LS, Darnell D, Davis J, et al. Brain abnormalities in patients with hyperimmunoglobulin E syndrome. Pediatrics. 2007;119(5):e1121–5. doi: 10.1542/peds.2006-2649. [DOI] [PubMed] [Google Scholar]
- 7.de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam scan study. J Neurol Neurosurg Psychiatry. 2001;70(1):9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longstreth WT, Jr, Arnold AM, Beauchamp NJ, Jr, Manolio TA, Lefkowitz D, Jungreis C, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the cardiovascular health study. Stroke. 2005;36(1):56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 9.Ovbiagele B, Saver JL. Cerebral white matter hyperintensities on MRI: current concepts and therapeutic implications. Cerebrovasc Dis. 2006;22(2–3):83–90. doi: 10.1159/000093235. [Rev] [DOI] [PubMed] [Google Scholar]
- 10.Gharib AM, Pettigrew RI, Elagha A, Hsu A, Welch P, Holland SM, et al. Coronary abnormalities in hyper-IgE recurrent infection syndrome: depiction at coronary MDCT angiography. Am J Roentgenol. 2009;193(6):W478–81. doi: 10.2214/AJR.09.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurth T, Mohamed S, Maillard P, Zhu YC, Chabriat H, Mazoyer B, et al. Headache, migraine, and structural brain lesions and function: population based epidemiology of vascular ageing-MRI study. BMJ. 2011;342:c7357. doi: 10.1136/bmj.c7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tzarouchi LC, Tsifetaki N, Konitsiotis S, Zikou A, Astrakas L, Drosos A, et al. CNS involvement in primary Sjogren syndrome: assessment of gray and white matter changes with MRI and voxel-based morphometry. AJR Am J Roentgenol. 2011;197(5):1207–12. doi: 10.2214/AJR.10.5984. [DOI] [PubMed] [Google Scholar]
- 13.Morgen K, Martin R, Stone RD, Grafman J, Kadom N, McFarland HF, et al. FLAIR and magnetization transfer imaging of patients with post-treatment Lyme disease syndrome. Neurology. 2001;57(11):1980–5. doi: 10.1212/wnl.57.11.1980. [DOI] [PubMed] [Google Scholar]
- 14.Marquez dela Plata C, Ardelean A, Koovakkattu D, Srinivasan P, Miller A, Phuong V, et al. Magnetic resonance imaging of diffuse axonal injury: quantitative assessment of white matter lesion volume. J Neurotrauma. 2007;24(4):591–8. doi: 10.1089/neu.2006.0214. [DOI] [PubMed] [Google Scholar]
- 15.Moore BD, Slopis JM, Schomer D, Jackson EF, Levy BM. Neurol. 1996;46(6):1660–8. doi: 10.1212/wnl.46.6.1660. [Res Support Non-US Gov’t] [DOI] [PubMed] [Google Scholar]
- 16.North K. Neurofibromatosis type 1. Am J Med Genet. 2000;97(2):119–27. doi: 10.1002/1096-8628(200022)97:2<119::aid-ajmg3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Wechsler D. Wechsler adult intelligence scale. 3. San Antonio: Psychological Corporation; 1997. [Google Scholar]
- 18.Arnau RC, Thompson B. Second-order confirmatory factor analysis of the WAIS-III. Wechsler adult intelligence scale. Assessment. 2000;7(3):237–46. doi: 10.1177/107319110000700304. [DOI] [PubMed] [Google Scholar]
- 19.Delis DC, Kramer JH, Kaplan E, Ober BA. CVLT-II California verbal learning test second edition adult version manual. San Antonio: The Psychological Corporation; 2000. [Google Scholar]
- 20.Donders J. A confirmatory factor analysis of the California Verbal Learning Test–Second Edition (CVLT-II) in the standardization sample. Assessment. 2008;15(2):123–31. doi: 10.1177/1073191107310926. [DOI] [PubMed] [Google Scholar]
- 21.Woods SP, Delis DC, Scott JC, Kramer JH, Holdnack JA. The California verbal learning test–second edition: test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Arch Clin Neuropsychol. 2006;21(5):413–20. doi: 10.1016/j.acn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler memory scale. 3. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 23.Lo AH, Humphreys M, Byrne GJ, Pachana NA. Test-retest reliability and practice effects of the Wechsler memory scale-III. J Neuropsychol. 2012;6(2):212–31. doi: 10.1111/j.1748-6653.2011.02023.x. [DOI] [PubMed] [Google Scholar]
- 24.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system examiner’s manual. San Antonio: The Psychological Corporation; 2001. [Google Scholar]
- 25.Mitchell M, Miller LS. Prediction of functional status in older adults: the ecological validity of four Delis-Kaplan executive function system tests. J Clin Exp Neuropsychol. 2008;30(6):683–90. doi: 10.1080/13803390701679893. [DOI] [PubMed] [Google Scholar]
- 26.Roth RM, Isquith PK, Gioia GA, Widows M. Development of the behavior rating inventory of executive function-adult version. Arch Clin Neuropsychol. 2005;20(7):906. [Meeting Abstract] [Google Scholar]
- 27.Malloy P, Grace J. A review of rating scales for measuring behavior change due to frontal systems damage. Cogn Behav Neurol. 2005;18(1):18–27. doi: 10.1097/01.wnn.0000152232.47901.88. [DOI] [PubMed] [Google Scholar]
- 28.Robertson IH, Ward T, Ridgeway V. Test of everyday attention-preliminary norms, reliablity, and validity. J Clin Exp Neuropsychol. 1993;15(3):413. [Meet Abstr] [Google Scholar]
- 29.Chan RC. Attentional deficits in patients with closed head injury: a further study to the discriminative validity of the test of everyday attention. Brain Inj. 2000;14(3):227–36. doi: 10.1080/026990500120709. [DOI] [PubMed] [Google Scholar]
- 30.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 31.Dagnan D, Chadwick P, Trower P. Psychometric properties of the hospital anxiety and depression scale with a population of members of a depression self-help group. Br J Med Psychol. 2000;73(Pt 1):129–37. doi: 10.1348/000711200160255. [DOI] [PubMed] [Google Scholar]
- 32.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The functional assessment of cancer-therapy scale-development and validation of the general use. J Clin Oncol. 1993;11(3):570–9. doi: 10.1200/JCO.1993.11.3.570. [Artic] [DOI] [PubMed] [Google Scholar]
- 33.Victorson D, Barocas J, Song J, Cella D. Reliability across studies from the functional assessment of cancer therapy-general (FACT-G) and its subscales: a reliability generalization. Qual Life Res. 2008;17(9):1137–46. doi: 10.1007/s11136-008-9398-2. [DOI] [PubMed] [Google Scholar]
- 34.Roth RM, Isquith PK, Gioia GA. behavior rating of executive function-adult version. Lutz, FL: Psychological Assessment Resources, Inc; 2005. [Google Scholar]
- 35.Fazekas F, Kleinert R, Offenbacher H, Payer F, Schmidt R, Kleinert G, et al. The morphologic correlate of incidental punctate white matter hyperintensities on MR images. AJNR Am J Neuroradiol. 1991;12(5):915–21. [PMC free article] [PubMed] [Google Scholar]
- 36.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldmann R, Denecke J, Grenzebach M, Schuierer G, Weglage J. Neurofibromatosis type 1: motor and cognitive function and T2-weighted MRI hyperintensities. Neurology. 2003;61(12):1725–8. doi: 10.1212/01.wnl.0000098881.95854.5f. [DOI] [PubMed] [Google Scholar]
- 38.Hofman KJ, Harris EL, Bryan RN, Denckla MB. Neurofibromatosis type 1: the cognitive phenotype. J Pediatr. 1994;124(4):S1–8. doi: 10.1016/s0022-3476(05)83163-4. [DOI] [PubMed] [Google Scholar]
- 39.Goh WH, Khong PL, Leung CS, Wong VC. T2-weighted hyperintensities (unidentified bright objects) in children with neurofibromatosis 1: their impact on cognitive function. J Child Neurol. 2004;19(11):853–8. doi: 10.1177/08830738040190110201. [DOI] [PubMed] [Google Scholar]
- 40.Holm EA, Esmann S, Jemec GBE. The handicap caused by atopic dermatitis—sick leave and job avoidance. J Eur Acad Dermatol Venereol. 2006;20(3):255–9. doi: 10.1111/j.1468-3083.2006.01416.x. [DOI] [PubMed] [Google Scholar]
- 41.NIMH. NIMH; http://www.nimh.nih.gov/statistics. cited 2013 January 2. [Google Scholar]
- 42.van Os-Medendorp H, Eland-de Kok PC, Grypdonck M, Bruijnzeel-Koomen CA, Ros WJ. Prevalence and predictors of psychosocial morbidity in patients with chronic pruritic skin diseases. J Eur Acad Dermatol Venereol. 2006;20(7):810–7. doi: 10.1111/j.1468-3083.2006.01647.x. [DOI] [PubMed] [Google Scholar]
- 43.Schmitt J, Romanos M, Pfennig A, Leopold K, Meurer M. Psychiatric comorbidity in adult eczema. Br J Dermatol. 2009;161(4):878–83. doi: 10.1111/j.1365-2133.2009.09309.x. [DOI] [PubMed] [Google Scholar]
- 44.Cho CH, Jung SW, Park JY, Song KS, Yu KI. Is shoulder pain for three months or longer correlated with depression, anxiety, and sleep disturbance? J Should Elb Surg/Am Should Elbow Surg. 2013;22(2):222–8. doi: 10.1016/j.jse.2012.04.001. [et al] [DOI] [PubMed] [Google Scholar]
- 45.Gandolphe MC, Nandrino JL, Hancart S, Vosgien V. Autobiographical memory and differentiation of schematic models in substance-dependent patients. J Behav Ther Exp Psychiatry. 2013;44(1):114–21. doi: 10.1016/j.jbtep.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 46.Nahon S, Lahmek P, Durance C, Olympie A, Lesgourgues B, Colombel JF, et al. Risk factors of anxiety and depression in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18(11):2086–91. doi: 10.1002/ibd.22888. [DOI] [PubMed] [Google Scholar]
- 47.Cella DF. Manual of the Functional Assessment of Chronic Illness Therapy (FACIT Scales) Version 4.1. Evanston: Evanston Northwestern Healthcare; 2004. [Google Scholar]
- 48.Freeman AF, Avila EM, Shaw PA, Davis J, Hsu AP, Welch P, et al. Coronary artery abnormalities in Hyper-IgE syndrome. J Clin Immunol. 2011;31(3):338–45. doi: 10.1007/s10875-011-9515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]